Cardiotoxicity Induced by Anticancer Therapies: A Call for Integrated Cardio-Oncology Practice
Abstract
1. Introduction
2. Therapeutic Agents Associated with Cardiotoxicity
2.1. Chemotherapy-Induced Cardiotoxicity
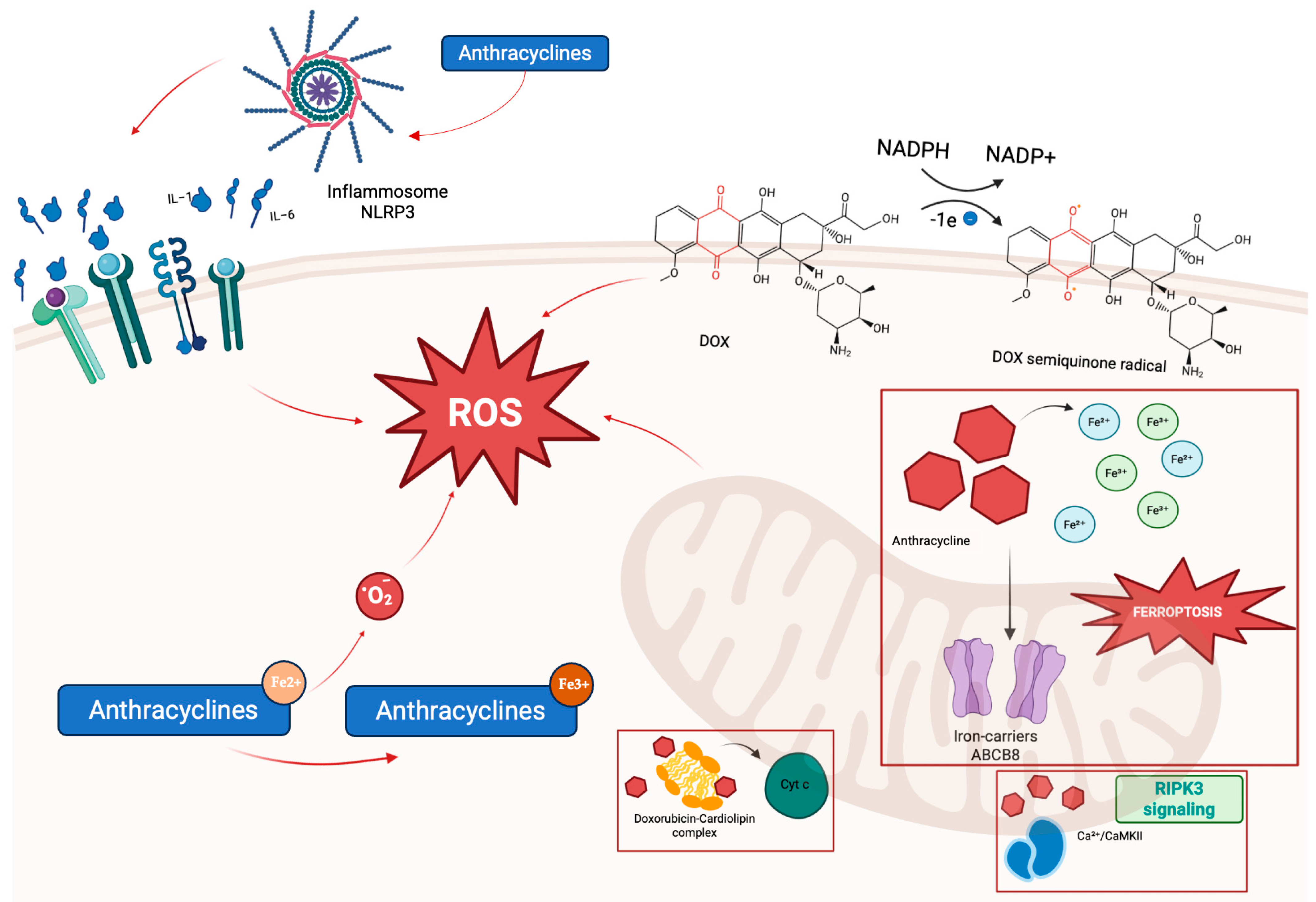
2.1.1. Anthracyclines
- Mitochondrial iron overload, resulting from chelation of intracellular iron and interference with iron regulatory proteins, including ferritin and the mitochondrial iron exporter ATP-binding cassette, subfamily B, member 8 (ABCB8), which promotes ferroptosis, a regulated cell death dependent on iron and lipid peroxidation. Doxorubicin also downregulates glutathione peroxidase 4 (GPx4), further facilitating ferroptotic injury [29].
- Cardiolipin disruption, as doxorubicin binds cardiolipin, destabilizing its association with cytochrome c and impairing mitochondrial membrane integrity and apoptotic signaling [7].
- Activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) via receptor-interacting protein kinase 3 (RIPK3) signaling, leading to opening of the mitochondrial permeability transition pore (mPTP) and necroptotic cell death, demonstrated in experimental mouse studies [24].
2.1.2. Fluoropirimidin
2.1.3. Taxanes
2.1.4. Alkylating Drugs
2.2. Cardiotoxicity Induced by Targeted Therapy
2.2.1. Anti-HER2
2.2.2. Tyrosine Kinase Inhibitors (TKIs)
2.3. Cardiotoxicity Induced by Hormonal Agents
2.4. Immuno-Related Cardiovascular Adverse Events (irAEs)
2.5. Cardiotoxicity Induced by Proteasome Inhibitors
2.6. Cardiotoxicity by New Classes of Drugs
3. Diagnostic Strategies and Risk Stratification
3.1. Baseline Risk Stratification
3.2. Cancer-Therapy-Related Risk Stratification
3.3. Electrocardiogram (ECG)
3.4. Echocardiography
3.5. Serum Biomarkers
3.6. Advanced Diagnostic Strategies
4. Management of Cardiotoxicity and Surveillance Programs
4.1. Pharmacological Cardioprotection
| Drug Class | Mechanism of Action | Primary Indication | Evidence Level | Guideline Recommendations |
|---|---|---|---|---|
| RAS Inhibitors (ACEi/ARBs) | Afterload reduction, anti-remodeling, neurohormonal attenuation | Elevated troponin post-anthracycline; high-risk patients | Mixed: Cardinale 2006 [129]; PRADA [130]; SAFE [134] | ESC 2021: consider in patients with LVEF drop ≥ 10% and to a value lower than 50% (Class IIa, LOE B) [47]; AHA 2022: uncertain benefit (Class IIb) [161] |
| Beta-Blockers | Sympathetic blockade, antioxidant effects, improved diastolic function | High-risk or early subclinical dysfunction; combined with RAS inhibitors | Mixed: CECCY [131]; Cardiac CARE [132]; meta-analyses [133] | ESC 2021: preferably carvedilol if LVEF < 50% [47]; ESMO 2020: may be considered in high-risk patients (Class IIb) [114]; AHA 2022: uncertain benefit (Class IIb) [161] |
| SGLT2i | Mitochondrial protection, anti-inflammatory, AMPK activation | Pre-existing HFrEF, high cardiotoxic risk therapies, early dysfunction | Growing: preclinical, Bhalraam 2025 [137], Greco 2025 [138] | Recommended in HFrEF and high-risk therapy patients; emerging role in early dysfunction [138] |
| Dexrazoxane | Topoisomerase II inhibition, iron chelation | High cumulative anthracycline dose | Strong: Swain 2003 [147]; Lipshultz 1995 [148] | ESC 2022: high and very high CV toxicity risk when anthracycline chemotherapy is indicated (Class IIa, LOE B) [10] |
| MRAs (Spironolactone, Eplerenone) | Aldosterone blockade, anti-fibrotic effects | Reduced LVEF or overt heart failure with concurrent indication | Limited: small trials/observational studies | No formal guideline recommendation; consider in HFrEF per general HF guidelines [47] |
| Statins | Anti-inflammatory, endothelial function improvement | Under investigation for primary prevention in anthracycline therapy | Weak/modest: meta-analysis; Raisi-Estabragh 2024 [140] | No official recommendation; not routinely recommended pending further evidence |
| ARNIs (Sacubitril/ Valsartan) | Neprilysin inhibition, natriuretic peptide enhancement, and RAAS inhibition | Under investigation for prevention of cardiotoxicity during breast cancer | Emerging: PRADAII [141]; ongoing MAINSTREAM trial [142] | No formal guideline recommendation; consider per general HF guidelines [47] |
| Ivabradine/RIC | Heart rate reduction/ischemic preconditioning | Not recommended; insufficient evidence | Negative/experimental: Rizk 2025 [143]; Moreno-Arciniegas 2024 [144] | Not recommended; negative trials and lack of benefit shown |
4.2. Cardiac Surveillance Protocols
5. The Role of an Integrated Cardio-Oncology Approach
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, F.; Xu, T.; Zang, F.; Luo, Y.; Pan, D. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms, Clinical Management and Innovative Treatment. Drug Des. Dev. Ther. 2024, 18, 4089–4116. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Cleary, S.; Rosen, S.D.; Gilbert, D.C.; Langley, R.E. Cardiovascular Health: An Important Component of Cancer Survivorship. BMJ Oncol. 2023, 2, e000090. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.M.; Anzevino, S.; Rao, R. Cardiovascular Disease in Cancer Survivors. Postgrad. Med. J. 2017, 93, 82–90. [Google Scholar] [CrossRef]
- Mulder, F.I.; Horváth-Puhó, E.; van Es, N.; Pedersen, L.; Büller, H.R.; Cronin-Fenton, D.; Christiansen, C.F.; Bøtker, H.E.; Bhaskaran, K.; Sørensen, H.T. Risk of Cardiovascular Disease in Cancer Survivors after Systemic Treatment: A Population-Based Cohort Study. JACC CardioOncology 2025, 7, 360–378. [Google Scholar] [CrossRef]
- Sturgeon, K.M.; Deng, L.; Bluethmann, S.M.; Zhou, S.; Trifiletti, D.M.; Jiang, C.; Kelly, S.P.; Zaorsky, N.G. A Population-Based Study of Cardiovascular Disease Mortality Risk in US Cancer Patients. Eur. Heart J. 2019, 40, 3889–3897. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.B.; Bongiovanni, C.; Da Pra, S.; Miano, C.; Sacchi, F.; Lauriola, M.; D’Uva, G. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms and Strategies for Cardioprotection. Front. Cardiovasc. Med. 2022, 9, 847012. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Ewer, S.M. Erratum: Cardiotoxicity of Anticancer Treatments. Nat. Rev. Cardiol. 2015, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Zito, C.; Manganaro, R.; Ciappina, G.; Spagnolo, C.C.; Racanelli, V.; Santarpia, M.; Silvestris, N.; Carerj, S. Cardiotoxicity Induced by Immune Checkpoint Inhibitors: What a Cardio-Oncology Team Should Know and Do. Cancers 2022, 14, 5403. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.E.J.; Guler, I.; Duchenne, J.; Janssens, S.; Van Aelst, L.N.L. Predictability of Cardiotoxicity: Experience of a Belgian Cardio-Oncology Clinic. Int. J. Cardiol. 2022, 363, 119–122. [Google Scholar] [CrossRef]
