Synthesis and Biological Screening of 4-Benzyl-2H-phthalazine Derivatives
Abstract
: Preparation of 4-benzyl-2-substituted phthalazin-1-one derivatives 2-8 is reported. Condensation of 4-benzyl-1-chlorophthalazine (9) with a series of different nucleophiles gave 4-benzylphthalazin-1-ylamino derivatives (10-13 and 16) and 4-amino-2-[N′-(4-benzylphthalazin-1-yl)-hydrazino]-6-arylpyrimidine-5-carbonitriles (14a,b). Interaction of 9 with ambident anions was also studied. 5-Benzyl-6,6a,12-triazobenzo[a]-anthracen-7-one (15) is obtained from 9 and anthranilic acid derivatives. Treatment of 16 with (EtO)3CH/Ac2O under reflux afforded the corresponding ethoxymethylene derivative 17, while aqueous ammonium hydroxide treatment afforded carboxamide derivative 18. The structures of the newly synthesized derivatives were confirmed by their elemental analysis, IR, 1H NMR, 13C NMR and mass spectral studies. Antimicrobial activities of some selected compounds were also studied and some of these were found to exhibit promising effects against Gram-positive and Gram-negative bacteria and fungi.1. Introduction
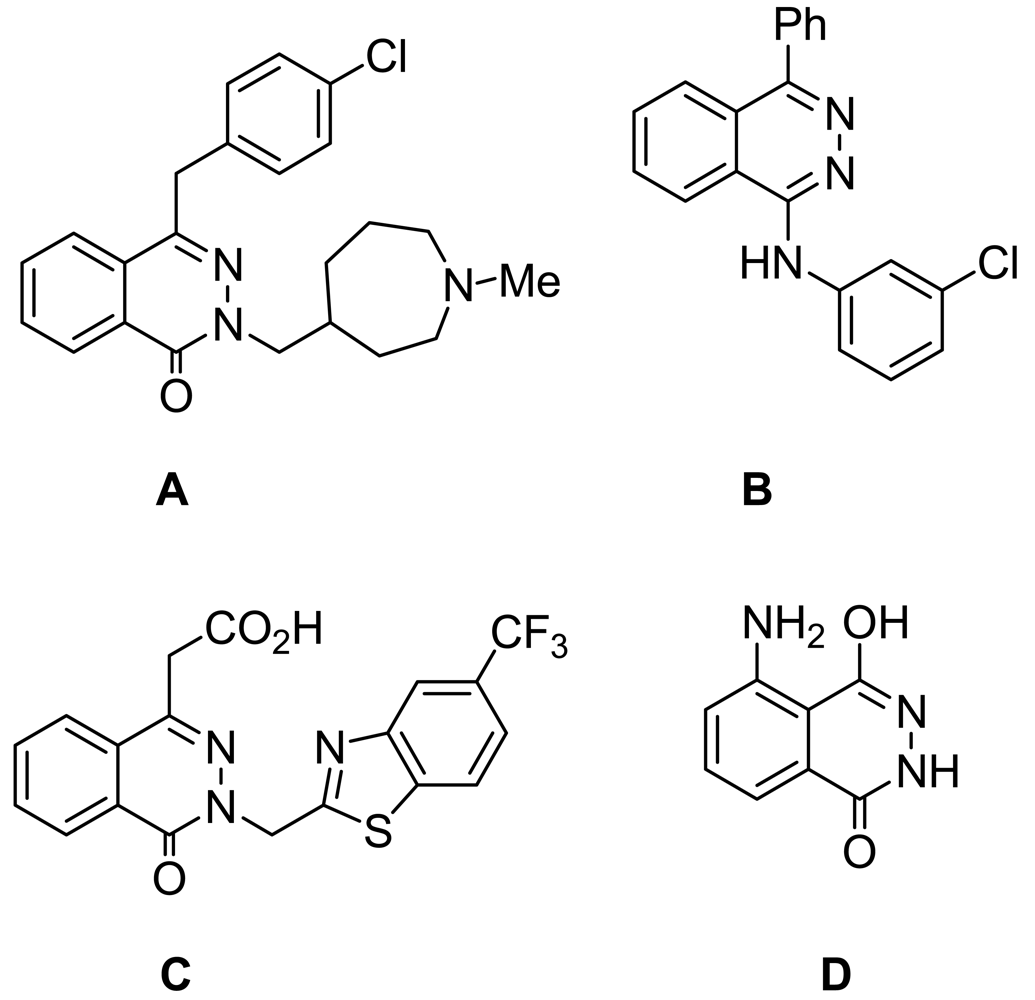
The phthalazine derivative azelastine (A, Figure 1) is an antihistamine used in the treatment of allergic rhinitis [1]. Newer agents are more selective inhibitors of the cGMP-inhibited phosphor diesterase (PDE) and casn be exemplified by phthalazine derivatives like MY5445 (B, Figure 1) [2-5]. Zopolrestat (C, Figure 1) is a phthalazinone derivative that has been in clinical trials; it inhibits aldose reductase and has potential use in the prevention of retinopathy, neuropathy, and cataract formation in diabetes [6]. The chemiluminescence reactions of luminol (D, Figure 1) and related phthalazines have found analytical applications, particularly in biological systems where the inherent signal strength and low signal noise ratio contribute to sensitivity. The hydrogen peroxide/luminol system has been used for the on-line determination by chemiluminescence of nitric oxide in isolated organ perfusates [7-10].
Phthalazine derivatives have been widely applied as therapeutic agents due to their anticonvulsant, cardiotonic, vasorelaxant and anti-inflammatory properties [11-17] in addition to having antimicrobial activity [18].
