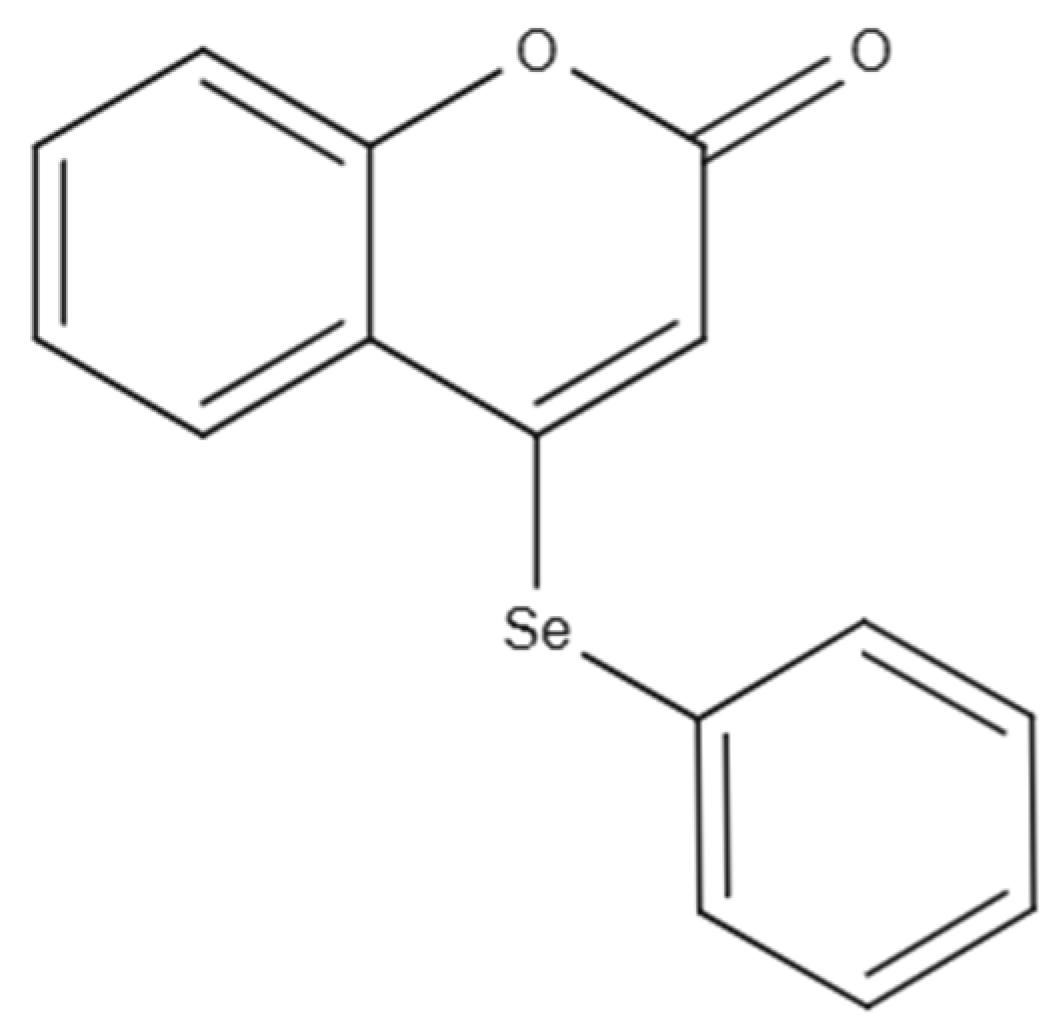
Preclinical Evaluation of Nanoemulsion and Polymeric Nanocapsule Delivery Systems of 4-(Phenylselanyl)-2H-Chromen-2-One for Rheumatoid Arthritis and Comorbidities
Abstract
1. Introduction
2. Results
2.1. Nanocapsule Suspension and Nanoemulsion Physicochemical Characterization
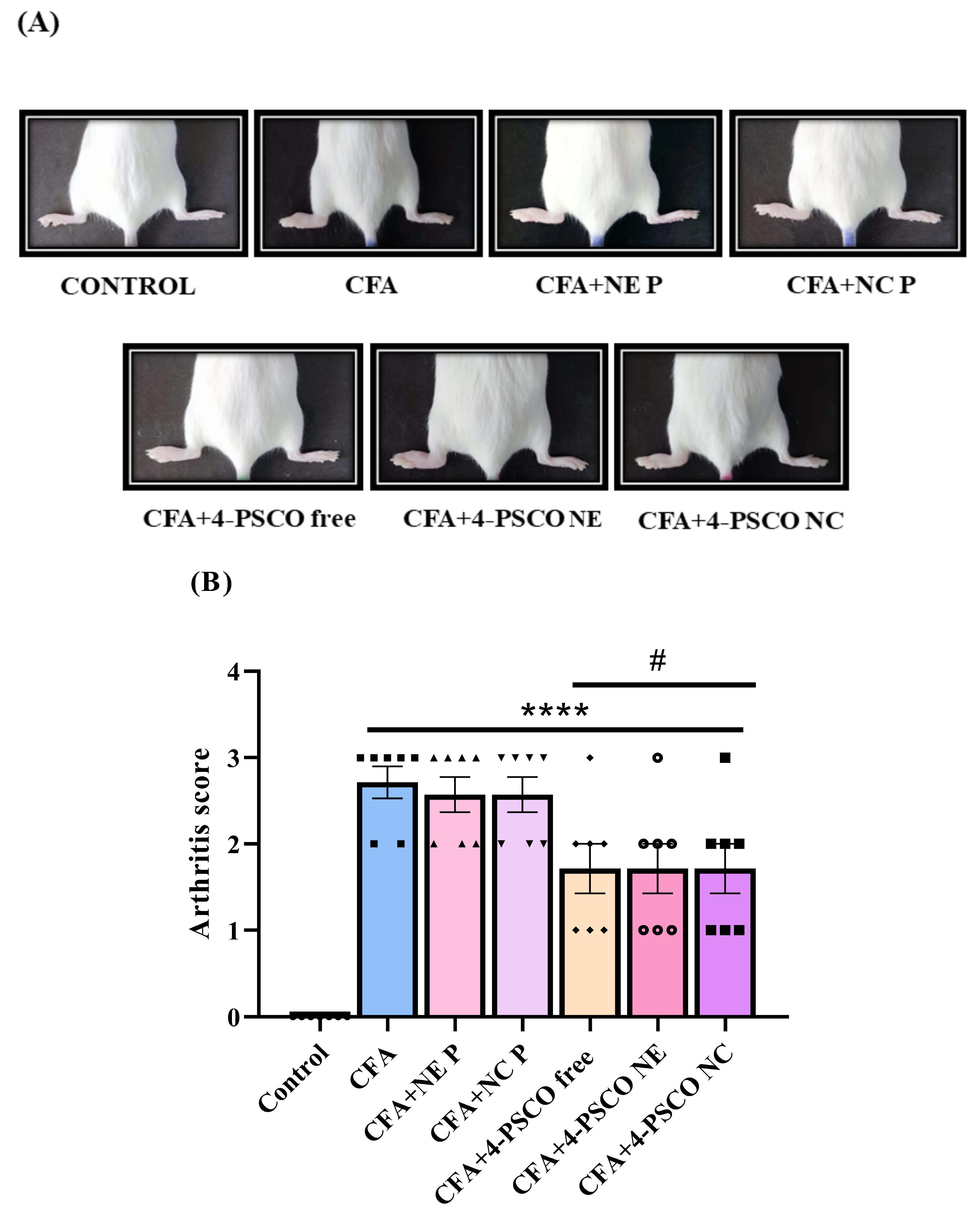
2.2. Therapeutic Effects of 4-PSCO in Free or Nanoencapsulated Form on the Arthritis Severity of Arthritis in CFA-Induced RA
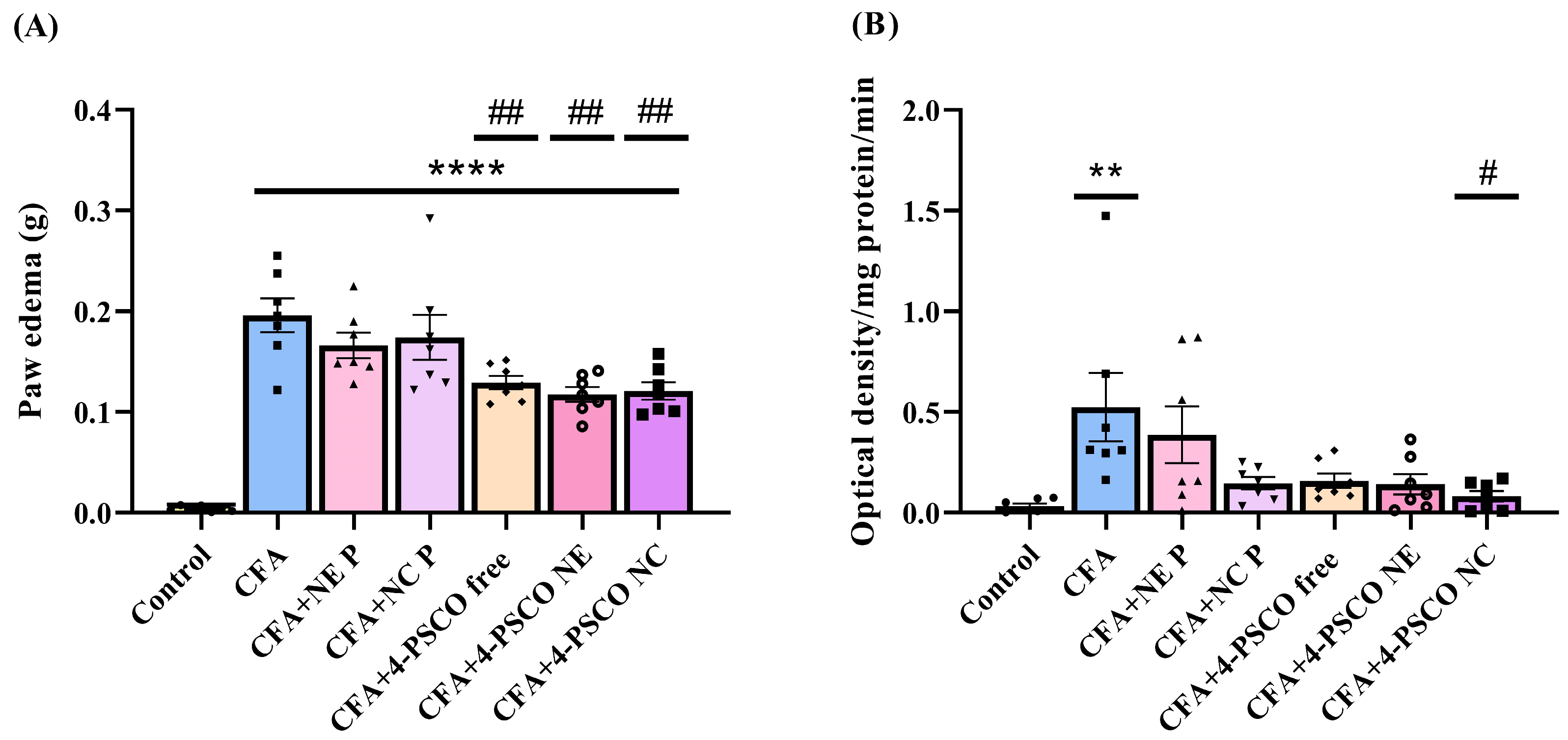
2.3. Mitigation of RA Symptoms Through the Anti-Inflammatory Effects of 4-PSCO in Free or Nanoencapsulated Form
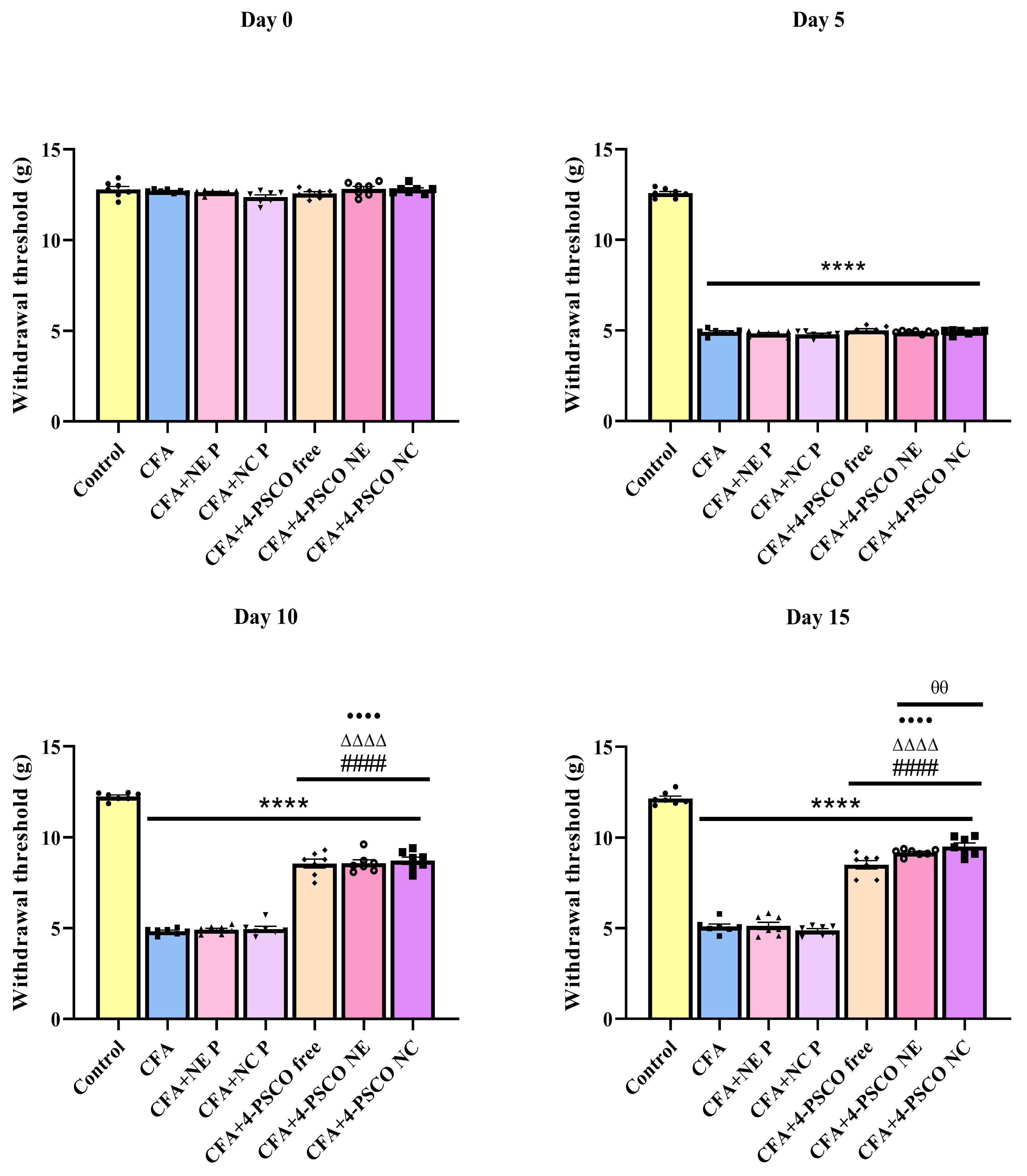
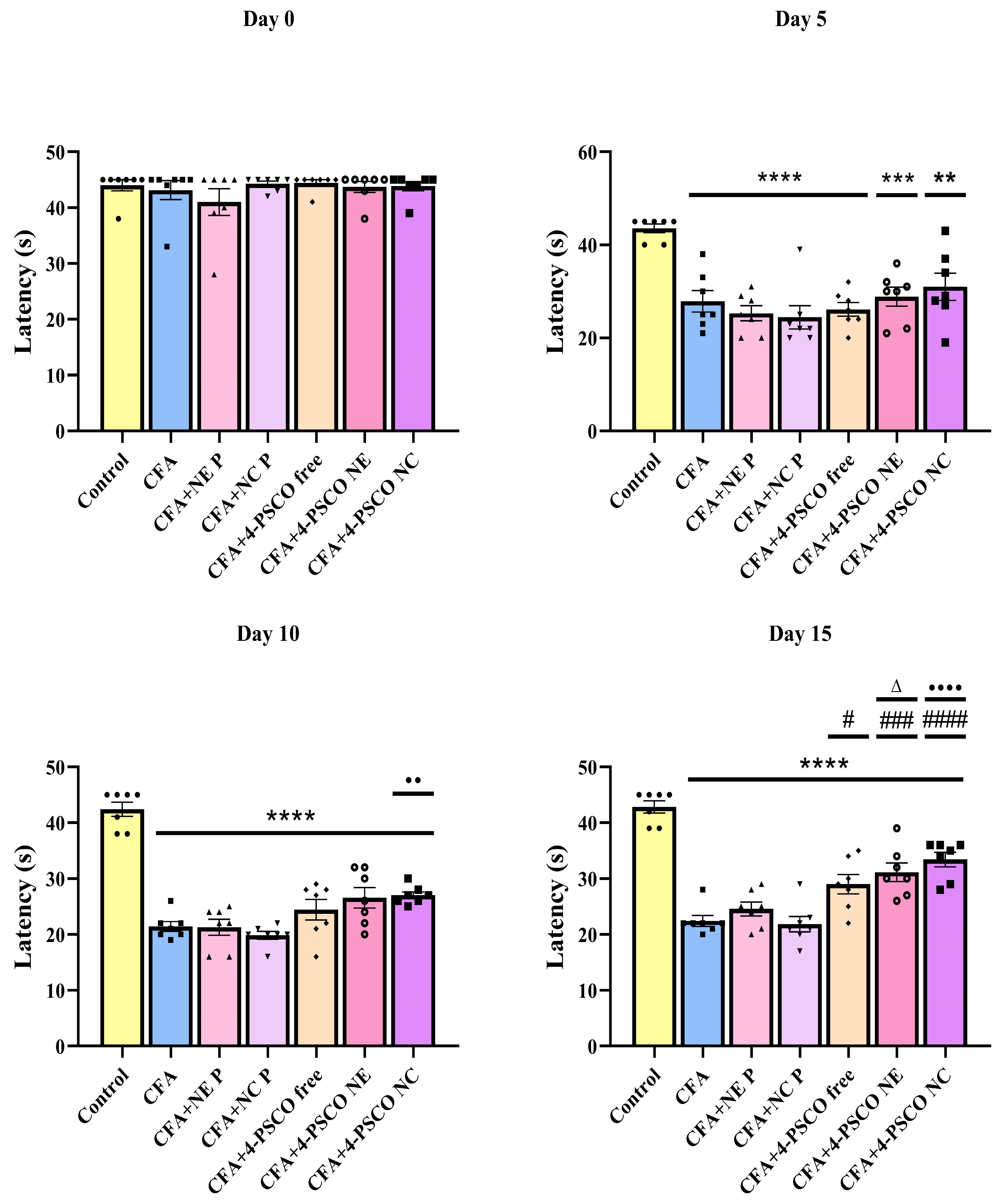
2.4. Administration of 4-PSCO in Free or Nanoencapsulated Form Attenuates the Mechanical and Thermal Nociception Induced by CFA in Mice
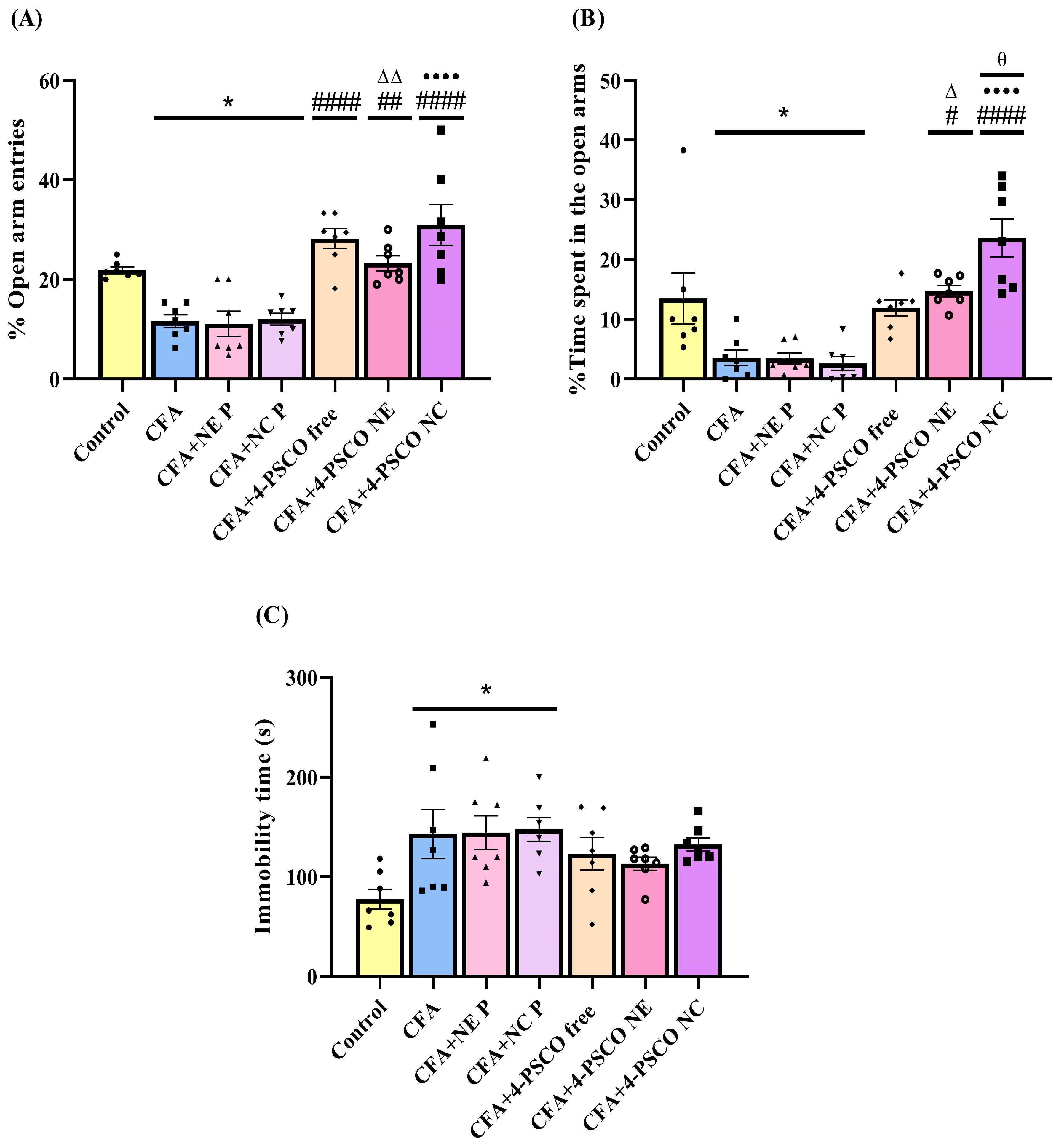
2.5. CFA Caused Depression-like and Anxiety-like Behaviors in Animals: Therapeutic Potential of 4-PSCO in Free or Nanoencapsulated Form
2.6. Effects of 4-PSCO in Free or Nanoencapsulated Form on AChE and Na+, K+-ATPase Activities
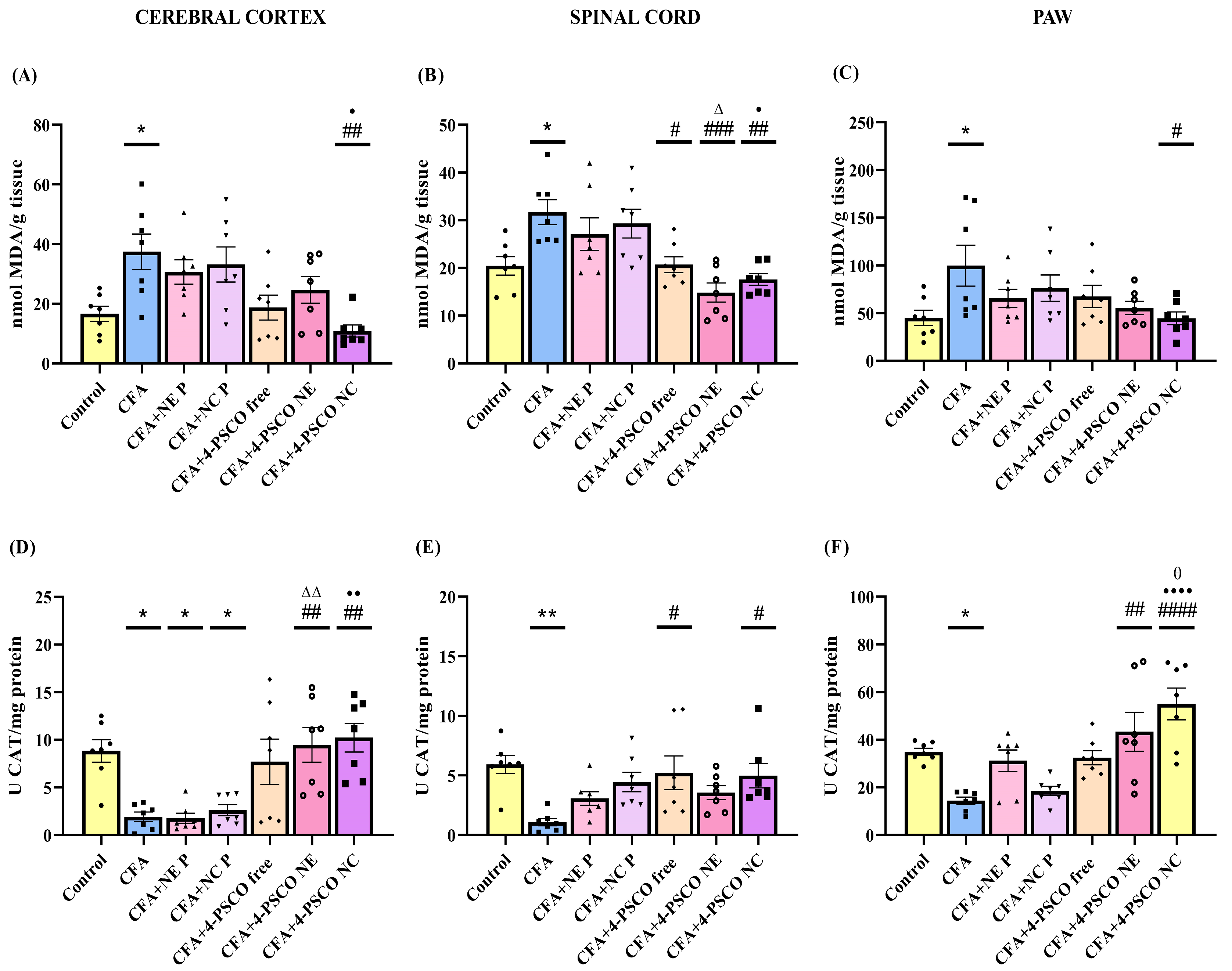
2.7. Effect of 4-PSCO in Free or Nanoencapsulated Form on Oxidative Stress in CFA-Induced AR and Comorbidities
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Approval
4.2. Drugs and Reagents
4.3. Preparation of Nanocapsule Suspensions and Nanoemulsion Containing 4-PSCO
4.4. Analytical Method for the 4-PSCO Quantification
4.5. Physicochemical Characterization of the Nanocapsule Suspension and Nanoemulsion
4.6. In Vitro Release Profile of 4-PSCO from Polymeric Nanocapsules and Nanoemulsions
4.7. Experimental Design of In Vivo Evaluations
4.8. Clinical Manifestations of RA
4.8.1. Arthritis Score
4.8.2. Assessment of Polyarthritis
4.9. Behavioral Tests
4.9.1. Von Frey Test
4.9.2. Hot-Plate Test
4.9.3. Locomotor and Exploratory Domain Assessment
4.10. Evaluation of Emotional Responses
4.10.1. Assessment of Anxiety-Related Behavior
4.10.2. Assessment of Depression-Related Behavior
4.11. Ex Vivo Assays
4.11.1. Tissue Processing for Biochemical Analyses
4.11.2. Inflammatory Parameters
Paw Edema
MPO Assay
4.11.3. Oxidative Stress Parameters
TBARS Levels
CAT Activity
4.11.4. AChE Activity
4.11.5. Na+, K+-ATPase Activity
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RA | Rheumatoid arthritis |
| 4-PSCO | 4-(phenylselanyl)-2H-chromen-2-one |
| GPM | Good Manufacturing Practices |
| PCL | Poly(ℇ-caprolactone) |
| MCT | Medium chain triglycerides |
| i.pl. | Intraplantar |
| i.g. | Intragastric |
| 4-PSCO NC | Nanocapsule suspensions containing 4-PSCO |
