In Vivo Antihypertensive and Ex Vivo Vasodilatory Studies of Taxifolin
Abstract
1. Introduction
2. Result
2.1. Network Pharmacology and Molecular Docking Analysis
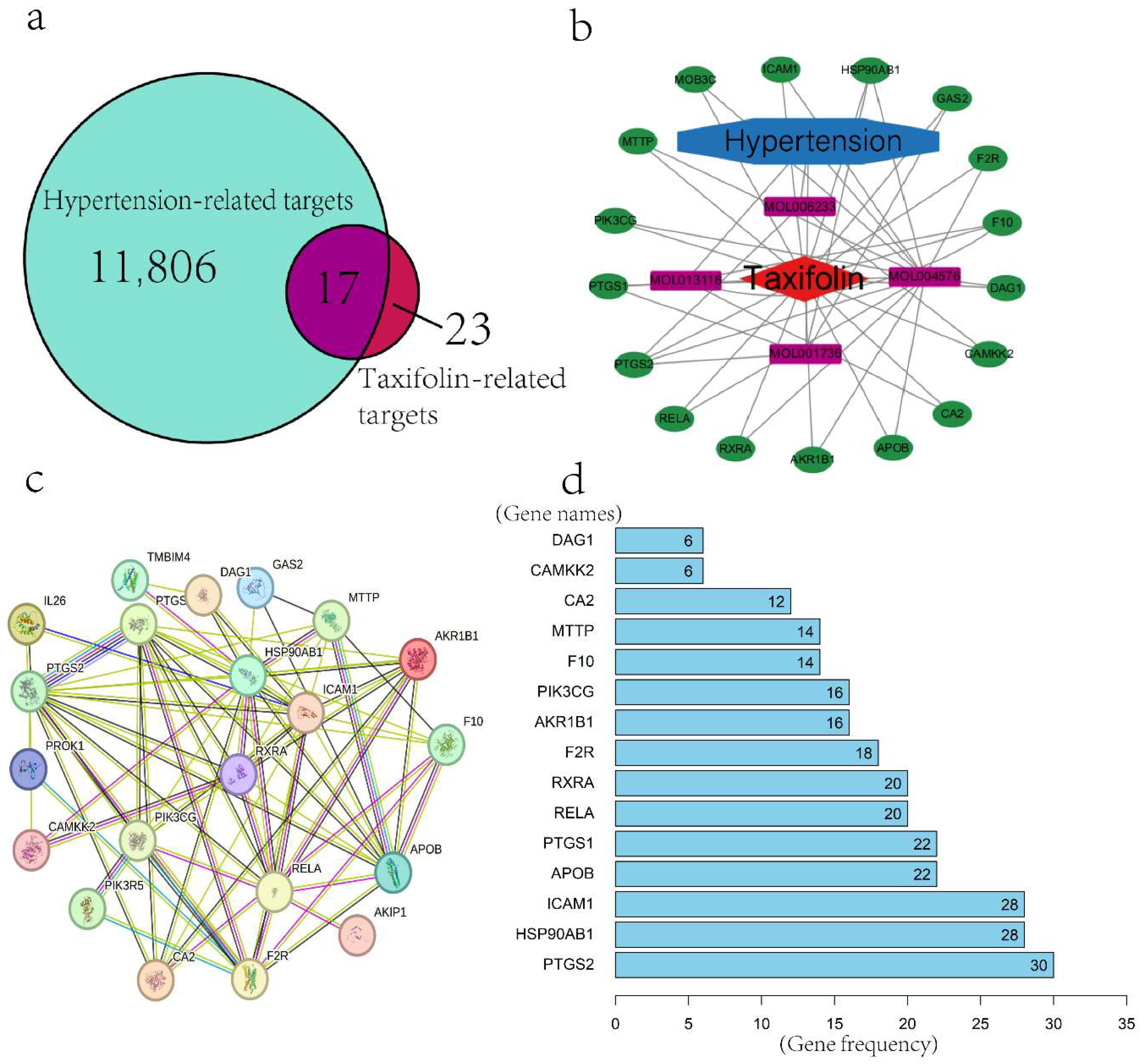
2.1.1. Screening of Active Components and Targets of Taxifolin
2.1.2. Construction of Drug–Disease–Target Network
2.1.3. Protein–Protein Interaction (PPI) Network and Core Gene Screening
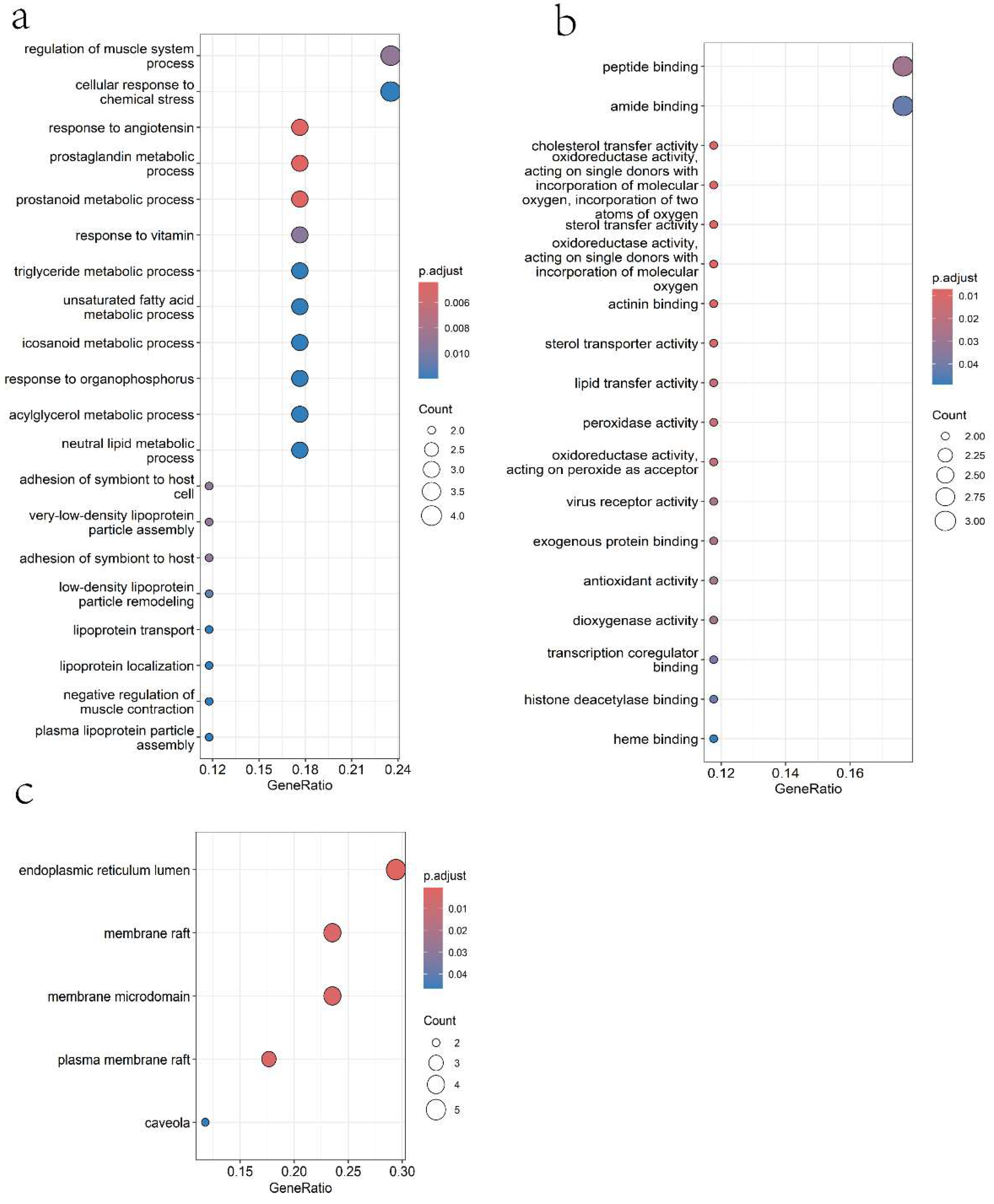
2.1.4. Gene Ontology (GO) Analysis
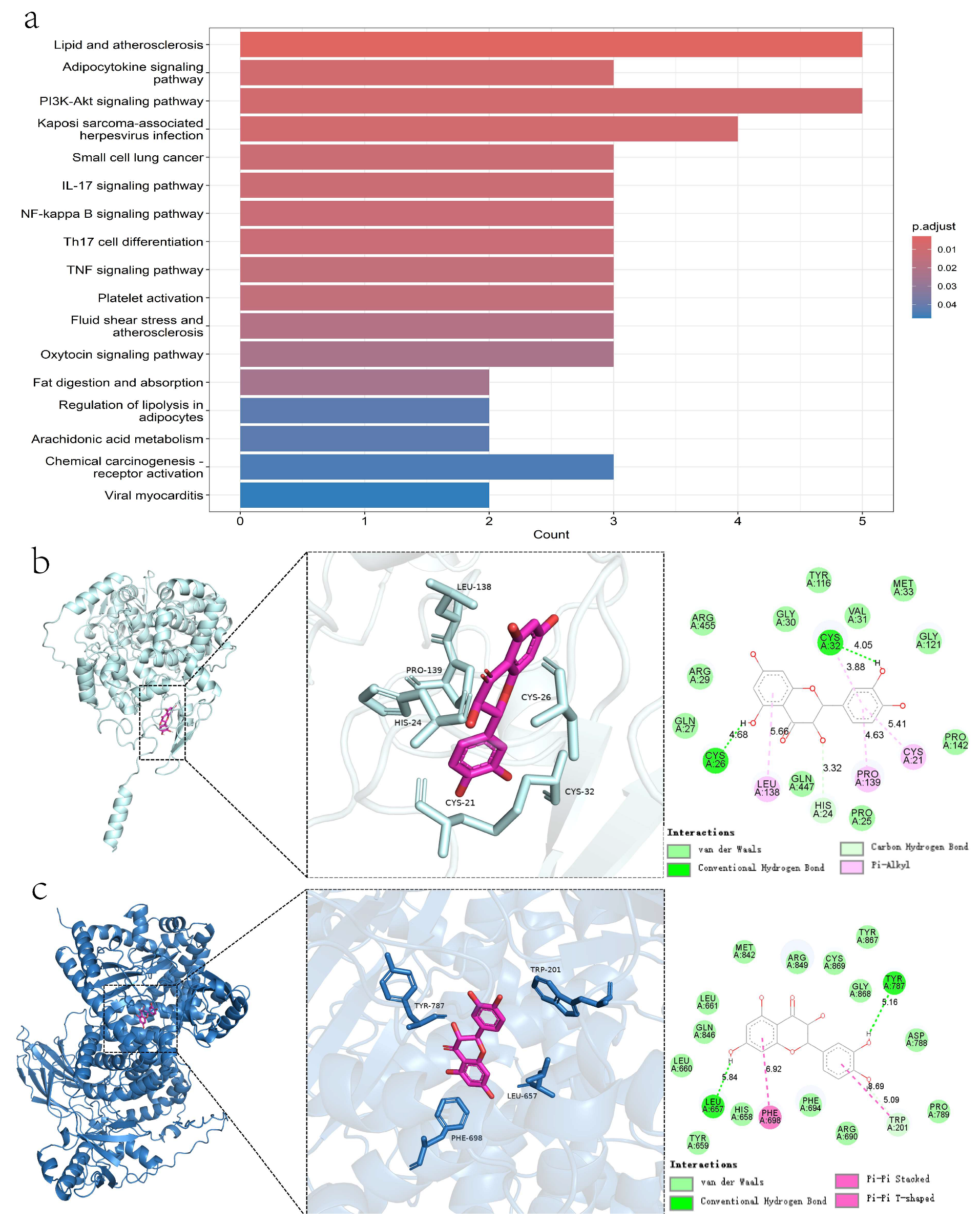
2.1.5. KEGG Pathway Enrichment Analysis
2.1.6. Molecular Docking
2.2. The Vasorelaxant Effects of Taxifolin Were Evaluated Using Isolated Rat Aortic Rings
2.3. Taxifolin Significantly Increased Nitric Oxide (NO) Levels and Activated Multiple Vasodilatory Pathways in Spontaneously Hypertensive Rats (SHRs)
2.4. In Vivo Antihypertensive Effects of Taxifolin on Spontaneously Hypertensive Rats (SHRs)
2.4.1. Antihypertensive Effect of Taxifolin on Spontaneously Hypertensive Rats
2.4.2. Toxicological Evaluation of Taxifolin
3. Discussion
4. Materials and Methods
4.1. Animal
4.2. Network Analysis of Taxifolin’s Potential Mechanisms Against Hypertension
4.3. Experimental Procedures for Assessing Taxifolin’s Vasorelaxant Mechanisms in Rat Aortic Rings
4.4. Ex Vivo Incubation of Rat Aortic Tissues for Mechanistic Analysis of Vasodilatory Signaling Pathways
4.5. In Vivo Evaluation of Taxifolin’s Effects and Toxicity in Spontaneously Hypertensive Rats
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soleimani, H.; Nasrollahizadeh, A.; Nasrollahizadeh, A.; Razeghian, I.; Molaei, M.M.; Hakim, D.; Nasir, K.; Al-Kindi, S.; Hosseini, K. Cardiovascular disease burden in the North Africa and Middle East region: An analysis of the global burden of disease study 1990–2021. BMC Cardiovasc. Disord. 2024, 24, 712. [Google Scholar] [CrossRef]
- Smith, A.; Tucker, K.L.; Barnes, R.K.; Drakesmith, C.W.; Agwunobi, A.; Bateman, P.A.; Forbes, A.; de Lusignan, S.; Ford, G.A.; Fujiwara, T.; et al. A service evaluation of the implementation of a novel digital intervention for hypertension self-monitoring and management system in primary care (SHIP): Protocol for a mixed methods study. BMC Cardiovasc. Disord. 2024, 24, 707. [Google Scholar] [CrossRef]
- Ching, S.M.; Lee, K.W.; Yusof Khan, A.H.K.; Devaraj, N.K.; Cheong, A.T.; Yap, S.F.; Hoo, F.K.; Wan Sulaiman, W.A.; Loh, W.C.; Chong, S.H.; et al. Prevalence and factors associated with peripheral neuropathy in a setting of retail pharmacies in Malaysia—A cross-sectional study. PLoS ONE 2024, 19, e0307093. [Google Scholar] [CrossRef]
- Taheri, S. Waking up to sleep extension for cardiometabolic health. Sleep 2024, 47, zsae016. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.A.; Jeong, S.Y.; Sohn, I.; Kim, M.S.; Kang, J.H.; Paik, E.S.; Lee, Y.Y.; Choi, C.H. Impact of Angiotensin Receptor Blockers, Beta Blockers, Calcium Channel Blockers and Thiazide Diuretics on Survival of Ovarian Cancer Patients. Cancer Res. Treat. 2020, 52, 645–654. [Google Scholar] [CrossRef]
