Combination Therapy with Olmesartan and Amlodipine in the Treatment of Hypertension
Abstract
:1. Introduction
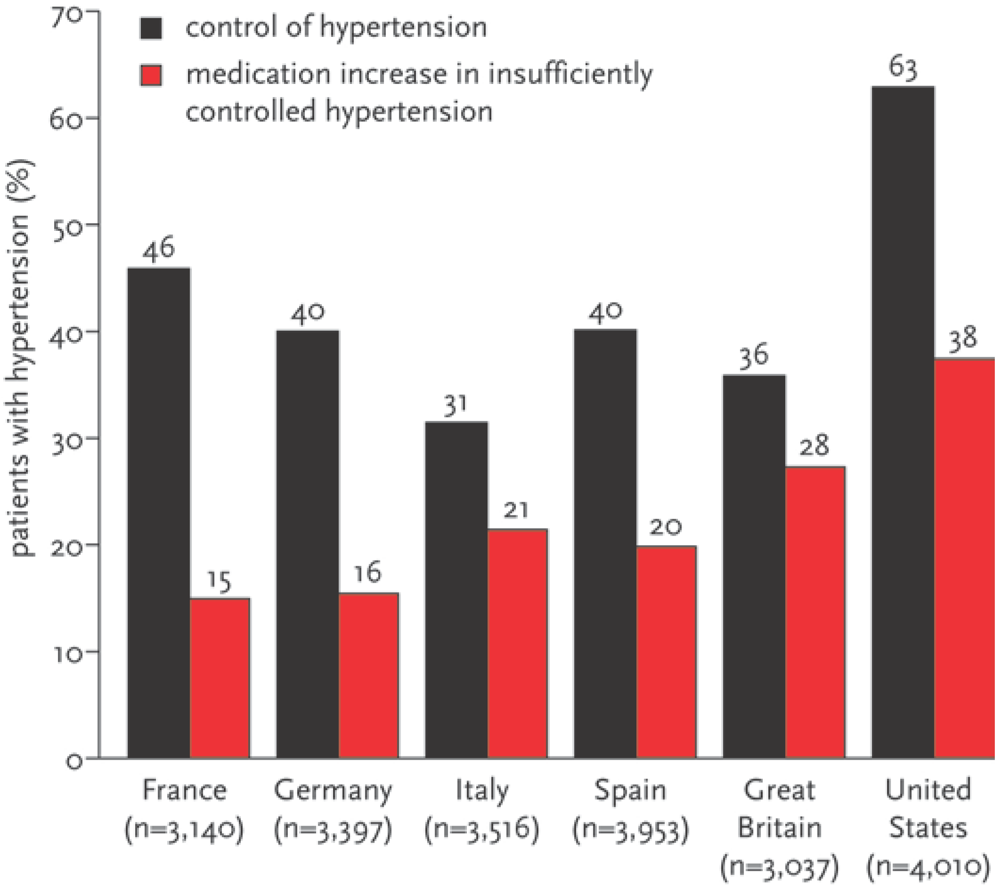
- —
- promoting a healthy lifestyle and identifying cardiovascular risk factors and concomitant diseases that could influence the prognosis and be significant in the treatment;
- —
- identifying demonstrable causes of high blood pressure;
- —
- evaluating the possible presence of organ damage and cardiovascular diseases.
| Blood pressure classification | Systolic | Diastolic |
|---|---|---|
| normal | <120 mmHg | and <80 mmHg |
| prehypertension | 120–139 mmHg | or 80–89 mmHg |
| stage 1 hypertension | 140–159 mmHg | or 90–99 mmHg |
| stage 2 hypertension | ≥160 mmHg | or ≥ 100 mmHg |

2. The Combination with Olmesartan and Amlodipine (the COACH study)
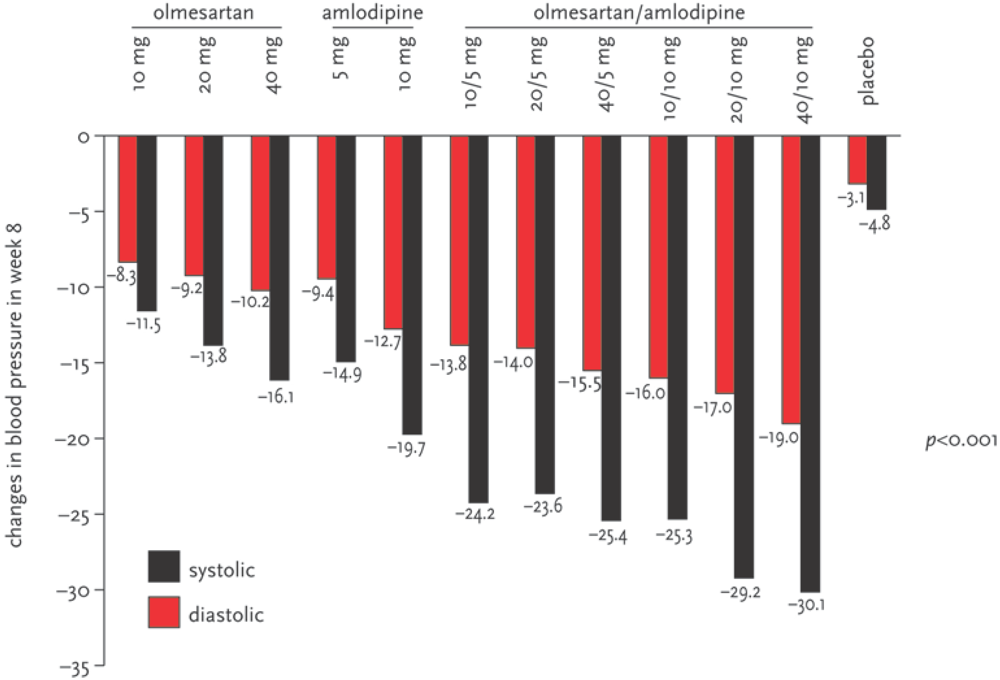
3. Discussion
4. Conclusions
Acknowledgements
Disclaimer
References and Notes
- Ong, K.L.; Cheung, B.M.; Man, Y.B.; Lau, C.P.; Lam, S.L. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension 2007, 49, 69–75. [Google Scholar] [PubMed]
- Wang, Y.R.; Alexander, G.C.; Stafford, R.S. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch. Intern. Med. 2007, 167, 141–147. [Google Scholar]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Materson, B.J.; Oparil, S.; Wright, J.T.; Roccella, E.J. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of Joint National Committee on Prevention, Detection, Evaluation. and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Petro, R.; Collins, R. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Lopez, V.A.; Franklin, S.S.; Tang, S.; Wong, N.D. Coronary heart disease events, preventable by control of blood pressure and lipids in US adults with hypertension. J. Clin. Hypertens. 2007, 9, 436–443. [Google Scholar]
- Okonofua, E.C.; Simpson, K.N.; Jesri, A.; Rehman, S.U.; Durkalski, V.L.; Egan, B.M. Therapeutic inertia is an impediment to achieving the healthy people 2010 blood pressure control goals. Hypertension 2006, 47, 345–351. [Google Scholar]
- Okken, V.S.; Niemeijer, M.G.; Dijkstra, A.; Baarsman, M.W.; Said, S.; Hoogenberg, K.; Orfgen, H.; Ottens, S.; Cleophas, T. The effect of physical, social and psychological factors on drug compliance in patients with mild hypertension. Neth. Heart J. 2008, 16, 197–200. [Google Scholar]
- Dählof, B.; Sever, P.S.; Poulter, N.R.; Wedel, H.; Gareth Weevers, D.; Caulfield, M.; Collins, R.; Kjeldsen, S.E.; Kristinsson, A.; McInnes, G.; Mehlsen, J.; Miettinen, M.; O’Brien, E.; Ostergren, J. ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendrolumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial- Blood Pressure Lowering Arm (ASCOT-BPLA): A multicentre randomized controlled trial. Lancet 2005, 366, 895–906. [Google Scholar]