- Narayan, V.; Ky, B. Common Cardiovascular Complications of Cancer Therapy: Epidemiology, Risk Prediction, and Prevention. Annu. Rev. Med. 2018, 69, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Tetterton-Kellner, J.; Jensen, B.C.; Nguyen, J. Navigating Cancer Therapy Induced Cardiotoxicity: From Pathophysiology to Treatment Innovations. Adv. Drug Deliv. Rev. 2024, 211, 115361. [Google Scholar] [CrossRef]
- Mudd, T.W.; Khalid, M.; Guddati, A.K. Cardiotoxicity of Chemotherapy and Targeted Agents. Am. J. Cancer Res. 2021, 11, 1132–1147. [Google Scholar] [PubMed]
- Van Der Zanden, S.Y.; Qiao, X.; Neefjes, J. New Insights into the Activities and Toxicities of the Old Anticancer Drug Doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Henriksen, P.A. Anthracycline Cardiotoxicity: An Update on Mechanisms, Monitoring and Prevention. Heart 2018, 104, 971–977. [Google Scholar] [CrossRef]
- Damiani, R.M.; Moura, D.J.; Viau, C.M.; Caceres, R.A.; Henriques, J.A.P.; Saffi, J. Pathways of Cardiac Toxicity: Comparison between Chemotherapeutic Drugs Doxorubicin and Mitoxantrone. Arch. Toxicol. 2016, 90, 2063–2076. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, T.; Qiu, B.; Zhang, Y.; Zhou, Y.; Yu, H.; Zhang, J.; Liu, L.; Yuan, L.; Yang, G.; et al. Risk Factors for Anthracycline-Induced Cardiotoxicity. Front. Cardiovasc. Med. 2021, 8, 736854. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.Y.; Wang, J.J. Pharmacogenetics of Chemotherapy-Induced Cardiotoxicity. Curr. Oncol. Rep. 2018, 20, 52. [Google Scholar] [CrossRef]
- Krajinovic, M.; Elbared, J.; Drouin, S.; Bertout, L.; Rezgui, A.; Ansari, M.; Raboisson, M.-J.; Lipshultz, S.E.; Silverman, L.B.; Sallan, S.E.; et al. Polymorphisms of ABCC5 and NOS3 Genes Influence Doxorubicin Cardiotoxicity in Survivors of Childhood Acute Lymphoblastic Leukemia. Pharmacogenomics J. 2016, 16, 530–535. [Google Scholar] [CrossRef]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.-E.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020, 9, e018403. [Google Scholar] [CrossRef]
- Nishi, M.; Wang, P.; Hwang, P.M. Cardiotoxicity of Cancer Treatments: Focus on Anthracycline Cardiomyopathy. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2648–2660. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Cui, M.; Jin, L.; Wang, Y.; Lv, F.; Liu, Y.; Zheng, W.; Shang, H.; Zhang, J.; et al. CaMKII Is a RIP3 Substrate Mediating Ischemia- and Oxidative Stress–Induced Myocardial Necroptosis. Nat. Med. 2016, 22, 175–182. [Google Scholar] [CrossRef]
- Hutchins, E.; Yang, E.H.; Stein-Merlob, A.F. Inflammation in Chemotherapy-Induced Cardiotoxicity. Curr. Cardiol. Rep. 2024, 26, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Hinton, A.; Claypool, S.M.; Neikirk, K.; Senoo, N.; Wanjalla, C.N.; Kirabo, A.; Williams, C.R. Mitochondrial Structure and Function in Human Heart Failure. Circ. Res. 2024, 135, 372–396. [Google Scholar] [CrossRef]
- Christidi, E.; Brunham, L.R. Regulated Cell Death Pathways in Doxorubicin-Induced Cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Guo, T.; Wang, Z.; Zhao, Y. The Role of Iron in Doxorubicin-Induced Cardiotoxicity: Recent Advances and Implication for Drug Delivery. J. Mater. Chem. B 2021, 9, 4793–4803. [Google Scholar] [CrossRef]
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.; et al. Mitochondria-Dependent Ferroptosis Plays a Pivotal Role in Doxorubicin Cardiotoxicity. JCI Insight 2023, 8, e169756. [Google Scholar] [CrossRef]
- Xie, S.; Sun, Y.; Zhao, X.; Xiao, Y.; Zhou, F.; Lin, L.; Wang, W.; Lin, B.; Wang, Z.; Fang, Z.; et al. An Update of the Molecular Mechanisms Underlying Anthracycline Induced Cardiotoxicity. Front. Pharmacol. 2024, 15, 1406247. [Google Scholar] [CrossRef] [PubMed]
- Szponar, J.; Ciechanski, E.; Ciechanska, M.; Dudka, J.; Mandziuk, S. Evolution of Theories on Doxorubicin-Induced Late Cardiotoxicity-Role of Topoisomerase. Int. J. Mol. Sci. 2024, 25, 13567. [Google Scholar] [CrossRef]
- Blandino, G.; Valenti, F.; Sacconi, A.; Di Agostino, S. Wild Type- and Mutant P53 Proteins in Mitochondrial Dysfunction: Emerging Insights in Cancer Disease. Semin. Cell Dev. Biol. 2020, 98, 105–117. [Google Scholar] [CrossRef]
- Hoshino, A.; Mita, Y.; Okawa, Y.; Ariyoshi, M.; Iwai-Kanai, E.; Ueyama, T.; Ikeda, K.; Ogata, T.; Matoba, S. Cytosolic P53 Inhibits Parkin-Mediated Mitophagy and Promotes Mitochondrial Dysfunction in the Mouse Heart. Nat. Commun. 2013, 4, 2308. [Google Scholar] [CrossRef]
- Narezkina, A.; Narayan, H.K.; Zemljic-Harpf, A.E. Molecular Mechanisms of Anthracycline Cardiovascular Toxicity. Clin. Sci. Lond. Engl. 1979 2021, 135, 1311–1332. [Google Scholar] [CrossRef] [PubMed]
- Skaggs, C.; Nick, S.; Patricelli, C.; Bond, L.; Woods, K.; Woodbury, L.; Oxford, J.T.; Pu, X. Effects of Doxorubicin on Extracellular Matrix Regulation in Primary Cardiac Fibroblasts from Mice. BMC Res. Notes 2023, 16, 340. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, V.; Varzideh, F.; Wilson, S.; Kansakar, U.; Jankauskas, S.; Santulli, G. Doxorubicin-Induced Cardiotoxicity: A Comprehensive Update. J. Cardiovasc. Dev. Dis. 2025, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Zaha, V.G.; Hamad, E.; Woodard, P.K.; Rimner, A.; Chang, J.Y.; Chun, S.G.; Donington, J.; Edelman, M.J.; Gubens, M.A.; et al. American Radium Society Appropriate Use Criteria on Cardiac Toxicity Prevention and Management After Thoracic Radiotherapy. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2024, 19, 1654–1667. [Google Scholar] [CrossRef]
- Patil, S.; Pingle, S.-R.; Shalaby, K.; Kim, A.S. Mediastinal Irradiation and Valvular Heart Disease. Cardio-Oncol. Lond. Engl. 2022, 8, 7. [Google Scholar] [CrossRef]
- Lestuzzi, C.; Mascarin, M.; Coassin, E.; Canale, M.L.; Turazza, F. Cardiologic Long-Term Follow-Up of Patients Treated With Chest Radiotherapy: When and How? Front. Cardiovasc. Med. 2021, 8, 671001. [Google Scholar] [CrossRef]
- Wilson, J.; Jun Hua, C.; Aziminia, N.; Manisty, C. Imaging of the Acute and Chronic Cardiovascular Complications of Radiation Therapy. Circ. Cardiovasc. Imaging 2025, 18, e017454. [Google Scholar] [CrossRef]
- Schiffer, W.; Pedersen, L.N.; Lui, M.; Bergom, C.; Mitchell, J.D. Advances in Screening for Radiation-Associated Cardiotoxicity in Cancer Patients. Curr. Cardiol. Rep. 2023, 25, 1589–1600. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Ameri, P.; de Boer, R.A.; D’Alessandra, Y.; Russo, M.; Sorriento, D.; Ciccarelli, M.; Kiss, B.; Bertrand, L.; Dawson, D.; et al. Cardiac Dysfunction in Cancer Patients: Beyond Direct Cardiomyocyte Damage of Anticancer Drugs: Novel Cardio-Oncology Insights from the Joint 2019 Meeting of the ESC Working Groups of Myocardial Function and Cellular Biology of the Heart. Cardiovasc. Res. 2020, 116, 1820–1834. [Google Scholar] [CrossRef]
- Jurczyk, M.; Król, M.; Midro, A.; Kurnik-Łucka, M.; Poniatowski, A.; Gil, K. Cardiotoxicity of Fluoropyrimidines: Epidemiology, Mechanisms, Diagnosis, and Management. J. Clin. Med. 2021, 10, 4426. [Google Scholar] [CrossRef]