- Azaredo Raposo, M.; Inacio Cazeiro, D.; Guimaraes, T.; Lousada, N.; Freitas, C.; Brito, J.; Martins, S.; Resende, C.; Dorfmuller, P.; Luis, R.; et al. Pulmonary arterial hypertension: Navigating the pathways of progress in diagnosis, treatment, and patient care. Rev. Port. Cardiol. 2024, 43, 699–719. [Google Scholar] [CrossRef]
- Fang, Z.; Raza, U.; Song, J.; Lu, J.; Yao, S.; Liu, X.; Zhang, W.; Li, S. Systemic aging fuels heart failure: Molecular mechanisms and therapeutic avenues. ESC Heart Fail. 2024, 12, 1059–1080. [Google Scholar] [CrossRef]
- Xu, S.; Han, X.; Wang, X.; Yu, Y.; Qu, C.; Liu, X.; Yang, B. The role of oxidative stress in aortic dissection: A potential therapeutic target. Front. Cardiovasc. Med. 2024, 11, 1410477. [Google Scholar] [CrossRef] [PubMed]
- Tunctan, B.; Korkmaz, B.; Sari, A.N.; Kacan, M.; Unsal, D.; Serin, M.S.; Buharalioglu, C.K.; Sahan-Firat, S.; Cuez, T.; Schunck, W.H.; et al. Contribution of iNOS/sGC/PKG pathway, COX-2, CYP4A1, and gp91(phox) to the protective effect of 5,14-HEDGE, a 20-HETE mimetic, against vasodilation, hypotension, tachycardia, and inflammation in a rat model of septic shock. Nitric Oxide 2013, 33, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.A.; Je, J.G.; Hwang, J.; Jeon, Y.J.; Ryu, B. Ecklonia cava Extract and Its Derivative Dieckol Promote Vasodilation by Modulating Calcium Signaling and PI3K/AKT/eNOS Pathway in In Vitro and In Vivo Models. Biomedicines 2021, 9, 438. [Google Scholar] [CrossRef]
- Bernatova, I.; Bartekova, M. Molecular Aspects of Cardiometabolic Diseases: From Etiopathogenesis to Potential Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 5841. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yang, R.; Xiong, X.; Yuan, Y.; Zhang, L.; Hua, C.; Wu, M.; Xiao, Y.; Zhang, R.; Liu, J.; et al. Taxifolin Inhibits Platelet Activation and Thrombosis by Regulating the PI3K/Akt and MAPK Signaling Pathways. Mol. Nutr. Food Res. 2025, 69, e70129. [Google Scholar] [CrossRef]
- Liskova, S.; Cacanyiova, S.; Cebova, M.; Berenyiova, A.; Kluknavsky, M.; Micurova, A.; Valachova, K.; Soltes, L.; Bernatova, I. Taxifolin Reduces Blood Pressure via Improvement of Vascular Function and Mitigating the Vascular Inflammatory Response in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2023, 24, 12616. [Google Scholar] [CrossRef]
- Flores, J.; Nugent, K. Sodium, the Vascular Endothelium, and Hypertension: A Narrative Review of Literature. Cardiol. Rev. 2025. [Google Scholar] [CrossRef] [PubMed]
- Golan, S.; Entin-Meer, M.; Semo, Y.; Maysel-Auslender, S.; Mezad-Koursh, D.; Keren, G.; Loewenstein, A.; Barak, A. Gene profiling of human VEGF signaling pathways in human endothelial and retinal pigment epithelial cells after anti VEGF treatment. BMC Res. Notes 2014, 7, 617. [Google Scholar] [CrossRef]
- Kim, D.I.; Kim, S.R.; Kim, H.J.; Lee, S.J.; Lee, H.B.; Park, S.J.; Im, M.J.; Lee, Y.C. PI3K-γ inhibition ameliorates acute lung injury through regulation of IκBα/NF-κB pathway and innate immune responses. J. Clin. Immunol. 2012, 32, 340–351. [Google Scholar] [CrossRef]
