3.1. Chemistry. General Procedures
All solvents, chemicals, and reagents were obtained commercially and used without purification. Purity tests of the products was performed by the TLC method on silica gel 60 F254 plates (Merck). Melting points were determined in the capillary tube using melting point apparatus (Stuart Scientific) and are uncorrected. Infrared (IR) spectra were recorded on a FTIR spectrophotometer (8400S, Shimadzu), 1H-NMR and 13C-NMR spectra were recorded on a JEOL JNM 500 spectrometer, using TMS as internal standard, and high resolution mass spectra (HRMS) were measured with a Waters LCT Premier XE (ESI-TOF) system in positive mode.
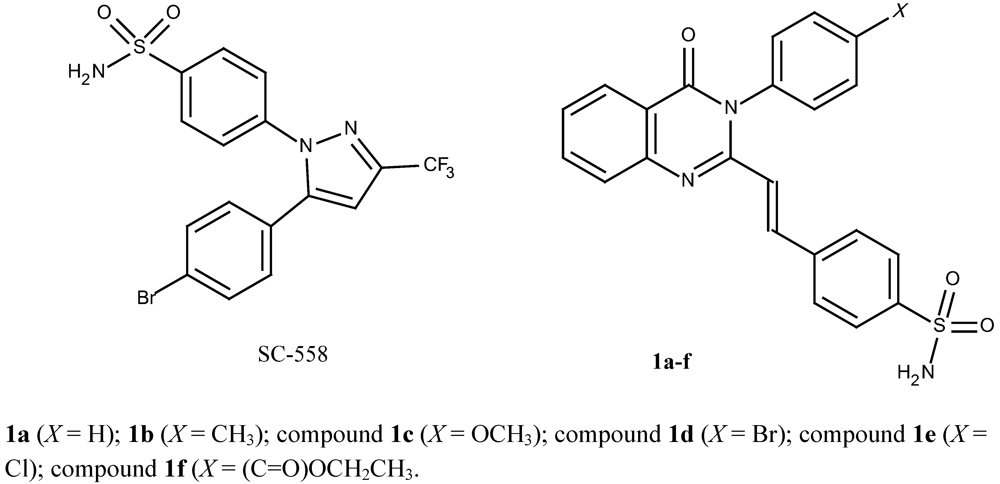
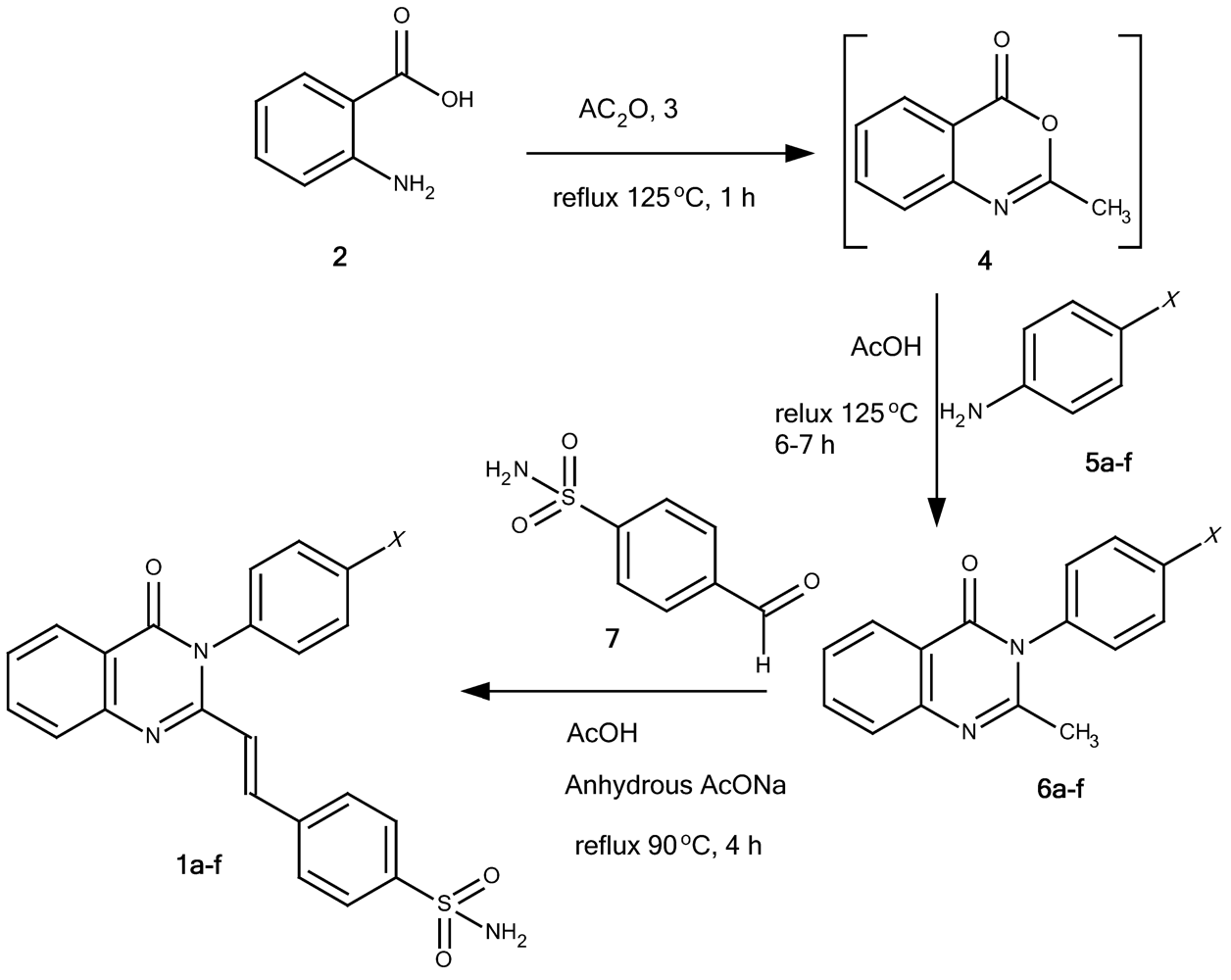
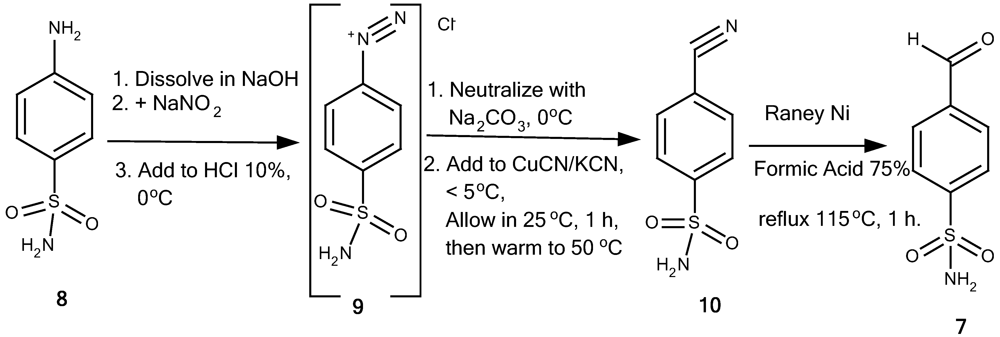
4-[(E)-2-(4-oxo-3-phenyl-3,4-quinazolin-2-yl)ethenyl]benzene-1-sulfonamide (1a): A mixture of 2-methyl-3-phenyl-4(3H)-quinazolinone (6a; 0.98 g, 5 mmol), 4-formylbenzenesulfonamide (7; 1.02 g, 5.5 mmol) and anhydrous sodium acetate (1 g) were dissolved in glacial acetic acid (6 mL) and refluxed at 100 °C. The progress of the reaction was monitored by TLC. After the reaction was completed, the reaction mixture was poured onto cold water and filtered off through a Buchner funnel. The solid product was washed with cold