- Deac, A.L.; Burz, C.C.; Bocsan, I.C.; Buzoianu, A.D. Fluoropyrimidine-Induced Cardiotoxicity. World J. Clin. Oncol. 2020, 11, 1008–1017. [Google Scholar] [CrossRef]
- Shiga, T.; Hiraide, M. Cardiotoxicities of 5-Fluorouracil and Other Fluoropyrimidines. Curr. Treat. Options Oncol. 2020, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, F.C.A.; de Almeida Barbosa, A.B.; Sano, V.K.T.; Kelly, F.A.; Burbano, R.M.R. Pharmacogenetics of DPYD and Treatment-Related Mortality on Fluoropyrimidine Chemotherapy for Cancer Patients: A Meta-Analysis and Trial Sequential Analysis. BMC Cancer 2024, 24, 1210. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Wong, C.-K.; Ho, I.; Choo, A.; Lau, R.; Ma, T.-F.; Chiu, A.C.H.-O.; Lam, T.-H.; Lin, M.; Leung, R.W.-H.; Chor-Cheung Tam, F.; et al. Cardiovascular Safety of 5-Fluorouracil and Capecitabine in Colorectal Cancer Patients: Real-World Evidence. Cardio-Oncol. Lond. Engl. 2025, 11, 3. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Lu, S.; Zeng, M.; Liu, L.; Dai, Q.; Yin, R. Adverse Event Profile of Albumin-Bound Paclitaxel: A Real-World Pharmacovigilance Analysis. Front. Pharmacol. 2024, 15, 1448144. [Google Scholar] [CrossRef] [PubMed]
- Batra, A.; Patel, B.; Addison, D.; Baldassarre, L.A.; Desai, N.; Weintraub, N.; Deswal, A.; Hussain, Z.; Brown, S.-A.; Ganatra, S.; et al. Cardiovascular Safety Profile of Taxanes and Vinca Alkaloids: 30 Years FDA Registry Experience. Open Heart 2021, 8, e001849. [Google Scholar] [CrossRef]
- Beavers, C.J.; Rodgers, J.E.; Bagnola, A.J.; Beckie, T.M.; Campia, U.; Di Palo, K.E.; Okwuosa, T.M.; Przespolewski, E.R.; Dent, S.; American Heart Association Clinical Pharmacology Committee and Cardio-Oncology Committee of the Council on Clinical Cardiology and Council on Genomic and Precision Medicine; et al. Cardio-Oncology Drug Interactions: A Scientific Statement From the American Heart Association. Circulation 2022, 145, e811–e838. [Google Scholar] [CrossRef]
- Avagimyan, A.; Pogosova, N.; Kakturskiy, L.; Sheibani, M.; Challa, A.; Kogan, E.; Fogacci, F.; Mikhaleva, L.; Vandysheva, R.; Yakubovskaya, M.; et al. Doxorubicin-Related Cardiotoxicity: Review of Fundamental Pathways of Cardiovascular System Injury. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2024, 73, 107683. [Google Scholar] [CrossRef]
- Alexandre, J.; Moslehi, J.J.; Bersell, K.R.; Funck-Brentano, C.; Roden, D.M.; Salem, J.-E. Anticancer Drug-Induced Cardiac Rhythm Disorders: Current Knowledge and Basic Underlying Mechanisms. Pharmacol. Ther. 2018, 189, 89–103. [Google Scholar] [CrossRef]
- Morrow, A.J.; Cameron, A.C.; Payne, A.R.; White, J.; Lang, N.N. Cisplatin Related Cardiotoxicity—Acute and Chronic Cardiovascular Morbidity in a Testicular Cancer Survivor. Scott. Med. J. 2020, 65, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Li, J.; He, Z.; Boswell, S.A.; Chung, M.; You, F.; Han, S. Three Tyrosine Kinase Inhibitors Cause Cardiotoxicity by Inducing Endoplasmic Reticulum Stress and Inflammation in Cardiomyocytes. BMC Med. 2023, 21, 147. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, X.; Li, J. Personalized Trastuzumab-Induced Cardiac Dysfunction Risk Stratification and Surveillance. JACC CardioOncology 2025, 7, 216–218. [Google Scholar] [CrossRef]
- Senechal, I.; Andres, M.S.; Tong, J.; Perone, Y.; Ramalingam, S.; Nazir, M.S.; Rosen, S.D.; Turner, N.; Ring, A.; Lyon, A.R. Late Cardiotoxicity Related to HER2-Targeted Cancer Therapy. Cardio-Oncol. Lond. Engl. 2024, 10, 14. [Google Scholar] [CrossRef]
- Zagami, P.; Trapani, D.; Nicolò, E.; Corti, C.; Valenza, C.; Criscitiello, C.; Curigliano, G.; Carey, L.A. Cardiotoxicity of Agents Used in Patients With Breast Cancer. JCO Oncol. Pract. 2024, 20, 38–46. [Google Scholar] [CrossRef]
- Liu, J.; Meng, Z.; Yidan, X. Cardiotoxicity of HER2-Targeted Drugs When Combined with Other Drugs: A Systematic and Meta-Analysis of Randomized Controlled Trials. Cardiovasc. Toxicol. 2024, 24, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Torelli, S.; Agnihotri, V.; Zhu, H.; Wang, Z.; Cheng, P.; Rhee, J.-W. Clinical Relevance and Mechanistic Underpinnings of Tyrosine Kinase Inhibitor Associated Cardiotoxicities. Curr. Treat. Options Cardiovasc. Med. 2025, 27, 10. [Google Scholar] [CrossRef]
- Wang, X.; Chen, R.; Liu, J.; Wang, E.; Luo, H. Liver Injury Related to Vascular Endothelial Growth Factor Tyrosine Kinase Inhibitors: A Pharmacovigilance Analysis of the USA FDA Adverse Event Reporting System (FAERS) Database. Expert Opin. Drug Saf. 2025. epub ahead of print. [Google Scholar] [CrossRef]
- Mihalcea, D.; Memis, H.; Mihaila, S.; Vinereanu, D. Cardiovascular Toxicity Induced by Vascular Endothelial Growth Factor Inhibitors. Life 2023, 13, 366. [Google Scholar] [CrossRef]
- Cohen, J.B.; Brown, N.J.; Brown, S.-A.; Dent, S.; van Dorst, D.C.H.; Herrmann, S.M.; Lang, N.N.; Oudit, G.Y.; Touyz, R.M.; American Heart Association Council on Hypertension; et al. Cancer Therapy-Related Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2023, 80, e46–e57. [Google Scholar] [CrossRef] [PubMed]
- Gaydarski, L.; Petrova, K.; Stanchev, S.; Pelinkov, D.; Iliev, A.; Dimitrova, I.N.; Kirkov, V.; Landzhov, B.; Stamenov, N. Morphometric and Molecular Interplay in Hypertension-Induced Cardiac Remodeling with an Emphasis on the Potential Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 4022. [Google Scholar] [CrossRef]
- Chu, T.F.; Rupnick, M.A.; Kerkela, R.; Dallabrida, S.M.; Zurakowski, D.; Nguyen, L.; Woulfe, K.; Pravda, E.; Cassiola, F.; Desai, J.; et al. Cardiotoxicity Associated with Tyrosine Kinase Inhibitor Sunitinib. Lancet 2007, 370, 2011–2019. [Google Scholar] [CrossRef]
- Deng, Z.; Xiao, S.; He, Y.-Y.; Guo, Y.; Tang, L.-J. Sorafenib-Induced Cardiovascular Toxicity: A Cause for Concern. Chem. Biol. Interact. 2025, 410, 111388. [Google Scholar] [CrossRef]
- Haguet, H.; Douxfils, J.; Chatelain, C.; Graux, C.; Mullier, F.; Dogné, J.-M. BCR-ABL Tyrosine Kinase Inhibitors: Which Mechanism(s) May Explain the Risk of Thrombosis? TH Open 2018, 2, e68–e88. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.-H.; Zhang, R.; Lu, X.-C.; Yang, B.; Fan, H.; Zhu, H.-L. Imatinib-Induced Decompensated Heart Failure in an Elderly Patient with Chronic Myeloid Leukemia: Case Report and Literature Review. J. Geriatr. Cardiol. JGC 2012, 9, 411–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kobara, M.; Nessa, N.; Toba, H.; Nakata, T. Induction of Autophagy Has Protective Roles in Imatinib-Induced Cardiotoxicity. Toxicol. Rep. 2021, 8, 1087–1097. [Google Scholar] [CrossRef]
