Design and Characterization of Thermosensitive Niosomes as Platforms for Daunorubicin Delivery
Abstract
1. Introduction
2. Results and Discussion
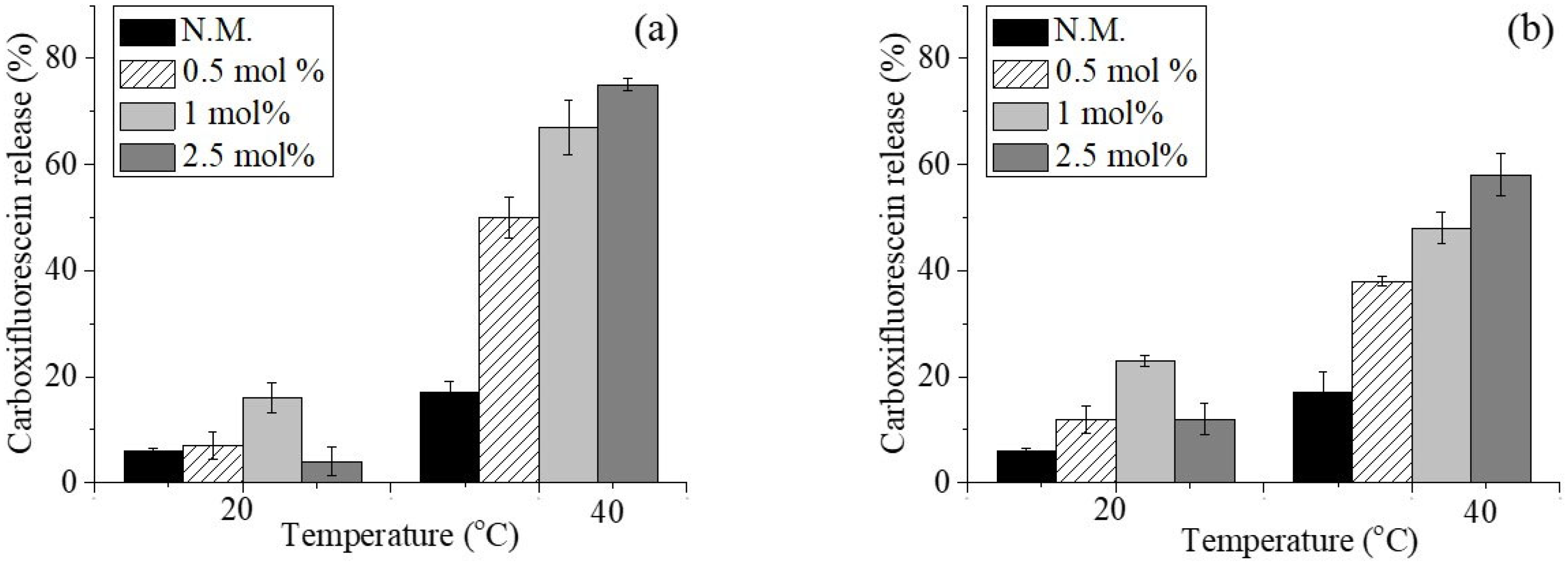
2.1. Preparation and Characterization of Conventional and Copolymer-Modified Blank Niosomes—Preliminary Studies
| Sample Code | Cloud Point (°C) | Tgc (°C) |
|---|---|---|
| PiPOX [24] | 39 | 52 |
| PETEGA [25] | 37 | −57 |
2.2. Preparation and Characterization of Temperature-Sensitive Daunorubicin Hydrochloride-Loaded Niosomes
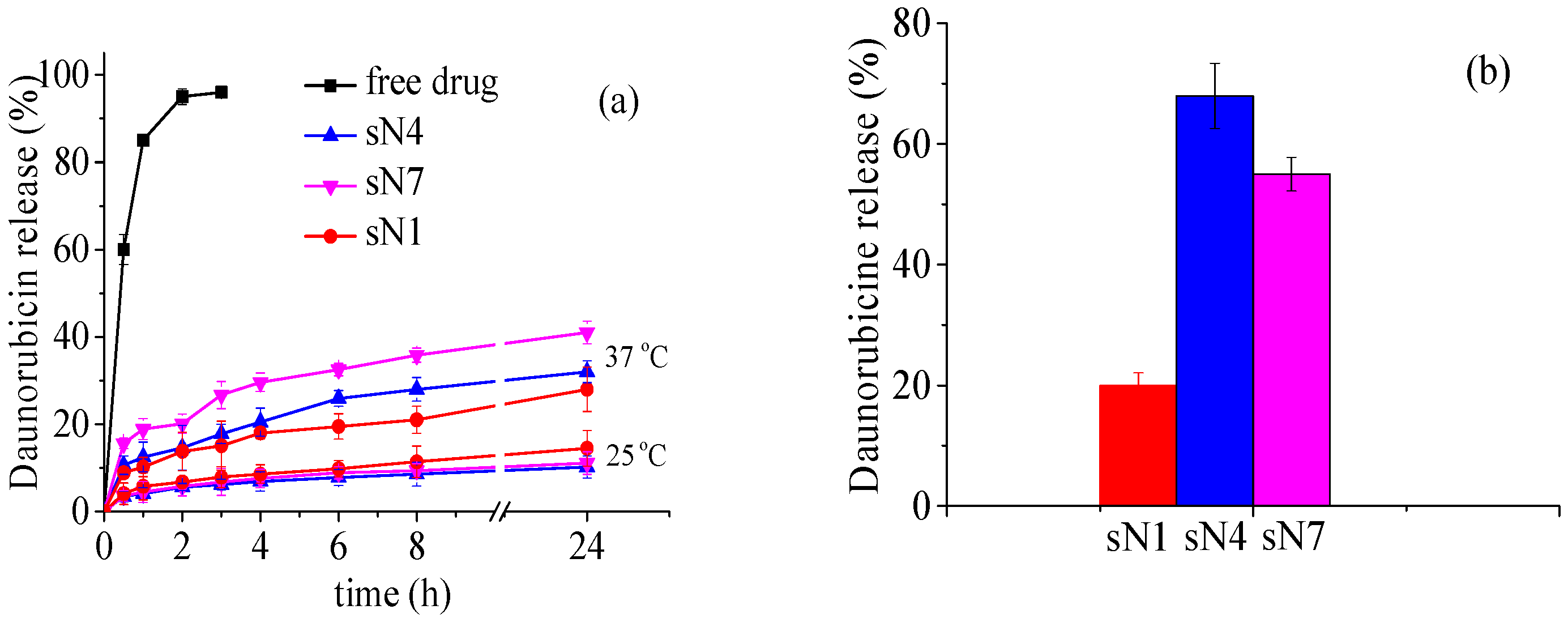
2.3. In Vitro Daunorubicin Release
2.4. Storage Stability of Niosomes
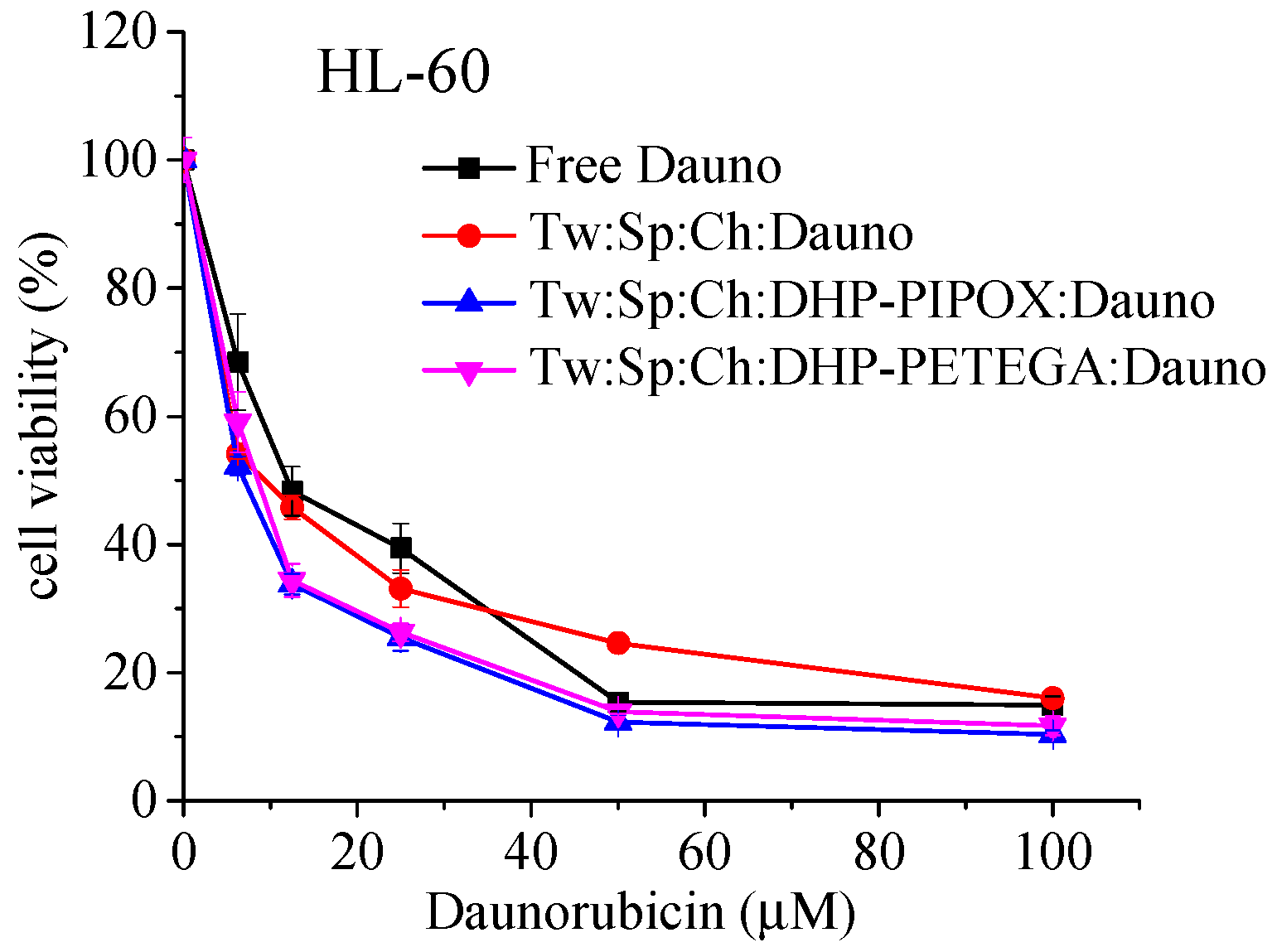
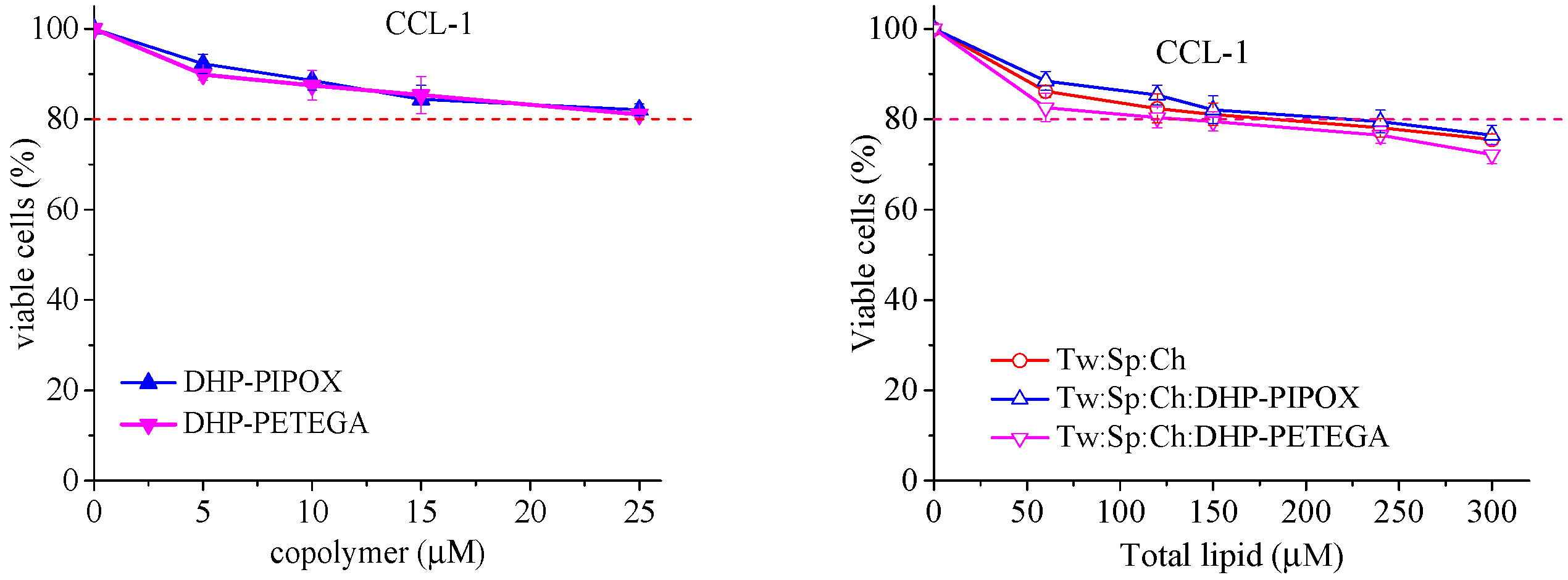
2.5. In Vitro Cytotoxicity Evaluation
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of Copolymers
3.2.2. Preparation of Empty and Daunorubicin-Loaded Niosomes
Preparation of Empty and 5(6)-Carboxyfluorescein-Loaded Niosomes
Preparation of Daunorubicin Hydrochloride-Loaded Niosomes
3.3. Characterization of Niosomes
3.3.1. Morphology
3.3.2. DLS Analysis
3.3.3. Entrapment Efficacy (EE)
3.4. Evaluation of Thermal Responsiveness of Niosomes
3.5. In Vitro Daunorubicin Release
3.6. Stability Evaluation
3.7. Assessment of Cytotoxicity of Daunorubicin-Loaded Conventional and Copolymer-Modified Thermoresponsive Niosomes
3.7.1. Cell Lines and Culture Conditions
3.7.2. MTT Colorimetric Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DHP-PiPOX | 1,3-dihexadecyl-propane-2-ol-poly(2-isopropyl-2-oxazoline |
| DHP-PETEGA | 1,3-dihexadecyl-propane-2-ol-poly(ethoxytriethylene glycol acrylate |
| HL-60 | acute myelocyte leukemia-derived cells |
| CCL-1 | normal mouse fibroblasts |
| Cryo-TEM | cryogenic transmission electron microscopy |
