Losartan Protects Against Radiation-Induced Testicular Damage by Modulating Oxidative Stress, Testosterone Levels, and Metabolic Profile
Abstract
1. Introduction
2. Results
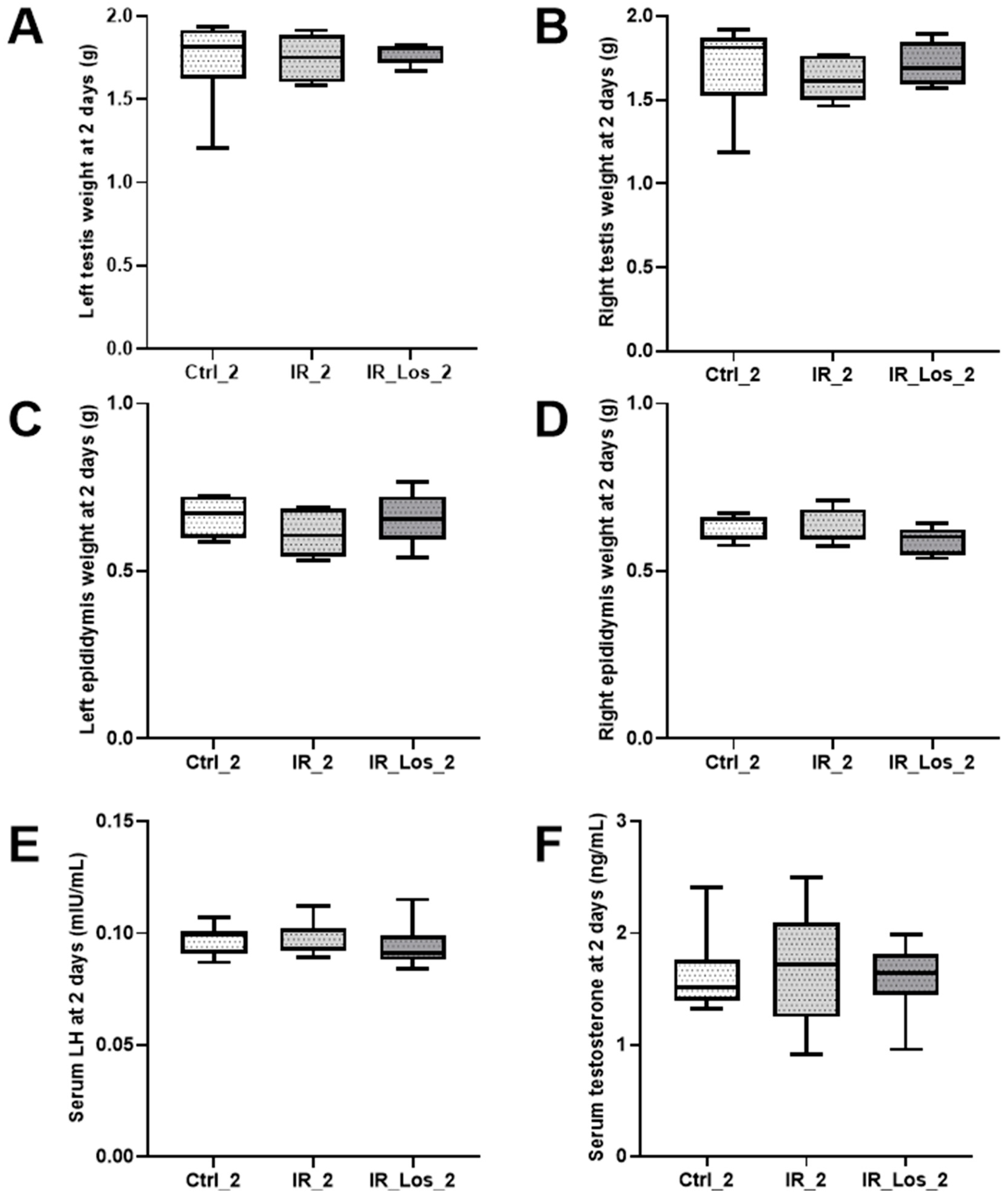
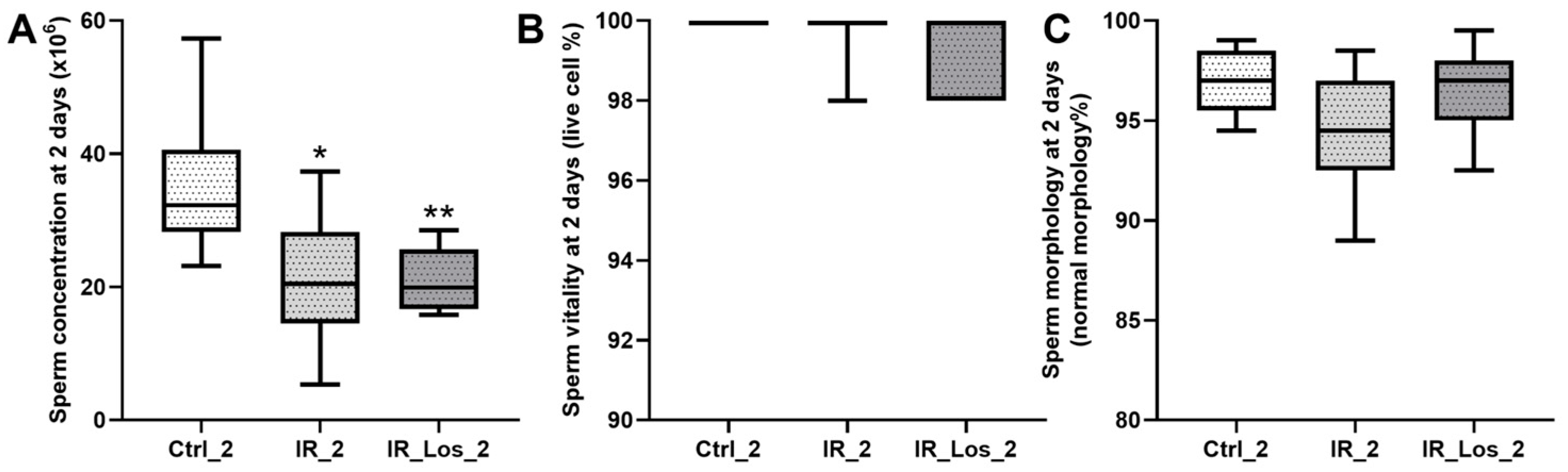
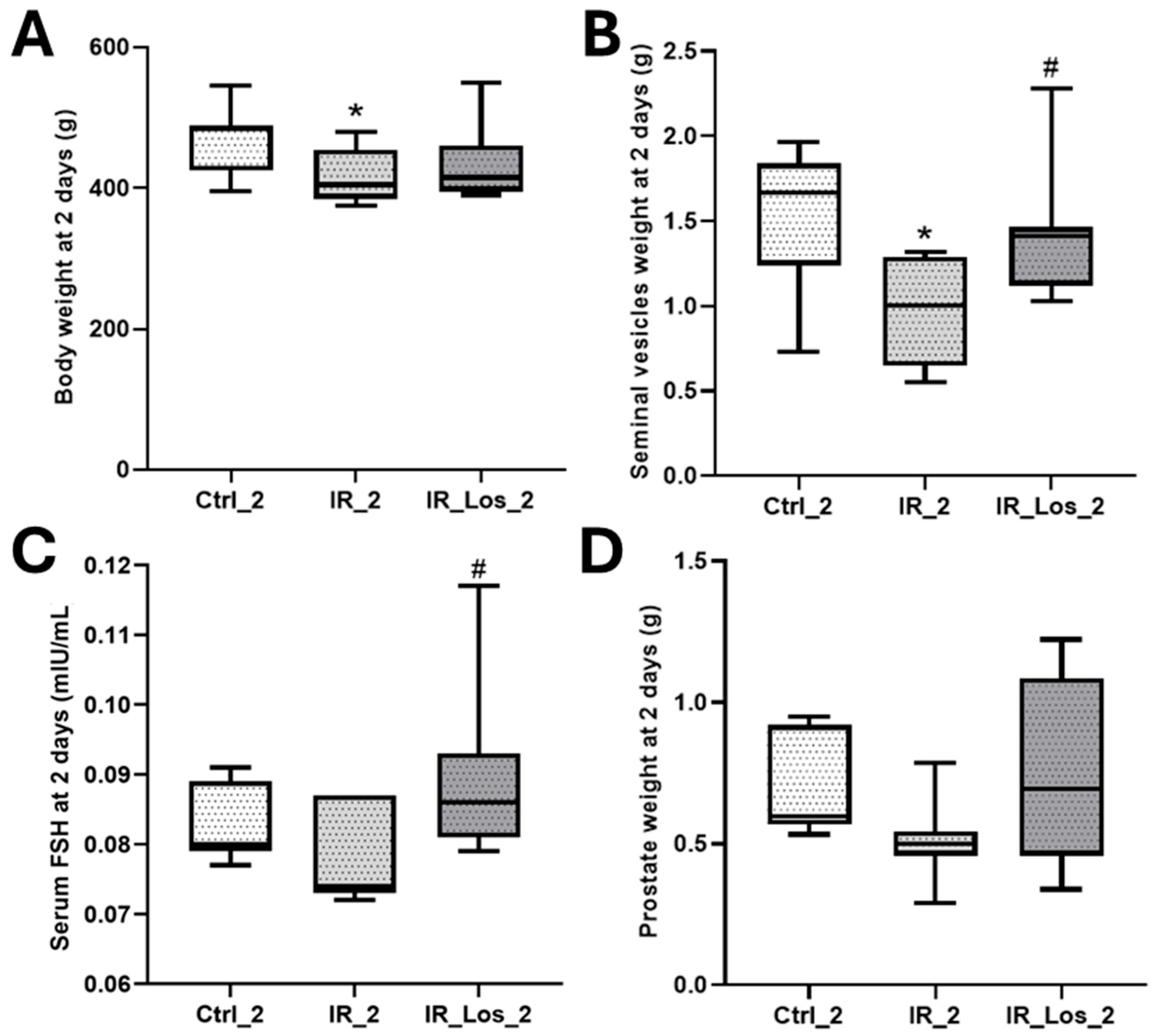
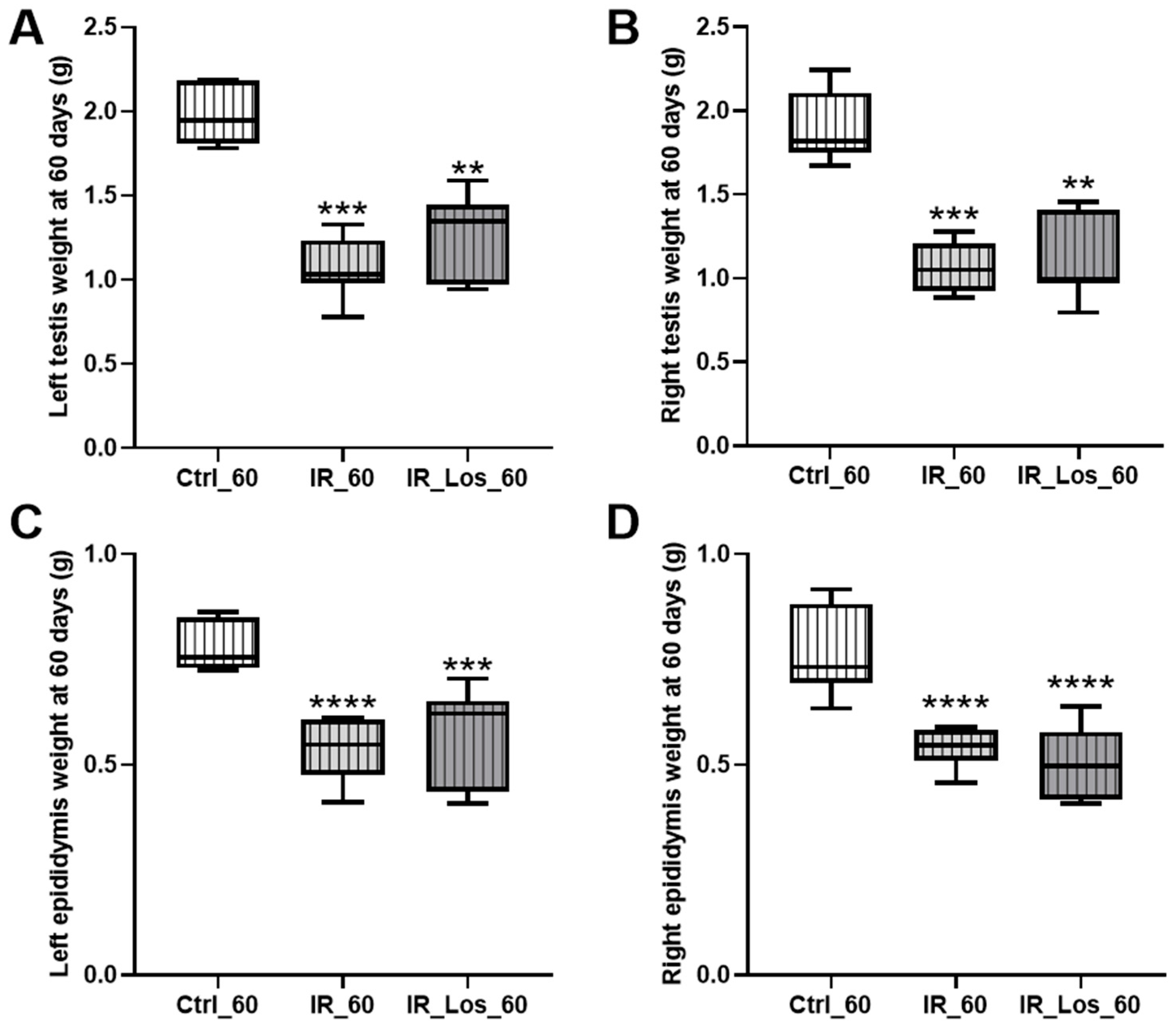
2.1. Losartan Prevented Weight Loss in Rats Two Days After Radiotherapy but Not Sperm Quality Decline
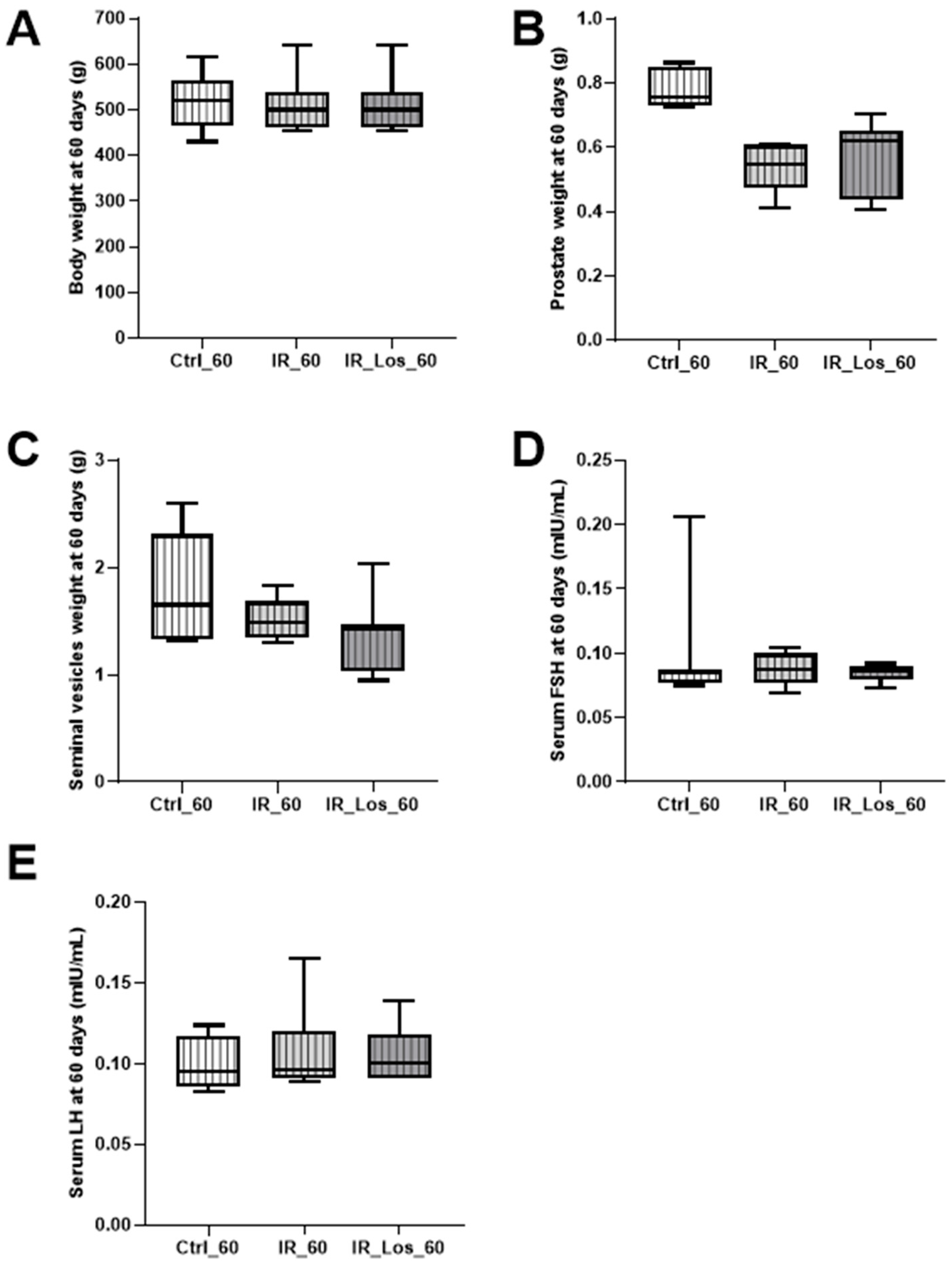
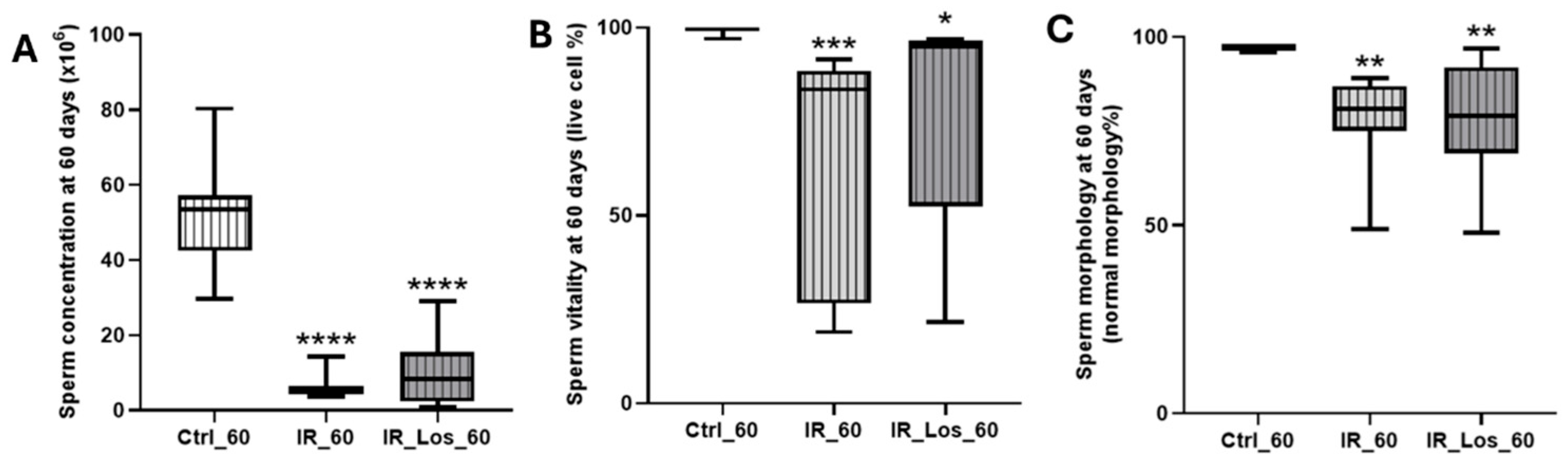
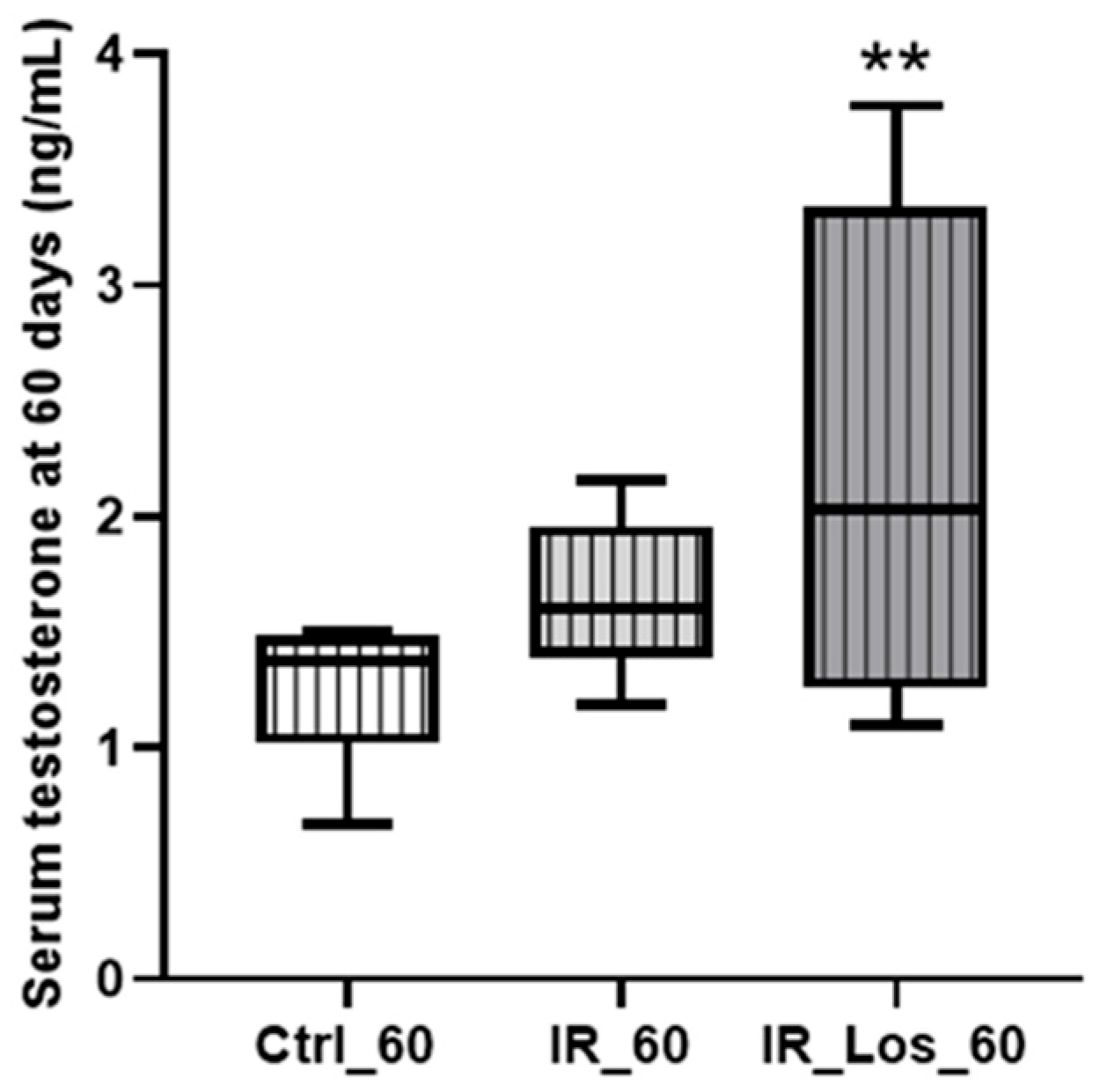
2.2. Losartan Increased Serum Testosterone in Rats Late Post-Radiotherapy but Did Not Fully Restore Sperm Quality
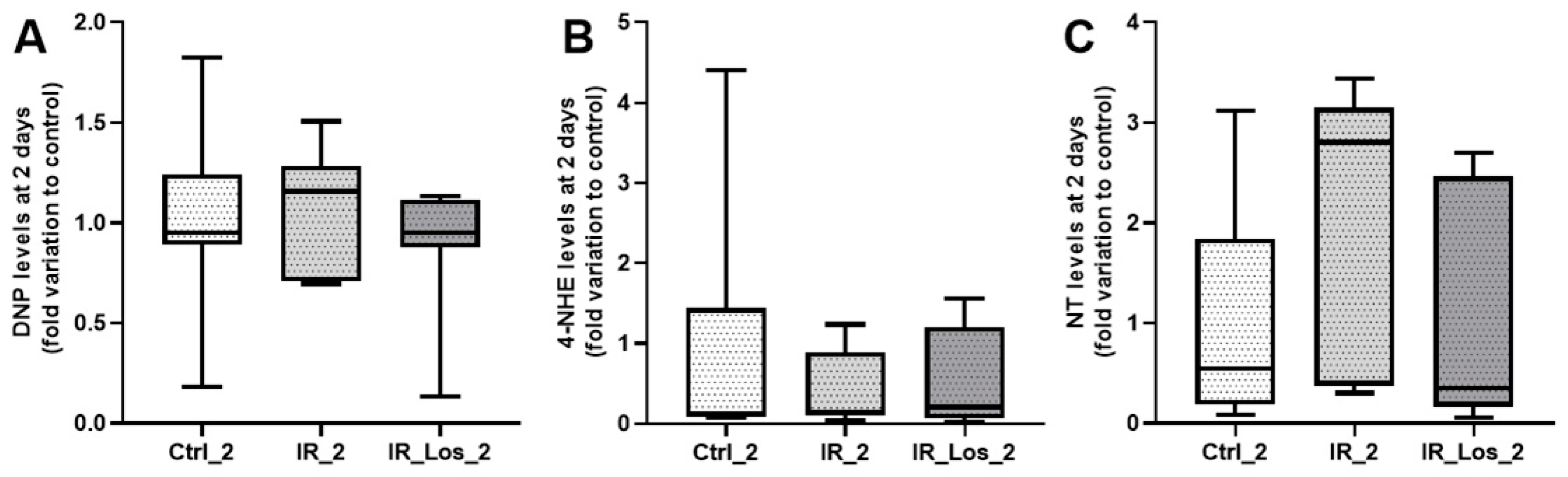
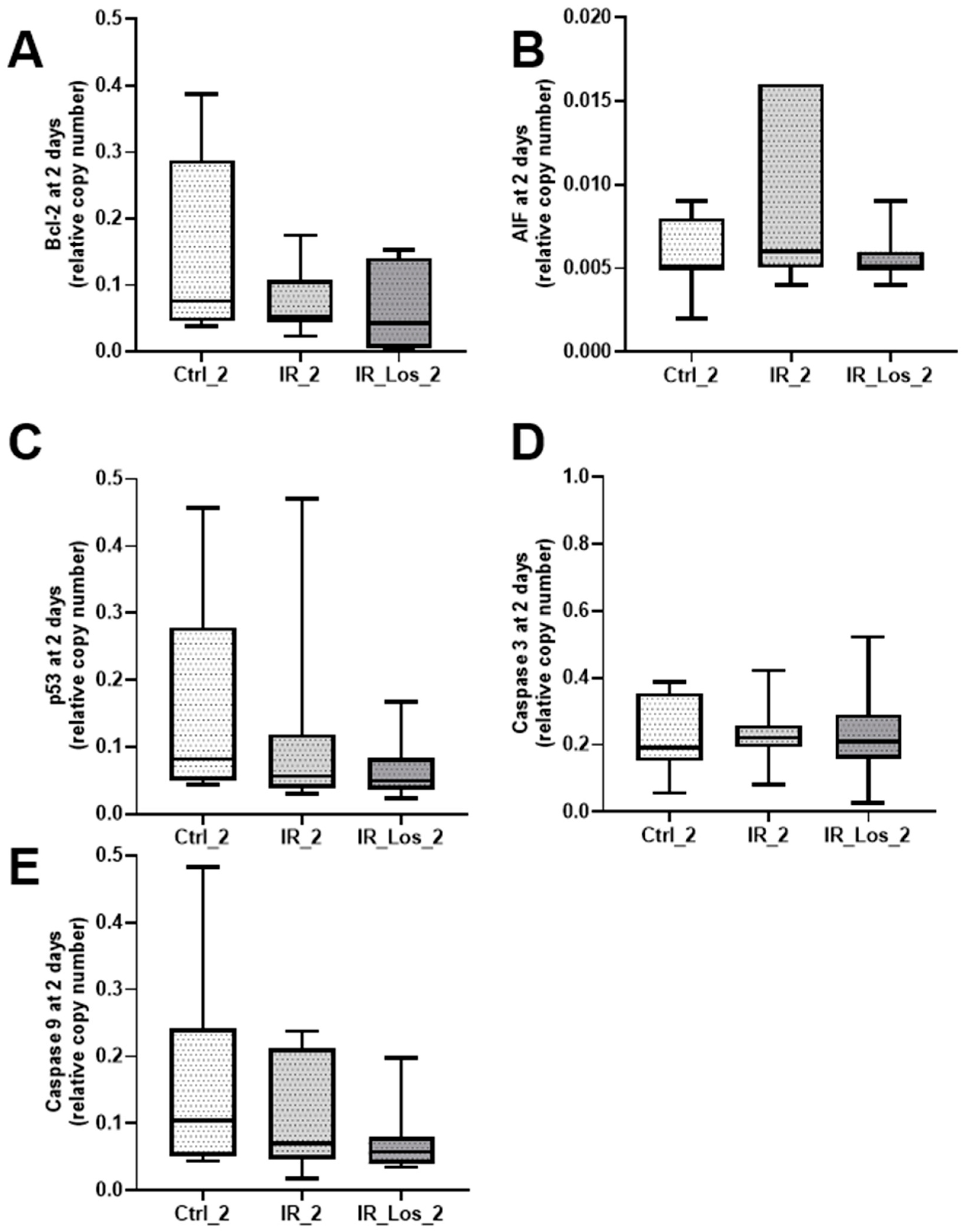
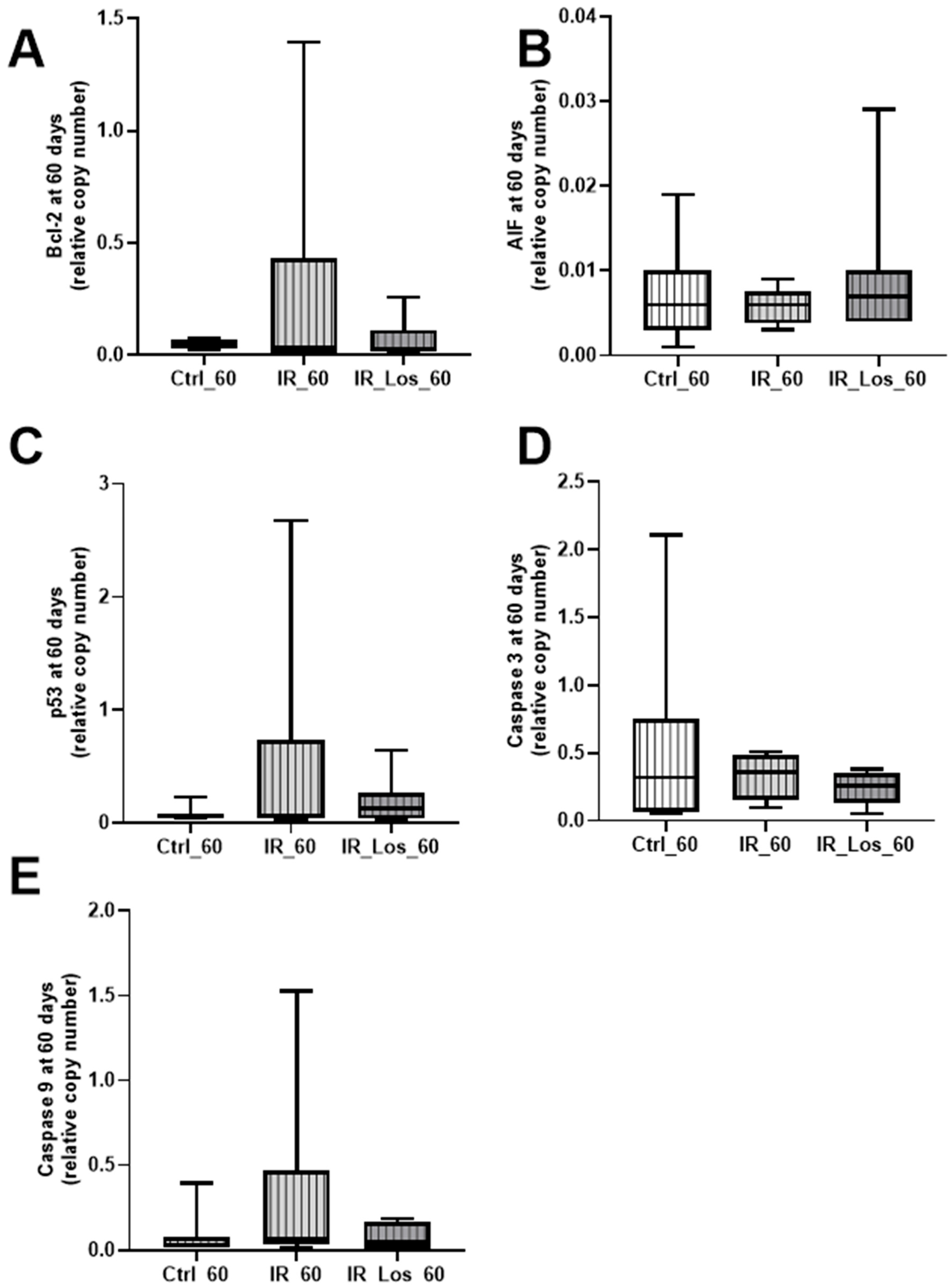
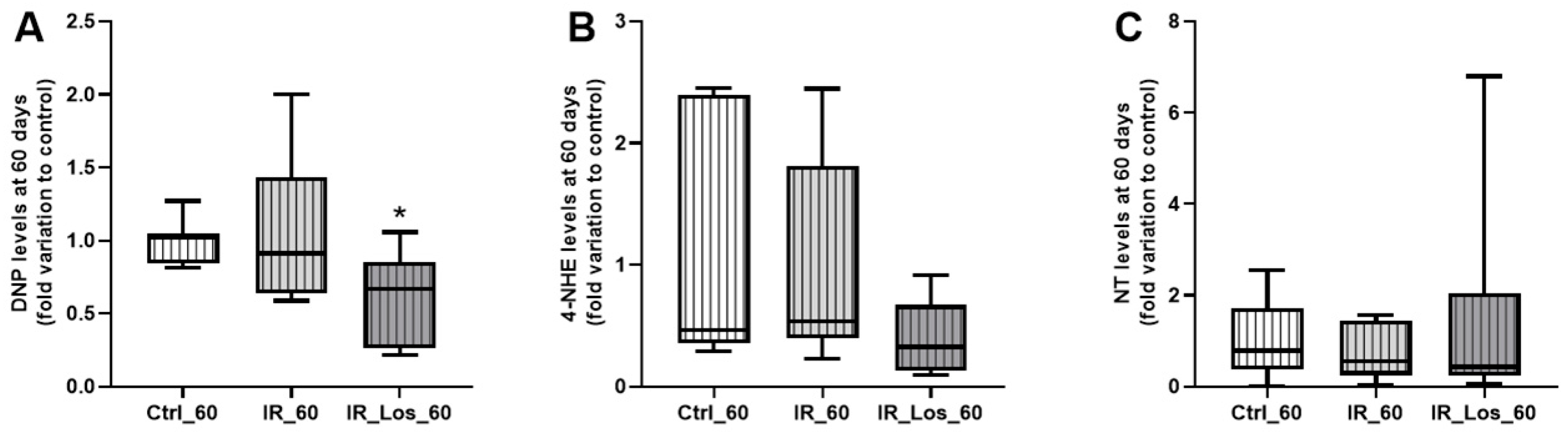
2.3. Losartan Failed to Mitigate Acute Post-Radiotherapy Testicular Oxidative Stress-Related Damage, but Significantly Reduced Late Effects on Protein Carbonylation in the Testes of Rats
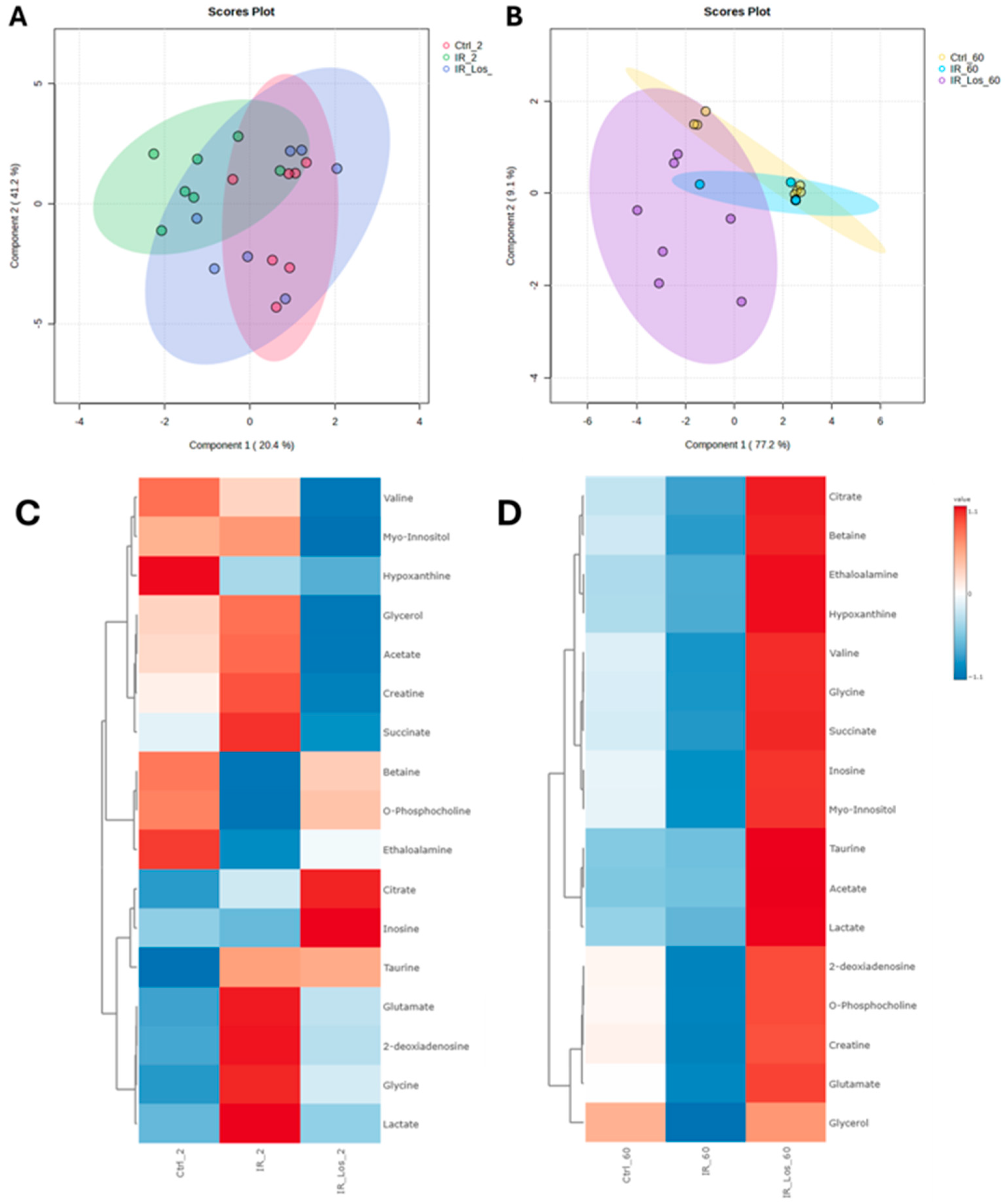
2.4. Losartan Mitigates Testicular Metabolic Perturbation Induced by Radiotherapy in a Time-Dependent Manner
3. Discussion
4. Materials and Methods
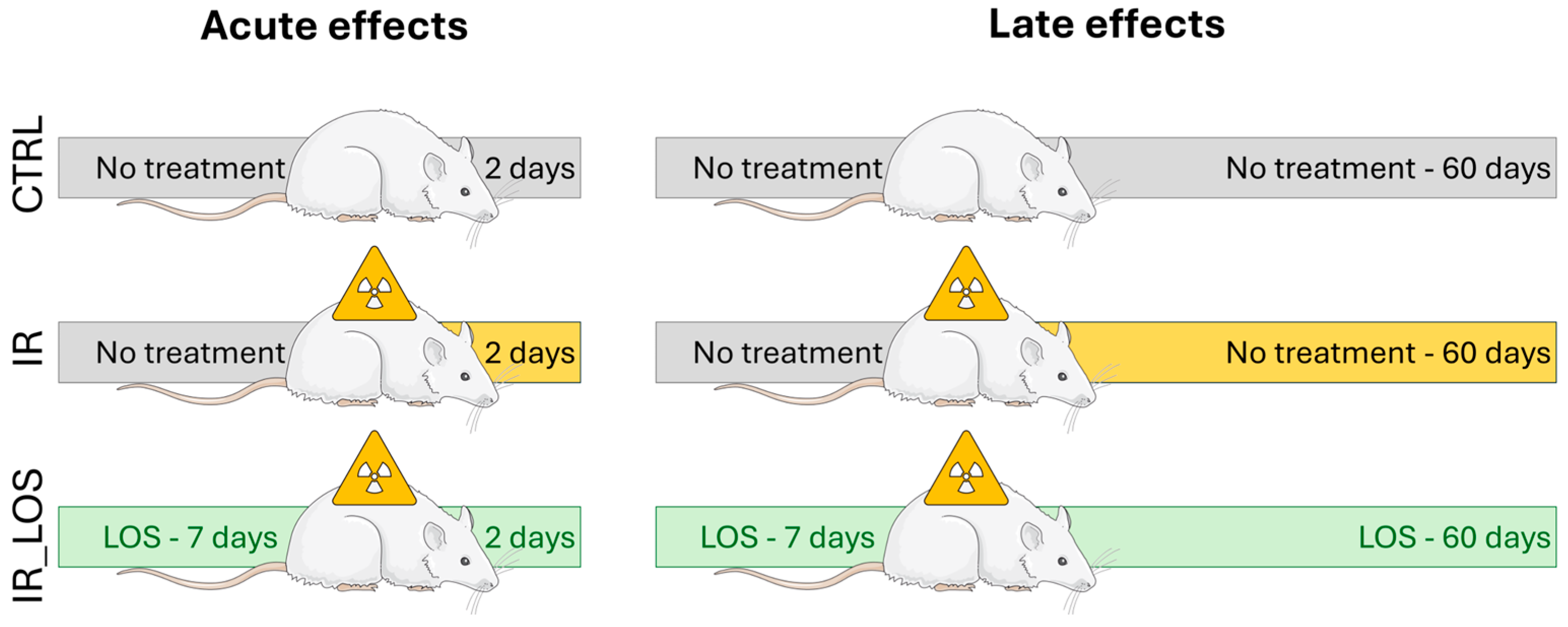
4.1. Animal Model and Experimental Design
4.2. Biometric and Sperm Quality Characterization
4.3. Polymerase Chain Reaction
4.4. Determination of Oxidative Stress-Related Markers
4.5. Determination of Testis Metabolome
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2013, 25, 578–585. [Google Scholar] [CrossRef]
- Yumura, Y.; Takeshima, T.; Komeya, M.; Karibe, J.; Kuroda, S.; Saito, T. Long-Term Fertility Function Sequelae in Young Male Cancer Survivors. World J. Mens. Health 2023, 41, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Schover, L.R.; Brey, K.; Lichtin, A.; Lipshultz, L.I.; Jeha, S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J. Clin. Oncol. 2002, 20, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Schover, L.R.; Rybicki, L.A.; Martin, B.A.; Bringelsen, K.A. Having children after cancer: A pilot survey of survivors’ attitudes and experiences. Cancer 1999, 86, 697–709. [Google Scholar] [CrossRef]
- Georgakopoulos, I.; Kouloulias, V.; Ntoumas, G.N.; Desse, D.; Koukourakis, I.; Kougioumtzopoulou, A.; Kanakis, G.; Zygogianni, A. Radiotherapy and Testicular Function: A Comprehensive Review of the Radiation-Induced Effects with an Emphasis on Spermatogenesis. Biomedicines 2024, 12, 1492. [Google Scholar] [CrossRef]
