Phlorizin Alleviates Depression-like Behaviors via Gut Microbiota Reprogramming-Induced Methionine to Inhibit Neuroinflammation in Mice Hippocampus
Abstract
1. Introduction
2. Results
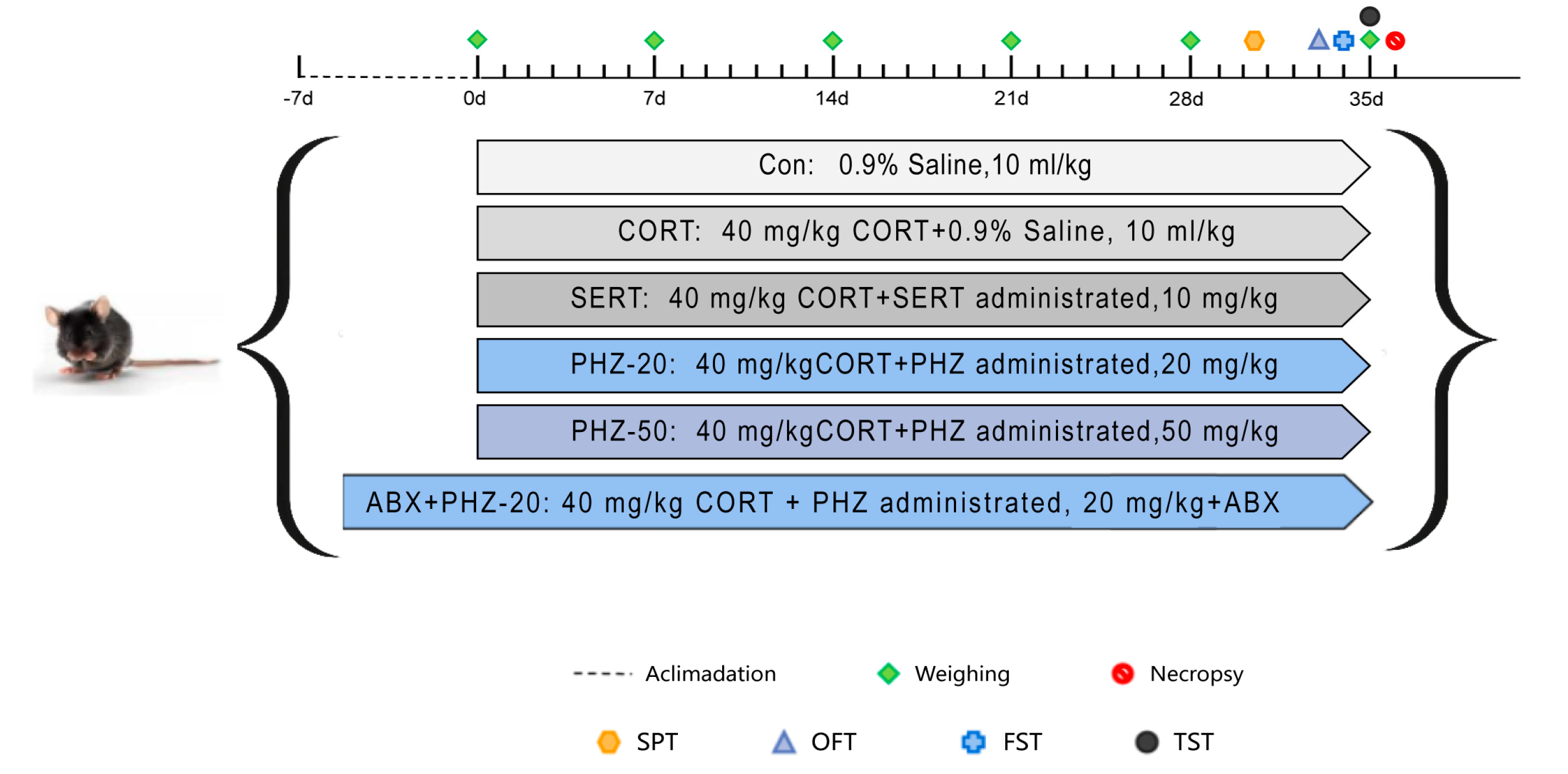
2.1. Oral PHZ Improves CORT Mice’s Weight and Behavior
2.2. PHZ Decreased the Neuroinflammation in CORT Mice
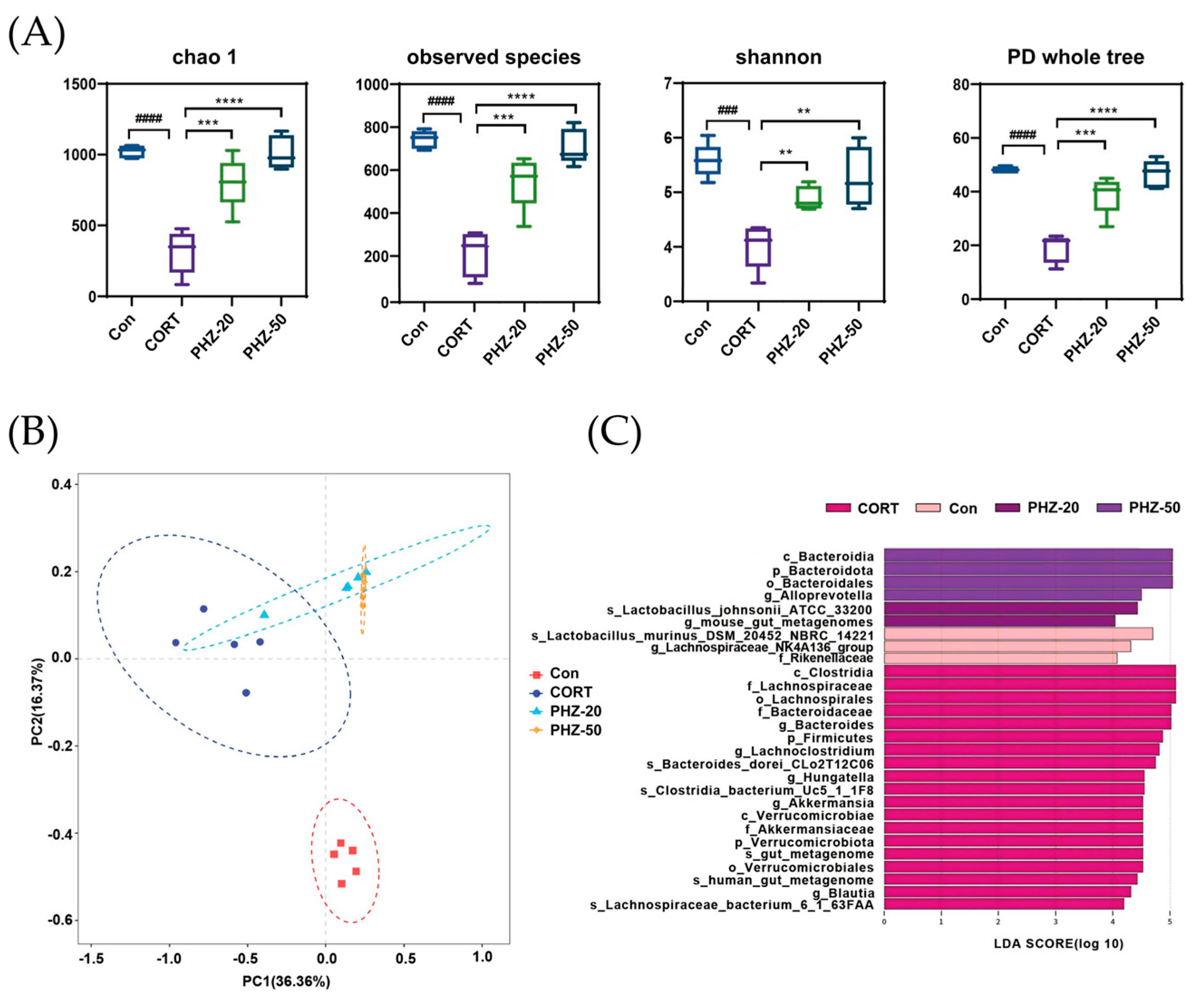
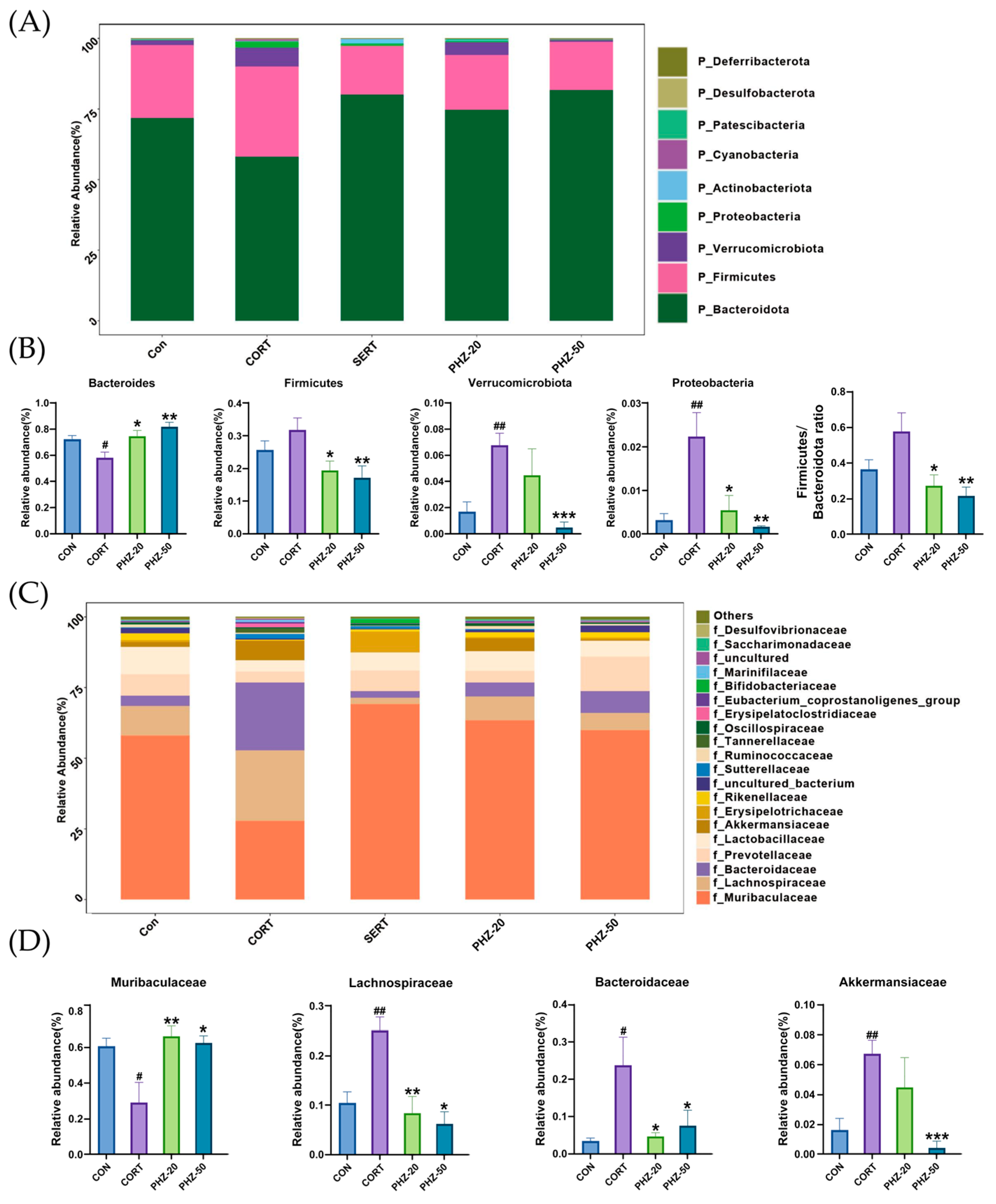
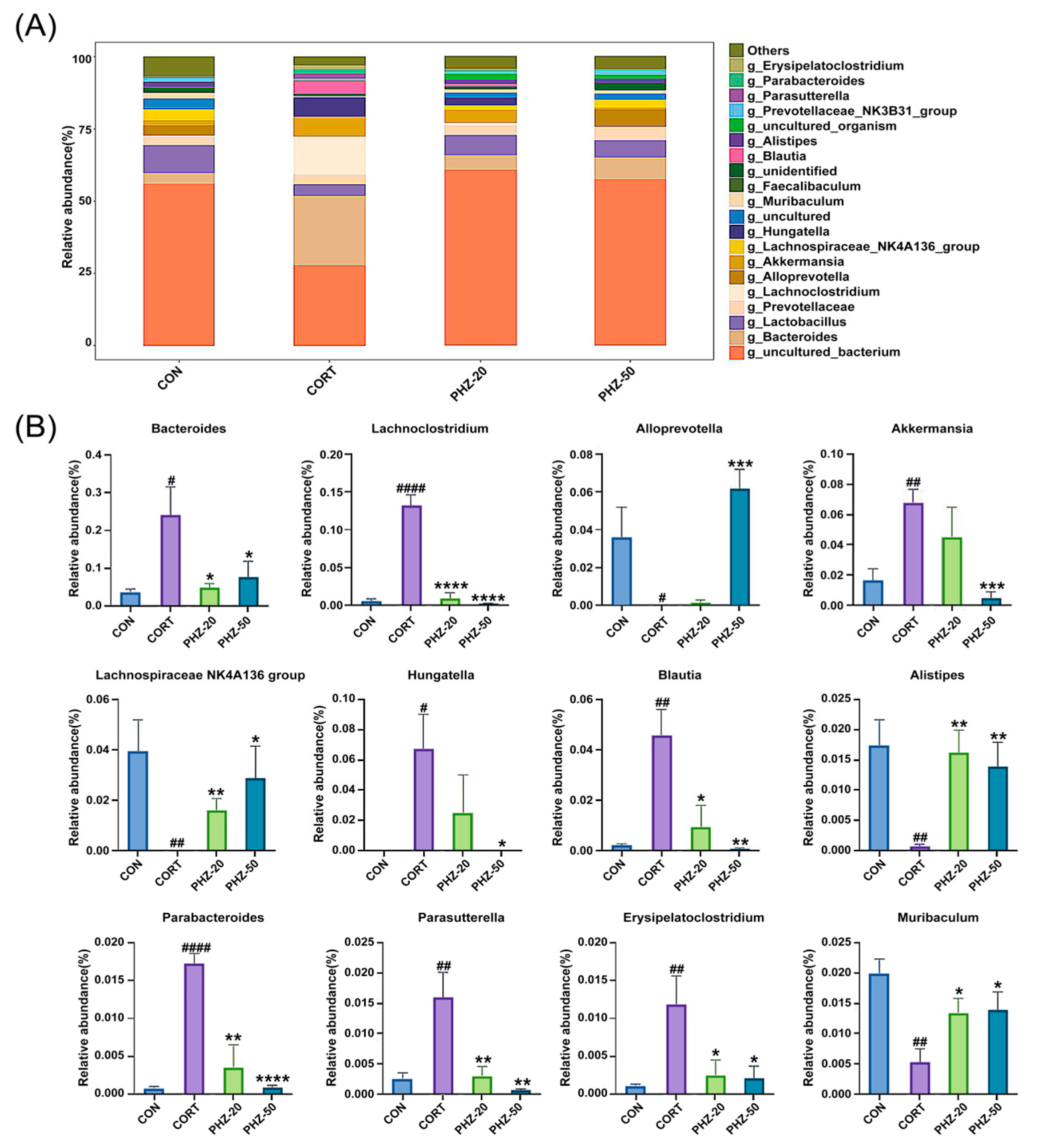
2.3. PHZ Restored the Gut Microbiota of CORT Mice
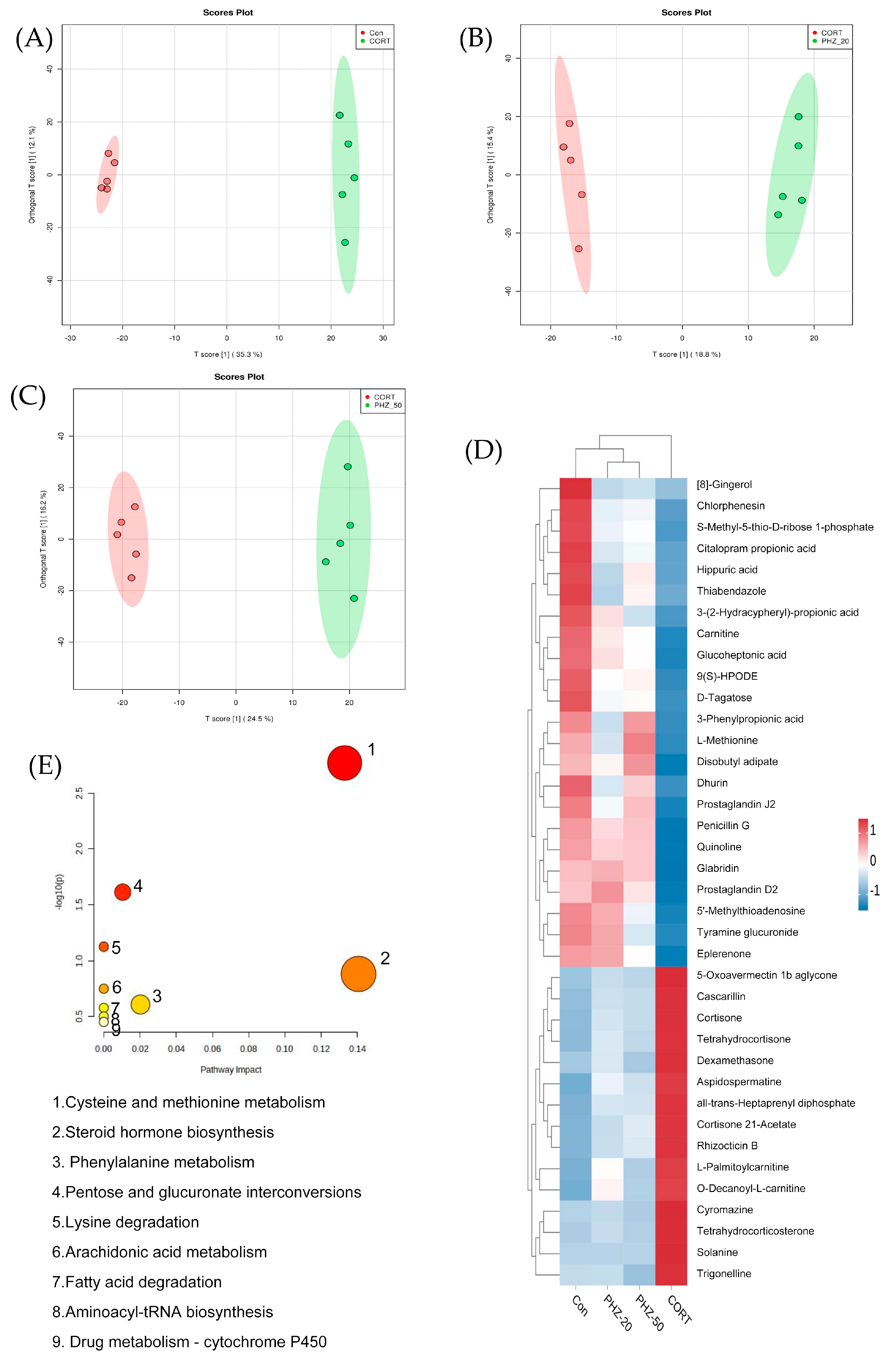
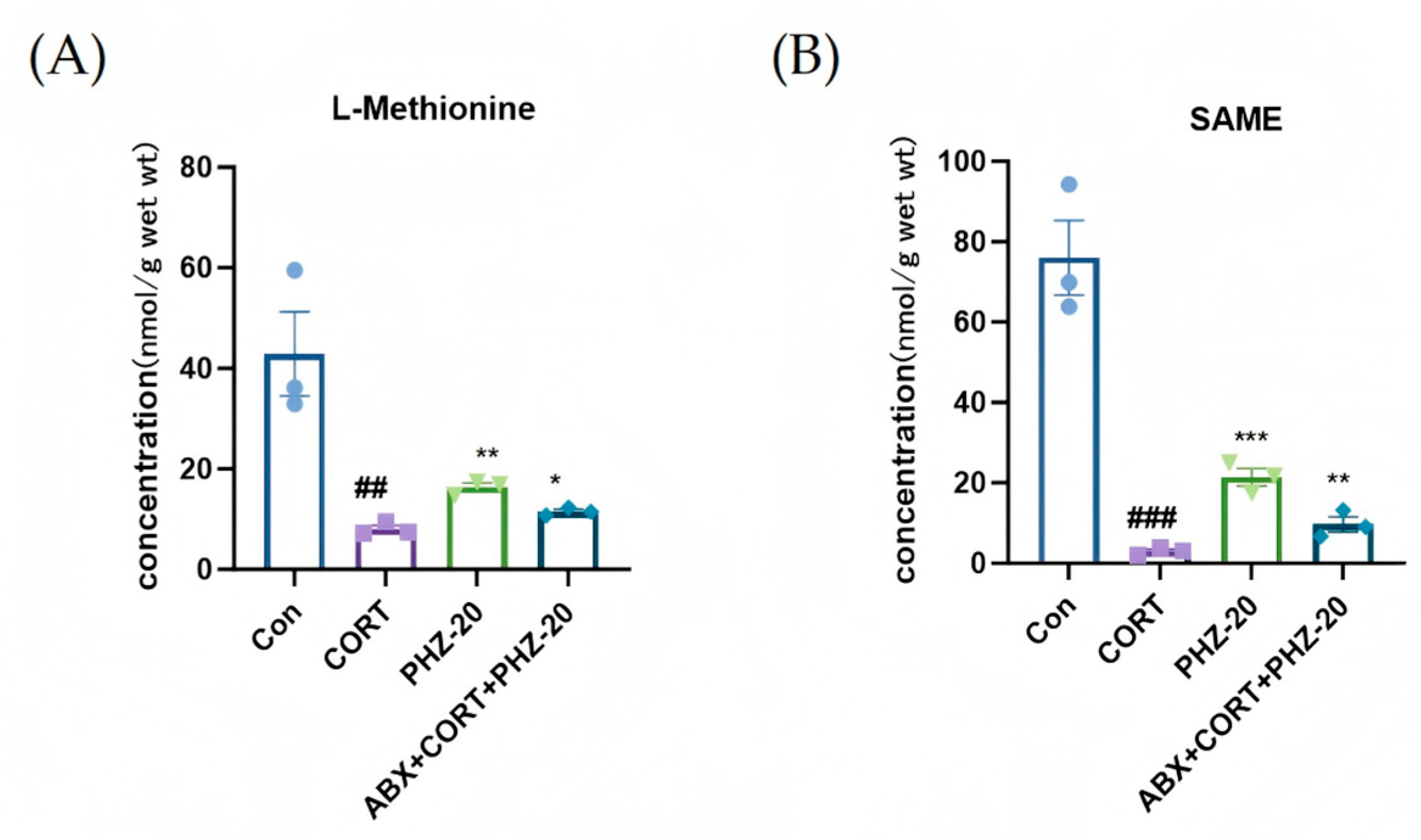
2.4. PHZ Changed the Metabolism in CORT Mice
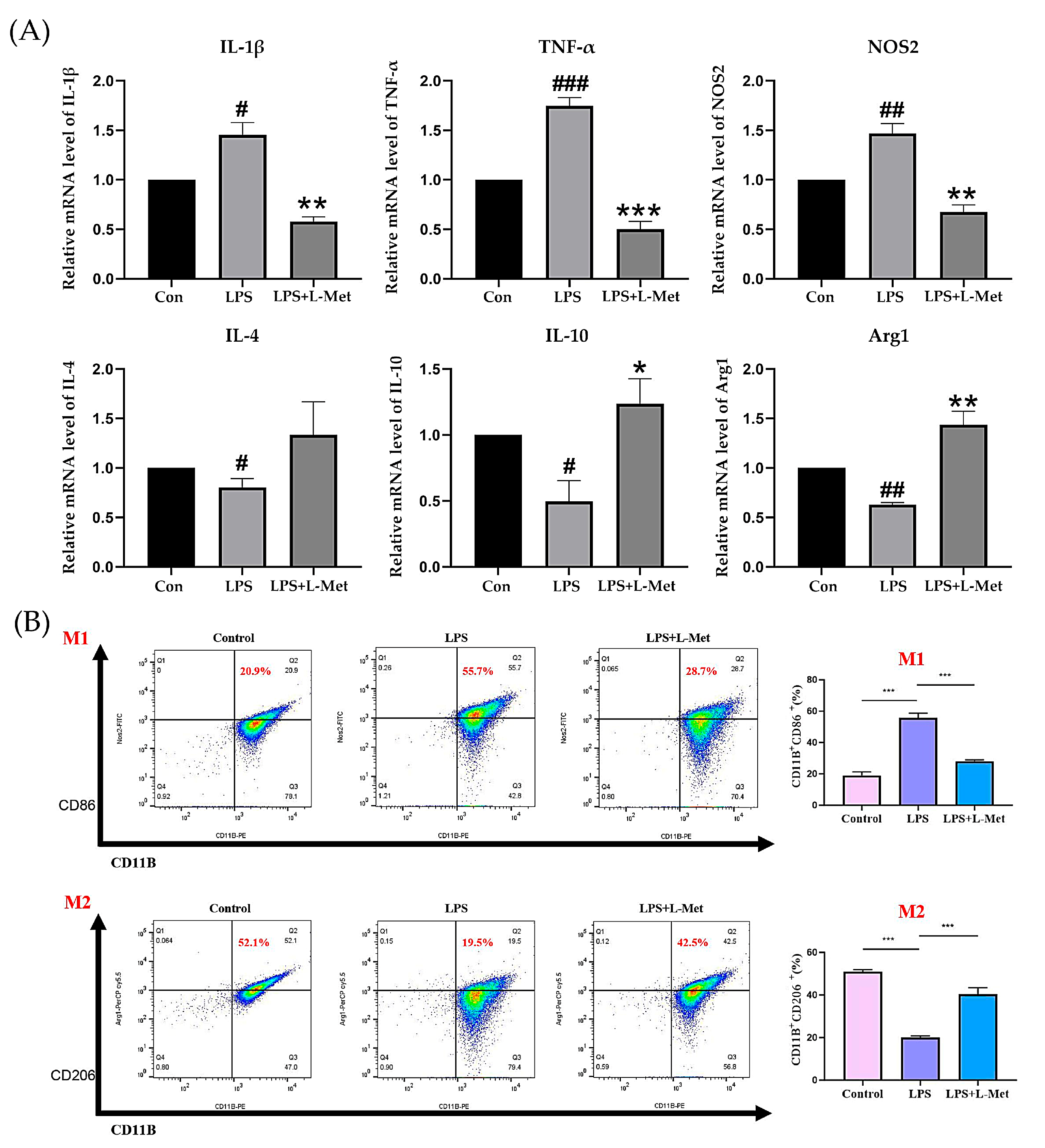
2.5. Met Modulates LPS-Induced Inflammation and Promotes M2 Polarization in BV2 Microglial Cells
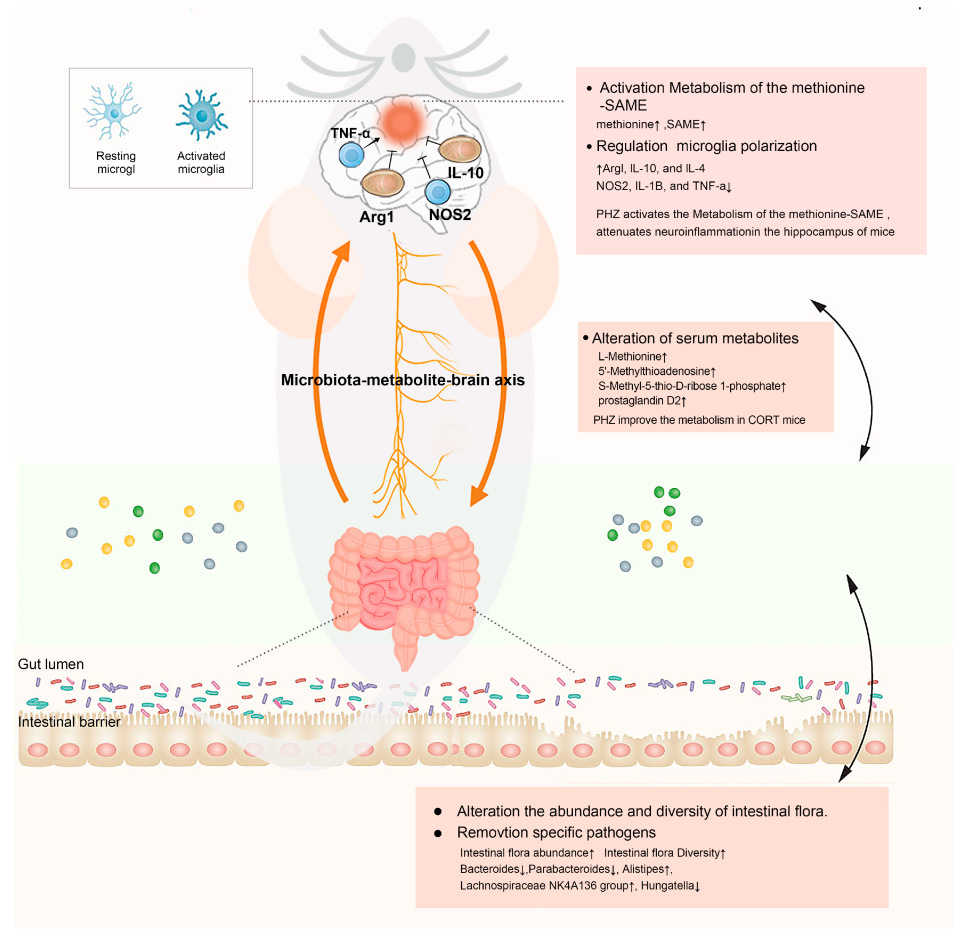
2.6. Integrative Effect of PHZ on the Microbiome–Metabolism–Brain Axis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Effect of PHZ on Weight and Behavior in CORT-Induced Depression Mice
4.2.1. Depression Modeling and Drug Administration
4.2.2. Behavioral Testing
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Immunofluorescence
4.5. Western Blot
4.6. 16S rRNA Gene Sequencing
4.7. Metabolomics Analysis
4.7.1. UPLC-Q-TOF/MS Detection Analysis
4.7.2. Differential Metabolites Identification and Data Analysis
4.8. Methionine-Targeted Metabolomics Analysis
4.9. Cell Viability Assay
4.10. Cell Culture and Treatment
4.11. Quantitative Real-Time PCR
4.12. Flow Cytometry
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABx | antibiotic mixture |
| CCK-8 | Cell Counting Kit-8 assay |
| CORT | corticosterone |
| ELISA | enzyme-linked immunosorbent assay |
| EP | Eppendorf |
| F/B | Firmicutes/Bacteroidetes |