| DLS | dynamic light scattering |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Tw60 | polyoxyethylene sorbitan monostearate |
| Sp60 | sorbitan monostearate |
| Ch | cholesterol |
References
- Aparajay, P.; Dev, A. Functionalized niosomes as a smart delivery device in cancer and fungal infection. Eur. J. Pharm. Sci. 2022, 168, 106052. [Google Scholar] [CrossRef]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Mazzotta, E.; Tavano, L.; Muzzalupo, R. Thermo-Sensitive Vesicles in Controlled Drug Delivery for Chemotherapy. Pharmaceutics 2018, 10, 150. [Google Scholar] [CrossRef]
- Tavano, L.; Oliviero Rossi, C.; Picci, N.; Muzzalupo, R. Spontaneous temperature-sensitive Pluronic(®) based niosomes: Triggered drug release using mild hyperthermia. Int. J. Pharm. 2016, 511, 703–708. [Google Scholar] [CrossRef]
- Hemati, M.; Haghiralsadat, F.; Jafary, F.; Moosavizadeh, S.; Moradi, A. Targeting cell cycle protein in gastric cancer with CDC20siRNA and anticancer drugs (doxorubicin and quercetin) co-loaded cationic PEGylated nanoniosomes. Int. J. Nanomed. 2019, 14, 6575–6585. [Google Scholar] [CrossRef]
- Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Doxorubicin and other anthracyclines in cancers: Activity, chemoresistance and its overcoming. Mol. Aspects Med. 2023, 93, 101205. [Google Scholar] [CrossRef]
- Bindusri, C.H.; Devi, P.G.; Chowdary, Y.A.; Veerendranadh, Y.V. Statistical Study on Daunorubicin. Int. J. Pharm. Sci. 2025, 3, 742–771. [Google Scholar] [CrossRef]
- Callies, S.; De Alwis, D.P.; Mehta, A.; Burgess, M.; Aarons, L. Population pharmacokinetic model for daunorubicin and daunorubicinol co-administered with zosuquidar.3HCl (LY335979). Cancer Chemother. Pharmacol. 2004, 54, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.-J.; Höffken, K.; Possinger, K. Kompendium Internistische Onkologie, Teil 1–2; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar] [CrossRef]