- Wang, Y.G.; Dedkova, E.N.; Ji, X.; Blatter, L.A.; Lipsius, S.L. Phenylephrine acts via IP3-dependent intracellular NO release to stimulate L-type Ca2+ current in cat atrial myocytes. J. Physiol. 2005, 567, 143–157. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Li, Z.; Sang, Y.; Niu, Y.; Zhang, Q.; Ding, H.; Yin, S. Fucoidan from Undaria pinnatifida prevents vascular dysfunction through PI3K/Akt/eNOS-dependent mechanisms in the l-NAME-induced hypertensive rat model. Food Funct. 2016, 7, 2398–2408. [Google Scholar] [CrossRef]
- Narang, D.; Kerr, P.M.; Baserman, J.; Tam, R.; Yang, W.; Searle, G.; Manning-Fox, J.E.; Paulsen, I.M.; Kozuska, J.L.; MacDonald, P.E.; et al. Triton X-100 inhibits L-type voltage-operated calcium channels. Can. J. Physiol. Pharmacol. 2013, 91, 316–324. [Google Scholar] [CrossRef]
- Lin, A.; Piao, H.-L.; Zhuang, L.; Sarbassov, D.D.; Ma, L.; Gan, B. FoxO transcription factors promote AKT Ser473 phosphorylation and renal tumor growth in response to pharmacologic inhibition of the PI3K-AKT pathway. Cancer Res. 2014, 74, 1682–1693. [Google Scholar] [CrossRef]
- Lee, C.-W.; Lin, C.-C.; Luo, S.-F.; Lee, H.-C.; Lee, I.-T.; Aird, W.C.; Hwang, T.-L.; Yang, C.-M. Tumor necrosis factor-α enhances neutrophil adhesiveness: Induction of vascular cell adhesion molecule-1 via activation of Akt and CaM kinase II and modifications of histone acetyltransferase and histone deacetylase 4 in human tracheal smooth muscle cells. Mol. Pharmacol. 2008, 73, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Role of Dietary Polyphenols in the Activity and Expression of Nitric Oxide Synthases: A Review. Antioxidants 2023, 12, 147. [Google Scholar] [CrossRef]
- Qi, D.H.; Ma, H.; Chen, Y.Y.; Wang, K.X.; Ding, M.M.; Hao, Y.L.; Guo, Y.; Kong, L.B. Research progress in mechanism of puerarin in treating vascular dementia. Zhongguo Zhong Yao Za Zhi 2023, 48, 5993–6002. [Google Scholar] [CrossRef]
- Mann, G.E.; Rowlands, D.J.; Li, F.Y.; de Winter, P.; Siow, R.C. Activation of endothelial nitric oxide synthase by dietary isoflavones: Role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc. Res. 2007, 75, 261–274. [Google Scholar] [CrossRef]
- Sakkinen, H.; Luosujarvi, H.; Karvonen, T.; Mustonen, E.; Moilanen, A.-M.; Aro, J.; Napankangas, J.; Ruskoaho, H.; Rysa, J. Transcriptional cofactor dyxin mediates hypertrophic response in the heart during angiotensin II-induced hypertension. J. Physiol. Pharmacol. 2023, 74, 623–632. [Google Scholar] [CrossRef]
- Ballesteros-Martinez, C.; Rodrigues-Diez, R.; Beltran, L.M.; Moreno-Carriles, R.; Martinez-Martinez, E.; Gonzalez-Amor, M.; Martinez-Gonzalez, J.; Rodriguez, C.; Cachofeiro, V.; Salaices, M.; et al. Microsomal prostaglandin E synthase-1 is involved in the metabolic and cardiovascular alterations associated with obesity. Br. J. Pharmacol. 2022, 179, 2733–2753. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Anthanont, P.; Asztalos, B.F. High-density lipoprotein metabolism, composition, function, and deficiency. Curr. Opin. Lipidol. 2014, 25, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Genest, J.; Schwertani, A.; Choi, H.Y. Membrane microdomains and the regulation of HDL biogenesis. Curr. Opin. Lipidol. 2018, 29, 36–41. [Google Scholar] [CrossRef]