| 4-PSCO NE | Nanoemulsion containing 4-PSCO |
| PDI | Polydispersity index |
| EE% | Encapsulation efficiency |
| HPLC | High-performance liquid chromatography |
| DLS | Dynamic light scattering |
| NE P | Placebo nanoemulsion |
| NC P | Placebo nanocapsule |
| CFA | Complete Freund’s Adjuvant |
| OFT | Open field test |
| EPM | Elevated plus-maze |
| TST | Tail suspension test |
| VF | Von Frey |
| HP | Hot Plate |
| S1 | Supernatant fraction |
| MPO | Myeloperoxidase |
| P2 | Pellet |
| TBARS | Thiobarbituric acid reactive substances |
| CAT | Catalase |
| NOx | Nitrite and nitrate |
| AChE | Acetylcholinesterase |
| CNS | Central nervous system |
| PNS | Peripheral nervous system |
| BBB | Blood–brain barrier |
| Ach | Acetylcholine |
| α7nAChRs | α7 subunit of nicotinic acetylcholine receptors |
References
- Radu, A.F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Bae, S.-C.; Thomas, R.; Deane, K.D.; Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591–602. [Google Scholar] [CrossRef]
- Prasad, P.; Verma, S.; Surbhi Ganguly, N.K.; Chaturvedi, V.; Mittal, S.A. Rheumatoid arthritis: Advances in treatment strategies. Mol. Cell. Biochem. 2022, 478, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Wang, P.; Xu, J.; Hou, W.; Xu, P.; Xu, K.; Liu, L. Immunomodulatory role of T helper cells in rheumatoid arthritis. Bone Jt. Res. 2022, 11, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, X.; Liu, S.; Wang, Q.; Wang, Y.; Hou, S.; Wang, J.; Zhang, Y. Global, regional, and national epidemiology of rheumatoid arthritis among people aged 20–54 years from 1990 to 2021. Sci. Rep. 2025, 15, 10736. [Google Scholar] [CrossRef] [PubMed]
- Perpetual, U.; Maharaj, A.; Adebajo, A. A review on the epidemiology of rheumatoid arthritis: An update and trends from current literature. Best Pract. Res. Clin. Rheumatol. 2025, 39, 102036. [Google Scholar] [CrossRef]
- Choy, E.H.S.; Calabrese, L.H. Neuroendocrine and neurophysiological effects of interleukin 6 in rheumatoid arthritis. Rheumatology 2017, 57, 1885–1895. [Google Scholar] [CrossRef]
- Moosavian, S.P.; Paknahad, Z.; Habibagahi, Z. A randomized, double-blind, placebo-controlled clinical trial, evaluating the garlic supplement effects on some serum biomarkers of oxidative stress, and quality of life in women with rheumatoid arthritis. Int. J. Clin. Pract. 2020, 74, e13498. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Zen, M.; Arru, F.; Giorgi, V.; Choy, E.A. Residual pain in rheumatoid arthritis: Is it a real problem? Autoimmun. Rev. 2023, 22, 103423. [Google Scholar] [CrossRef]