- Shaban, N.; Kamashev, D.; Emelianova, A.; Buzdin, A. Targeted Inhibitors of EGFR: Structure, Biology, Biomarkers, and Clinical Applications. Cells 2023, 13, 47. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Lin, S.; Huang, J.; Ruan, Z. Advancements in Understanding Cardiotoxicity of EGFR- TKIs in Non-Small Cell Lung Cancer Treatment and Beyond. Front. Pharmacol. 2024, 15, 1404692. [Google Scholar] [CrossRef]
- Solomon, B.J.; Liu, G.; Felip, E.; Mok, T.S.K.; Soo, R.A.; Mazieres, J.; Shaw, A.T.; De Marinis, F.; Goto, Y.; Wu, Y.-L.; et al. Lorlatinib Versus Crizotinib in Patients with Advanced ALK-Positive Non–Small Cell Lung Cancer: 5-Year Outcomes From the Phase III CROWN Study. J. Clin. Oncol. 2024, 42, 3400–3409. [Google Scholar] [CrossRef]
- Jia, J.; Tian, C.; Han, W.; Ma, Q. Real-World Assessment of Thromboembolic Risk Associated with Tamoxifen. Sci. Rep. 2025, 15, 27820. [Google Scholar] [CrossRef]
- Muniyan, S.; Xi, L.; Datta, K.; Das, A.; Teply, B.A.; Batra, S.K.; Kukreja, R.C. Cardiovascular Risks and Toxicity—The Achilles Heel of Androgen Deprivation Therapy in Prostate Cancer Patients. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188383. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on Cancer Treatments and Cardiovascular Toxicity Developed under the Auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- El-Taji, O.; Taktak, S.; Jones, C.; Brown, M.; Clarke, N.; Sachdeva, A. Cardiovascular Events and Androgen Receptor Signaling Inhibitors in Advanced Prostate Cancer: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024, 10, 874–884. [Google Scholar] [CrossRef]
- Li, D.; Chai, S.; Wang, H.; Dong, J.; Qin, C.; Du, D.; Wang, Y.; Du, Q.; Liu, S. Drug-Induced QT Prolongation and Torsade de Pointes: A Real-World Pharmacovigilance Study Using the FDA Adverse Event Reporting System Database. Front. Pharmacol. 2023, 14, 1259611. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Reddy, A.; Sekar, A. Enzalutamide Induced Non-Ischemic Cardiomyopathy. A Case Report and Review of Literature on Anti-Androgen Therapy-Related Cardiovascular Events. Cardio-Oncology 2023, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shen, G.; Li, S.; Ma, F.; Li, H.; Tang, Y.; Li, Y.; Li, Z.; Zhu, Z.; Qiu, T.; et al. Cardiovascular Events Associated with CDK4/6 Inhibitors: A Safety Meta-Analysis of Randomized Controlled Trials and a Pharmacovigilance Study of the FAERS Database. Am. J. Cardiovasc. Drugs 2025, 25, 373–388. [Google Scholar] [CrossRef]
- Michel, L.; Rassaf, T.; Totzeck, M. Cardiotoxicity from Immune Checkpoint Inhibitors. IJC Heart Vasc. 2019, 25, 100420. [Google Scholar] [CrossRef] [PubMed]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 Have Opposing Effects on the Response of T Cells to Stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory Mechanisms of PD-1/PD-L1 in Cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Cadeddu, C.; Di Lisi, D.; Femminò, S.; Madonna, R.; Mele, D.; Monte, I.; Novo, G.; Penna, C.; Pepe, A.; et al. From Molecular Mechanisms to Clinical Management of Antineoplastic Drug-Induced Cardiovascular Toxicity: A Translational Overview. Antioxid. Redox Signal. 2019, 30, 2110–2153. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 1721. [Google Scholar] [CrossRef]
- Dolladille, C.; Akroun, J.; Morice, P.-M.; Dompmartin, A.; Ezine, E.; Sassier, M.; Da-Silva, A.; Plane, A.-F.; Legallois, D.; L’Orphelin, J.-M.; et al. Cardiovascular Immunotoxicities Associated with Immune Checkpoint Inhibitors: A Safety Meta-Analysis. Eur. Heart J. 2021, 42, 4964–4977. [Google Scholar] [CrossRef] [PubMed]
- Frascaro, F.; Bianchi, N.; Sanguettoli, F.; Marchini, F.; Meossi, S.; Zanarelli, L.; Tonet, E.; Serenelli, M.; Guardigli, G.; Campo, G.; et al. Immune Checkpoint Inhibitors-Associated Myocarditis: Diagnosis, Treatment and Current Status on Rechallenge. J. Clin. Med. 2023, 12, 7737. [Google Scholar] [CrossRef]
- Jiménez-Alejandre, R.; Ruiz-Fernández, I.; Martín, P. Pathophysiology of Immune Checkpoint Inhibitor-Induced Myocarditis. Cancers 2022, 14, 4494. [Google Scholar] [CrossRef]
- Serzan, M.; Rapisuwon, S.; Krishnan, J.; Chang, I.C.; Barac, A. Takotsubo Cardiomyopathy Associated With Checkpoint Inhibitor Therapy: Endomyocardial Biopsy Provides Pathological Insights to Dual Diseases. JACC CardioOncology 2021, 3, 330–334. [Google Scholar] [CrossRef]
- Quagliariello, V.; Bisceglia, I.; Berretta, M.; Iovine, M.; Canale, M.L.; Maurea, C.; Giordano, V.; Paccone, A.; Inno, A.; Maurea, N. PCSK9 Inhibitors in Cancer Patients Treated with Immune-Checkpoint Inhibitors to Reduce Cardiovascular Events: New Frontiers in Cardioncology. Cancers 2023, 15, 1397. [Google Scholar] [CrossRef] [PubMed]
- Zarifa, A.; Kim, J.W.; Lopez-Mattei, J.; Palaskas, N.; Iliescu, C.; Kim, P.Y. Cardiac Toxicities Associated with Immune Checkpoints Inhibitors: Mechanisms, Manifestations and Management. Korean Circ. J. 2021, 51, 579. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.; Won, T.; Daoud, A.; Čiháková, D. Immune Checkpoint Inhibitors Associated Cardiovascular Immune-Related Adverse Events. Front. Immunol. 2024, 15, 1340373. [Google Scholar] [CrossRef] [PubMed]
- Ciappina, G.; Ottaiano, A.; Santorsola, M.; Esposito, E.; De Luca, F.; Giorgi, C.; Zito, C.; Capra, A.P.; Carroccio, P.; Maurea, N.; et al. Impact of Proton Pump Inhibitor Use on Progression-Free and Overall Survival in Cancer Patients Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis of Recent Studies. Cancers 2025, 17, 2228. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Makris, N.; Laina, A.; Theodorakakou, F.; Briasoulis, A.; Trougakos, I.P.; Dimopoulos, M.-A.; Kastritis, E.; Stamatelopoulos, K. Cardiovascular Toxicity of Proteasome Inhibitors: Underlying Mechanisms and Management Strategies: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncology 2023, 5, 1–21. [Google Scholar] [CrossRef]
- Efentakis, P.; Psarakou, G.; Varela, A.; Papanagnou, E.D.; Chatzistefanou, M.; Nikolaou, P.-E.; Davos, C.H.; Gavriatopoulou, M.; Trougakos, I.P.; Dimopoulos, M.A.; et al. Elucidating Carfilzomib’s Induced Cardiotoxicity in an In Vivo Model of Aging: Prophylactic Potential of Metformin. Int. J. Mol. Sci. 2021, 22, 10956. [Google Scholar] [CrossRef]
- Rahman, M.R.; Ball, S.; Paz, P.; Elmassry, M.; Vutthikraivit, W.; Bandyopadhyay, D.; Lavie, C.J.; Fonarow, G.C. Heart Failure with Carfilzomib in Patients with Multiple Myeloma: A Meta-Analysis of Randomized Controlled Trials. J. Card. Fail. 2021, 27, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Buck, B.; Kellett, E.; Addison, D.; Vallakati, A. Carfilzomib-Induced Cardiotoxicity: An Analysis of the FDA Adverse Event Reporting System (FAERS). J. Saudi Heart Assoc. 2022, 34, 134–141. [Google Scholar] [CrossRef]