- Isik, O.A.; Cizmecioglu, O. Rafting on the Plasma Membrane: Lipid Rafts in Signaling and Disease. Adv. Exp. Med. Biol. 2023, 1436, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Crul, T.; Maleth, J. Endoplasmic Reticulum-Plasma Membrane Contact Sites as an Organizing Principle for Compartmentalized Calcium and cAMP Signaling. Int. J. Mol. Sci. 2021, 22, 4703. [Google Scholar] [CrossRef]
- Ibrahim, M.; Derbyshire, E.R.; Soldatova, A.V.; Marletta, M.A.; Spiro, T.G. Soluble guanylate cyclase is activated differently by excess NO and by YC-1: Resonance Raman spectroscopic evidence. Biochemistry 2010, 49, 4864–4871. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Czyz, A.; Nalecz, M.J. ATP-regulated potassium channel blocker, glibenclamide, uncouples mitochondria. Pol. J. Pharmacol. 1997, 49, 49–52. [Google Scholar] [PubMed]
- Siri-Angkul, N.; Kamp, T.J. Cardiac L-type calcium channel regulation by Leucine-Rich Repeat-Containing Protein 10. Channels 2024, 18, 2355121. [Google Scholar] [CrossRef]
- Omokawa, S.; Tamai, S.; Ohneda, Y.; Mizumoto, S.; Yamamoto, S. A histopathological study on the femoral head necrosis in spontaneously hypertensive rats (SHR). Nihon Seikeigeka Gakkai Zasshi 1992, 66, 69–82. [Google Scholar] [PubMed]
- Ding, L.; Jia, C.; Zhang, Y.; Wang, W.; Zhu, W.; Chen, Y.; Zhang, T. Baicalin relaxes vascular smooth muscle and lowers blood pressure in spontaneously hypertensive rats. Biomed. Pharmacother. 2019, 111, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Chakrabarti, S.; Pereira, T.J.; Oka, T.; Levasseur, J.; Beker, D.; Zordoky, B.N.; Morton, J.S.; Nagendran, J.; Lopaschuk, G.D.; et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 1723–1733. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Jiang, X.; Ruan, Y.; Wang, J.; Qi, C. The potential mechanism of Guizhi Fuling Wan effect in the treatment of cervical squamous cell carcinoma: A bioinformatics analysis investigation. Medicine 2024, 103, e37153. [Google Scholar] [CrossRef]
- Tao, W.; Xu, X.; Wang, X.; Li, B.; Wang, Y.; Li, Y.; Yang, L. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J. Ethnopharmacol. 2013, 145, 1–10. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 2012, 13, 6964–6982. [Google Scholar] [CrossRef]
- Wu, X.; Cao, S.; Zou, Y.; Wu, F. Traditional Chinese Medicine studies for Alzheimer’s disease via network pharmacology based on entropy and random walk. PLoS ONE 2023, 18, e0294772. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wang, Z.; Yang, J.; Pan, P.; Shi, T.; Xu, S.; Lan, J.; Hao, Z.; Yang, A.; Chen, L.; et al. Mechanism of Ligusticum wallichii-Borneol in the Treatment of Cerebral Ischemic Stroke in Rats Based On Network Pharmacology, Molecular Docking, and Experimental Verification. Chem. Biodivers. 2025, 22, e202401893. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, J.; Chen, X.; Zhang, X.H. Exploring the mechanism of luteolin improving immune and inflammatory responses in systemic sclerosis based on systems biology and cell experiments. Int. Immunopharmacol. 2024, 138, 112587. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Zhou, Q.; Zhang, P.; Xie, Y.Q.; Yang, Z.Y.; Tan, W.H.; Khan, A.; Duan, W.G.; Zhou, Z.H.; Liu, L. Integrating multi-level interactive network and in vivo/vitro studies to explore the protective mechanism of Ampelopsis grossedentata in hyperuricemia. Fitoterapia 2024, 172, 105718. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, J.; Zhou, Y. Network pharmacology analysis of molecular targets and related mechanisms of Guizhi decoction in treating of menopausal syndrome. Medicine 2022, 101, e29453. [Google Scholar] [CrossRef]