| FST | forced swimming test |
| HFFD | high-fat, high-fructose diet |
| Ig | immunoglobulin |
| IL | interleukin |
| L-Met | L-methionine |
| LPS | lipopolysaccharide |
| OPLS-DA | Orthogonal Projections to Latent Structures Discriminant Analysis |
| PHZ | Phlorizin |
| SAMe | S-adenosylmethionine |
| SPT | sucrose preference test |
| SSRI | selective serotonin reuptake inhibitors |
| TNF | tumor necrosis factor |
| TST | tail suspension test |
| UPLC-Q-TOF/MS | ultra-high-performance liquid chromatography integrated with quadruple time-of-flight mass spectrometry |
| VIP | Variable Importance in Projection |
| MGB | microbiota–gut–brain |
| RAs | relative abundances |
References
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Collishaw, S.; Pine, D.S.; Thapar, A.K. Depression in adolescence. Lancet 2012, 379, 1056–1067. [Google Scholar] [CrossRef]
- WHO. Depression and other common mental disorders. In Global Health Estimates; WHO: Geneva, Switzerland, 2017. [Google Scholar] [CrossRef]
- Fava, G.A.; Cosci, F.; Guidi, J.; Rafanelli, C. The deceptive manifestations of treatment resistance in depression: A new look at the problem. Psychother. Psychosom. 2020, 89, 265–273. [Google Scholar] [CrossRef]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-gut-brain axis: New therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef]
- Trzeciak, P.; Herbet, M. Role of the intestinal microbiome, intestinal barrier and psychobiotics in depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rodriguez, E.M.; Watson, J.; Reyes, J.; Trivedi, M.; Beurel, E. Th17 cells sense microbiome to promote depressive-like behaviors. Microbiome 2023, 11, 92. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, J.; Gong, L.; Liu, F.; Zhao, H.; Yan, Z.; Li, Y.; Zhang, J.; Xiao, M.; Mu, J. Microbiota-derived short-chain fatty acids may participate in post-stroke depression by regulating host’s lipid metabolism. J. Psychiatr. Res. 2023, 161, 426–434. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Giusti, P. Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: A review. CNS Neurol. Disord. Drug Targets 2014, 13, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.Y.; Liang, L.F.; Shi, T.L.; Shen, Z.Q.; Yin, S.Y.; Zhang, J.R.; Li, W.; Mi, W.L.; Wang, Y.Q.; Zhang, Y.Q.; et al. Microglia-derived interleukin-6 triggers astrocyte apoptosis in the hippocampus and mediates depression-like behavior. Adv. Sci. 2025, 12, e2412556. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wei, Y.; Hashimoto, K. Brain–gut–microbiota axis in depression: A historical overview and future directions. Brain Res. Bull. 2022, 182, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Zhang, S.; Rao, J.; Zhao, J.; Huang, H.; Liu, Y.; Ding, Y.; Liu, Y.; Ma, Y.; Zhang, S.; et al. Phlorizin, an important glucoside: Research progress on its biological activity and mechanism. Molecules 2024, 29, 741. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The Bioavailability, Extraction, Biosynthesis and Distribution of Natural Dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Chen, H.; Dong, L.; Chen, X.; Ding, C.; Hao, M.; Peng, X.; Zhang, Y.; Zhu, H.; Liu, W. Anti-aging effect of phlorizin on D-galactose–induced aging in mice through antioxidant and anti-inflammatory activity, prevention of apoptosis, and regulation of the gut microbiota. Exp. Gerontol. 2022, 163, 111769. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Liu, S.; Hu, C.; Meng, Y. Phlorizin ameliorates cognitive and behavioral impairments via the microbiota-gut-brain axis in high-fat and high-fructose diet-induced obese male mice. Brain Behav. Immun. 