water, recrystallized from suitable solvents and dried in vacuum oven at 85 °C for 1 hour to provide 1a as pale yellow crystalline powder (57.9% yield, recrystallized from acetonitril-water, and washed with cold ethanol), m.p. 277-278 °C. IR (KBr), □max, cm-1: 3,362, 3,234 (primary sulfonamide N-H streching), 3,049 (aromatic/alkene C-H streching), 1,672 (C=O lactam), 1,556 (C=C), 1,340, 1,165 (sulfonamide asymmetric and symmetric SO2 streching). 1H-NMR (DMSO-d6), δ/ppm: 8.16 (1H, dd, J = 7.1, 1.9 Hz, 5-Hquinazolinone), 7.88 (1H, td, J = 7.1, 1.9 Hz, 7-Hquinazolinone), 7.87 (1H, d, J = 15.5 Hz, 2-Htrans ethenyl), 7.80 (1H, d, J = 7.8 Hz, 8-Hquinazolinone) 7.74 (2H, d, J = 8.4 Hz, 2”,6”-HAr), 7.53-7.63 (6H, m, overlap of 6-Hquinazolinone, 3”,5”; 2’,6’; and 4’-HAr), 7.48 (2H, d, J = 8.45 Hz, 3’,5’-HAr), 7.39 (2H, s broad, NH2 sulfonamide), and 6.43 (1H, d, J = 15.5 Hz, 1-Htrans ethenyl). 13C-NMR (DMSO-d6, 125 MHz, TMS), δ/ppm: 161.2 (C(=O)-N), C-4quinazolinone, 151.1 (N-C=N-, C-2quinazolinone ), 147.3 (CPhe-N=C, C-9quinazolinone), 144.5 (C-1”Phe-1”-sulfonamide), 138.0, 137.1, 136.8, 134.9, 130.0, 129.7, 129.3, 127.9, 127.3, 126.9, 126.5, 126.3, 122.6, and 120.8 (C aromatic and C ethenyl). HRESIMS (m/z): found 404.1046 ([M+H]+), calculated masses of C22H18N3O3S: 404.1069 (error 5.7 ppm).