- Britle, A.J. Anthracyclines and cardiotoxicity. Clin. Oncol. (R. Coll. Radiol.) 2000, 12, 146–152. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Xiao, Q.; Fan, L.; Huang, D.; Chen, W.; He, W. Liposomes and liposome-like nanoparticles: From anti-fungal infection to the COVID-19 pandemic treatment. Asian J. Pharm. Sci. 2022, 17, 817–837. [Google Scholar] [CrossRef]
- Balasubramaniam, A.; Kumar, V.A.; Pillai, K.S. Formulation and in vivo evaluation of niosome-encapsulated daunorubicin hydrochloride. Drug Dev. Ind. Pharm. 2002, 28, 1181–1193. [Google Scholar] [CrossRef]
- Liu, F.R.; Jin, H.; Wang, Y.; Chen, C.; Li, M.; Mao, S.J.; Wang, Q.; Li, H. Anti-CD123 antibody-modified niosomes for targeted delivery of daunorubicin against acute myeloid leukemia. Drug Deliv. 2017, 24, 882–890. [Google Scholar] [CrossRef]
- Alrbyawi, H.; Boddu, S.H.S.; Poudel, I.; Annaji, M.; Mita, N.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Cardiolipin for Enhanced Cellular Uptake and Cytotoxicity of Thermosensitive Liposome-Encapsulated Daunorubicin toward Breast Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 11763. [Google Scholar] [CrossRef]
- Yaramiri, A.; Asalh, R.A.; Asalh, M.A.; AlSawaftah, N.; Abuwatfa, W.H.; Husseini, G.A. A Comprehensive Review of Smart Thermosensitive Nanocarriers for Precision Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 7322. [Google Scholar] [CrossRef] [PubMed]