2025, 123, 193–210. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Stilling, R.M.; Van De Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Balcheva-Sivenova, Z.P.; Georgiev, M.I. Metabolomics and health: From nutritional crops and plant-based pharmaceuticals to profiling of human biofluids. Cell. Mol. Life Sci. 2021, 78, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, X.; Song, X.; Yang, Y.; Wang, X.; Xu, G.; Wang, T.; Liu, Y.; Fan, Z.; Song, G. Effects of Fzd6 on intestinal flora and neuroinflammation in lipopolysaccharide-induced depression-like mice. J. Affect. Disord. 2025, 372, 160–172. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, X.; Yan, F.; Zhang, W.; Zhao, S.; Song, Y.; Wang, S.; Zhu, Z.; Wang, Y.; Liu, X. Quinoa saponin ameliorates lipopolysaccharide-induced behavioral disorders in mice by inhibiting neuroinflammation, modulating gut microbiota, and counterbalancing intestinal inflammation. J. Agric. Food Chem. 2025, 73, 4700–4715. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, R.; Deng, Q.; Wang, W.; Cao, C.; Yu, C.; Li, S.; Shi, L.; Tian, J. S-adenosylmethionine improves cognitive impairment in D-galactose-induced brain aging by inhibiting oxidative stress and neuroinflammation. J. Chem. Neuroanat. 2023, 128, 102232. [Google Scholar] [CrossRef]
- Kwon, M.-S. Advanced therapeutic strategies targeting microglia: Beyond neuroinflammation. Arch. Pharm. Res. 2022, 45, 618–630. [Google Scholar] [CrossRef]