- Wesley, C.D.; Sansonetti, A.; Neutel, C.H.G.; Krüger, D.N.; De Meyer, G.R.Y.; Martinet, W.; Guns, P.-J. Short-Term Proteasome Inhibition: Assessment of the Effects of Carfilzomib and Bortezomib on Cardiac Function, Arterial Stiffness, and Vascular Reactivity. Biology 2024, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Jeong, J.; Heo, K.-N.; Koh, Y.; Lee, J.-Y. Real-World Incidence and Risk Factors of Bortezomib-Related Cardiovascular Adverse Events in Patients with Multiple Myeloma. Blood Res. 2024, 59, 3. [Google Scholar] [CrossRef]
- El-Cheikh, J.; Moukalled, N.; Malard, F.; Bazarbachi, A.; Mohty, M. Cardiac Toxicities in Multiple Myeloma: An Updated and a Deeper Look into the Effect of Different Medications and Novel Therapies. Blood Cancer J. 2023, 13, 83. [Google Scholar] [CrossRef]
- Akram, F.; Ali, A.M.; Akhtar, M.T.; Fatima, T.; Shabbir, I.; Ul Haq, I. The Journey of Antibody-Drug Conjugates for Revolutionizing Cancer Therapy: A Review. Bioorg. Med. Chem. 2025, 117, 118010. [Google Scholar] [CrossRef]
- Pondé, N.; Ameye, L.; Lambertini, M.; Paesmans, M.; Piccart, M.; De Azambuja, E. Trastuzumab Emtansine (T-DM1)-Associated Cardiotoxicity: Pooled Analysis in Advanced HER2-Positive Breast Cancer. Eur. J. Cancer 2020, 126, 65–73. [Google Scholar] [CrossRef]
- D’Arienzo, A.; Verrazzo, A.; Pagliuca, M.; Napolitano, F.; Parola, S.; Viggiani, M.; Caputo, R.; Puglisi, F.; Giuliano, M.; Del Mastro, L.; et al. Toxicity Profile of Antibody-Drug Conjugates in Breast Cancer: Practical Considerations. EClinicalMedicine 2023, 62, 102113. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. DESTINY-Breast03 Trial Investigators. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Gómez Pardo, P.; et al. Overall Survival with Sacituzumab Govitecan in Hormone Receptor-Positive and Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer (TROPiCS-02): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet 2023, 402, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. ASCENT Clinical Trial Investigators. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- De Bousser, E.; Callewaert, N.; Festjens, N. T Cell Engaging Immunotherapies, Highlighting Chimeric Antigen Receptor (CAR) T Cell Therapy. Cancers 2021, 13, 6067. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Dani, S.S.; Yang, E.H.; Zaha, V.G.; Nohria, A. Cardiotoxicity of T-Cell Antineoplastic Therapies: JACC: CardioOncology Primer. JACC CardioOncology 2022, 4, 616–623. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Conroy, R. Estimation of Ten-Year Risk of Fatal Cardiovascular Disease in Europe: The SCORE Project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- SCORE2 Working Group; ESC Cardiovascular Risk Collaboration. SCORE2 Risk Prediction Algorithms: New Models to Estimate 10-Year Risk of Cardiovascular Disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- SCORE2-Diabetes Working Group; The ESC Cardiovascular Risk Collaboration. SCORE2-Diabetes: 10-Year Cardiovascular Risk Estimation in Type 2 Diabetes in Europe. Eur. Heart J. 2023, 44, 2544–2556. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by Representatives of 10 Societies and by Invited Experts) Developed with the Special Contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.-L. The Frailty Syndrome: Definition and Natural History. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of Cardiac Disease in Cancer Patients throughout Oncological Treatment: ESMO Consensus Recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy: A Report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef]
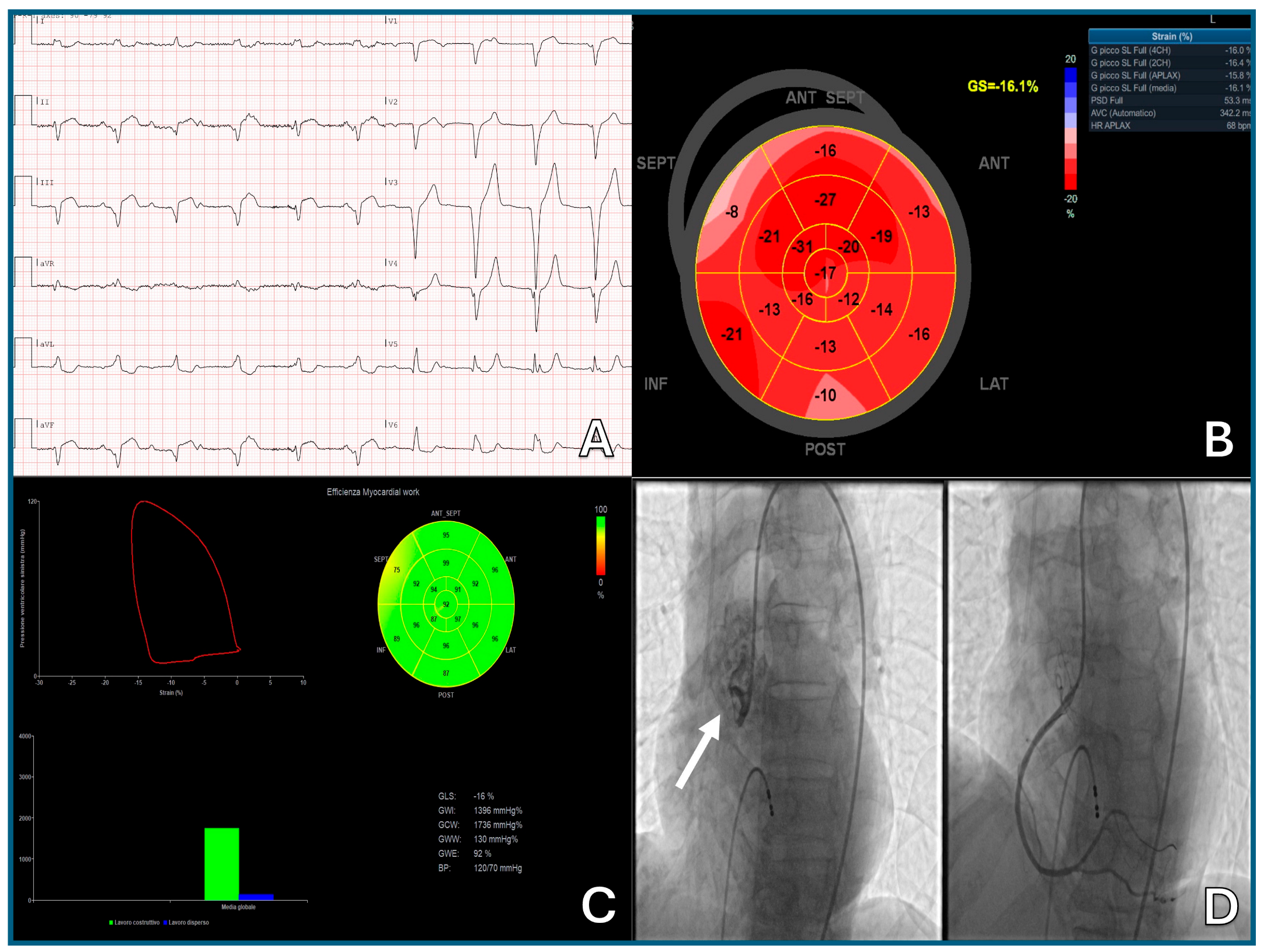
- Thavendiranathan, P.; Poulin, F.; Lim, K.-D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of Myocardial Strain Imaging by Echocardiography for the Early Detection of Cardiotoxicity in Patients During and After Cancer Chemotherapy. J. Am. Coll. Cardiol. 2014, 63, 2751–2768. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Negishi, T.; Somerset, E.; Negishi, K.; Penicka, M.; Lemieux, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; et al. Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. J. Am. Coll. Cardiol. 2021, 77, 392–401. [Google Scholar] [CrossRef]
- Negishi, T.; Thavendiranathan, P.; Penicka, M.; Lemieux, J.; Murbraech, K.; Miyazaki, S.; Shirazi, M.; Santoro, C.; Cho, G.-Y.; Popescu, B.A.; et al. Cardioprotection Using Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. JACC Cardiovasc. Imaging 2023, 16, 269–278. [Google Scholar] [CrossRef]
- Chaganti, B.T.; Negishi, K.; Okajima, K. Role of Myocardial Strain Imaging in Cancer Therapy–Related Cardiac Dysfunction. Curr. Cardiol. Rep. 2022, 24, 739–748. [Google Scholar] [CrossRef]
- Cardinale, D.; Sandri, M.T.; Martinoni, A.; Tricca LabTech, A.; Civelli, M.; Lamantia, G.; Cinieri, S.; Martinelli, G.; Cipolla, C.M.; Fiorentini, C. Left Ventricular Dysfunction Predicted by Early Troponin I Release after High-Dose Chemotherapy. J. Am. Coll. Cardiol. 2000, 36, 517–522. [Google Scholar] [CrossRef]
- Cardinale, D.; Sandri, M.T.; Colombo, A.; Colombo, N.; Boeri, M.; Lamantia, G.; Civelli, M.; Peccatori, F.; Martinelli, G.; Fiorentini, C.; et al. Prognostic Value of Troponin I in Cardiac Risk Stratification of Cancer Patients Undergoing High-Dose Chemotherapy. Circulation 2004, 109, 2749–2754. [Google Scholar] [CrossRef]
- Cornell, R.F.; Ky, B.; Weiss, B.M.; Dahm, C.N.; Gupta, D.K.; Du, L.; Carver, J.R.; Cohen, A.D.; Engelhardt, B.G.; Garfall, A.L.; et al. Prospective Study of Cardiac Events During Proteasome Inhibitor Therapy for Relapsed Multiple Myeloma. J. Clin. Oncol. 2019, 37, 1946–1955. [Google Scholar] [CrossRef]
- Murtagh, G.; Januzzi, J.L.; Scherrer-Crosbie, M.; Neilan, T.G.; Dent, S.; Ho, J.E.; Appadurai, V.; McDermott, R.; Akhter, N. Circulating Cardiovascular Biomarkers in Cancer Therapeutics-Related Cardiotoxicity: Review of Critical Challenges, Solutions, and Future Directions. J. Am. Heart Assoc. 2023, 12, e029574. [Google Scholar] [CrossRef]
- Wang, X.; Hegde, S.M. Leveraging the Power of Radiomics to Predict Heart Failure: New Frontiers in Cardio-Oncology. Int. J. Cardiovasc. Imaging 2024, 40, 1161–1162. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the Management of Chronic Coronary Syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Novo, G.; Santoro, C.; Manno, G.; Di Lisi, D.; Esposito, R.; Mandoli, G.E.; Evola, V.; Pastore, M.C.; Sperlongano, S.; D’Andrea, A.; et al. Usefulness of Stress Echocardiography in the Management of Patients Treated with Anticancer Drugs. J. Am. Soc. Echocardiogr. 2021, 34, 107–116. [Google Scholar] [CrossRef]
- Bharadwaj, A.; Potts, J.; Mohamed, M.O.; Parwani, P.; Swamy, P.; Lopez-Mattei, J.C.; Rashid, M.; Kwok, C.S.; Fischman, D.L.; Vassiliou, V.S.; et al. Acute Myocardial Infarction Treatments and Outcomes in 6.5 Million Patients with a Current or Historical Diagnosis of Cancer in the USA. Eur. Heart J. 2020, 41, 2183–2193. [Google Scholar] [CrossRef]