2. Results and Discussion
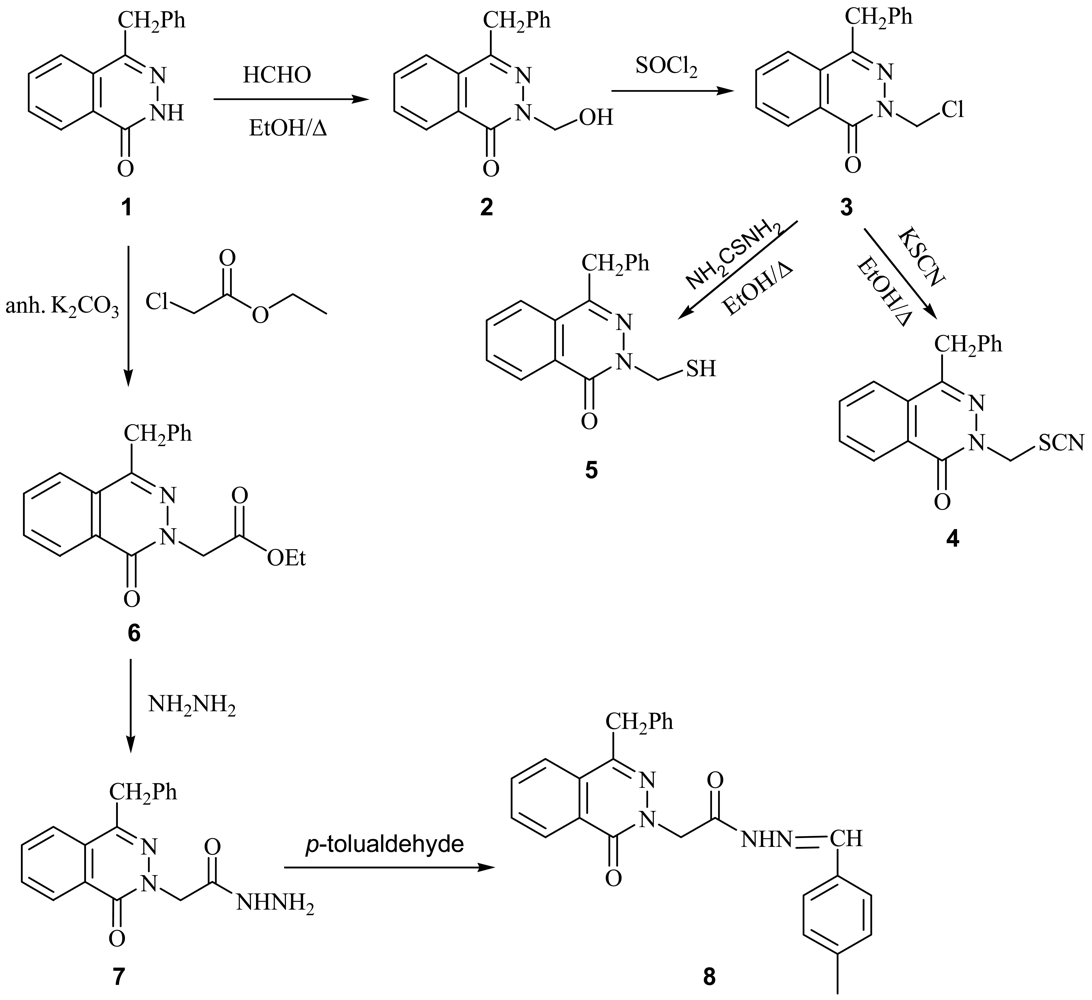
4-Benzyl-2H-phthalazin-1-one (1) [19] was used as a starting material for this investigation. Reaction of compound 1 with formalin in ethanol afforded 4-benzyl-2-hydroxymethyl-2H-phthalazin-1-one (2). Treatment of 2 with thionyl chloride afforded the corresponding 4-benzyl-2-chloromethyl-2H-phthalazin-1-one (3). Interaction of 3 with alcoholic potassium thiocyanate yield the corresponding 4-benzyl-2-thiocyanatomethyl-2H-phthalazin-1-one (4), while with ethanolic thiourea it afforded 4-benzyl-2-mercaptomethyl-2H-phthalazin-1-one (5). Treatment of 1 with ethyl chloroacetate in the presence of anhydrous K2CO3 afforded the corresponding ethyl (2-(4-benzyl-1-oxo-1H-phthalazin-2-yl)acetate (6). Reaction of 6 with hydrazine hydrate gave the corresponding (4-benzyl-1-oxo-1H-phthalazin-2-yl)acetic acid hydrazide (7) which was reacted with p-tolualdehyde to give the corresponding (4-benzyl-1-oxo-1H-phthalazin-2-yl) acetic acid (4-methylbenzylidene) hydrazide (8) (Scheme 1).
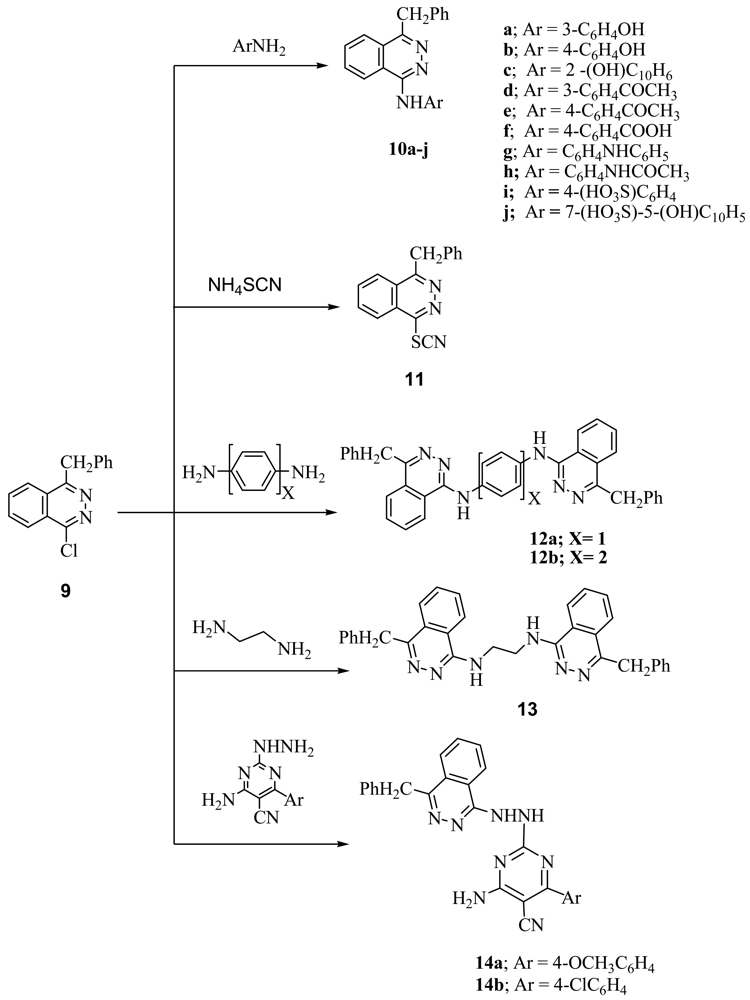
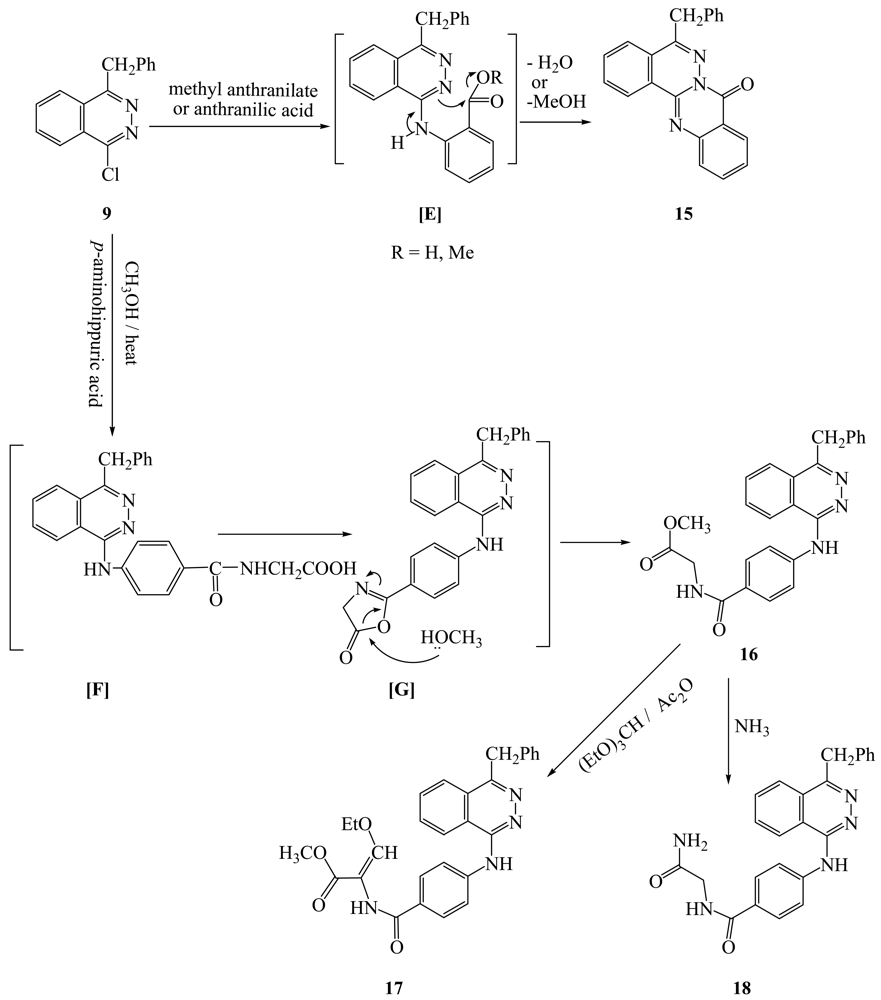
4-Benzyl-1-chlorophthalazine (9) [19] was used as starting martial from preparation of new 1,4-disubstuted phthalazine derivatives. Thus, interaction of 9 with equimolar amount of aminophenols, aminoacetophenones and p-aminobenzoic acid gave the corresponding 4-benzylphthalazin-1-ylamino derivatives (10a-f). Compound 9 also reacted with N-(4-aminophenyl)acetanilide under reflux to give the corresponding 4-(4-benzylphthalazin-1-ylamino)acetanilide (10g), in the case of p-phenylene-diamine and/or benzidine but in the case of p-aminodiphenyl amine the only isolable product is 4-(4-benzylphthalazin-1-ylamino)diphenylamine (10h). Treatment of 9 with sulfanilic acid and/or J-acid (2-amino-5-naphthol-7-sulfonic acid) gave the corresponding 4-(4-benzylphthala-zin-1-ylamino)benzenesulfonic acid (10i) and/or 7-(4-benzylphthalazin-1-ylamino)-4-hydroxynaphthalene-2-sulfonic acid (10j), while 9 with ammonium thiocyanate gave the corresponding 4-benzylphthalazin-1-ylthiocyanate (11). Interaction of 9 with dibasic aromatic amines in a 1:2 ratio under fusion conditions afforded the corresponding N,N′-bis(4-benzyl-phthalazin-1-yl)-1,4-phenylenediamine and/or N,N′-bis(4-benzylphthalazin-1-yl)biphenyl-4,4′-diamines (12a,b). Also, 9 was reacted with ethylenediamine in boiling ethanol to give N,N′-bis(4-benzylphthalazin-1-yl)ethane-1,2-diamine (13), However, interaction of 9 with 2-hydrazino-4-amino-5-cyano-6-arylpyrimidine gave the corresponding pyrimidine-5-carbonitrile derivatives 14a,b respectively (Scheme 2). Interaction of 9 with methyl anthranilate and/or anthranilic acid gave anthracen-7-one derivative 15 as the only isolable product. The formation of 15 was explained by the formation of intermediate [E] which undergoes intramolecular ring closure to the final product 15 (Scheme 3).