- Okada, K.; Fujisawa, M. Recovery of Spermatogenesis Following Cancer Treatment with Cytotoxic Chemotherapy and Radiotherapy. World J. Mens. Health 2019, 37, 166–174. [Google Scholar] [CrossRef]
- Wallace, W.H.B. Oncofertility and preservation of reproductive capacity in children and young adults. Cancer 2011, 117, 2301–2310. [Google Scholar] [CrossRef]
- Colpi, G.; Contalbi, G.; Nerva, F.; Sagone, P.; Piediferro, G. Testicular function following chemo–radiotherapy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, S2–S6. [Google Scholar] [CrossRef]
- Hermann, R.M.; Henkel, K.; Christiansen, H.; Vorwerk, H.; Hille, A.; Hess, C.F.; Schmidberger, H. Testicular dose and hormonal changes after radiotherapy of rectal cancer. Radiother. Oncol. 2005, 75, 83–88. [Google Scholar] [CrossRef]
- Kim, E.-J.; Prayoga, A.H.; Ha, J.; Kang, D.G.; Yang, J.; Kang, S.; Kim, J.-M.; Ahn, B.; Cao, D.L.; Yun, S.P. Cumulative Low-Dose-Rate Radiation Induces Oxidative Stress, Apoptosis, and Fibrosis in Mouse Testis. Antioxidants 2025, 14, 1028. [Google Scholar] [CrossRef]
- Liang, X.; Zheng, S.; Cui, J.; Yu, D.; Yang, G.; Zhou, L.; Wang, B.; Cai, L.; Li, W. Alterations of microRNA expression in the liver, heart, and testis of mice upon exposure to repeated low-dose radiation. Dose-Response 2018, 16, 1559325818799561. [Google Scholar] [CrossRef]
- Brydøy, M.; Fosså, S.D.; Klepp, O.; Bremnes, R.M.; Wist, E.A.; Wentzel-Larsen, T.; Dahl, O. Paternity following treatment for testicular cancer. J. Natl. Cancer Inst. 2005, 97, 1580–1588. [Google Scholar] [CrossRef]
- Hsiao, W.; Stahl, P.J.; Osterberg, E.C.; Nejat, E.; Palermo, G.D.; Rosenwaks, Z.; Schlegel, P.N. Successful treatment of postchemotherapy azoospermia with microsurgical testicular sperm extraction: The Weill Cornell experience. J. Clin. Oncol. 2011, 29, 1607–1611. [Google Scholar] [CrossRef]
- Katz, D.J.; Kolon, T.F.; Feldman, D.R.; Mulhall, J.P. Fertility preservation strategies for male patients with cancer. Nat. Rev. Urol. 2013, 10, 463–472. [Google Scholar] [CrossRef]
- García, A.; Herrero, M.B.; Holzer, H.; Tulandi, T.; Chan, P. Assisted reproductive outcomes of male cancer survivors. J. Cancer Surviv. 2015, 9, 208–214. [Google Scholar] [CrossRef]