- Uda, M.; Hashimoto, M.; Uozumi, R.; Torii, M.; Fujii, T.; Tanaka, M.; Furu, M.; Ito, H.; Terao, C.; Yamamoto, W.; et al. Factors associated with anxiety and depression in rheumatoid arthritis patients: A cross-sectional study. Adv. Rheumatol. 2021, 61, 65. [Google Scholar] [CrossRef]
- Jones Amaowei, E.E.; Anwar, S.; Kavanoor Sridhar, K.; Shabbir, K.; Mohammed, E.H.; Bahar, A.R.; Talpur, A.S.; Bhat, S.; Zafar, S.; Qadar, L.T. Correlation of Depression and Anxiety With Rheumatoid Arthritis. Cureus 2022, 14, e23137. [Google Scholar] [CrossRef]
- Marrie, R.A.; Hitchon, C.A.; Walld, R.; Patten, S.B.; Bolton, J.M.; Sareen, J.; Walker, J.R.; Singer, A.; Lix, L.M.; El-Gabalawy, R.; et al. Increased Burden of Psychiatric Disorders in Rheumatoid Arthritis. Arthritis Care Res. 2018, 70, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Mahajan, N.; Abrol, S.; Raina, A. Anxiety and depression are common in rheumatoid arthritis and correlate with poor quality of life in Indian patients. Reumatologia 2021, 59, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Geng, Y.; Han, Z.; Qin, W.; Zhou, L.; Ding, Y.; Zhang, Z.; Sun, G. Self-reported sleep disturbance is significantly associated with depression, anxiety, self-efficacy, and stigma in Chinese patients with rheumatoid arthritis. Psychol. Health Med. 2022, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Malmström, V.; Catrina, A.I.; Klareskog, L. The immunopathogenesis of seropositive rheumatoid arthritis: From triggering to targeting. Nat. Rev. Immunol. 2016, 17, 60–75. [Google Scholar] [CrossRef]
- Taylor, P.C. Update on the diagnosis and management of early rheumatoid arthritis. Clin. Med. (Lond.) 2020, 20, 561–564. [Google Scholar] [CrossRef]
- Kaźmierczak, M.; Tudaj, O.; Kalas, W.; Sajewicz, K.; Dulęba, J.; Kalisiak, J.; Musiał, M.; Hobot, M.; Mazur, A.; Kin, F. The relationship between rheumatoid arthritis (RA) and psychiatric disorder. Qual. Sport 2025, 43, 2450–3118. [Google Scholar] [CrossRef]
- Howarth Aa Poole, D. Assessment and management of chronic pain. Nurs. Stand. 2019, 34, 75–82. [Google Scholar] [CrossRef]
- Sari, M.; Mota Ferreira, L.; Costa Prado, V.; Wayne Nogueira, C.; Cruz, L. Nano-based formulations as an approach for providing a novel identity for organoselenium compounds. Eur. J. Pharm. Biopharm. 2022, 178, 69–81. [Google Scholar] [CrossRef]
- Pinto, E.; Olivia, S.; D’Haese, C.; Nysten, B.; Machado, F.P.; Rocha, L.M.; de Souza, T.M.; Beloqui, A.; Machado, R.R.; Araújo, R.S. Poly-ɛ-caprolactone nanocapsules loaded with copaiba essential oil reduce inflammation and pain in mice. Int. J. Pharm. 2023, 642, 123147. [Google Scholar] [CrossRef]