- Xie, X.; Xiao, Q.; Xiong, Z.; Yu, C.; Zhou, J.; Fu, Z. Crocin-I Ameliorates the Disruption of Lipid Metabolism and Dysbiosis of the Gut Microbiota Induced by Chronic Corticosterone in Mice. Food Funct. 2019, 10, 6779–6791. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, G.; Shao, B.; Zhou, H.; Xu, C.; Yan, F.; Wang, L.; Chen, G.; Li, J.; Fu, X. Gut Microbiota Dysbiosis Induced by Intracerebral Hemorrhage Aggravates Neuroinflammation in Mice. Front. Microbiol. 2021, 12, 647304. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress 2019, 22, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Huang, S.; Luo, Z.; Zhou, S.; Zhang, C.; Zhu, Y.; Yang, J.; Li, L. Radix Bupleuri aqueous extract attenuates MK801-induced schizophrenia-like symptoms in mice: Participation of intestinal flora. Biomed. Pharmacother. 2024, 172, 116267. [Google Scholar] [CrossRef]
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C.; et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J. Neuroinflamm. 2021, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Shanahan, F.; O’Mahony, L.; O’Mahony, C.; Coakley, M.; Hart, O.; Lawlor, P.; Quigley, E.M.; Kiely, B.; et al. Metabolic activity of the enteric microbiota influences the fatty acid composition of murine and porcine liver and adipose tissues. Am. J. Clin. Nutr. 2009, 89, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.A.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef]
- Sarkisova, K.Y.; Gabova, A.V.; Fedosova, E.A.; Shatskova, A.B.; Narkevich, V.B.; Kudrin, V.S. Antidepressant and anxiolytic effects of L-methionine in the WAG/Rij rat model of depression comorbid with absence epilepsy. Int. J. Mol. Sci. 2023, 24, 12425. [Google Scholar] [CrossRef]