- Meistrich, M.L. Risks of genetic damage in offspring conceived using spermatozoa produced during chemotherapy or radiotherapy. Andrology 2020, 8, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Zhang, Y.; Qiu, H.; Zheng, J.; Xue, J.; Jin, J.; Ni, F.; Zhang, C.; Chen, C.; et al. Effects of paternal ionizing radiation exposure on fertility and offspring’s health. Reprod. Med. Biol. 2024, 23, e12567. [Google Scholar] [CrossRef]
- Spadella, M.A.; Silva, E.J.R.; Chies, A.B.; Almeida, L.A. Insights Into Antioxidant Strategies to Counteract Radiation-Induced Male Infertility. Antioxid. Redox Signal 2024, 40, 776–801. [Google Scholar] [CrossRef] [PubMed]
- Klaus, R.; Niyazi, M.; Lange-Sperandio, B. Radiation-induced kidney toxicity: Molecular and cellular pathogenesis. Radiat. Oncol. 2021, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.C.; Dunlay, R.P.; Lazartigues, E.; Zhang, Y.; Sharma, R.V.; Engelhardt, J.F.; Davisson, R.L. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ. Res. 2004, 95, 532–539. [Google Scholar] [CrossRef]
- Zimnol, A.; Spicker, N.; Balhorn, R.; Schröder, K.; Schupp, N. The NADPH oxidase isoform 1 contributes to angiotensin II-mediated DNA damage in the kidney. Antioxidants 2020, 9, 586. [Google Scholar] [CrossRef]
- Ferreira-Machado, S.C.; Rocha, N.D.N.; Mencalha, A.L.; De Melo, L.D.B.; Salata, C.; Ribeiro, A.F.; Torres, T.D.S.; Mandarim-De-Lacerda, C.A.; Canary, P.C.; Peregrino, A.A.D.F. Up-regulation of angiotensin-converting enzyme and angiotensin II type 1 receptor in irradiated rats. Int. J. Radiat. Biol. 2010, 86, 880–887. [Google Scholar] [CrossRef]
- Pinter, M.; Kwanten, W.J.; Jain, R.K. Renin-Angiotensin System Inhibitors to Mitigate Cancer Treatment-Related Adverse Events. Clin. Cancer Res. 2018, 24, 3803–3812. [Google Scholar] [CrossRef]
- Gianzo, M.; Subirán, N. Regulation of Male Fertility by the Renin-Angiotensin System. Int. J. Mol. Sci. 2020, 21, 7943. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.L.M.; Alves, M.G.; Chies, A.B.; Spadella, M.A. Losartan Attenuates Radiation-Induced Damage on Testes and Accelerates Tubular Regeneration. Front. Reprod. Health 2022, 4, 904804. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Meistrich, M.L.; Wilson, G.; Shetty, G.; Marcelli, M.; McPhaul, M.J.; Morris, P.L.; Wilkinson, M.F. Irradiation selectively inhibits expression from the androgen-dependent Pem homeobox gene promoter in sertoli cells. Endocrinology 2001, 142, 1567–1577. [Google Scholar] [CrossRef]