- Fonseca, C.; Prado, V.; Paltian, J.; Kazmierczak, J.C.; Schumacher, R.F.; Henrique, M.; Cordeiro, L.; Franzen, A.; Alexandre, F.; Oliboni, S.; et al. 4-(Phenylselanyl)-2H-chromen-2-one-Loaded Nanocapsule Suspension—A Promising Breakthrough in Pain Management: Comprehensive Molecular Docking, Formulation Design, and Toxicological and Pharmacological Assessments in Mice. Pharmaceutics 2024, 16, 269. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Kozak, A.; Lavrih, E.; Mikhaylov, G.; Turk, B.; Vasiljeva, O. Navigating the Clinical Landscape of Liposomal Therapeutics in Cancer Treatment. Pharmaceutics 2025, 17, 276. [Google Scholar] [CrossRef]
- Alamos-Musre, S.; Beltrán-Chacana, D.; Moyano, J.; Márquez-Miranda, V.; Duarte, Y.; Miranda-Rojas, S.; Olguín, Y.; Fuentes, J.A.; González-Nilo, D.; Otero, M.C. From Structure to Function: The Promise of PAMAM Dendrimers in Biomedical Applications. Pharmaceutics 2025, 17, 927. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Ahmed, N.; Muhammad, A.; Shah, K.U.; Mir, M.; Rehman, A.U. A macromolecule infliximab loaded reverse nanomicelles-based transdermal hydrogel: An innovative approach against rheumatoid arthritis. Biomater. Adv. 2025, 167, 214093. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Sari, M.; Ferreira, L.; Zborowski, V.; Paulo Cervi, V.; Brüning, C.A.; Nogueira, C.W. p,p′-Methoxyl-diphenyl diselenide-loaded polymeric nanocapsules are chemically stable and do not induce toxicity in mice. Eur. J. Pharm. Biopharm. 2017, 117, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres Mdel, P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Iacia, M.V.; Mendes, M.E.; Vieira, K.C.; Ruiz, G.C.; Constantino, C.J.; Martin, C.; Job, A.; Nai, G.; Eller, L. Evaluation of curcumin nanoemulsion effect to prevent intestinal damage. Int. J. Pharm. 2024, 650, 123683. [Google Scholar] [CrossRef]
- Rashidi, L. Different nano-delivery systems for delivery of nutraceuticals. Food Biosci. 2021, 43, 101258. [Google Scholar] [CrossRef]
- Jasim, S.; Mustafa, Y. A review on coumarin backbone: An attractive scaffold for promising bioactive compounds. Iraqi J. Pharm. 2022, 18, 104–125. [Google Scholar] [CrossRef]
- Ristovski, J.; Minorics, R.; Bartha, S.; Janković, N.; Zupkó, I. The evaluation of the anticancer activity of the Biginelli hybrids and pharmacokinetic profiling based on their retention parameters. J. Mol. Struct. 2022, 1254, 132373. [Google Scholar] [CrossRef]