- Stahl, S.M. Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr. 2007, 12, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Alachkar, A.; Agrawal, S.; Baboldashtian, M.; Nuseir, K.; Salazar, J.; Agrawal, A. L-Methionine Enhances Neuroinflammation and Impairs Neurogenesis: Implication for Alzheimer’s Disease. J. Neuroimmunol. 2022, 366, 577843. [Google Scholar] [CrossRef]
- Wu, X.; Han, Z.; Liu, B.; Yu, D.; Sun, J.; Ge, L.; Tang, W.; Liu, S. Gut microbiota contributes to the methionine metabolism in host. Front. Microbiol. 2022, 13, 1065668. [Google Scholar] [CrossRef]
- Li, W.; Jia, Y.; Gong, Z.; Dong, Z.; Yu, F.; Fu, Y.; Jiang, C.; Kong, W. Ablation of the gut microbiota alleviates high-methionine diet-induced hyperhomocysteinemia and glucose intolerance in mice. npj Sci. Food 2023, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Gan, L.-H.; Liang, X.-N.; Xu, Z.-H.; Hu, T.-Y.; Li, X.-Y.; Tang, Y.-L.; Wang, J.; Liu, Y.-Q. Association of plasma homocysteine with cognitive impairment in patients with Parkinson’s disease. Front. Aging Neurosci. 2024, 16, 1434943. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-T.; Zhang, Y.; Xiang, C.-G.; Yang, T.; Wang, X.-H.; Lu, Q.-K.; Lu, H.-M.; Fan, C.; Feng, C.-L.; Yang, X.-Q.; et al. Methionine-choline deficient diet deteriorates DSS-induced murine colitis through disturbance of gut microbes and infiltration of macrophages. Acta Pharmacol. Sin. 2024, 45, 1912–1925. [Google Scholar] [CrossRef]
- Bolaños, C.A.; Willey, M.D.; Maffeo, M.L.; Powers, K.D.; Kinka, D.W.; Grausam, K.B.; Henderson, R.P. Antidepressant treatment can normalize adult behavioral deficits induced by early-life exposure to methylphenidate. Biol. Psychiatry 2008, 63, 309–316. [Google Scholar] [CrossRef]
- Song, A.-Q.; Gao, B.; Fan, J.-J.; Zhu, Y.-J.; Zhou, J.; Wang, Y.-L.; Xu, L.-Z.; Wu, W.-N. NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J. Neuroinflamm. 2020, 17, 178. [Google Scholar] [CrossRef]