Treatment of 9 with p-aminohippuric acid in boiling methanol/TEA, gave the corresponding methyl ester derivative 16 was obtained as the only isolable product. The formation of 16 can be explained by the nucleophilic transformation into benzylphthalazin-1-ylamino derivative [F] as intermediate which undergoes intramolecular cyclization into oxazolone intermediate [G] then reacted with a molecule of methanol to give the methyl ester derivative 16 as the final product (Scheme 3).
Treatment of 16 with (EtO)3CH/Ac2O under reflux afforded the corresponding ethoxymethylene derivative 17. Treatment of 16 with aqueous ammonium hydroxide under reflux in dioxane in the presence of triethylamine afforded the corresponding carboxamide derivative 18 respectively (Scheme 3).
3. Experimental
3.1. General
Melting points were determined on a Stuart melting point apparatus and are uncorrected. IR spectra were recorded in KBr using a FT-IR 5300 spectrometer and Perkin Elmer spectrum RXIFT-IR system (ν, cm−1). The 1H NMR at (300 MHz) and 13C NMR spectra (75 MHz) were recorded in CDCl3 or DMSO-d6 on a Varian Mercury VX-300 NMR spectrometer. Chemical shifts (δ) are related to that of the solvent. Mass spectra were measured on a Shimadzu GMMS-QP-1000 EX mass spectrometer at 70 eV. The elemental analyses were performed at the Microanalytical Center, Cairo University. Cairo (Egypt).
4-Benzyl-2-hydroxymethyl-2H-phthalazin-1-one (2). A mixture of compound 1 (0.23 g, 1.0 mmol) and formaldehyde solution 38% (0.8 mL) in ethanol (30 mL) was refluxed for 3 hours. The solvent was evaporated under vacuum, and then water (25 mL) was added. The solid obtained was filtered off and recrystallized from ethanol. Colourless crystals, 90%, 0.19 g. mp 115-116 °C; IR (νmax, cm−1): 3334 (OH), 1640 (CO). 1H NMR (CDCl3): δH 4.13 (t, J = 8.4 Hz, 1H, OH), 4.31 (s, 2H, CH2Ph), 5.69 (d, J = 8.4 Hz, 2H, CH2OH), 7.30 (m, 5H, Ph-H), 7.71 (m, 3H, phthalazinyl-3H), 8.45 (m, 1H, phthalazinyl-1H); the singlet at 4.134 was cancelled by D2O. 13C NMR (DMSO-d6) δ 37.3 (CH2), 60.1 (CH2OH), 125.7 (C4-Ar), 128 (C5), 128.3 (C8), 128.8 (C3-Ar, C5-Ar), 129.1 (C2-Ar, C6-Ar), 130.2 (C5a), 130.3 (C8a), 131.2 (C7), 132.3 (C6), 137.2 (C1-Ar), 155.1 (C4) and 160.1 (CO). MS, m/z (%) = 266 (M+, 10.25), 235 (100) together with other peaks at 207 (5.74), 178 (20.38), 149 (15.82), 130 (9.19), 91 (12.03), 57 (17.77); Anal. Calcd for C16H14N2O2 (266.10): C, 72.16; H, 5.30; N, 10.52%. Found: C, 71.89; H, 5.02; N, 9.96%.