- Sonego, J.M.; De, S.I.; Szajnman, S.H.; Gallo-Rodriguez, C.; Rodríguez, J.B. Organoselenium Compounds: Chemistry and Applications in Organic Synthesis. Chem. A Eur. J. 2023, 29, e202300030. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.L.; Gratieri, T.; Cunha-Filho, M.; Gelfuso, G.M. Polymeric nanocapsules: A review on design and production methods for pharmaceutical purpose. Methods 2022, 199, 54–66. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Sun, R.; Han, S.; Yang, Z.; Teng, L. Microfluidics for nano-drug delivery systems: From fundamentals to industrialization. Acta Pharm. Sin. B 2023, 13, 3277–3299. [Google Scholar] [CrossRef]
- Washington, K.E.; Kularatne, R.N.; Karmegam, V.; Biewer, M.C.; Stefan, M.C. Recent advances in aliphatic polyesters for drug delivery applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 9, e1446. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 85–126. [Google Scholar] [CrossRef]
- Bhadran, A.; Shah, T.K.; Babanyinah, G.K.; Polara, H.; Taslimy, S.; Biewer, M.C.; Stefan, M.C. Recent Advances in Polycaprolactones for Anticancer Drug Delivery. Pharmaceutics 2023, 15, 1977. [Google Scholar] [CrossRef]
- Seyednejad, H.; Ghassemi, A.H.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J. Control. Release 2011, 152, 168–176. [Google Scholar] [CrossRef]
- Kreua-ongarjnukool, N.; Soomherun, N.; Thumsing Niyomthai, S.; Chumnanvej, S. Aliphatic Polyester Nanoparticles for Drug Delivery Systems. In Smart Drug Delivery; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef]
- Li, M.; Kang, W.; Li, Z.; Yang, H.; Jia, R.; He, Y.; Kang, X.; Zheng, Z.; Wang, Y.; Sarsenbekuly, B.; et al. Stability of oil-in-water (O/W) nanoemulsions and its oil washing performance for enhanced oil recovery. Phys. Fluids 2021, 33, 072002. [Google Scholar] [CrossRef]
- Venturini, C.G.; Bruinsmann, F.A.; Oliveira, C.P.; Contri, R.V.; Pohlmann, A.R.; Guterres, S.S. Vegetable Oil-Loaded Nanocapsules: Innovative Alternative for Incorporating Drugs for Parenteral Administration. J. Nanosci. Nanotechnol. 2016, 16, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Voon, F.-L.; Sulaiman, M.R.; Akhtar, M.N.; Idris, M.F.; Akira, A.; Perimal, E.K.; Israf, D.A.; Ming-Tatt, L. Cardamonin (2′,4′-dihydroxy-6′-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur. J. Pharmacol. 2017, 794, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Quintão, N.L.M.; Pastor, M.V.D.; Antonialli Cde-Souza da Silva, G.F.; Rocha, L.W.; Berté, T.E.; de Souza, M.M.; Meyre-Silva, C.; Lucinda-Silva, R.M.; Bresolin, T.M.B.; Cechinel Filho, V. Aleurites moluccanus and its main active constituent, the flavonoid 2″-O-rhamnosylswertisin, in experimental model of rheumatoid arthritis. J. Ethnopharmacol. 2019, 235, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The Etiology of Rheumatoid Arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Strzepa, A.; Pritchard, K.A.; Dittel, B.N. Myeloperoxidase: A new player in autoimmunity. Cell. Immunol. 2017, 317, 1–8. [Google Scholar] [CrossRef]