- Gugleva, V.; Michailova, V.; Mihaylova, R.; Momekov, G.; Zaharieva, M.M.; Najdenski, H.; Petrov, P.; Rangelov, S.; Forys, A.; Trzebicka, B.; et al. Formulation and evaluation of hybrid niosomal in situ gel for intravesical co-delivery of curcumin and gentamicin sulfate. Pharmaceutics 2022, 14, 747. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Singhsa, P.; Suksiriworapong, J.; Chantasart, D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int. J. Pharm. 2012, 423, 303–311. [Google Scholar] [CrossRef]
- Yeo, L.K.; Olusanya, T.O.B.; Chaw, C.S.; Elkordy, A.A. Brief effect of a small hydrophobic drug (cinnarizine) on the physicochemical characterisation of niosomes produced by thin-film hydration and microfluidic methods. Pharmaceutics 2018, 10, 185. [Google Scholar] [CrossRef]
- Xi, L.; Li, C.; Wang, Y.; Gong, Y.; Su, F.; Li, S. Novel Thermosensitive Polymer-Modified Liposomes as Nano-Carrier of Hydrophobic Antitumor Drugs. J. Pharm. Sci. 2020, 109, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Abuwatfa, W.H.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Thermosensitive polymers and thermo-responsive liposomal drug delivery systems. Polymers 2022, 14, 925. [Google Scholar] [CrossRef]
- Damera, D.P.; Nag, A. Tuning the phase transition temperature of hybrid Span60-L64 thermoresponsive niosomes: Insights from fluorescence and Raman spectroscopy. J. Mol. Liq. 2021, 340, 117110. [Google Scholar] [CrossRef]
- Toncheva, N.; Tsvetanov, C.; Rangelov, S.; Trzebicka, B.; Dworak, A. Hydroxyl end-functionalized poly(2-isopropyloxazoline)s used as nano-sized colloidal templates for preparation of hollow polymeric nanocapsules. Polymer 2013, 54, 5166–5173. [Google Scholar] [CrossRef]
- Toncheva-Moncheva, N.; Dimitrov, P.; Tsvetanov, C.; Trzebicka, B.; Dworak, A.; Rangelov, S. Formation of mesoglobules in aqueous media from thermo-sensitive poly(ethoxytriethyleneglycol acrylate). Polym. Bull. 2011, 67, 1335–1346. [Google Scholar] [CrossRef]