4-Benzyl-2-chloromethyl-2H-phthalazin-1-one (3). A mixture of compound 2 (0.26 g, 1.0 mmol) and thionyl chloride (5 mL) was refluxed for 1 hour. The solid obtained was filtered off and recrystallized from benzene. Colourless crystals, 87%, 0.12 g. mp 125-127 °C; IR (νmax, cm−1): 3046 (C-H aromatic), 1662 (CO). MS, m/z (%) = 284 (M+, 43.4), M+2 (12.7), 249 (100) together with other peaks at 220 (34.9), 178 (17.9), 130 (29.2), 91 (80.7), 51 (28.9); Anal. Calcd for C16H13ClN2O (284.07): C, 67.49; H, 4.60; N, 9.84%. Found: C, 66.90; H, 4.08; N, 9.11%.
4-Benzyl-2-thiocyanatomethyl-2H-phthalazin-1-one (4). A mixture of compound 3 (0.28 g, 1.0 mmol) and potassium thiocyanate (1.0 mmol) in ethanol (30 mL) was refluxed for 3 hours. The solvent was evaporated under vacuum. The solid obtained was filtered off and recrystallized from ethanol. White crystals, 85%, 0.17 g. mp 155-156 °C; IR (νmax, cm−1): 2158 (SCN), 1664 (CO). MS, m/z (%) = 307 (M+, 0.7), 249 (56.3), 130 (12.8) and 91 (100); Anal. Calcd for C17H13N3OS (307.36): C, 66.43; H, 4.26; N, 13.67%. Found: C, 66.22; H, 3.98; N, 13.50%.
4-Benzyl-2-mercaptomethyl-2H-phthalazin-1-one (5). A mixture of compound 3 (0.28 g, 1.0 mmol) and thiourea (1.0 mmol) in ethanol (30 mL) was refluxed for 3 h hours. The solvent was evaporated under vacuum. The solid obtained was filtered off and recrystallized from ethanol. White crystals, 80%, 0.10 g. mp 145-146 °C; IR (νmax, cm−1): 2750 (SH), 1650 (CO). MS, m/z (%) = 282 (M+, 17.2), 249 (78.2), 235 (18.1), 132 (27.3), 130 (23.5), 91 (100) 51 (32.8); Anal. Calcd for C16H14N2OS (282.36): C, 68.06; H, 5.00; N, 9.92%. Found: C, 67.45; H, 4.67; N, 9.22%.
Ethyl (4-benzyl-1-oxo-1H-phthalazin-2-yl)acetate (6). A mixture of compound 1 (0.23 g, 10 mmol) and ethyl chloroacetate (2 mL) and anhydrous K2CO3 (0.13 g, 1.0 mmol) was refluxed for 4 hours. The solvent was evaporated under vacuum, then water (50 mL) was added. The solid obtained was filtered off and recrystallized from pet. ether and chloroform, respectively. Violet crystals, 65%, 0.13 g. mp 76-78 °C; IR (νmax, cm−1): 2978 (CH aliphatic), 1744, 1648 (CO). MS, m/z (%) = 322 (M+, 37.9) and base peak at 248 together with other peak at 249 (97.9), 221 (8.2), 220 (21.5), 219 (24.3), 193 (4.6), 102 (14.6), 91 (98.4), 76 (11.4); Anal. Calcd for C19H18N2O3 (322.12): C, 70.79; H, 5.63; N, 8.69%. Found: C, 70.36; H, 5.44; N, 8.52%.
(4-Benzyl-1-oxo-1H-phthalazin-2-yl)acetic acid hydrazide (7). A mixture of compound 6 (0.32 g, 10 mmol) and hydrazine hydrate (0.8 mL) in ethanol (30 mL) was refluxed for 3 hours. The solvent was evaporated under vacuum, then water (25 mL) was added. The solid obtained was filtered off and recrystallized from ethanol. White crystals, 86%, 0.18 g. mp 130-132 °C; IR (νmax, cm−1): 3326 (NH), 1684 (CO, carboxylic acid hydrazide), 1642 (CO, phthalazinyl). 1H NMR (CDCl3): δH 14.29 (s, 2H, CH2Ph), 4.75 (s, 2H, NCH2CO), 7.29 (m, 5H, Ar-H), 7.81 (m, 5H, phthalazinyl-3H and NH2), 8.25 (m, 1H, phthalazinyl-H) and 9.30 (s, 1H, CONH; cancelled by D2O). MS, m/z (%) = 308 (M+, 0), 278 (7.2), 277 (23.4), 249 (44.9), 130 (15.3), 91 (100); Anal. Calcd for C17H16N4O (308.33): C, 66.22; H, 5.23; N, 18.17%. Found: C, 66.09; H, 5.12; N, 17.89%.
3.2. General Procedure for the Synthesis of Benzylphthalazin-1-ylamino Derivatives 10a-h
A mixture of compound 9 (0.25g, 10 mmol) and aromatic amine (10 mmol) in ethanol (30 mL) was refluxed for 3 hours. The solvent was evaporated under vacuum. The solid obtained was filtered off to give crude products
(4-Benzylphthalazin-1-ylamino)phenols (10a,b). Compound 10a Yellow crystals, 90%, 0.17 g. mp 240-242 °C; IR (νmax, cm−1): 3258 (NH and OH phenolic) as broad peak. MS, m/z (%) = 307 327 (M+, 42.2) and the base peak at 326 together with other peaks at 325 (84.3), 91 (62.6); Anal. Calcd for C21H17N3O (327.10): C, 77.04; H, 5.23; N, 12.84%. Found: C, 76.83; H, 5.02; N, 12.56%. Compound 10b: Yellow crystals, 80%, 0.12 g. mp 236-237 °C; IR (νmax, cm−1): 3268 (NH and OH phenolic) as broad peak. MS, m/z (%) = 327 (M+, 40.0) and the base peak at 326 together with other peaks at 325 (86.7), 91 (50.8); Anal. Calcd for C21H17N3O (327.10): C, 77.04; H, 5.23; N, 12.84%. Found: C, 76.75; H, 5.01; N, 12.59%.