- Mititelu, R.R.; Pădureanu, R.; Băcănoiu, M.; Pădureanu, V.; Docea, A.O.; Calina, D.; Barbulescu, A.L.; Buga, A.M. Inflammatory and Oxidative Stress Markers—Mirror Tools in Rheumatoid Arthritis. Biomedicines 2020, 8, 125. [Google Scholar] [CrossRef]
- La, R.; Zhou, L.; Yin, Y.; Lu, L.; Li, L.; Jiang, D.; Huang, L.; Wu, Q. Association between oxidative balance score and rheumatoid arthritis in female: A cross-sectional study. BMC Women’s Health 2024, 24, 225. [Google Scholar] [CrossRef]
- Lei, Q.; Yang, J.; Li, L.; Zhao, N.; Lu, C.; Lu, A.; He, X. Lipid metabolism and rheumatoid arthritis. Front. Immunol. 2023, 14, 1190607. [Google Scholar] [CrossRef]
- Shim, J.H.; Stavre, Z.; Gravallese, E.M. Bone loss in rheumatoid arthritis: Basic mechanisms and clinical implications. Calcif. Tissue Int. 2018, 102, 533–546. [Google Scholar] [CrossRef]
- Marques-da-Silva, D.; Lagoa, R. Rafting on the Evidence for Lipid Raft-like Domains as Hubs Triggering Environmental Toxicants’ Cellular Effects. Molecules 2023, 28, 6598. [Google Scholar] [CrossRef]
- Süß, P.; Hoffmann, A.; Rothe, T.; Ouyang, Z.; Baum, W.; Staszewski, O.; Schett, G.; Prinz, M.; Krönke, G.; Glass, C.K.; et al. Chronic Peripheral Inflammation Causes a Region-Specific Myeloid Response in the Central Nervous System. Cell Rep. 2020, 30, 4082–4095.e6. [Google Scholar] [CrossRef]
- Tyagi, V.; Singh, V.K.; Sharma, P.K.; Singh, V. Essential oil-based nanostructures for inflammation and rheumatoid arthritis. J. Drug Deliv. Sci. Technol. 2020, 60, 101983. [Google Scholar] [CrossRef]
- da Fonseca, L.J.S.; Nunes-Souza, V.; Goulart, M.O.F.; Rabelo, L.A. Oxidative Stress in Rheumatoid Arthritis: What the Future Might Hold regarding Novel Biomarkers and Add-On Therapies. Oxidative Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Akhter, S.; Irfan, H.M.; Alamgeer Jahan, X.; Shahzad, M.; Latif, M.B. Nerolidol: A potential approach in rheumatoid arthritis through reduction of TNF-α, IL-1β, IL-6, NF-kB, COX-2 and antioxidant effect in CFA-induced arthritic model. Inflammopharmacology 2022, 30, 537–548. [Google Scholar] [CrossRef] [PubMed]
- López-Armada, M.J.; Fernández-Rodríguez, J.A.; Blanco, F.J. Mitochondrial Dysfunction and Oxidative Stress in Rheumatoid Arthritis. Antioxidants 2022, 11, 1151. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Sun, M.; Guo, Y.; Xia, W.; Qiao, S.; Tao, Y.; Fang, Y.; Zhang, Q.; Zhu, Y.; Yalikun, Y.; et al. Cholinergic dysfunction-induced insufficient activation of alpha7 nicotinic acetylcholine receptor drives the development of rheumatoid arthritis through promoting protein citrullination via the SP3/PAD4 pathway. Acta Pharm. Sin. B 2023, 13, 1600–1615. [Google Scholar] [CrossRef]