- Li, M.; Jiang, S.; Simon, J.; Paßlick, D.; Frey, M.L.; Wagner, M.; Mailänder, V.; Crespy, D.; Landfester, K. Brush conformation of polyethylene glycol determines the stealth effect of nanocarriers in the low protein adsorption regime. Nano Lett. 2021, 21, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- El-Far, S.W.; Abo El-Enin, H.A.; Abdou, E.M.; Nafea, O.E.; Abdelmonem, R. Targeting colorectal cancer cells with niosome systems loaded with two anticancer drug models: Comparative in vitro and anticancer studies. Pharmaceuticals 2022, 15, 816. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart drug delivery systems for precise cancer therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Cheng, Y.; Weng, S.; Yu, L.; Zhu, N.; Yang, M.; Yuan, Y. The role of hyperthermia in the multidisciplinary treatment of malignant tumors. Integr. Cancer Ther. 2019, 18, 1534735419876345. [Google Scholar] [CrossRef] [PubMed]
- Ritwiset, A.; Krongsuk, S.; Johns, J.R. Molecular structure and dynamical properties of niosome bilayers with and without cholesterol incorporation: A molecular dynamics simulation study. Appl. Surf. Sci. 2016, 380, 23–31. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2017.
- Dmitrov, E.; Toncheva-Moncheva, N.; Bakardzhiev, P.; Forys, A.; Doumanov, J.; Mladenova, K.; Petrova, S.; Trzebicka, B.; Rangelov, S. Nucleic acid-based supramolecular structures: Vesicular spherical nucleic acids from a non-phospholipid nucleolipid. Nanoscale Adv. 2022, 4, 3793–3803. [Google Scholar] [CrossRef]
- Konstantinov, S.M.; Eibl, H.; Berger, M.R. BCR-ABL influences the antileukaemic efficacy of alkylphosphocholines. Br. J. Haematol. 1999, 107, 365–374. [Google Scholar] [CrossRef] [PubMed]

| Sample Code | Polymer (mol%) | Dh (nm) ± SD | PDI ± SD | ζ-Potential (mV) ± SD | |||