- Choi, J.E.; Park, D.-M.; Chun, E.; Choi, J.J.; Seo, J.H.; Kim, S.; Son, J.; Do, M.; Kim, S.Y.; Park, Y.-C.; et al. Control of stress-induced depressive disorders by So-ochim-tang-gamibang, a Korean herbal medicine. J. Ethnopharmacol. 2017, 196, 141–150. [Google Scholar] [CrossRef]
- Gu, Y.; Ye, T.; Tan, P.; Tong, L.; Ji, J.; Gu, Y.; Shen, Z.; Shen, X.; Lu, X.; Huang, C. Tolerance-inducing effect and properties of innate immune stimulation on chronic stress-induced behavioral abnormalities in mice. Brain Behav. Immun. 2021, 91, 451–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Guo, Y.; Xu, L.; Wang, H. Phlorizin exerts potent effects against aging induced by D-galactose in mice and PC12 cells. Food Funct. 2021, 12, 2148–2160. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Guo, H.; Wang, Q.; Liu, C.; Ge, S.; Yan, B. The role and mechanism of NLRP3 inflammasome-mediated astrocyte activation in dehydrocorydaline against CUMS-induced depression. Front. Pharmacol. 2022, 13, 1008249. [Google Scholar] [CrossRef]
- Cao, L.-H.; Zhao, Y.-Y.; Bai, M.; Geliebter, D.; Geliebter, J.; Tiwari, R.; He, H.-J.; Wang, Z.-Z.; Jia, X.-Y.; Li, J.; et al. Mechanistic studies of gypenosides in microglial state transition and its implications in depression-like behaviors: Role of TLR4/MyD88/NF-κB signaling. Front. Pharmacol. 2022, 13, 838261. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.L.; Quan, H.F.; Yan, L.; Pei, X.Y.; Wang, R.; Peng, X.D. Effects of betaine on LPS-stimulated activation of microglial M1/M2 phenotypes by suppressing TLR4/NF-κB pathways in N9 cells. Molecules 2019, 24, 367. [Google Scholar] [CrossRef]




| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| IL-1β | TTTGAAGTTGACGGACCCCAA | CACAGCTTCTCCACAGCCACA |