- Keever, K.R.; Cui, K.; Casteel, J.L.; Singh, S.; Hoover, D.B.; Williams, D.L.; Pavlov, V.A.; Yakubenko, V.P. Cholinergic signaling via the α7 nicotinic acetylcholine receptor regulates the migration of monocyte-derived macrophages during acute inflammation. J. Neuroinflamm. 2024, 21, 3. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Padilha, G.; Birmann, P.T.; Domingues, M.; Kaufman, T.S.; Savegnago, L.; Silveira, C.C. Convenient Michael addition/β-elimination approach to the synthesis of 4-benzyl- and 4-aryl-selenyl coumarins using diselenides as selenium sources. Tetrahedron Lett. 2017, 58, 985–990. [Google Scholar] [CrossRef]
- Ferreira, L.; Cervi, V.; Henrique, M.; Barbieri, A.; Ramos, A.; Copetti, P.; Gerson Nascimento, K.; Nadal, J.; Farago, P.V.; Sagrillo, M.; et al. Diphenyl diselenide loaded poly(ε-caprolactone) nanocapsules with selective antimelanoma activity: Development and cytotoxic evaluation. Mater. Sci. Eng. C Biomim. Mater. Sens. Syst. 2018, 91, 1–9. [Google Scholar] [CrossRef]
- Saccol, C.; Cervi, V.; Blume, J.C.; Menezes, Á.; Apel, M.; Saldanha, L.; Tasca, T.; Cruz, L. Xanthan-carrageenan film containing sesame seed oil: A nanocomposite pharmaceutical platform for trichomoniasis treatment. Int. J. Biol. Macromol. 2024, 257, 128701. [Google Scholar] [CrossRef] [PubMed]
- Omorogbe, O.; Ajayi, A.M.; Ben-Azu, B.; Oghwere, E.E.; Adebesin, A.; Aderibigbe, A.O.; Okubena, O.; Umukoro, S. Jobelyn® attenuates inflammatory responses and neurobehavioural deficits associated with complete Freund-adjuvant-induced arthritis in mice. Biomed. Pharmacother. 2018, 98, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, M.; Li, M.; Du, Y.; Duan, S.; Huang, Y.; Lu, Y.; Zhang, J.; Wang, T.; Fu, F. Ginsenoside Rg1 attenuates adjuvant-induced arthritis in rats via modulation of PPAR-γ/NF-κB signal pathway. Oncotarget 2017, 8, 55384–55393. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.A.; Ahmed, O.M.; Fahim, H.I.; Ali, T.M.; Elesawy, B.H.; Ashour, M.B. Potency of Bone Marrow-Derived Mesenchymal Stem Cells and Indomethacin in Complete Freund’s Adjuvant-Induced Arthritic Rats: Roles of TNF-α, IL-10, iNOS, MMP-9, and TGF-β1. Stem Cells Int. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Alamri, F.F.; Shoyaib, A.A.; Biggers, A.; Jayaraman, S.; Guindon, J.; Karamyan, V.T. Applicability of the grip strength and automated von Frey tactile sensitivity tests in the mouse photothrombotic model of stroke. Behav. Brain Res. 2018, 336, 250–255. [Google Scholar] [CrossRef]