7-(4-Benzylphthalazin-1-ylamino)naphthalen-2-ol (10c). Green crystals, 70%, 0.09 g. mp 242-243 °C; IR (νmax, cm−1): 3272 (OH and NH) as broad peak. 1H NMR (DMSO-d6): δH 24.65 (s, 2H, CH2Ph), 7.08-7.33, 7.55-7.58, 8.09-8.11 (ms, 9H, Ar-H), 7.38 (d, J = 7.8 Hz, 1H, Ar-H), 7.78 (d, J = 9 Hz, 1H, Ar-H), 7.86 (d, J = 9 Hz, 1H, Ar-H), 8.14 (d, J = 1.8 Hz, 1H, Ar-H), 8.16 (s, 1H, NH, exchangeable with D2O), 8.33 (d, J = 1.8 Hz, 1H, Ar-H), 9.04 (d, J = 7.8 Hz, 1H, Ar-H) and 9.96 (s, 1H, phenolic-OH, exchangeable with D2O).; Anal. Calcd for C25H19N3O (377.13): C, 79.55; H, 5.07; N, 11.13%. Found: C, 79.32; H, 4.87; N, 10.92%.
1-[(4-Benzylphth-alazin-1-ylamino)phenyl]ethanones (10d,e). Compound 10d: White crystals, 75%, 0.11 g. mp 225-226 °C; IR (νmax, cm−1): 3390 (NH), 1662 (CO). MS, m/z (%) = 353 (73.56) and the base peak at 352 together with other peaks at 311 (3.7), 310 (11.97), 262 (10.56), 220 (5.37), 219 (4.82) and 91 (54.14); Anal. Calcd for C23H19N3O (353.05): C, 78.16; H, 5.42; N, 11.89%. Found: C, 77.88; H, 5.23; N, 11.64%. Compound 10e: Yellow crystals, 80%, 0.13 g. mp 260-261 °C; IR (νmax, cm−1): 3312 (NH), 1670 (CO). 1H NMR (DMSO-d6): δH 2.57 (s, 3H, CH3CO), 4.75 (s, 2H, CH2Ph), 7.21-7.27 (m, 2H, Ar-H), 7.31 and 7.40 (AB-q, J = 7.2 Hz, 4H, Ar-H), 8.01 (s, 1H, NH), 8.10-8.18 (m, 5H, Ar-H) and 8.39, 8.94 (2d, J = 7.8 Hz, 2H, Ar-H); Anal. Calcd for C23H19N3O (353.23): C, 78.18; H, 5.42; N, 11.89%. Found: C, 77.97; H, 5.18; N, 11.69%.
4-(4-Benzylphthalazin-1-ylamino)benzoic acid (10f). Yellow crystals, 85%, 0.16 g. mp 280-281 °C; IR (νmax, cm−1): 3224 (NH and OH), 1676 (CO). MS, m/z (%) = 355 (38.4) and base peak at 354 together with other peaks at 310 (3), 234 (5.3), 204 (9.9), 219 (6.9), 204 (9.9), 178 (10.1), 102 (15.9), 91 (68.3), 90 (60.2), 77 (30.6) and 76 (29.7); Anal. Calcd for C22H17N3O2 (355.16): C, 74.35; H, 4.82; N, 11.82%. Found: C, 74.14; H, 4.57; N, 11.60%.
4-(4-Benzylphthalazin-1-ylamino)diphenyl amine (10g). Green crystals, 70%, 0.13 g. mp 215-217 °C; IR (νmax, cm−1): 3276 (NH). MS, m/z (%) = 402 (M+, 46.5), 117 (88.7), 92 (60.32), 76 (28.37), 91 (18.88), 57 (87.49), 56 (29.88) & 55 (100); Anal. Calcd for C27H22N4 (402.02): C, 80.57; H, 5.51; N, 13.92%. Found: C, 80.44; H, 5.49; N, 13.75%.
4-(4-benzylphthalazin-1-ylamino)acetanilide (10h). Yellow crystals, 80%, 0.18 g. mp > 340 °C; IR (νmax, cm−1): 3428 (NH), 1620 (CO). MS, m/z (%) = 368 (M+, 12.94), 235 (10.25), 149 (88.35), 117 (78.7), 92 (63.32), 76 (28.37), 91 (18.88), 57 (87.49), 56 (29.88) and 55 (100); Anal. Calcd for C23H20N4O (368.17): C, 74.98; H, 5.47; N, 15.21%. Found: C, 74.80; H, 5.32; N, 15.12%.
3.3. General Method for the Synthesis of Sulfonic Acid Derivatives 10i, j
A mixture of compound 9 (0.25 g, 1.0 mmol) in ethanol (30 mL) sulfanilic acid or (2-amino-5-naphthol-7-sulfonic acid) (1.0 mmol) and 2 drops of triethylamine (TEA) was refluxed for 3 hours. The solvent was evaporated under vacuum. The solid obtained was filtered off and recrystallized from dioxane.
4-(4-Benzylphthalazin-1-ylamno)benzenesulfonic acid (10i). Yellow crystals, 80%, 0.17 g. mp > 340 °C; IR (νmax, cm−1): 3402 (OH), 3256 (NH). MS, m/z (%) = 391 (M+, 4.7), 327 (18.12), 311 (72.15), 310 (95.64), 235 (40.60), 219 (89.60), 193 (100), 92 (57.72) and 91 (16.78). Anal. Calcd for C21H17N3O3S (391.09): C, 64.43; H, 4.38; N, 10.73%. Found: C, 64.36; H, 4.28; N, 10.65%.
7-(4-benzylphthalazin-1-ylamino)-4-hydroxynaphthalene-2-sulfonic acid (10j). Yellow crystals, 65%, 0.13 g. mp > 340 °C; IR (νmax, cm−1): 3308 (OH), 3066 (NH). MS, m/z (%) = 457 (M+, 5.3), 455 (naphthsaltone cationic radical, 59.1), 222 (5.1), 235 (100), 203 (6.4), 193 (3.6) and 178 (15.8); Anal. Calcd for C25H19N3O4S (457): C, 65.63; H, 4.19; N, 9.18%. Found: C, 65.54; H, 3.97; N, 9.10%.
Synthesis of N,N′-bis(4-benzylphthalazin-1-yl)-1,4-phenylenediamine (11) and N,N′-bis(4-benzylphthalazin-1-yl)biphenyl-4,4′-diamine (12). A mixture of compound 9 (0.50 g, 20 mmol) and dibasic aromatic amines (10 mmol) in ethanol (30 mL) was refluxed for 3 hours. The solvent was evaporated under vacuum. The solid obtained was filtered off and recrystallized from dioxane. Compound 11 Yellow crystals, 70%, 0.13 g. mp 250-251 °C; IR (νmax, cm−1): 3344 (NH). MS, m/z (%) = 544 (M+, 0.74) and base peak at 325 together with other peaks at 234 (5.7), 219 (3.29), 106 (1.74), 91 (13.66), 76 (1.25); Anal. Calcd for C36H28N6 (544.27): C, 79.39; H, 5.18; N, 15.43%. Found: C, 79.08; H, 5.06; N, 15.22%. Compound 12: Yellow crystals, 70%, 0.12 g. mp > 340 °C; IR (νmax, cm−1): 3277 (NH). MS, m/z (%) = 620 (M+, O), M/2 (310, 9.3); the base peak at 401 and other peaks at 102 (1.8), 91 (17.1); Anal. Calcd for C42H32N6 (620.13): C, 81.27; H, 5.20; N, 13.54%. Found: C, 80.96; H, 5.04; N, 13.30%.