- Chemaitilly, W.; Liu, Q.; van Iersel, L.; Ness, K.K.; Li, Z.; Wilson, C.L.; Brinkman, T.M.; Klosky, J.L.; Barnes, N.; Clark, K.L.; et al. Leydig Cell Function in Male Survivors of Childhood Cancer: A Report From the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2019, 37, 3018–3031. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.M.; Iyengar, P. Implications of weight loss for cancer patients receiving radiotherapy. Curr. Opin. Support. Palliat. Care 2017, 11, 261–265. [Google Scholar] [CrossRef]
- Liu, C.; Wei, J.; Wang, X.; Zhao, Q.; Lv, J.; Tan, Z.; Xin, Y.; Jiang, X. Radiation-induced skin reactions: Oxidative damage mechanism and antioxidant protection. Front. Cell Dev. Biol. 2024, 12, 1480571. [Google Scholar] [CrossRef]
- Rosimont, M.; Kariyawasam, D.; Samara-Boustani, D.; Giani, E.; Beltrand, J.; Bolle, S.; Fresneau, B.; Puget, S.; Sainte-Rose, C.; Alapetite, C.; et al. Assessment of Puberty and Hypothalamic-Pituitary-Gonadal Axis Function After Childhood Brain Tumor Treatment. J. Clin. Endocrinol. Metab. 2023, 108, e823–e831. [Google Scholar] [CrossRef]
- Stern, E.; Ben-Ami, M.; Gruber, N.; Toren, A.; Caspi, S.; Abebe-Campino, G.; Lurye, M.; Yalon, M.; Modan-Moses, D. Hypothalamic-pituitary-gonadal function, pubertal development, and fertility outcomes in male and female medulloblastoma survivors: A single-center experience. Neuro-Oncology 2023, 25, 1345–1354. [Google Scholar] [CrossRef]
- Wei, C.; Crowne, E. The impact of childhood cancer and its treatment on puberty and subsequent hypothalamic pituitary and gonadal function, in both boys and girls. Best. Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101291. [Google Scholar] [CrossRef]
- Yumura, Y.; Takeshima, T.; Komeya, M.; Kuroda, S.; Saito, T.; Karibe, J. Fertility and sexual dysfunction in young male cancer survivors. Reprod. Med. Biol. 2022, 21, e12481. [Google Scholar] [CrossRef]
- Amirkhosravi, A.; Mirtajaddini Goki, M.; Heidari, M.R.; Karami-Mohajeri, S.; Iranpour, M.; Torshabi, M.; Mehrabani, M.; Mandegary, A.; Mehrabani, M. Combination of losartan with pirfenidone: A protective anti-fibrotic against pulmonary fibrosis induced by bleomycin in rats. Sci. Rep. 2024, 14, 8729. [Google Scholar] [CrossRef] [PubMed]