4-[(E)-2-{3-(4-methylphenyl)-4-oxo-3,4-dihydroquinazolin-2-yl}ethenyl]benzene-1-sulfonamide (1b): Compound 1b was prepared as a pale yellow crystalline powder from 2-methyl-3-(4-methylphenyl)-4(3H)-quinazolinone (6b) using the procedure described for 1a (35.8% yield, recrystallized from THF-ethanol, followed by chloroform), m.p. 244-245 °C. IR (KBr), □max, cm-1: 3,329, 3,265 (primary sulfonamide N-H streching), 3,063 (aromatic/alkene C-H streching), 1,691 (C=O lactam), 1,550 (C=C), 1,340, 1,165 (sulfonamide asymmetric and symmetric SO2 streching). 1H-NMR (DMSO-d6) δ/ppm: 8.14 (1H, dd, J = 7.8; 1.3 Hz, 5-Hquinazolinone), 7.90 (1H, td, J = 8.9, 1.9 Hz, 7-Hquinazolinone), 7.89 (1H, d, J = 15.5 Hz, 2-Htrans ethenyl), 7.80 (1H, d, J = 6 Hz, 8-Hquinazolinone), 7.78 (2H, d, J = 8.4 Hz, 2”,6”-HPhe-1”-sulfonamide), 7.56 (1H, td, J = 6.5; 1.3 Hz, 6-Hquinazolinone), 7.53 (2H, d, J = 8.5 Hz, 3”,5”-HPhe-1”-sulfonamide), 7.42 (2H, s, NH2sulfonamide), 7.37 (2H, d, J = 8.45 Hz, 2’,6’-HAr), 7,34 (2H, d, J = 7.8 Hz, 3’,5’-HAr), 6,43 (1H, d, J = 15.5 Hz, 1-Htrans ethenyl), 2,44 (3H, s, CH3-Ar). 13C-NMR (DMSO-d6) δ/ppm: 161.2 ((C(=O)-N), C-4quinazolinone), 151.1 (N-C=N-, C-2quinazolinone), 147.2 (CPhe-N=C, C-9quinazolinone), 144.5 (C-1”Phe-1”-sulfonamide), 138.7, 138.0, 137.1, 136.8, 134.9, 134.8, 130.1, 128.6, 127.9, 127.3, 126.8, 126.3, 122.6, 120.7 (C aromatic and C ethenyl), and 20.4 (CH3-Ar). HRESIMS (m/z): found 418.1237 ([M+H]+), calculated masses of C23H20N3O3S: 418.1225 (error 2.9 ppm).