|---|---|---|---|---|---|---|---|
| 25 °C | 40 °C | 25 °C | 40 °C | 25 °C | 40 °C | ||
| S1 | - | 133 ± 1.7 | 142 ± 10.7 | 0.3 ± 0.09 | 0.46 ± 0.05 | −11.2 ± 1.5 | −11.0 ± 2.1 |
| DHP-PiPOX-modified niosomes | |||||||
| S2 | 0.5 | 100 ± 2.5 | 115 ± 9.5 | 0.24 ± 0.06 | 0.24 ± 0.02 | −26.1 ± 0.07 | −18.5 ± 0.5 |
| S3 | 1 | 110 ± 6.9 | 126 ± 5.2 | 0.34 ± 0.08 | 0.27 ± 0.01 | −44.1 ± 0.35 | −42.2 ± 0.6 |
| S4 | 2.5 | 112 ± 5.3 | 136 ± 8.5 | 0.24 ± 0.05 | 0.31 ± 0.09 | −26.1 ± 0.01 | −19.2 ± 1.4 |
| DHP-PETEGA-modified niosomes | |||||||
| S5 | 0.5 | 94 ± 8.5 | 76.2 ± 9.7 | 0.32± 0.05 | 0.19± 0.02 | −12.8 ± 2.6 | −12.5 ± 0.4 |
| S6 | 1 | 100 ± 9.7 | 117 ± 15.6 | 0.28± 0.06 | 0.17 ± 0.2 | −15.1 ± 0.23 | −13.5 ± 0.35 |
| S7 | 2.5 | 119 ± 7.6 | 163 ± 4.2 | 0.27 ± 0.02 | 0.17 ± 0.05 | −12.5 ± 0.14 | −10.2 ± 0.78 |
| Sample Code | Encapsulation Efficacy (%) | |
|---|---|---|
| Passive Loading | Active Loading | |
| Tw60:Sp60:Ch:Dauno (sN1) | 18.9 ± 3.4 | 71 ± 2.8 |
| Tw60:Sp60:Ch:DHP-PIPOX:Dauno (sN4) | 16.8 ± 6.2 | 68.6 ± 4.1 |
| Tw60:Sp60:Ch:DHP-PETEGA:Dauno (sN7) | 16.2 ± 4.3 | 66.5 ± 3.2 |
| Composition | Copolymer (2.5 mol%) | Dauno:Surfactants (mol:mol) | Dh (nm) ± SD | PDI ± SD | ζ-Potential (mV) ± SD |
|---|---|---|---|---|---|
| sN1 | - | 2:15 | 157 ± 2.3 | 0.29 ± 0.05 | −10 ± 1.8 |
| sN4 | DHP-PiPOX | 2:15 | 155 ± 5.9 | 0.25 ± 0.04 | −22 ± 2.4 |
| sN7 | DHP-PETEGA | 2:15 | 158 ± 4.8 | 0.29 ± 0.02 | −11 ± 2.5 |
| Sample | Size (nm) | PDI | ζ Potential (mV) | EE (%) | |
|---|---|---|---|---|---|
| (sN1) | Initial | 157 ± 2.2 | 0.29 ± 0.05 | −10 ±1.8 | 71 ± 2.8 |
| After 1 month storage | 161 ± 2.2 | 0.31 ± 2.2 | −10.8 ± 3.4 | 66 ± 1.6 | |
| (sN4) | Initial | 155 ± 5.9 | 0.25 ± 0.04 | −22 ± 2.4 | 68.6 ± 4.1 |