- Pedrinelli, R.; Dell’Omo, G.; Mariani, M. Calcium channel blockers, postural vasoconstriction and dependent oedema in essential hypertension. J. Hum. Hypertens. 2001, 15, 455–461. [Google Scholar]
- Hollenberg, N.K. The renin-angiotensin system and cardiovascular disease. Blood Press. Suppl. 2000, 1, 5–8. [Google Scholar]
- Chalmers, J. The use of free and fixed drug combinations to improve hypertension control in our populations. Eur. Heart. J. 1999, 20, 1060–1061. [Google Scholar]
- Chow, S.C.; Liu, J.P. Combination trials. In Design and Analysis of Clinical Trials: Concepts and Methodologies, 2nd ed; Wiley: Hoboken, USA, 2004; pp. 270–283. [Google Scholar]
- Chrysant, S.G.; Melino, M.; Karki, S.; Lee, J.; Heyrman, R. The combination of olmesartan medoximil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin. Ther. 2008, 30, 587–604. [Google Scholar]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Lifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.; Kjeldsen, S.; Laurent, S.; Nakiewicz, K.; Ruilope, L.; Rynkiewicz, A.; Schmieder, R.; Struijker Boudier, H.; Zanchetti, A.; Vahanian, A.; Camm, J.; De Laterina, R.; Dean, V.; Dickstein, K.; Filippatos, G.; Funck-Brentano, C.; Hellemans, I.; Kristensen, S.; McGregor, K.; Sechten, U.; Silber, S.; Tendera, M.; Widinsky, P.; Zamorano, J.; Erdine, S.; Kiowski, W.; Agabiti, E.; Ambrosioni, E.; Lindholm, L.; Manolis, A.; Nilsson, P.; Redon, J.; Viigimaa, M.; Adamopoulos, G.; Bertoneu, V.; Clement, D.; Ruschitzka, F.; Famargo, J.; Van Zwieten, P.; Waeber, B.; Williams, B.; Zumorano, J.L. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). 2007 Guidelines for the management of arterial hypertension. Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [PubMed]
- Bangalore, S.; Kamalakkannan, G.; Parkar, S.; Messerli, F.H. Fixed-dose combinations improve medication compliance: A meta-analysis. Am. J. Med. 2007, 120, 713–719. [Google Scholar]
- Philipp, T.; Smith, T.R.; Glazer, R.; Wensing, M.; Yen, J.; Jin, J.; Scheider, H.; Pospiech, R. Two multicenter, 8 week, randomized, double-blind, placebo-controlled, parallel-group studies evaluating the efficacy and the tolerability of amlodipine and valsartan in combination and as monotherapy in adult patients with mild to moderate essential hypertension. Clin. Ther. 2007, 29, 563–580. [Google Scholar]
- Fogari, R.; Zoppi, A.; Derosa, G.; Mugekini, A.; Lazzaro, P.; Rinaldi, A.; Fogari, E.; Preti, P. Effect of valsartan addition to amlodipine on ankle oedema and subcutaneous tissue pressure in hypertensive patients. J. Hum. Hypertens. 2007, 21, 220–224. [Google Scholar]