N,N′-bis(4-benylphthalazin-1-yl)ethane-1,2-diamine (13). A mixture of compound 9 (0.50 g, 20 mmol) and ethylenediamine (10 mmol) in absolute ethanol (30 mL) anhydrous K2CO3 was refluxed for 4 hours. The obtained was cooled to room temperature, then diluted with water. The solid obtained was filtered off and recrystallized from ethanol. White crystals, 60%, 0.21 g. mp 234-235 °C; IR (νmax, cm−1): 3316, 3260 (NH) 2930, 2870 (C-H aliphatic). 1H NMR (DMSO-d6): δH 3.93 (s, 4H, CH2-CH2), 4.48 (s, 4H, 2CH2Ph), 7.14-7.32 (m, 10H, Ar-H), 7.78-7.28 (m, 4H, Ar-H), 7.93-7.90 (br s, 2H, NH; exchangeable with D2O), 8.00-8.021(m, 2H, Ar-H) and 8.26-8.29 (m, 2H, Ar-H); Anal. Calcd for C32H28N6 (496.60): C, 77.39; H, 5.68; N, 16.92%. Found: C, 76.29; H, 5.45; N, 16.65%.
3.4. General Method for the Synthesis of Hydrazino Pyrimidine Derivatives 14a,b
A mixture of compound 9 (0.25 g, 10 mmol) and 2-hydrazino-4-amino-5-cyano-6-arylpyrimidine (10 mmol) in n-butanol (30 mL) was refluxed for 3 hours. The solvent was evaporated under vacuum. The solid obtained was filtered off and recrystallized from dioxane.
4-Amino-2-[N′-(4-benzylphthalazin-1-yl)hydrazino]-6-(4-methoxyphenyl)-pyrimidine-5-carbonitrile (14a). White crystals, 75%, 0.16 g. mp 294-295 °C; IR (νmax, cm−1): 3450, 3210 (NH), 2206 (CN), 1634 (C=N). 1H NMR (DMSO-d6): δH 3.84 (s, 3H, OCH3), 4.58 (s, 2H, CH2Ph), 7.10 and 7.13 (2d, J = 8.4 Hz), 2H, Ar-H), 7.17 and 7.22 (2d, J = 7.8 Hz, 2H, Ar-H), 7.37 and 7.57 (2d, J = 7.8 Hz, 4H, Ar-H), 8.18 and 8.46 (2d, J = 8.1 Hz, 2H, Ar-H), 8.684 (s, 1H, Ar-H), 6.99 and 7.88 (m, 2H, Ar-H), 6.250 (br s, 2H, NH2), 8.90 (br s, 2H, 2-NH); Anal. Calcd for C27H22N8 (474.02): C, 68.34; H, 4.67; N, 23.612%. Found: C, 68.34; H, 4.55; N, 23.54%.
4-Amino-2-[N′-(4-benzylphthalazin-1-yl)hydrazino]-6-(4-chorophenyl)pyramidine-5-carbonitrile (14b). White crystals, 80%, 0.19 g. mp 274-275 °C; IR (νmax, cm−1): 3380, 3270 (NH), 2192 (CN), 1638 (C=N). MS, m/z (%) = 478 (M+, and M+2), 462 (19.22), 368 (14.61), 236 (12.68), 275 (75.31), 138 (36.35), 128 (46.12), 111 (29.49), 91(100); Anal. Calcd for C26H19ClN8 (478.01): C, 65.20; H, 4.00; N, 23.40%. Found: C, 65.08; H, 3.87; N, 23.29%.
5-Benzyl-6,6a,12-triazobenzo[a]anthracen-7-one (15). A mixture of compound 9 (0.25 g, 10 mmol) and methyl anthranilate or anthranilic acid (10 mmol) in absolute ethanol (30 mL) and 2 drops of triethylamine (TEA) was refluxed for 3 hours. The solvent was evaporated under vacuum. The solid obtained was filtered off and recrystallized from dioxane. Yellow crystals, 80%, 0.16 g. mp 176-178 °C; IR (νmax, cm−1): 1704 (CO); MS, m/z (%) = 337 (M+, 66.92), 336 (100) and 91 (13.09); Anal. Calcd for C22H15N3O (337.12): C, 78.32; H, 4.48; N, 12.46%. Found: C, 78.19; H, 4.37; N, 12.30%.
[4-(4-Benzylphthalazin-1-ylamino)benzoylamino]acetic acid methyl ester (16). A mixture of compound 9 (0.25 g, 10 mmol) and p-aminohippuric acid (10 mmol) in methanol (30 mL) and three drops of TEA was refluxed for 3 hours. The solvent was evaporated under vacuum. The solid obtained was filtered off and recrystallized from dioxane. Yellow crystals, 90%, 0.18 g. mp 165-167 °C; IR (νmax, cm−1): 3110, 3328 (NH), 1752 (CO), 1642 (CONH). 1H NMR (DMSO-d6): δH 3.67 (s, 3H, OCH3), 3.95 (d, J = 5.4 Hz), 1H, non equivalent CH2 protons), 4.04 (d, J = 5.4 Hz), 1H, non equivalent CH2 protons), 4.73 (s, 2H, PhCH2), 7.88 and 8.006 (2d, J = 8.7 Hz, 2H, Ar-H), 8.44 (d, J = 7.8 Hz, 1H, Ar-H), three sets of multipletes at (7.24-7.30), (8.14-8.28), (8.93-9.01) (6H, Ar-H and NH), 7.33 and 7.41 (AB-q, J = 7.2Hz, 4H, Ar-H), 10.80 (hump, 1H, NH, exchangeable by D2O).; MS, m/z (%) = 426 (M+, 48.2), 425 (100), 338 (27.8), 310 (5.4), 219 (2.7), 128 (5.4) and 91 (37.2).; Anal. Calcd for C15H22N4O3 (426.46): C, 70.41; H, 5.20; N, 13.14%. Found: C, 70.25; H, 5.07; N, 12.87%.
Methyl 2-(4-(4-benzylphthalazin-1-ylamino)benzamido)-3-ethoxyacrolate (17). A mixture of compound 16 (0.42 g, 1.0 mmol) and triethyl orthoformate (0.14 g, 1.0 mmol) in acetic anhydride (10 mL) was refluxed for 5 hours, and then allowed to cool at room temperature and diluted with water (20 mL). The solid obtained was filtered off and recrystallized from dioxane Yellow crystals, 60%, 0.17 g. mp 175-177 °C; IR (νmax, cm−1): 3060 (C-H olefinic) 3366 (NH), 2942 (C-H aliphatic), 1752, 1666 (CO). 1H NMR (DMSO-d6): δH 2.08 (t, 3H, CH2CH3), 3.64 (s, 3H, COOCH3), 4.01 (q, 2H, CH2CH3), 4.75 (s, 2H, CH2Ph), (7.17-8.38) (sets of multiplets, 15H, Ar-H and 2NH), 8.97 (s, 1H, =CH); Anal. Calcd for C28H26N4O4 (482.52): C, 69.70; H, 5.43 N, 11.49%. Found: C, 69.63; H, 5.23; N, 11.49%.