| After 1 month storage | 151 ± 7.5 | 0.29 ± 0.04 | −24 ± 3.8 | 66.7 ± 2.2 | |
| (sN7) | Initial | 158 ± 4.8 | 0.29 ± 0.02 | −11 ± 2.5 | 66.5 ± 3.2 |
| After 1 month storage | 162 ± 5.4 | 0.32 ± 0.06 | −10.9 ± 2.2 | 62.1 ± 4.2 | |
| Sample | HL-60 |
|---|---|
| Free daunorubicin | 12.14 ± 2.15 |
| sN1 | 9.85 ± 3.01 |
| sN4 | 6.91 ± 2.42 |
| sN7 | 8.54 ± 1.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugleva, V.; Ahchiyska, K.; Drakalska-Sersemova, E.; Mihaylova, R.; Toncheva-Moncheva, N.; Dimitrov, E.; Aleksandrov, K.; Forys, A.; Trzebicka, B.; Momekova, D. Design and Characterization of Thermosensitive Niosomes as Platforms for Daunorubicin Delivery. Pharmaceuticals 2025, 18, 1375. https://doi.org/10.3390/ph18091375
Gugleva V, Ahchiyska K, Drakalska-Sersemova E, Mihaylova R, Toncheva-Moncheva N, Dimitrov E, Aleksandrov K, Forys A, Trzebicka B, Momekova D. Design and Characterization of Thermosensitive Niosomes as Platforms for Daunorubicin Delivery. Pharmaceuticals. 2025; 18(9):1375. https://doi.org/10.3390/ph18091375
Chicago/Turabian StyleGugleva, Viliana, Katerina Ahchiyska, Elena Drakalska-Sersemova, Rositsa Mihaylova, Natalia Toncheva-Moncheva, Erik Dimitrov, Krum Aleksandrov, Aleksander Forys, Barbara Trzebicka, and Denitsa Momekova. 2025. "Design and Characterization of Thermosensitive Niosomes as Platforms for Daunorubicin Delivery" Pharmaceuticals 18, no. 9: 1375. https://doi.org/10.3390/ph18091375
APA StyleGugleva, V., Ahchiyska, K., Drakalska-Sersemova, E., Mihaylova, R., Toncheva-Moncheva, N., Dimitrov, E., Aleksandrov, K., Forys, A., Trzebicka, B., & Momekova, D. (2025). Design and Characterization of Thermosensitive Niosomes as Platforms for Daunorubicin Delivery. Pharmaceuticals, 18(9), 1375. https://doi.org/10.3390/ph18091375