- Dal-Ros, S.; Oswald-Mammosser, M.; Pestrikova, T.; Schott, C.; Boehm, N.; Bronner, C.; Chataigneau, T.; Gény, B.; Schini-Kerth, V.B. Losartan prevents portal hypertension-induced, redox-mediated endothelial dysfunction in the mesenteric artery in rats. Gastroenterology 2010, 138, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Massiera, F.; Quignard-Boulange, A.; Ailhaud, G.; Voy, B.H.; Wasserman, D.H.; Moustaid-Moussa, N. Overproduction of angiotensinogen from adipose tissue induces adipose inflammation, glucose intolerance, and insulin resistance. Obesity 2012, 20, 48–56. [Google Scholar] [CrossRef]
- Lagou, V.; Manios, Y.; Moran, C.N.; Bailey, M.E.; Grammatikaki, E.; Oikonomou, E.; Ioannou, E.; Moschonis, G.; Wilson, R.H.; Pitsiladis, Y.P. Developmental changes in adiposity in toddlers and preschoolers in the GENESIS study and associations with the ACE I/D polymorphism. Int. J. Obes. 2007, 31, 1052–1060. [Google Scholar] [CrossRef]
- Riera-Fortuny, C.; Real, J.T.; Chaves, F.J.; Morales-Suárez-Varela, M.; Martínez-Triguero, M.L.; Morillas-Ariño, C.; Hernández-Mijares, A. The relation between obesity, abdominal fat deposit and the angiotensin-converting enzyme gene I/D polymorphism and its association with coronary heart disease. Int. J. Obes. 2005, 29, 78–84. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, A.D.; Krause, E.G.; Kim, D.H.; Sakai, R.R.; Seeley, R.J.; Woods, S.C. The effect of angiotensin-converting enzyme inhibition using captopril on energy balance and glucose homeostasis. Endocrinology 2009, 150, 4114–4123. [Google Scholar] [CrossRef]
- Takahashi, N.; Li, F.; Hua, K.; Deng, J.; Wang, C.H.; Bowers, R.R.; Bartness, T.J.; Kim, H.S.; Harp, J.B. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab. 2007, 6, 506–512. [Google Scholar] [CrossRef]
- Araki, K.; Masaki, T.; Katsuragi, I.; Tanaka, K.; Kakuma, T.; Yoshimatsu, H. Telmisartan prevents obesity and increases the expression of uncoupling protein 1 in diet-induced obese mice. Hypertension 2006, 48, 51–57. [Google Scholar] [CrossRef]
- Cassis, L.; Helton, M.; English, V.; Burke, G. Angiotensin II regulates oxygen consumption. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R445–R453. [Google Scholar] [CrossRef]