2-(4-(4-Benzylphthalazin-1-ylamino)benzamido)acetamide (18). To compound 16 (0.42 g, 1.0 mmol) and distilled water and/or ammonium hydroxide, two drops of triethylamine (TEA) were added, and the mixture was refluxed in dioxane (15 mL) for 2 hours. The solvent was evaporated under vacuum. The solid obtained was filtered off and recrystallized from dioxane. Yellow crystals, 75%, 0.19 g. mp 240-242 °C; IR (νmax, cm−1): 3272, 3354(NH), 1734, 1640 (CO); MS, m/z (%) = 411 (55.9), 394 (20.7), 355 (59), 354 (100), 338 (48.0), 246 (32.8); Anal. Calcd for C24H21N5O2 (411): C, 70.06; H, 5.14; N, 17.02%. Found: C, 69.89; H, 5.07; N, 16.93%.
3.5. Antibacterial Activity
The newly synthesized compounds were screened for their antimicrobial activities in vitro against two species of Gram-negative bacteria Pseudomonas aeruginosa (MTCC 741); Escherichia coli (NCTC-10410); and four Gram-positive bacteria, Bacillus cereus (ATGG 14579); Bacillus subtilis (MTCC 441); Bacillus sphaericus (MTCC 11); Staphylococcus (MTCC 96); and two fungus, Aspergillus ochraceus Wilhelm (AUCC-230); Penicillium chrysogenum Thom (AUCC-530). The activities of these compounds were tested using the disc diffusion method [20]. For bacteria and the paper disk diffusion method [21] for fungi. The area of zone of inhibition was measured using Ampicillin; tetracycline and norfloxacin (30 μg mL−1) as standard antibiotic and mycostatin (30 μg mL−1) was used as a reference antifungal. The tested compounds were dissolved in N,N-dimethylformamide (DMF) to give a solution of 1 mg mL−1. The inhibition zones were measured in millimeters at the end of an incubation period of 48 hours at 28 °C. N,N-dimethylformamide (DMF) showed no inhibition zone. Test results are shown in Table 1
3.6. Antibacterial Activities
The screening results indicate that compounds 2–8 and 11 show weaker inhibitory activity than the standard drugs, while compounds 15–18 are moderately inhibitory, compared to the standard drugs. Compounds 10a–j, 12, 13 and 14a,b showed nearly the same inhibition activity asn antibacterial activity (Table 1). It clear that decomposition of chloride atom at C-1 of 4-benzyl-1-chlorophthalazine with N-nucleophiles is responsible for the antimicrobial activities.
4. Conclusions
The results from this screening demonstrated that replacement of the hydrogen atom attached to the phthalazine nucleus at N-1 with amino derivatives (compounds 10a-j), diimino derivatives (compounds 12a,b-13) and pyrimidine derivatives (14a,b) resulted in spectrum if moderate antibacterial activity against all tested Gram positive and Gram negative fungi.
| Compd. No. a | Inhibition zone diameter in mm | |||||||
|---|---|---|---|---|---|---|---|---|
| Gram-negative bacteria | Gram-positive bacteria | Fungi | ||||||
| PA | EC | BC | BS | BS | SC | AOW | PCT | |
| 1 | 10 | 12 | 10 | 14 | 12 | 10 | 11 | 13 |
| 2 | 14 | 10 | 12 | 11 | 10 | 13 | 10 | 11 |
| 3 | 10 | 10 | 14 | 12 | 11 | 10 | 14 | 10 |
| 4 | 13 | 10 | 12 | 13 | 10 | 12 | 10 | 12 |
| 5 | 11 | 14 | 10 | 13 | 14 | 13 | 12 | 13 |
| 6 | 14 | 10 | 13 | 10 | 12 | 14 | 11 | 14 |
| 7 | 10 | 12 | 15 | 12 | 10 | 11 | 10 | 11 |
| 8 | 12 | 13 | 10 | 14 | 10 | 14 | 10 | 10 |
| 10a | 16 | 15 | 14 | 15 | 15 | 16 | 17 | 15 |
| 10b | 18 | 19 | 18 | 17 | 15 | 19 | 14 | 17 |
| 10c | 16 | 16 | 19 | 15 | 18 | 14 | 17 | 18 |
| 10d | 15 | 17 | 16 | 19 | 17 | 15 | 16 | 14 |
| 10e | 17 | 16 | 14 | 17 | 19 | 16 | 19 | 19 |
| 10f | 16 | 15 | 17 | 18 | 17 | 14 | 15 | 17 |
| 10g | 18 | 15 | 15 | 16 | 16 | 17 | 18 | 16 |
| 10h | 19 | 18 | 14 | 15 | 17 | 15 | 14 | 19 |
| 10i | 17 | 16 | 17 | 19 | 18 | 15 | 17 | 15 |
| 10j | 18 | 15 | 19 | 16 | 18 | 15 | 16 | 14 |
| 11 | 12 | 10 | 14 | 12 | 10 | 14 | 10 | 11 |
| 12a | 20 | 22 | 20 | 19 | 20 | 22 | 20 | 20 |
| 12b | 20 | 23 | 22 | 24 | 24 | 20 | 20 | 20 |
| 13 | 22 | 20 | 23 | 22 | 23 | 20 | 24 | 22 |
| 14a | 23 | 22 | 24 | 23 | 22 | 24 | 23 | 23 |
| 14b | 24 | 20 | 20 | 20 | 22 | 24 | 20 | 22 |
| 15 | 19 | 22 | 23 | 22 | 24 | 20 | 20 | 21 |
| 16 | 18 | 17 | 18 | 17 | 19 | 18 | 16 | 17 |
| 17 | 17 | 19 | 16 | 19 | 17 | 19 | 15 | 16 |
| 18 | 15 | 18 | 15 | 17 | 18 | 16 | 18 | 15 |
| Ampicillin | 22 | 22 | 22 | 22 | 22 | 22 | - | - |
| Tetracycline | 20 | 20 | 20 | 20 | 20 | 20 | - | - |
| Norfloxacin | 25 | 25 | 25 | 25 | 25 | 25 | - | - |
| Mycostatin | - | - | - | - | - | - | 20 | 20 |
ac = 1 mg mL−1 of new compounds in DMF. Microorganisms: Pseudomonas aeruginosa (MTCC 741); Escherichia coli (NCTC-10410); Bacillus cereus (ATGG 14579); Bacillus subtilis (MTCC 441); Bacillus sphaericus (MTCC 11); Staphylococcus (MTCC 96); Aspergillus ochraceus Wilhelm (AUCC-230); Penicillium chrysogenum Thom (AUCC-530).