| IL-4 | ATCGGCATTTTGAACGAGGTCACA | CGAAGCACCTTGGAAGCCCTA |
| IL-10 | TTACCTGGTAGAAGTGATGCCC | GACACCTTGGTCTTGGAGCTTA |
| Arg-1 | AGGAAAGCTGGTCTGCTGGAA | AGATGCTTCCAACTGCCAGAC |
| NOS2 | GGGCTGTCACGGAGATCAATG | GCCCGGTACTCATTCTGCATG |
| TNF-α | ACCACGCTCTTCTGTCTACT | AGGAGGTTGACTTTCTCCTG |
| β-actin | CTGAGAGGGAAATCGTGCGT | CCACAGGATTCCATACCCAAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Chen, J.; Zhang, X.; Zhang, X.; Fu, Y.; Jiang, H.; Yin, T.; Zhang, Y.; Li, X.; Hu, M.; et al. Phlorizin Alleviates Depression-like Behaviors via Gut Microbiota Reprogramming-Induced Methionine to Inhibit Neuroinflammation in Mice Hippocampus. Pharmaceuticals 2025, 18, 1395. https://doi.org/10.3390/ph18091395
Li L, Chen J, Zhang X, Zhang X, Fu Y, Jiang H, Yin T, Zhang Y, Li X, Hu M, et al. Phlorizin Alleviates Depression-like Behaviors via Gut Microbiota Reprogramming-Induced Methionine to Inhibit Neuroinflammation in Mice Hippocampus. Pharmaceuticals. 2025; 18(9):1395. https://doi.org/10.3390/ph18091395
Chicago/Turabian StyleLi, Lingling, Jianxin Chen, Xinyu Zhang, Xuya Zhang, Yan Fu, Hong Jiang, Tianxing Yin, Yali Zhang, Xue Li, Mengyuan Hu, and et al. 2025. "Phlorizin Alleviates Depression-like Behaviors via Gut Microbiota Reprogramming-Induced Methionine to Inhibit Neuroinflammation in Mice Hippocampus" Pharmaceuticals 18, no. 9: 1395. https://doi.org/10.3390/ph18091395
APA StyleLi, L., Chen, J., Zhang, X., Zhang, X., Fu, Y., Jiang, H., Yin, T., Zhang, Y., Li, X., Hu, M., & Lu, Y. (2025). Phlorizin Alleviates Depression-like Behaviors via Gut Microbiota Reprogramming-Induced Methionine to Inhibit Neuroinflammation in Mice Hippocampus. Pharmaceuticals, 18(9), 1395. https://doi.org/10.3390/ph18091395