- Yamamoto, R.; Akazawa, H.; Fujihara, H.; Ozasa, Y.; Yasuda, N.; Ito, K.; Kudo, Y.; Qin, Y.; Ueta, Y.; Komuro, I. Angiotensin II type 1 receptor signaling regulates feeding behavior through anorexigenic corticotropin-releasing hormone in hypothalamus. J. Biol. Chem. 2011, 286, 21458–21465. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Moustaid-Moussa, N. The adipose tissue renin-angiotensin system and metabolic disorders: A review of molecular mechanisms. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 379–390. [Google Scholar] [CrossRef]
- Izard, M.A. Leydig cell function and radiation: A review of the literature. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1995, 34, 1–8. [Google Scholar] [CrossRef]
- Brauner, R.; Czernichow, P.; Cramer, P.; Schaison, G.; Rappaport, R. Leydig-cell function in children after direct testicular irradiation for acute lymphoblastic leukemia. N. Engl. J. Med. 1983, 309, 25–28. [Google Scholar] [CrossRef]
- Sklar, C.A.; Robison, L.L.; Nesbit, M.E.; Sather, H.N.; Meadows, A.T.; Ortega, J.A.; Kim, T.; Hammond, G.D. Effects of radiation on testicular function in long-term survivors of childhood acute lymphoblastic leukemia: A report from the Children Cancer Study Group. J. Clin. Oncol. 1990, 8, 1981–1987. [Google Scholar] [CrossRef]
- Sarafoglou, K.; Boulad, F.; Gillio, A.; Sklar, C. Gonadal function after bone marrow transplantation for acute leukemia during childhood. J. Pediatr. 1997, 130, 210–216. [Google Scholar] [CrossRef]
- Shiraishi, K.; Yoshida, K.; Fujimiya, T.; Naito, K. Angiotensin II dependent testicular fibrosis and effects on spermatogenesis after vasectomy in the rat. J. Urol. 2003, 170, 2104–2108. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, A.; Zhao, W.; Wickström, S.; Arnér, E.S.J.; Kiessling, R. Reactive oxygen species: Janus-faced molecules in the era of modern cancer therapy. J. Immunother. Cancer 2024, 12, e009409. [Google Scholar] [CrossRef]
- Pechlivanova, D.; Krumova, E.; Kostadinova, N.; Mitreva-Staleva, J.; Grozdanov, P.; Stoynev, A. Protective effects of losartan on some type 2 diabetes mellitus-induced complications in Wistar and spontaneously hypertensive rats. Metab. Brain Dis. 2020, 35, 527–538. [Google Scholar] [CrossRef]
- Farooqui, Z.; Banday, A.A. Angiotensin 1–7 exerts antioxidant effects, suppresses Mammalian Target of Rapamycin (mTOR) signaling, and inhibits apoptosis in renal proximal tubular cells. Peptides 2024, 172, 171136. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, C.; Zatz, R. Linking oxidative stress, the renin-angiotensin system, and hypertension. Hypertension 2011, 57, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Heeba, G.H. Angiotensin II receptor blocker, losartan, ameliorates gentamicin-induced oxidative stress and nephrotoxicity in rats. Pharmacology 2011, 87, 232–240. [Google Scholar] [CrossRef]