- Leonetti, G.; Magnani, B.; Pessina, A.C.; Rappelli, A.; Trimarco, B.; Zanchetti, A. Tolerability of long-term treatment with lercanidipine versus amlodipine and lacidipine in elderly hypertension. Am. J. Hypertens. 2002, 15, 932–940. [Google Scholar] [PubMed]
- Kloner, R.A.; Weinberger, M.; Pool, J.L.; Chrysant, S.; Prasad, R.; Harris, S.; Zyczynski, I.; Leidy, N.; Nichelson, E. Comparison of Candesartan and Amlodipine for Safety, Tolerability and Efficacy (CASTLE) study investigators. Comparative effects of candesartan cilexetil and amlodipine in patients with mild systemic hypertension. Am. J. Cardiol. 2001, 87, 727–731. [Google Scholar]
- Julius, S.; Kjeldsen, S.E.; Weber, M.; Ekman, S.; Hansson, L.; Hua, T.; Laragh, J.; McInnes, G.; Mitchell, L.; Plat, F.; Schork, A.; Smith, B.; Zanchetti, A. VALUE Trial Group. Outcomes in hypertensive patients at high cardiovascular risk treatment with regimens based on valsartan or amlodipine. The VALUE randomized trial. Lancet 2004, 363, 2022–2031. [Google Scholar] [PubMed]
- Volpe, M.; Brommer, P.; Haag, U.; Miele, C. Efficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: A randomized double blind parallel group multicenter trial. Clin. Drug Invest. 2009, 29, 11–25. [Google Scholar]
- Mourad, J.; Le Jeune, S. Effective systolic blood pressure reduction with olmesartan medoxomil/amlodipine combination therapy: post hoc analysis of data from a randomized double blind parallel group multicenter study. Clin. Drug Invest. 2009, 29, 419–425. [Google Scholar] [CrossRef]
- Barrios, V.; Brommer, P.; Haag, U.; Calderon, A.; Escobar, C. Olmesartan medoxomil plus amlodipine increases efficacy in patients with moderate to severe hypertension after monotherapy: a randomized double blind multicenter study. Clin. Drug Invest. 2009, 29, 427–439. [Google Scholar]
- Jamerson, K.; Weber, M.A.; Bakris, G.L.; Dahlof, B.; Pitt, B.; Shi, V.; Heaster, A.; Gupte, J.; Gatlin, M.; Velazquez, E. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N. Engl. J. Med. 2008, 359, 2417–2428. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Niemeijer, M.G.; Cleophas, T.J. Combination Therapy with Olmesartan and Amlodipine in the Treatment of Hypertension. Pharmaceuticals 2009, 2, 125-133. https://doi.org/10.3390/ph2030125
Niemeijer MG, Cleophas TJ. Combination Therapy with Olmesartan and Amlodipine in the Treatment of Hypertension. Pharmaceuticals. 2009; 2(3):125-133. https://doi.org/10.3390/ph2030125
Chicago/Turabian StyleNiemeijer, Menco G., and Ton J. Cleophas. 2009. "Combination Therapy with Olmesartan and Amlodipine in the Treatment of Hypertension" Pharmaceuticals 2, no. 3: 125-133. https://doi.org/10.3390/ph2030125
APA StyleNiemeijer, M. G., & Cleophas, T. J. (2009). Combination Therapy with Olmesartan and Amlodipine in the Treatment of Hypertension. Pharmaceuticals, 2(3), 125-133. https://doi.org/10.3390/ph2030125