- Woolfe, G.; Macdonald, A.D. The evaluation of the analgesic action of pethidine hydrochloride (DEMEROL). J. Pharmacol. Exp. Ther. 1944, 80, 300–307. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The open-field test: A critical review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Suzuki, K.; Ota, H.; Sasagawa, S.; Sakatani, T.; Fujikura, T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 1983, 132, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]




| Parameters | |||||
|---|---|---|---|---|---|
| Average Diameter (nm) | PDI a | ZP b (mV) | pH | 4-PSCO content (%) | |
| Nanocapsules (NC) | |||||
| 4-PSCO NC | 251 ± 13 | 0.19 ± 0.05 | −11.4 ± 0.2 | 5.4 ± 0.0 | 101.9 ± 1.8 |
| NC P | 234 ± 3 | 0.16 ± 0.01 | −11.0 ± 1.5 | 5.3 ± 0.1 | - c |
| Nanoemulsions (NE) | |||||
| 4-PSCO NE | 205 ± 2 | 0.10 ± 0.01 | −24.3 ± 0.3 | 5.4 ± 0.0 | 100.5 ± 0.7 |
| NE P | 208 ± 5 | 0.13 ± 0.02 | −32.01 ± 2 ** | 5.4 ± 0.1 | - c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Fonseca, C.A.R.; Prado, V.C.; Cruz, L.; da Rocha, V.M.E.; Wille, A.P.B.; Reis, A.S.d.; Kazmierczak, J.C.; Schumacher, R.F.; Wilhelm, E.A. Preclinical Evaluation of Nanoemulsion and Polymeric Nanocapsule Delivery Systems of 4-(Phenylselanyl)-2H-Chromen-2-One for Rheumatoid Arthritis and Comorbidities. Pharmaceuticals 2025, 18, 1379. https://doi.org/10.3390/ph18091379
da Fonseca CAR, Prado VC, Cruz L, da Rocha VME, Wille APB, Reis ASd, Kazmierczak JC, Schumacher RF, Wilhelm EA. Preclinical Evaluation of Nanoemulsion and Polymeric Nanocapsule Delivery Systems of 4-(Phenylselanyl)-2H-Chromen-2-One for Rheumatoid Arthritis and Comorbidities. Pharmaceuticals. 2025; 18(9):1379. https://doi.org/10.3390/ph18091379
Chicago/Turabian Styleda Fonseca, Caren Aline Ramson, Vinicius Costa Prado, Letícia Cruz, Vanessa Macedo Esteves da Rocha, Ana Paula Bonato Wille, Angélica Schiavom dos Reis, Jean Carlo Kazmierczak, Ricardo Frederico Schumacher, and Ethel Antunes Wilhelm. 2025. "Preclinical Evaluation of Nanoemulsion and Polymeric Nanocapsule Delivery Systems of 4-(Phenylselanyl)-2H-Chromen-2-One for Rheumatoid Arthritis and Comorbidities" Pharmaceuticals 18, no. 9: 1379. https://doi.org/10.3390/ph18091379
APA Styleda Fonseca, C. A. R., Prado, V. C., Cruz, L., da Rocha, V. M. E., Wille, A. P. B., Reis, A. S. d., Kazmierczak, J. C., Schumacher, R. F., & Wilhelm, E. A. (2025). Preclinical Evaluation of Nanoemulsion and Polymeric Nanocapsule Delivery Systems of 4-(Phenylselanyl)-2H-Chromen-2-One for Rheumatoid Arthritis and Comorbidities. Pharmaceuticals, 18(9), 1379. https://doi.org/10.3390/ph18091379