- Du, X.; Di, Z.; Liu, Y.; Zhi, W.; Liu, Y.; Zhang, H.; Liu, F. Identification of Constituents and Exploring the Mechanism for Toutongning Capsule in the Treatment of Migraine. Evid.-Based Complement. Alternat Med. 2022, 2022, 5528845. [Google Scholar] [CrossRef]
- Darko, L.K.S.; Broni, E.; Amuzu, D.S.Y.; Wilson, M.D.; Parry, C.S.; Kwofie, S.K. Computational Study on Potential Novel Anti-Ebola Virus Protein VP35 Natural Compounds. Biomedicines 2021, 9, 1796. [Google Scholar] [CrossRef]
- Shi, T.; Hou, C.; Duan, Y.; Li, Y.; Liu, W.; Huang, P.; Zhou, Y.; Yu, S.; Song, L. Mechanism of Smilax china L. in the treatment of intrauterine adhesions based on network pharmacology, molecular docking and experimental validation. BMC Complement. Med. Ther. 2024, 24, 150. [Google Scholar] [CrossRef]
- Duan, Z.L.; Wang, Y.J.; Lu, Z.H.; Tian, L.; Xia, Z.Q.; Wang, K.L.; Chen, T.; Wang, R.; Feng, Z.Y.; Shi, G.P.; et al. Wumei Wan attenuates angiogenesis and inflammation by modulating RAGE signaling pathway in IBD: Network pharmacology analysis and experimental evidence. Phytomedicine 2023, 111, 154658. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, Y.; Liao, L.; Yu, L.; Yuan, P.; Zhang, J. Network Pharmacology and Transcriptomics Reveal the Mechanism of GuaLouQuMaiWan in Treatment of Type 2 Diabetes and Its Active Small Molecular Compound. J. Diabetes Res. 2022, 2022, 2736504. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Tew, W.Y.; Tan, C.S.; Qiu, Q.; Zhou, R.; Wang, X.; Yap, Y.P.; Xu, W.; Xu, W.; Teh, L.K. Investigation of synergistic interaction of sinensetin, eupatorin, and 3′-hydroxy-5,6,7,4′-tetramethoxyflavone in vasodilation efficacy. Hypertens. Res. 2024, 47, 3193–3199. [Google Scholar] [CrossRef]
- Ch’ng, Y.S.; Loh, Y.C.; Tan, C.S.; Ahmad, M.; Asmawi, M.Z.; Wan Omar, W.M.; Yam, M.F. Vasorelaxant properties of Vernonia amygdalina ethanol extract and its possible mechanism. Pharm. Biol. 2017, 55, 2083–2094. [Google Scholar] [CrossRef]
- Ch’ng, Y.S.; Loh, Y.C.; Tan, C.S.; Ahmad, M.; Asmawi, M.Z.; Wan Omar, W.M.; Yam, M.F. Vasodilation and Antihypertensive Activities of Swietenia macrophylla (Mahogany) Seed Extract. J. Med. Food 2018, 21, 289–301. [Google Scholar] [CrossRef]
- Yam, M.F.; Tan, C.S.; Shibao, R. Vasorelaxant effect of sinensetin via the NO/sGC/cGMP pathway and potassium and calcium channels. Hypertens. Res. 2018, 41, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.C.; Tan, C.S.; Ch’ng, Y.S.; Ahmad, M.; Ng, C.H.; Yam, M.F. Overview of Signaling Mechanism Pathways Employed by BPAid in Vasodilatory Activity. J. Med. Food 2017, 20, 1201–1213. [Google Scholar] [CrossRef]