- Leo, I.; Vidula, M.; Bisaccia, G.; Procopio, M.C.; Licordari, R.; Perotto, M.; La Vecchia, G.; Miaris, N.; Bravo, P.E.; Bucciarelli-Ducci, C. The Role of Advanced Cardiovascular Imaging Modalities in Cardio-Oncology: From Early Detection to Unravelling Mechanisms of Cardiotoxicity. J. Clin. Med. 2023, 12, 4945. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Sandri, M.T.; Lamantia, G.; Colombo, N.; Civelli, M.; Martinelli, G.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Prevention of High-Dose Chemotherapy–Induced Cardiotoxicity in High-Risk Patients by Angiotensin-Converting Enzyme Inhibition. Circulation 2006, 114, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.; Heck, S.L.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; Von Knobelsdorff-Brenkenhoff, F.; Bratland, Å.; Storås, T.H.; et al. Prevention of Cardiac Dysfunction during Adjuvant Breast Cancer Therapy (PRADA): A 2 × 2 Factorial, Randomized, Placebo-Controlled, Double-Blind Clinical Trial of Candesartan and Metoprolol. Eur. Heart J. 2016, 37, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.S.; Ayub-Ferreira, S.M.; De Barros Wanderley, M.R.; Das Dores Cruz, F.; Gonçalves Brandão, S.M.; Rigaud, V.O.C.; Higuchi-dos-Santos, M.H.; Hajjar, L.A.; Kalil Filho, R.; Hoff, P.M.; et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity. J. Am. Coll. Cardiol. 2018, 71, 2281–2290. [Google Scholar] [CrossRef]
- Henriksen, P.A.; Hall, P.; MacPherson, I.R.; Joshi, S.S.; Singh, T.; Maclean, M.; Lewis, S.; Rodriguez, A.; Fletcher, A.; Everett, R.J.; et al. Multicenter, Prospective, Randomized Controlled Trial of High-Sensitivity Cardiac Troponin I–Guided Combination Angiotensin Receptor Blockade and Beta-Blocker Therapy to Prevent Anthracycline Cardiotoxicity: The Cardiac CARE Trial. Circulation 2023, 148, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Li, J.T.; Duddy, A.M.; Cardona, M.; Pasupuleti, V.; Hernandez, A.V. Efficacy and Harms Associated with Beta-Blockers for Cardiotoxicity in Cancer Patients Undergoing Chemotherapy: A Systematic Review and Meta-Analysis. Arch. Med. Sci. 2024, 21, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Becherini, C.; Martella, F.; Del Bene, M.R.; Saieva, C.; Bacci, C.; Coltelli, L.; Pilato, G.; Visani, L.; Salvestrini, V.; et al. Cardioprotection in Patients with Anthracycline-Treated Breast Cancer: Final Analysis from the 2 × 2 Randomized, Placebo-Controlled, Double-Blind SAFE Trial. ESMO Open 2025, 10, 105116. [Google Scholar] [CrossRef] [PubMed]
- Dabour, M.S.; George, M.Y.; Daniel, M.R.; Blaes, A.H.; Zordoky, B.N. The Cardioprotective and Anticancer Effects of SGLT2 Inhibitors. JACC CardioOncology 2024, 6, 159–182. [Google Scholar] [CrossRef]
- Quagliariello, V.; Berretta, M.; Bisceglia, I.; Giacobbe, I.; Iovine, M.; Barbato, M.; Maurea, C.; Canale, M.L.; Paccone, A.; Inno, A.; et al. In the Era of Cardiovascular–Kidney–Metabolic Syndrome in Cardio-Oncology: From Pathogenesis to Prevention and Therapy. Cancers 2025, 17, 1169. [Google Scholar] [CrossRef]
- Bhalraam, U.; Veerni, R.B.; Paddock, S.; Meng, J.; Piepoli, M.; López-Fernández, T.; Tsampasian, V.; Vassiliou, V.S. Impact of Sodium–Glucose Cotransporter-2 Inhibitors on Heart Failure Outcomes in Cancer Patients and Survivors: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2025. epub ahead of print. [Google Scholar] [CrossRef]
- Greco, A.; Canale, M.L.; Quagliariello, V.; Oliva, S.; Tedeschi, A.; Inno, A.; De Biasio, M.; Bisceglia, I.; Tarantini, L.; Maurea, N.; et al. SGLT2 Inhibitors in Cancer Patients: A Comprehensive Review of Clinical, Biochemical, and Therapeutic Implications in Cardio-Oncology. Int. J. Mol. Sci. 2025, 26, 4780. [Google Scholar] [CrossRef]
- Wiseman, L.R.; Spencer, C.M. Dexrazoxane: A Review of Its Use as a Cardioprotective Agent in Patients Receiving Anthracycline-Based Chemotherapy. Drugs 1998, 56, 385–403. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Murphy, A.C.; Ramalingam, S.; Scherrer-Crosbie, M.; Lopez-Fernandez, T.; Reynolds, K.L.; Aznar, M.; Lin, A.E.; Libby, P.; Cordoba, R.; et al. Cardiovascular Considerations Before Cancer Therapy. JACC CardioOncology 2024, 6, 631–654. [Google Scholar] [CrossRef]
- Omland, T.; Heck, S.L.; Holte, E.; Mecinaj Lilleaasen, A.; Gynnild, M.N.; Fagerland, M.W.; Vinje-Jakobsen, V.; Næs, A.-K.L.; Blix, E.S.; Larsen, A.I.; et al. Sacubitril-Valsartan and Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy: The PRADA II Randomized Clinical Trial. Circulation 2025. epub ahead of print.. [Google Scholar] [CrossRef]
- Tajstra, M.; Dyrbuś, M.; Rutkowski, T.; Składowski, K.; Sosnowska-Pasiarska, B.; Góźdź, S.; Radecka, B.; Staszewski, M.; Majsnerowska, A.; Myrda, K.; et al. Sacubitril/Valsartan for Cardioprotection in Breast Cancer (MAINSTREAM): Design and Rationale of the Randomized Trial. ESC Heart Fail. 2023, 10, 3174–3183. [Google Scholar] [CrossRef]
- Rizk, S.I.; Costa, I.B.S.D.S.; Cruz, C.B.B.V.; Pileggi, B.; De Almeida Andrade, F.T.; Gonzalez, T.B.; Bittar, C.S.; Fukushima, J.T.; Quintao, V.C.; Osawa, E.A.; et al. Randomized, Placebo-Controlled, Triple-Blind Clinical Trial of Ivabradine for the Prevention of Cardiac Dysfunction During Anthracycline-Based Cancer Therapy. J. Am. Heart Assoc. 2025, 14, e039745. [Google Scholar] [CrossRef]
- Moreno-Arciniegas, A.; García, A.; Kelm, M.; D’Amore, F.; Da Silva, M.G.; Sánchez-González, J.; Sánchez, P.L.; López-Fernández, T.; Córdoba, R.; Asteggiano, R.; et al. RESILIENCE Trial Investigators. Rationale and Design of RESILIENCE: A Prospective Randomized Clinical Trial Evaluating Remote Ischaemic Conditioning for the Prevention of Anthracycline Cardiotoxicity. Eur. J. Heart Fail. 2024, 26, 2213–2222. [Google Scholar] [CrossRef]
- Lynce, F.; Barac, A.; Geng, X.; Dang, C.; Yu, A.F.; Smith, K.L.; Gallagher, C.; Pohlmann, P.R.; Nunes, R.; Herbolsheimer, P.; et al. Prospective Evaluation of the Cardiac Safety of HER2-Targeted Therapies in Patients with HER2-Positive Breast Cancer and Compromised Heart Function: The SAFE-HEaRt Study. Breast Cancer Res. Treat. 2019, 175, 595–603. [Google Scholar] [CrossRef]
- Díaz-Balboa, E.; Peña-Gil, C.; Rodríguez-Romero, B.; Cuesta-Vargas, A.I.; Lado-Baleato, O.; Martínez-Monzonís, A.; Pedreira-Pérez, M.; Palacios-Ozores, P.; López-López, R.; González-Juanatey, J.R.; et al. Exercise-Based Cardio-Oncology Rehabilitation for Cardiotoxicity Prevention during Breast Cancer Chemotherapy: The ONCORE Randomized Controlled Trial. Prog. Cardiovasc. Dis. 2024, 85, 74–81. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive Heart Failure in Patients Treated with Doxorubicin: A Retrospective Analysis of Three Trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Lipsitz, S.R.; Mone, S.M.; Goorin, A.M.; Sallan, S.E.; Sanders, S.P.; Orav, E.J.; Gelber, R.D.; Colan, S.D. Female Sex and Higher Drug Dose as Risk Factors for Late Cardiotoxic Effects of Doxorubicin Therapy for Childhood Cancer. N. Engl. J. Med. 1995, 332, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Drill, E.; Dang, C.T.; Liu, J.E.; Steingart, R.M.; Yu, A.F. Cardiac Outcomes of Trastuzumab Therapy in Patients with HER2-Positive Breast Cancer and Reduced Left Ventricular Ejection Fraction. Breast Cancer Res. Treat. 2019, 175, 239–246. [Google Scholar] [CrossRef]