4-[(E)-2-{3-(4-methoxyphenyl)-4-oxo-3,4-dihydroquinazolin-2-yl}ethenyl]benzene-1-sulfonamide (1c): Compound 1c was prepared from 2-methyl-3-(4-methoxyphenyl)-4(3H)-quinazolinone (6c) using the procedure described for 1a as a pale yellow crystalline powder (45.5% yield, recrystallized from ethanol), m.p. 228-229 °C. IR (KBr), □max, cm-1: 3,365, 3,246 (primary sulfonamide N-H streching), 3,080 (aromatic/alkene C-H streching), 1,675 (C=O lactam), 1,555 (C=C), 1,338, 1,163 (sulfonamide asymmetric and symmetric SO2 streching) and 1,250 (Ar-O-Al ether). 1H-NMR (DMSO-d6) δ/ppm: 8.14 (1H, dd, J = 7.8; 1.3 Hz, 5-Hquinazolinone), 7.88 (1H, td, J = 7.0; 1.9 Hz, 7-Hquinazolinone), 7,90 (1H, d, J = 15.6 Hz, 2-Htrans ethenyl), 7.77-7.80 (3H, overlap, d, J = 8.4 Hz, HAr), 7.55 (1H, t, J = 7.9 Hz, 6-Hquinazolinone), 7.58 (2H, d, J = 8.5 Hz (3”,5”-HPhe-1”-sulfonamide), 7,39 (2H, d, J = 6.5 Hz, (2’,6’-HPhe-4’-OMe), 7.34 (2H, d, J = 7.2 Hz, (3’,5’-HPhe-4’-OMe)), 7,42 (2H, s, NH2sulfonamide), 6,51 (1H, d, J = 15.6 Hz, 1-Htrans ethenyl) and 3.86 (3H, s, CH3O-). 13C-NMR (DMSO-d6) δ/ppm: 161.4 ((C(=O)-N), C-4quinazolinone), 159.5 (C-4’Phe-O), 151.5 (N-C=N-, C-2quinazolinone), 147.3 (CPhe-N=C, C-9quinazolinone), 144.5 (C-1”Phe-1”-sulfonamide), 138.0, 136.9, 134.7, 130.0, 129.2, 127.9, 127.3, 126,8, 126.5, 126,3, 122.7, 120.7, 114.8 (C aromatic and C ethenyl), and 55.4 (methoxy). HRESIMS (m/z): found 434.1176 ([M+H]+), calculated masses of C23H20N3O4S: 434.1175 (error 0.2 ppm).
4-[(E)-2-{3-(4-bromophenyl)-4-oxo-3,4-dihydroquinazolin-2-yl}ethenyl]benzene-1-sulfonamide (1d): Compound 1d was prepared from 2-methyl-3-(4-bromophenyl)-4(3H)-quinazolinone (6d) using the procedure described for 1a as a pale yellow crystalline powder (41.2% yield, recrystallized from ethanol), m.p. 211-212 °C. IR (KBr), □max, cm-1: 3,335, 3,236 (primary sulfonamide N-H streching), 3,095 (aromatic/alkene C-H streching), 1,683 (C=O lactam), 1,556 (C=C), 1,338, 1,165 (sulfonamide asymmetric and symmetric SO2 streching). 1H-NMR (DMSO-d6) δ/ppm: 8.14 (1H, dd, J = 7.8; 1.3 Hz, 5-Hquinazolinone), 7.88 (1H, td, J = 7.0; 1.3 Hz, 7-Hquinazolinone), 7.93 (1H, d, J = 15.0 Hz, 1H, 2-Htrans ethenyl), 7.78-7.83 (5H, m, overlap, HAr), 7.63 (2H, d, J = 8.5 Hz, 2’,6’-H Phe-4’-Br), 7.57 (1H, t, J = 7.8 Hz, 6-Hquinazolinone), 7.48 (2H, d, J = 7.2 Hz, 3’,5’-H Phe-4’-Br), 7.4 (2H, s, NH2 sulfonamide), and 6.5 (1H, d, J = 15.0 Hz, 1-Htrans ethenyl). 13C-NMR (DMSO-d6) δ/ppm: 161.1 ((C(=O)-N), C-4quinazolinone), 150.9 (N-C=N-, C-2quinazolinone), 147.2 (CPhe-N=C, C-9quinazolinone), 144.6 (C-1”Phe-1”-sulfonamide), 137.9, 137.4, 136.1, 134.9, 132.7, 131.3, 128.1, 127.3, 126.9, 126.3, 120.6, 122.5, 122.4 (C aromatic and C ethenyl). HRESIMS (m/z): found 482.0164 ([M+H]+, 95%) and 484.0157 ([M+H]+, 100%), calculated masses of C22H17N3O3SBr: 482.0174 (error 2.1 ppm).