- Tanaka, M.; Kaji, K.; Nishimura, N.; Asada, S.; Koizumi, A.; Matsuda, T.; Yorioka, N.; Tsuji, Y.; Fujinaga, Y.; Sato, S.; et al. Blockade of angiotensin II modulates insulin-like growth factor 1-mediated skeletal muscle homeostasis in experimental steatohepatitis. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119649. [Google Scholar] [CrossRef] [PubMed]
- Clermont, Y.; Leblond, C.P.; Messier, B. Duration of the cycle of the seminal epithelium of the rat. Arch. Anat. Microsc. Morphol. Exp. 1959, 48, 37–55. [Google Scholar]
- Spadella, M.A.; Alves, M.G.; Paiva, L.G.; Santos, L.L.M.; Chies, A.B. Standardization of experimental model of testicular injury induced by ionizing radiation in preclinical studies. In Advances in Medicine and Biology; Lv, B., Ed.; Nova Science Publisher: Hauppauge, NY, USA, 2022; Volume 193, pp. 77–119. [Google Scholar]
- Cavalim Vale, A.P.; Dos Santos, G.; da Silva, T.P.; Mansano, N.D.S.; Chies, A.B.; Chagas, E.F.B.; Spadella, M.A. Influence of the AT1 Receptor Antagonists Telmisartan and Losartan on Reproduction and Offspring After Paternal Exposure to Ionizing Radiation. Reprod. Sci. 2019, 26, 639–648. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
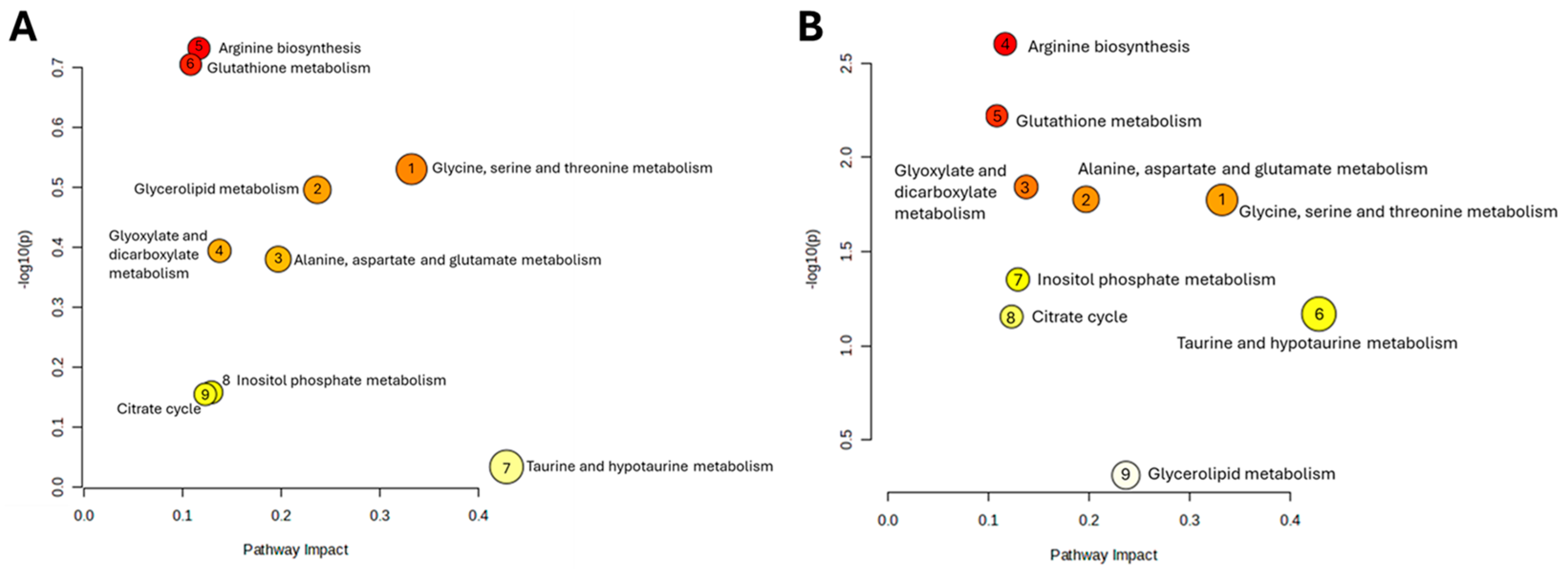
| Pathway Name | p | −log(p) | FDR | Impact |
|---|---|---|---|---|
| Arginine biosynthesis | 0.18561 | 0.7314 | 0.56164 | 0.11675 |
| Glutathione metabolism | 0.1975 | 0.70443 | 0.56164 | 0.10839 |
| Glycine, serine and threonine metabolism | 0.29519 | 0.5299 | 0.63904 | 0.3323 |
| Glycerolipid metabolism | 0.31952 | 0.4955 | 0.63904 | 0.23676 |
| Glyoxylate and dicarboxylate metabolism | 0.40388 | 0.39375 | 0.67083 | 0.13757 |
| Alanine, aspartate and glutamate metabolism | 0.41706 | 0.3798 | 0.67083 | 0.19712 |
| Inositol phosphate metabolism | 0.69537 | 0.15778 | 0.82811 | 0.12939 |
| Citrate cycle (TCA cycle) | 0.70071 | 0.15446 | 0.82811 | 0.12311 |
| Taurine and hypotaurine metabolism | 0.92575 | 0.033507 | 0.94219 | 0.42857 |
| Pathway Name | p | −log(p) | FDR | Impact |
|---|---|---|---|---|
| Arginine biosynthesis | 0.0024959 | 2.6028 | 0.021631 | 0.11675 |
| Glutathione metabolism | 0.0060079 | 2.2213 | 0.026034 | 0.10839 |