- Tew, W.Y.; Tan, C.S.; Yan, C.S.; Loh, H.W.; Wen, X.; Wei, X.; Yam, M.F. Evaluation of vasodilatory effect and antihypertensive effect of chrysin through in vitro and sub-chronic in vivo study. Biomed. Pharmacother. 2023, 157, 114020. [Google Scholar] [CrossRef] [PubMed]
- Tew, W.Y.; Tan, C.S.; Asmawi, M.Z.; Yam, M.F. Underlying mechanism of vasorelaxant effect exerted by 3,5,7,2′,4′-pentahydroxyflavone in rats aortic ring. Eur. J. Pharmacol. 2020, 880, 173123. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Yam, M.F. Mechanism of vasorelaxation induced by 3′-hydroxy-5,6,7,4′-tetramethoxyflavone in the rats aortic ring assay. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 561–569. [Google Scholar] [CrossRef]
- Tan, C.S.; Loh, Y.C.; Tew, W.Y.; Yam, M.F. Vasorelaxant effect of 3,5,4′-trihydroxy-trans-stilbene (resveratrol) and its underlying mechanism. Inflammopharmacology 2020, 28, 869–875. [Google Scholar] [CrossRef]
- Tan, C.S.; Loh, Y.C.; Ng, C.H.; Ch’ng, Y.S.; Asmawi, M.Z.; Ahmad, M.; Yam, M.F. Anti-hypertensive and vasodilatory effects of amended Banxia Baizhu Tianma Tang. Biomed. Pharmacother. 2018, 97, 985–994. [Google Scholar] [CrossRef]
- Tan, C.S.; Loh, Y.C.; Ch’ng, Y.S.; Ng, C.H.; Yeap, Z.Q.; Ahmad, M.; Asmawi, M.Z.; Yam, M.F. Vasorelaxant and chemical fingerprint studies of Citrus reticulatae pericarpium extracts. J. Ethnopharmacol. 2019, 232, 135–144. [Google Scholar] [CrossRef]
- Tan, C.S.; Ch’ng, Y.S.; Loh, Y.C.; Zaini Asmawi, M.; Ahmad, M.; Yam, M.F. Vasorelaxation effect of Glycyrrhizae uralensis through the endothelium-dependent Pathway. J. Ethnopharmacol. 2017, 199, 149–160. [Google Scholar] [CrossRef]
- Loh, Y.C.; Tan, C.S.; Ch’ng, Y.S.; Yeap, Z.Q.; Ng, C.H.; Yam, M.F. Overview of the Microenvironment of Vasculature in Vascular Tone Regulation. Int. J. Mol. Sci. 2018, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Templeton, E.M.; Pilbrow, A.P.; Kleffmann, T.; Pickering, J.W.; Rademaker, M.T.; Scott, N.J.A.; Ellmers, L.J.; Charles, C.J.; Endre, Z.H.; Richards, A.M.; et al. Comparison of SPEED, S-Trap, and In-Solution-Based Sample Preparation Methods for Mass Spectrometry in Kidney Tissue and Plasma. Int. J. Mol. Sci. 2023, 24, 6290. [Google Scholar] [CrossRef]
- Trindade, F.; Ferreira, A.F.; Saraiva, F.; Martins, D.; Mendes, V.M.; Sousa, C.; Gavina, C.; Leite-Moreira, A.; Manadas, B.; Falcao-Pires, I.; et al. Optimization of a Protocol for Protein Extraction from Calcified Aortic Valves for Proteomics Applications: Development of a Standard Operating Procedure. Proteomes 2022, 10, 30. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Choi, W.S.; Kwon, O.S.; Lee, J.S.; Son, S.Y.; Lee, C.H.; Lee, S.; Song, J.Y.; Lee, Y.J.; Lee, J.Y. Extract of Pinus densiflora needles suppresses acute inflammation by regulating inflammatory mediators in RAW264.7 macrophages and mice. Pharm. Biol. 2022, 60, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, J.; Guan, Y.; Cai, W.; Tang, W.; Luo, M. Effect of atorvastatin on LOX-1 and eNOS expression in collateral vessels of hypercholesterolemic rats. Nan Fang Yi Ke Da Xue Xue Bao J. Sourthern Med. Univ. 2019, 39, 1265–1272. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, D.L.; Zheng, Y.L.; Li, Y.X.; Huang, Y.J.; Jiang, Y.J.; Jin, R.J.; Li, J. Influence of electroacupuncture on ghrelin and the phosphoinositide 3-kinase/protein kinase B/endothelial nitric oxide synthase signaling pathway in spontaneously hypertensive rats. J. Integr. Med. 2022, 20, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jin, C.; Han, T.; Chen, J.; Ali Raza, M.; Li, B.; Wang, L.; Yan, H. Sinomenine promotes flap survival by upregulating eNOS and eNOS-mediated autophagy via PI3K/AKT pathway. Int. Immunopharmacol. 2023, 116, 109752. [Google Scholar] [CrossRef]
- Alanazi, W.A.; Alharbi, T.; El-Nagar, D.M.; Albogami, A.M.; Alswayyed, M. Dapagliflozin Mitigates Hypotension in Lipopolysaccharide-Induced Acute Inflammation Independent of Glycemia Level. Pharmaceutics 2023, 15, 1683. [Google Scholar] [CrossRef]
- Asiwe, J.N.; Ajayi, A.M.; Ben-Azu, B.; Fasanmade, A.A. Vincristine attenuates isoprenaline-induced cardiac hypertrophy in male Wistar rats via suppression of ROS/NO/NF-қB signalling pathways. Microvasc. Res. 2024, 155, 104710. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Tew, W.Y.; Jingying, C.; Yam, M.F. Vasorelaxant effect of 5,7,4′-Trihydroxyflavanone (Naringenin) via endothelium dependent, potassium and calcium channels in Sprague Dawley rats: Aortic ring model. Chem. Biol. Interact. 2021, 348, 109620. [Google Scholar] [CrossRef] [PubMed]
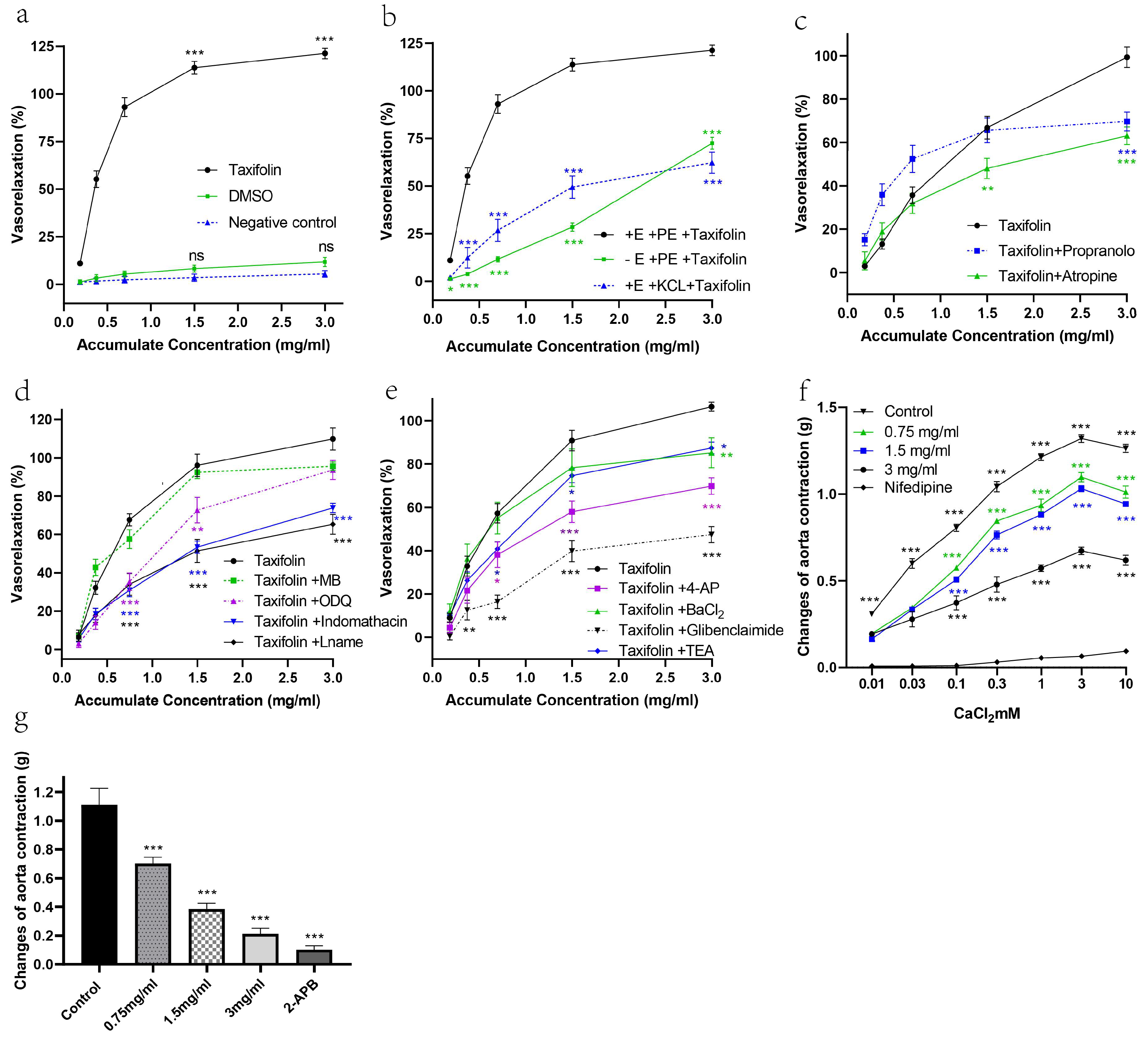


| Aortic Ring Condition/Antagonist | Taxifolin | |
|---|---|---|
| Rmax (%) | EC50 (mg/mL) | |
| KCl-induced vasoconstriction | 62.26 ± 5.59 *** | 0.58 ± 0.15 |
| Endothelium-intact aortic ring (Taxifolin) | 121.2 ± 2.82 | 0.31 ± 0.01 |
| TEA | 87.46 ± 2.76 * | 0.48 ± 0.15 |
| Propranolol | 69.7 ± 4.30 *** | 0.37 ± 0.11 |
| L-NAME | 65.33 ± 5.18 *** | 0.49 ± 0.17 |
| ODQ | 93.62 ± 4.99 | 0.59 ± 0.16 |
| Indomethacin | 73.82 ± 2.45 *** | 0.46 ± 0.15 |
| Atropine | 63.1 ± 4.10 *** | 0.50 ± 0.08 |
| Glibenclamide | 47.44 ± 3.71 *** | 0.60 ± 0.14 |
| 4-AP | 69.87 ± 3.81 *** | 0.50 ± 0.14 |
| Methylene blue | 95.62 ± 2.30 | 0.44 ± 0.09 |
| BaCl2 | 85.21 ± 5.9 ** | 0.42 ± 0.24 |
| DMSO | 11.75 ± 1.2 | N/A |
| Endothelium-denuded aortic ring | 72.47 ± 3.14 *** | 1.93 ± 0.02 |
| Taxifolin | |||||
|---|---|---|---|---|---|
| Parameters | Negative Control (1.25% DMSO) | Positive Control (Propranolol) | 15 mg/kg | 30 mg/kg | 60 mg/kg |
| Potassium (mmol/L) | 4.72 ± 0.18 | 4.69 ± 0.18 | 4.55 ± 0.07 | 4.63 ± 0.13 | 4.80 ± 0.19 |
| Platelet Count (×109/L) | 860.54 ± 75.32 | 746.22 ± 82.19 | 868.36 ± 78.42 | 839.95 ± 79.21 | 727.56 ± 81.72 |
| Sodium (mmol/L) | 146.13 ± 1.78 | 146.22 ± 1.72 | 145.16 ± 1.84 | 145.39 ± 1.68 | 145.51 ± 1.67 |
| Chloride (mmol/L) | 105.88 ± 1.23 | 105.65 ± 1.39 | 105.36 ± 1.26 | 105.50 ± 1.29 | 105.97 ± 1.36 |
| Creatinine (µmol/L) | 27.23 ± 1.34 | 26.92 ± 1.21 | 25.61 ± 1.01 | 26.25 ± 1.18 | 27.36 ± 1.29 |
| Blood Urea Nitrogen (mmol/L) | 6.10 ± 0.36 | 6.89 ± 0.39 | 6.25 ± 0.38 | 6.60 ± 0.41 | 5.27 ± 0.37 |
| High-Density Lipoprotein (mmol/L) | 1.00 ± 0.11 | 1.21 ± 0.13 | 1.09 ± 0.11 | 1.20 ± 0.11 | 1.13 ± 0.12 |
| Direct Bilirubin (µmol/L) | 0.97 ± 0.08 | 0.97 ± 0.06 | 0.87 ± 0.05 | 0.68 ± 0.05 | 0.50 ± 0.05 |