4-[(E)-2-{3-(4-chlorophenyl)-4-oxo-3,4-dihydroquinazolin-2-yl}ethenyl]benzene-1-sulfonamide (1e): Compound 1e was prepared from 2-methyl-3-(4-chlorophenyl)-4(3H)-quinazolinone (6e) using the the procedure described for 1a as a pale yellow crystalline powder (49.5% yield, recrystallized from THF-ethanol, followed chloroform), m.p. 248-249 °C. IR (KBr), □max, cm-1: 3340, 3200 (primary sulfonamide N-H streching), 3066 (aromatic/alkene C-H streching), 1672 (C=O lactam), 1552 (C=C), and 1330, 1166 (sulfonamide asymmetric and symmetric SO2 streching). 1H-NMR (DMSO-d6) δ/ppm: 8.14 (1H, dd, J = 7.8; 1.3 Hz, 5-Hquinazolinone), 7.88 (1H, td, J = 7.8; 1.3 Hz, 7-Hquinazolinone), 7.91 (1H, d, J = 15,0 Hz, 2-Htrans ethenyl), 7.8 (1H, t, J = 8 Hz, 6-Hquinazolinone), 7.79 (2H, d, J = 8.4 Hz, 2”,6”-HPhe-1”-sulfonamide), 7.68 (2H, d, J = 8.5 Hz, 3”,5”H-Phe-1”-sulfonamide), 7.63 (2H, d, J = 7.8 Hz, 2’,6’H-Phe-4’-Cl), 7.54 (2H, d, J = 10 Hz, 3’,5’-HPhe-4’-Cl), 7.4 (2H, s, NH2 sulfonamide), 7.57 (1H, d, J = 7.15 Hz, 8-Hquinazolinone), 6.5 (1H, d, J = 15.0 Hz, 1-Htrans ethenyl). 13C-NMR (DMSO-d6) δ/ppm: 161.2 ((C(=O)-N), C-4quinazolinone), 150.9 (N-C=N-, C-2quinazolinone), 147.2 (CPhe-N=C, C-9quinazolinone), 144.6 (C-1”Phe-1”-sulfonamide), 137.9, 137.4, 135.7, 134.9, 133.3, 130.9, 129.7, 128.1, 127.3, 126.9, 126.5, 126.3, 120.7 (C aromatic and C ethenyl). HRESIMS (m/z): found 438.0674 ([M+H]+, 100%), 440,0647 ([M+H]+, 40%), calculated masses of C22H17N3O3SCl: 438,0679 (error 1.1 ppm).
Ethyl 4-{4-okso-2-[(E)-2-(4-sulfamoylphenyl)ethenyl]-3,4-dihydroquinazolin-3-yl}benzoate (1f): Compound 1f was prepared from ethyl 4-(2-methyl-4-oxo-3,4-dihydroquinazolin-3-yl)benzoate (6f) using the procedure described for 1a as a pale yellow crystalline powder (50,2% yield, recrystallized from THF-ethanol, and washed with cold ethanol), m.p. 239-240 °C. IR (KBr), □max, cm-1: 3,331, 3,219 (primary sulfonamide N-H streching), 3,100 (aromatic/alkene C-H streching), 2,960-1,983 (alipatic C-H streching), 1,701 (C=O ester), 1,654 (C=O lactam), 1,556 (C=C), and 1,340, 1,165 (sulfonamide asymmetric and symmetric SO2 streching). 1H-NMR (DMSO-d6) δ/ppm: 8.15 (1H, dd, J = 8.4; 1.3 Hz, 5-Hquinazolinone), 8.17 (2H, d, J = 8.5 Hz, 2’,6’-Phe-1’-COOEt), 7.93 (1H, d, J = 15.5 Hz, 2-Htrans ethenyl), 7.91 (1H, td, J = 7.1; 1.9 Hz, 7-Hquinazolinone), 7.81 (1H, d, J = 7.8 Hz, 8-Hquinazolinone), 7.76 (2H, d, J = 8.5 Hz, 3”,5”-H-Phe-4”-sulfonamide), 7.66 (2H, d, J = 6.5 Hz, 3’,5’-HPhe-1’-COOEt), 7.61 (2H, d, J = 7.8 Hz, H-2”,6”-Phe-4”-sulfonamide ), 7.57 (1H, t, J = 8.5 Hz, 6-Hquinazolinone), 7.39 (2H, s, NH2 sulfonamide), 6.45 (1H, d, J = 15.5 Hz, 1-Htrans ethenyl). 13C-NMR (DMSO-d6) δ/ppm: 165.1 (C=O ester), 161.1 (C(=O)-N), C-4quinazolinone), 150.6 (N-C=N-, C-2quinazolinone), 147.2 (CPhe-N=C, C-9quinazolinone), 144.6 (C-1’-Phe-1’-COOEt), 141.0 (C-4”Phe-4”-sulfonamide), 137.6, 137.5, 134.9, 130.5, 130.4, 129.6, 128.1, 127.3, 127.0, 126.5, 126.2, 120.6 (C aromatic and C ethenyl), 61.1 (CH2-O-) and 14.1 (CH3-C). HRESIMS (m/z): found 476.1278 ([M+H]+), calculated masses of C25H22N3O5S: 476.1280 (error 0.4 ppm).