| Glyoxylate and dicarboxylate metabolism | 0.014336 | 1.8436 | 0.033617 | 0.13757 |
| Alanine, aspartate and glutamate metabolism | 0.016675 | 1.7779 | 0.033617 | 0.19712 |
| Glycine, serine and threonine metabolism | 0.016809 | 1.7745 | 0.033617 | 0.3323 |
| Inositol phosphate metabolism | 0.044451 | 1.3521 | 0.055034 | 0.12939 |
| Taurine and hypotaurine metabolism | 0.067794 | 1.1688 | 0.079131 | 0.42857 |
| Citrate cycle (TCA cycle) | 0.070001 | 1.1549 | 0.079131 | 0.12311 |
| Glycerolipid metabolism | 0.48687 | 0.31259 | 0.48687 | 0.23676 |
| Gene | Primer Sequence (5′-3′) | AT (°C) | Cycles |
|---|---|---|---|
| p53 | Sense: CTGCCCACCACAGCGACAGG Anti-sense: AGGAGCCAGGCCGTCACCAT | 60 | 35 |
| Casp-3 | Sense: AGGCCTGCCGAGGTACAGAGC Anti-Sense: CCGTGGCCACCTTCCGCTTA | 60 | 35 |
| Casp-9 | Sense: TGCAGGGTACGCCTTGTGCG Anti-Sense: CCTGATCCCGCCGAGACCCA | 60 | 35 |
| AIF | Sense: CGGCGGTGTGTGAAAAGAAA Anti-Sense: ATTTTGCCCCCTGATGGACC | 56 | 30 |
| BCL-2 | Sense: GGTGAACTGGGGGAGGATTG Anti-Sense: AGAGCGATGTTGTCCACCAG | 58 | 30 |
| β2-M | Sense: AGGTTTGAGGGGGAATGCTG Anti-sense: ATGAGTATGCCTGCCGTGTG | 58 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Spadella, M.A.; Moreira, R.J.; Braga, P.C.; Chies, A.B.; Oliveira, P.F.; Alves, M.G. Losartan Protects Against Radiation-Induced Testicular Damage by Modulating Oxidative Stress, Testosterone Levels, and Metabolic Profile. Pharmaceuticals 2026, 19, 76. https://doi.org/10.3390/ph19010076
Spadella MA, Moreira RJ, Braga PC, Chies AB, Oliveira PF, Alves MG. Losartan Protects Against Radiation-Induced Testicular Damage by Modulating Oxidative Stress, Testosterone Levels, and Metabolic Profile. Pharmaceuticals. 2026; 19(1):76. https://doi.org/10.3390/ph19010076
Chicago/Turabian StyleSpadella, Maria A., Rúben J. Moreira, Patrícia C. Braga, Agnaldo B. Chies, Pedro F. Oliveira, and Marco G. Alves. 2026. "Losartan Protects Against Radiation-Induced Testicular Damage by Modulating Oxidative Stress, Testosterone Levels, and Metabolic Profile" Pharmaceuticals 19, no. 1: 76. https://doi.org/10.3390/ph19010076
APA StyleSpadella, M. A., Moreira, R. J., Braga, P. C., Chies, A. B., Oliveira, P. F., & Alves, M. G. (2026). Losartan Protects Against Radiation-Induced Testicular Damage by Modulating Oxidative Stress, Testosterone Levels, and Metabolic Profile. Pharmaceuticals, 19(1), 76. https://doi.org/10.3390/ph19010076