References
- Ito, S.; Yamaguchi, K.; Komoda, Y. Structural confirmation of the nitration product of the 1(2H)-phthalazinone as the 2-Nitro-1(2H)-phthalazinone. Chem. Pharm. Bull. 1992, 40, 3327–3329. [Google Scholar]
- Haack, T.; Fattori, R.; Napoletano, M.; Pellacini, F.; Fronza, G.; Raffaini, G.; Ganazzoli, F. Phthalazine PDE IV inhibitors: Conformational study of some 6-methoxy-1,4-disubstituted derivatives. Bioorg. Med. Chem. 2005, 13, 4425–4433. [Google Scholar]
- Heinisch, G.; Frank, H. Progress in Medicinal Chemistry; Ellis, G.P., Luscombe, D.K., Eds.; Elsevier: Amesterdam, The Netherlands, 1990; Volume 27, pp. 1–49. [Google Scholar]
- Heinisch, G.; Frank, H. Progress in Medicinal Chemistry; Ellis, G.P., Luscombe, D.K., Eds.; Elsevier: Amesterdam, The Netherlands, 1992; Volume 29, pp. 141–1483. [Google Scholar]
- Melikian, A.; Schiewer, G.; Chambon, J.P.; Wermuth, C.G. Condensation of muscimol or thiomuscimol with aminopyridazines yields GABA-A antagonists. J. Med. Chem. 1992, 35, 4092–4197. [Google Scholar]
- Napoletano, M.; Norcini, G.; Pellacini, F.; Morazzoni, G.; Ferlenga, P.; Pradella, L. Phthalazine PDE4 inhibitiors. Part 2: The synthesis and biological evolution of 6-methoxy-1,4-disubstituted derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 33–37. [Google Scholar]
- Kikuchi, K.; Nagano, T.; Hayakawa, H.; Hirata, Y.; Hirobe, M. Detection of nitric oxide production from a perfused organ by a luminol-hydrogen peroxide system. Anal. Chem. 1999, 22, 5131–5136. [Google Scholar]
- Arakawa, H.; Ishida, J.; Yamaguchi, M.; Nakamura, M. Chemiluminescent products of reaction between α-Keto acids and 4,5-diaminophthalhydrazide. Chem. Pharm. Bull. 1990, 38, 3491–3499. [Google Scholar]
- Arakawa, H.; Ishida, J.; Yamaguchi, M.; Nakamura, M. New chemiluminogenic substrate for N-Acetyl-β-d-glucosaminidase, 4′-(6′-diethylamino-benzofuranyl)phthalylhydrazido-N-acetyl-β-d-glucosaminide. Chem. Pharm. Bull. 1991, 39, 411–419. [Google Scholar]
- Samoto, K.; Ohkura, Y. Ring expansion reaction of 1,3-dithiolanes and 1,3-oxathiolanes using tellurium tetrachloride. Chem. Lett. 1990, 19, 1323. [Google Scholar]
- Tsoungas, P.G.; Searcey, M. A convenient access to benzo-substituted phthalazines as potential precursors to DNA intercalators. Tetrahedron Lett. 2001, 42, 6589–6592. [Google Scholar]
- Sivakumar, R.; Gnanasam, S.K.; Ramachandran, S.; Leonard, J.T. Pharmacological evaluation of some new 1-substituted-4-hydroxy-phthalazines. Eur. J. Med. Chem. 2002, 37, 793–801. [Google Scholar]
- Coelho, A.; Sotelo, E.; Fraiz, N.M.; Yanez, M.; Laguna, R.; Canob, E.; Ravina, E. Pyridazines. Part 36: Synthesis and antiplatelet activity of 5-substituted-6-phenyl-3(2H)-pyridazinones. Bioorg. Med. Chem. Lett. 2004, 14, 321–324. [Google Scholar]
- Demirayak, S.; Karaburun, A.C.; Beis, R. Some pyrrole substituted aryl pyridazinone and phthalazinone derivatives and their antihypertensive activities. Eur. J. Med. Chem. 2004, 39, 1089–1095. [Google Scholar]
- Dogruer, D.S.; Sahin, M.F.; Unlu, S.; Ito, S. Studies on some 3(2H)-pyridazinone derivatives with antinociceptive activity. Arch. Pharm. 2000, 333, 79–86. [Google Scholar]
- Dogruer, D.S.; Sahin, M.F.; Kupeli, E.; Yesilada, E. Synthesis and analgesic and anti-inflammatory activity of new pyridazinones. Turk. J. Chem. 2003, 27, 727–738. [Google Scholar]
- Dogruer, D.S.; Sahin, M.F.; Kupeli, E.; Yesilada, E. Synthesis of new 2-[1(2H)-phthalazinon-2-yl]acetamide and 3-[1(2H)-phthalazinon-2-yl]propanamide derivatives as antinociceptive and anti-inflammatory agents. Arch. Pharm. 2004, 337, 303–310. [Google Scholar]
- Sonmez, M.M.; Berber, I.; Akbas, E. Synthesis, antibacterial and antifungal activity of some new pyridazinone metal complexes. Eur. J. Med. Chem. 2006, 41, 101–105. [Google Scholar]
- Condensed Pyridazines Including Cinnolines and Phthalazines; Castle, N.R., Ed.; Wiley-Intersicence: New York, NY, USA, 1973; p. 383.
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed.Approved Standard M7-A5; NCCLS: Wayne, PA, USA, 2000. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
El-Wahab, A.H.F.A.; Mohamed, H.M.; El-Agrody, A.M.; El-Nassag, M.A.; Bedair, A.H. Synthesis and Biological Screening of 4-Benzyl-2H-phthalazine Derivatives. Pharmaceuticals 2011, 4, 1158-1170. https://doi.org/10.3390/ph4081158
El-Wahab AHFA, Mohamed HM, El-Agrody AM, El-Nassag MA, Bedair AH. Synthesis and Biological Screening of 4-Benzyl-2H-phthalazine Derivatives. Pharmaceuticals. 2011; 4(8):1158-1170. https://doi.org/10.3390/ph4081158
Chicago/Turabian StyleEl-Wahab, Ashraf H.F. Abd, Hany M. Mohamed, Ahmed M. El-Agrody, Mohammed A. El-Nassag, and Ahmed H. Bedair. 2011. "Synthesis and Biological Screening of 4-Benzyl-2H-phthalazine Derivatives" Pharmaceuticals 4, no. 8: 1158-1170. https://doi.org/10.3390/ph4081158
APA StyleEl-Wahab, A. H. F. A., Mohamed, H. M., El-Agrody, A. M., El-Nassag, M. A., & Bedair, A. H. (2011). Synthesis and Biological Screening of 4-Benzyl-2H-phthalazine Derivatives. Pharmaceuticals, 4(8), 1158-1170. https://doi.org/10.3390/ph4081158