- Thuny, F.; Alexandre, J.; Salem, J.-E.; Mirabel, M.; Dolladille, C.; Cohen-Solal, A.; Cohen, A.; Ederhy, S.; Cautela, J. Management of Immune Checkpoint Inhibitor–Induced Myocarditis. JACC CardioOncology 2021, 3, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Tocchetti, C.G.; Galdiero, M.R.; Varricchi, G. Cardiac Toxicity in Patients Treated With Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1765–1767. [Google Scholar] [CrossRef]
- Ball, S.; Ghosh, R.K.; Wongsaengsak, S.; Bandyopadhyay, D.; Ghosh, G.C.; Aronow, W.S.; Fonarow, G.C.; Lenihan, D.J.; Bhatt, D.L. Cardiovascular Toxicities of Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2019, 74, 1714–1727. [Google Scholar] [CrossRef]
- Alvi, R.M.; Frigault, M.J.; Fradley, M.G.; Jain, M.D.; Mahmood, S.S.; Awadalla, M.; Lee, D.H.; Zlotoff, D.A.; Zhang, L.; Drobni, Z.D.; et al. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J. Am. Coll. Cardiol. 2019, 74, 3099–3108. [Google Scholar] [CrossRef]
- Rachow, T.; Schiffl, H.; Lang, S.M. Risk of Lung Cancer and Renin–Angiotensin Blockade: A Concise Review. J. Cancer Res. Clin. Oncol. 2021, 147, 195–204. [Google Scholar] [CrossRef]
- Scott, O.W.; TinTin, S.; Cavadino, A.; Elwood, J.M. Beta-Blocker Use and Breast Cancer Outcomes: A Meta-Analysis. Breast Cancer Res. Treat. 2024, 206, 443–463. [Google Scholar] [CrossRef]
- Nakai, Y.; Isayama, H.; Ijichi, H.; Sasaki, T.; Sasahira, N.; Hirano, K.; Kogure, H.; Kawakubo, K.; Yagioka, H.; Yashima, Y.; et al. Inhibition of Renin–Angiotensin System Affects Prognosis of Advanced Pancreatic Cancer Receiving Gemcitabine. Br. J. Cancer 2010, 103, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Kwanten, W.J.; Jain, R.K. Renin–Angiotensin System Inhibitors to Mitigate Cancer Treatment–Related Adverse Events. Clin. Cancer Res. 2018, 24, 3803–3812. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Huda, B.; Bhurka, F.; Patnaik, R.; Banerjee, Y. Molecular and Immunomodulatory Mechanisms of Statins in Inflammation and Cancer Therapeutics with Emphasis on the NF-κB, NLRP3 Inflammasome, and Cytokine Regulatory Axes. Int. J. Mol. Sci. 2025, 26, 8429. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Scully, R.E.; Lipsitz, S.R.; Sallan, S.E.; Silverman, L.B.; Miller, T.L.; Barry, E.V.; Asselin, B.L.; Athale, U.; Clavell, L.A.; et al. Assessment of Dexrazoxane as a Cardioprotectant in Doxorubicin-Treated Children with High-Risk Acute Lymphoblastic Leukaemia: Long-Term Follow-up of a Prospective, Randomised, Multicentre Trial. Lancet Oncol. 2010, 11, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lu, J.; Qi, J.; Yang, Q.; Zhao, H.; Liu, H.; Chen, Z.; Huang, L.; Ye, Y.; Xu, M.; et al. The Effect of SGLT2 Inhibition on Prostate Cancer: Mendelian Randomization and Observational Analysis Using Electronic Healthcare and Cohort Data. Cell Rep. Med. 2024, 5, 101688. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef] [PubMed]
- Battisti, N.M.L.; Andres, M.S.; Lee, K.A.; Ramalingam, S.; Nash, T.; Mappouridou, S.; Senthivel, N.; Asavisanu, K.; Obeid, M.; Tripodaki, E.-S.; et al. Incidence of Cardiotoxicity and Validation of the Heart Failure Association-International Cardio-Oncology Society Risk Stratification Tool in Patients Treated with Trastuzumab for HER2-Positive Early Breast Cancer. Breast Cancer Res. Treat. 2021, 188, 149–163. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) Clinical Practice Guideline on Immune Checkpoint Inhibitor-Related Adverse Events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef] [PubMed]
- Lentz, R.; Feinglass, J.; Ma, S.; Akhter, N. Risk Factors for the Development of Atrial Fibrillation on Ibrutinib Treatment. Leuk. Lymphoma 2019, 60, 1447–1453. [Google Scholar] [CrossRef]
- Mato, A.R.; Clasen, S.; Pickens, P.; Gashonia, L.; Rhodes, J.; Svoboda, J.; Hughes, M.; Nabhan, C.; Ali, N.; Schuster, S.; et al. Left Atrial Abnormality (LAA) as a Predictor of Ibrutinib-Associated Atrial Fibrillation in Patients with Chronic Lymphocytic Leukemia. Cancer Biol. Ther. 2018, 19, 1–2. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline Cardiovascular Risk Assessment in Cancer Patients Scheduled to Receive Cardiotoxic Cancer Therapies: A Position Statement and New Risk Assessment Tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in Collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Gawli, C.S.; Patil, B.R.; Nagpure, N.R.; Patil, C.R.; Kumar, A.; Patel, H.M. Prevalence of Osimertinib-Induced Cardiotoxicity in Non-Small Cell Lung Cancer Patients: A Systematic Review and Meta-Analysis. Lung Cancer 2025, 205, 108629. [Google Scholar] [CrossRef]
- Aguado, R.; Mitroi, C.; Calvo, V.; Collazo, A.; Blanco, M.; Martinez, M.; Garitaonaindia, Y.; Ospina, A.V.; Contreras, C.; Segovia, J.; et al. New Generation ALK-Inhibitors Burden in Lung Cancer Patients Cardiovascular Health: Analysis from Real World Data. Eur. Heart J. Suppl. 2025, 27 (Suppl. S6), suaf083.094. [Google Scholar] [CrossRef]
- Jerzak, K.J.; Sehdev, S.; Boileau, J.-F.; Brezden-Masley, C.; Califaretti, N.; Edwards, S.; Gordon, J.; Henning, J.-W.; LeVasseur, N.; Railton, C. Multidisciplinary Practical Guidance for Implementing Adjuvant CDK4/6 Inhibitors for Patients with HR-Positive, HER2-Negative Early Breast Cancer in Canada. Curr. Oncol. 2025, 32, 444. [Google Scholar] [CrossRef]
- Xia, W.; Khalil, R.A. Hormone Replacement Therapy and Cardiovascular Health in Postmenopausal Women. Int. J. Mol. Sci. 2025, 26, 5078. [Google Scholar] [CrossRef]
- Fradin, J.; Kim, F.J.; Lu-Yao, G.L.; Storozynsky, E.; Kelly, W.K. Review of Cardiovascular Risk of Androgen Deprivation Therapy and the Influence of Race in Men with Prostate Cancer. Cancers 2023, 15, 2316. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Guha, A.; Morgans, A.K.; Niazi, T.; Pinthus, J.H. Cardiovascular Risk in Prostate Cancer. JACC CardioOncology 2024, 6, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Li, H.; Wang, W.; Li, L.; Liu, B.; Lan, B.; Li, Q.; Yang, W.; Wang, J.; Ma, F. Investigating Cardiovascular Diseases Related to Endocrine Therapy in Hormone Receptor-Positive Early Breast Cancer: Insights from a Nationwide Real-World Study. Cardio-Oncology 2025, 11, 35. [Google Scholar] [CrossRef]
- Fung, K.; Imeson, J.; Cusano, F. The Clinical Significance of QT Prolongation Associated with Tamoxifen: A Review of the Literature. J. Oncol. Pharm. Pract. 2018, 24, 525–530. [Google Scholar] [CrossRef]