| Lymphocyte Percentage (%) | 74.12 ± 3.24 | 72.98 ± 3.64 | 74.09 ± 3.23 | 73.27 ± 3.19 | 73.54 ± 3.34 |
| Aspartate Aminotransferase (U/L) | 104.92 ± 11.38 | 121.45 ± 13.45 | 115.98 ± 12.98 | 121.48 ± 11.76 | 114.90 ± 12.43 |
| Gamma-Glutamyl Transferase (U/L) | 3.13 ± 0.50 | 2.84 ± 0.48 | 3.21 ± 0.46 | 3.04 ± 0.49 | 3.56 ± 0.51 |
| Alkaline Phosphatase (U/L) | 100.41 ± 8.29 | 110.34 ± 9.23 | 115.42 ± 8.79 | 109.51 ± 9.06 | 98.65 ± 9.03 |
| Lactate Dehydrogenase (U/L) | 350.15 ± 25.79 | 338.76 ± 27.12 | 342.67 ± 24.67 | 349.21 ± 25.12 | 358.21 ± 25.87 |
| Triglycerides (mmol/L) | 0.75 ± 0.09 | 0.84 ± 0.08 | 0.69 ± 0.07 | 0.82 ± 0.08 | 0.76 ± 0.09 |
| Cholesterol (mmol/L) | 1.51 ± 0.14 | 1.62 ± 0.15 | 1.43 ± 0.13 | 1.49 ± 0.14 | 1.53 ± 0.14 |
| Low-Density Lipoprotein (mmol/L) | 0.51 ± 0.08 | 0.45 ± 0.09 | 0.48 ± 0.07 | 0.54 ± 0.08 | 0.52 ± 0.08 |
| White Blood Cell Count (×109/L) | 8.53 ± 1.02 | 8.76 ± 1.10 | 8.91 ± 1.01 | 8.89 ± 1.08 | 8.65 ± 1.07 |
| Red Blood Cell Count (×1012/L) | 8.05 ± 0.54 | 8.11 ± 0.48 | 7.85 ± 0.52 | 7.93 ± 0.53 | 7.92 ± 0.52 |
| Hemoglobin Concentration (g/L) | 151.21 ± 12.52 | 134.57 ± 11.42 | 147.28 ± 10.84 | 148.20 ± 11.32 | 142.72 ± 10.84 |
| Hematocrit (L/L) | 0.42 ± 0.02 | 0.46 ± 0.01 | 0.39 ± 0.02 | 0.41 ± 0.02 | 0.46 ± 0.01 |
| Mean Corpuscular Volume (fL) | 52.31 ± 2.14 | 56.71 ± 2.03 | 50.19 ± 1.98 | 51.04 ± 1.97 | 57.99 ± 1.87 |
| Mean Platelet Volume (fL) | 6.33 ± 0.64 | 8.41 ± 0.56 | 6.98 ± 0.59 | 7.55 ± 0.57 | 7.15 ± 0.59 |
| Albumin/Globulin Ratio | 1.18 ± 0.10 | 1.50 ± 0.12 | 1.87 ± 0.13 | 1.73 ± 0.12 | 1.80 ± 0.13 |
| Total Protein (g/L) | 70.78 ± 5.32 | 75.81 ± 5.12 | 66.37 ± 4.89 | 72.51 ± 5.21 | 77.71 ± 5.32 |
| Albumin (g/L) | 38.5 ± 3.10 | 45.5 ± 3.01 | 43.3 ± 3.02 | 45.9 ± 3.19 | 49.9 ± 3.27 |
| Globulin (g/L) | 33.33 ± 2.45 | 30.33 ± 2.21 | 23.1 ± 2.01 | 26.6 ± 2.04 | 27.8 ± 2.03 |
| Total Bilirubin (µmol/L) | 3.92 ± 0.47 | 2.25 ± 0.48 | 2.71 ± 0.42 | 4.69 ± 0.47 | 3.81 ± 0.48 |
| Alanine Aminotransferase (U/L) | 55.60 ± 5.74 | 33.51 ± 6.15 | 42.21 ± 5.81 | 23.75 ± 5.94 | 43.13 ± 5.21 |
| Neutrophil Percentage (%) | 20.74 ± 2.16 | 22.14 ± 1.93 | 21.67 ± 2.24 | 21.98 ± 2.03 | 21.76 ± 2.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xu, X.; Tew, W.Y.; Ouyang, L.; Yang, X.; Loh, H.W.; Xu, W.; Xu, W.; Yam, M.F. In Vivo Antihypertensive and Ex Vivo Vasodilatory Studies of Taxifolin. Pharmaceuticals 2025, 18, 1420. https://doi.org/10.3390/ph18091420
Wang X, Xu X, Tew WY, Ouyang L, Yang X, Loh HW, Xu W, Xu W, Yam MF. In Vivo Antihypertensive and Ex Vivo Vasodilatory Studies of Taxifolin. Pharmaceuticals. 2025; 18(9):1420. https://doi.org/10.3390/ph18091420
Chicago/Turabian StyleWang, Xuye, Xiangyang Xu, Wan Yin Tew, Liyun Ouyang, Xiaoning Yang, Hui Wei Loh, Wen Xu, Wei Xu, and Mun Fei Yam. 2025. "In Vivo Antihypertensive and Ex Vivo Vasodilatory Studies of Taxifolin" Pharmaceuticals 18, no. 9: 1420. https://doi.org/10.3390/ph18091420
APA StyleWang, X., Xu, X., Tew, W. Y., Ouyang, L., Yang, X., Loh, H. W., Xu, W., Xu, W., & Yam, M. F. (2025). In Vivo Antihypertensive and Ex Vivo Vasodilatory Studies of Taxifolin. Pharmaceuticals, 18(9), 1420. https://doi.org/10.3390/ph18091420