- Mancuso, S.; Carlisi, M.; Sarocchi, M.; Napolitano, M.; Siragusa, S. Cardio-Oncology in Multiple Myeloma: Is It Time for a Specific Focus? Leuk. Lymphoma 2018, 59, 1764–1766. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.-B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- López-Fernández, T.; Farmakis, D.; Ameri, P.; Asteggiano, R.; De Azambuja, E.; Aznar, M.; Barac, A.; Bayes-Genis, A.; Bax, J.J.; Bergler-Klein, J.; et al. European Society of Cardiology Core Curriculum for cARDIO-ONCOLOGY. Eur. J. Heart Fail. 2024, 26, 754–771. [Google Scholar] [CrossRef]

| Molecular Target | Examples | Cardiovascular Toxicities (Incidence) | Mechanistic Notes |
|---|---|---|---|
| ERBB2 (HER2) | Trastuzumab, Pertuzumab, Lapatinib, Tucatinib, Afatinib, Neratinib, Dacomitinib | Heart failure: 2–5% (trastuzumab), up to 28% with anthracycline combination; LVEF reduction: 1–5% (TKIs) | Inhibition of NRG1-ERBB2/ERBB4 signaling → impaired cardiomyocyte survival, proliferation, and contractility; oxidative stress, mitochondrial dysfunction; reversible in most cases |
| VEGFRs, PDGFRs | Sunitinib, Sorafenib, Axitinib, Vandetanib, Cabozantinib, Lenvatinib, Pazopanib, Ponatinib, Regorafenib | Hypertension (up to 47%), LV dysfunction (≈28%), heart failure (≈8%) | VEGFR inhibition → ↓ NO → hypertension; capillary rarefaction → impaired myocardial perfusion; PDGFR blockade → pericyte loss → microvascular dysfunction |
| Bcr-Abl, c-Kit, c-Abl, PDGFRs | Imatinib | Heart failure (especially in elderly) | ER stress, mitochondrial damage, impaired cardiac progenitor cell function |
| Mutant Bcr-Abl, PDGFRs | Dasatinib, Nilotinib, Bosutinib, Ponatinib | Occlusive arterial disease ↑ risk | Designed to overcome BCR-ABL resistance; CV risk elevated vs. imatinib |
| EGFR | Erlotinib, Gefitinib, Afatinib, Osimertinib | QTc prolongation, arrhythmias, reduced LVEF, heart failure | PI3K pathway inhibition, HER2 inhibition, oxidative stress, mitochondrial dysfunction, autophagy/apoptosis dysregulation |
| ALK | Crizotinib, Ceritinib, Alectinib, Brigatinib, Lorlatinib | Sinus bradycardia, AV block, QTc prolongation, hypertension, hyperglycemia, dyslipidemia | Long-term CV effects; generally well-tolerated but important due to extended survival |
| Baseline CV Toxicity Risk Factors | Anthracycline Chemotherapy | HER2-Targeted Therapies | VEGF Inhibitors | BCR-ABL Inhibitors | Multiple Myeloma Therapies | RAF and MEK Inhibitors |
|---|---|---|---|---|---|---|
| Previous CVD | ||||||
| HF/cardiomyopathy/CTRCD | VH | VH | VH | H | VH | VH |
| Severe VHD | H | H | - | - | - | H |
| MI or PCI or CABG | H | H | VH | - | - | H |
| Stable angina | H | H | VH | - | - | H |
| Arterial vascular disease | - | - | VH | VH | VH | - |
| Abnormal ankle-brachial pressure index | - | - | - | H | - | - |
| PH | - | - | - | H | - | - |
| Arterial thrombosis with TKI | - | - | - | VH | - | - |
| Venous thrombosis (DVT/PE) | - | - | H | M2 | VH | - |
| Arrhythmia | - | M2 | M2 | M2 | M2 | M1 |
| QTc ≥ 480 ms | - | - | H | H | - | - |
| 450 ≤ QTc, 480 ms (men), 460 ≤ QTc < 480 ms (women) | - | - | M2 | M2 | - | - |
| Prior PI CV toxicity | - | - | - | - | VH | - |
| Prior IMiD CV toxicity | - | - | - | - | H | - |
| Cardiac imaging | ||||||
| LVEF < 50% | H | H | H | H | H | H |
| LVEF 50–54% | M2 | M2 | M2 | - | M2 | M2 |
| LV hypertrophy | - | - | - | - | M1 | - |
| Cardiac amyloidosis | - | - | - | - | VH | - |
| Cardiac biomarkers | ||||||
| Elevated baseline cTn | M1 | M2 | M1 | - | M2 | M2 |
| Elevated baseline NP | M1 | M2 | M1 | - | H | M2 |
| Age and CVRF | ||||||
| Age ≥ 80 years | H | H | - | - | - | M1 |
| Age 65–79 years | M2 | M2 | - | - | - | M1 |
| Age ≥ 75 years | - | - | H | H | H | M1 |
| Age 65–74 years | - | - | M1 | M2 | M1 | M1 |
| Age ≥ 60 years | - | - | - | M1 | - | - |
| CVD 10-year risk score > 20% | - | - | - | H | - | - |
| Hypertension | M1 | M1 | H | M2 | M1 | M2 |
| Chronic kidney disease | M1 | M1 | M1 | M1 | M1 | M1 |
| Proteinuria | - | - | M1 | - | - | - |
| DM | M1 | M1 | M1 | M1 | M1 | M1 |
| Hyperlipidemia | - | - | M1 | M1 | M1 | - |
| Family history of thrombophilia | - | - | - | M1 | M1 | - |
| Current cancer treatment | ||||||
| Dexamethasone > 160 mg/month | - | - | - | - | M1 | - |
| Includes anthracycline before HER2-targeted therapy | - | M1 | - | - | - | - |
| Previous exposure to | ||||||
| Anthracycline | H | M2 | H | - | H | H |
| Trastuzumab | - | VH | - | - | - | - |
| RT to left chest or mediastinum | H | M2 | M1 | - | M1 | M2 |
| Non-anthracycline chemotherapy | M1 | - | - | - | - | - |
| Lifestyle risk factors | ||||||
| Current smoker or significant smoking history | M1 | M1 | M1 | H | M1 | M1 |
| Obesity (BMI > 30 kg/m2) | M1 | M1 | M1 | M1 | M1 | M1 |
| Severity | Asymptomatic CTRCD | Symptomatic CTRCD |
|---|---|---|
| Mild | LVEF ≥ 50% AND new relative decline in GLS by >15% from baseline AND/OR new rise in cardiac biomarkers | Mild HF symptoms, no intensification of therapy required |
| Moderate | New LVEF reduction by ≥10 percentage points to an LVEF of 40–49% OR new LVEF reduction by <10 percentage points to an LVEF of 40–49% AND either new relative decline in GLS by >15% from baseline OR new rise in cardiac biomarkers | Need for outpatient intensification of diuretic and HF therapy |
| Severe | New LVEF reduction to < 40% | Requires hospitalization for heart failure |
| Very severe | ----- | Heart failure requiring inotropic support, mechanical circulatory assistance, or heart transplantation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciappina, G.; Colarusso, L.; Maiorana, E.; Ottaiano, A.; Franchina, T.; Picone, A.; Facchini, G.; Barraco, C.; Ieni, A.; Cusmà Piccione, M.; et al. Cardiotoxicity Induced by Anticancer Therapies: A Call for Integrated Cardio-Oncology Practice. Pharmaceuticals 2025, 18, 1399. https://doi.org/10.3390/ph18091399
Ciappina G, Colarusso L, Maiorana E, Ottaiano A, Franchina T, Picone A, Facchini G, Barraco C, Ieni A, Cusmà Piccione M, et al. Cardiotoxicity Induced by Anticancer Therapies: A Call for Integrated Cardio-Oncology Practice. Pharmaceuticals. 2025; 18(9):1399. https://doi.org/10.3390/ph18091399
Chicago/Turabian StyleCiappina, Giuliana, Luigi Colarusso, Enrica Maiorana, Alessandro Ottaiano, Tindara Franchina, Antonio Picone, Gaetano Facchini, Chiara Barraco, Antonio Ieni, Maurizio Cusmà Piccione, and et al. 2025. "Cardiotoxicity Induced by Anticancer Therapies: A Call for Integrated Cardio-Oncology Practice" Pharmaceuticals 18, no. 9: 1399. https://doi.org/10.3390/ph18091399
APA StyleCiappina, G., Colarusso, L., Maiorana, E., Ottaiano, A., Franchina, T., Picone, A., Facchini, G., Barraco, C., Ieni, A., Cusmà Piccione, M., Zito, C., & Berretta, M. (2025). Cardiotoxicity Induced by Anticancer Therapies: A Call for Integrated Cardio-Oncology Practice. Pharmaceuticals, 18(9), 1399. https://doi.org/10.3390/ph18091399










