Preparation and Application of Shen Ling Cao Composite Particles with Different Structures Based on Co-Spray Drying
Abstract
1. Introduction
2. Results
2.1. The Fundamental Physical Properties of the Powder
2.1.1. Analysis of Surface Morphology
2.1.2. FT-IR
2.1.3. Thermodynamic Properties
2.1.4. Fundamental Properties of Powder
2.2. The Functional Properties of Powders
2.2.1. Flowability
2.2.2. Analysis of Compactibility
2.3. Application of Composite Particles of DC
2.3.1. Tablet Structure
2.3.2. Tablet Properties
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Materials
4.2.1. Preparation of Particles
4.2.2. Physical Mixtures
4.3. Performance of Powder
4.3.1. Moisture Content (MC)
4.3.2. Angle of Repose (AR)
4.3.3. True Density (ρture)
4.3.4. Density, Carr’s Index (CI), and Hausner Ratio (HR)
4.3.5. Particle Size (D0.5), Particle Size Distribution (Span), and Uniformity
4.3.6. Surface Morphology
4.3.7. Texture Parameters
4.3.8. Specific Surface Area (SSA), Pore Volume (PV), and Pore Diameter (PD)
4.3.9. Fourier-Transform Infrared Radiation (FT-IR)
4.3.10. Thermogravimetric Analysis (TGA)
4.3.11. Differential Scanning Calorimetry (DSC)
4.3.12. Compactibility
4.4. Powder Compaction
4.4.1. Ejection Force and Upper Die Wall Force
4.4.2. Power and Energy
4.4.3. Internal Structural Morphology of the Tablets
4.4.4. Tablet Disintegration
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLC | Sen Ling Cao |
| MC | Moisture Content |
| TS | Tensile strength |
| CPs | Core–shell particles |
| PMs | Physical mixtures |
| Ps | Porous particles |
| PCPs | Porous core–shell composite particles |
| DC | Direct compression |
| NaHCO3 | Sodium bicarbonate |
| NH4HCO3 | Ammonium bicarbonate |
| MD | Maltodextrin |
| HPC EF | Hydroxypropyl cellulose EF |
| Mgst | Magnesium stearate |
| HPMC E3 | Hydroxypropyl methylcellulose E3 |
| PVP K30 | Polyvinylpyrrolidone K30 |
References
- Gao, W. Comprehensive summary of material basis—Laying a foundation for substances of medicine food homology development. Chin. Herb. Med. 2024, 16, 311–312. [Google Scholar]
- Salunke, S.; Liu, F.; Batchelor, H.; Walsh, J.; Turner, R.; Ju, T.R.; Tuleu, C. European Paediatric Formulation Initiative (EuPFI)-Formulating Ideas for Better Medicines for Children. AAPS PharmSciTech 2017, 18, 257–262. [Google Scholar] [CrossRef]
- Walsh, J.; Mills, S. Conference report: Formulating better medicines for children: 4th European Paediatric Formulation Initiative conference. Ther. Deliv. 2013, 4, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Benabbas, R.; Sanchez-Ballester, N.M.; Bataille, B.; Sharkawi, T.; Soulairol, I. Development and pharmaceutical performance of a novel co-processed excipient of alginic acid and microcrystalline cellulose. Powder Technol. 2021, 378, 576–584. [Google Scholar] [CrossRef]
- Košir, D.; Vrečer, F. The performance of HPMC matrix tablets using various agglomeration manufacturing processes. Drug Dev. Ind. Pharm. 2017, 43, 329–337. [Google Scholar] [CrossRef]
- Medarević, D.; Djuriš, J.; Krkobabić, M.; Ibrić, S. Improving Tableting Performance of Lactose Monohydrate by Fluid-Bed Melt Granulation Co-Processing. Pharmaceutics 2021, 13, 2165. [Google Scholar] [CrossRef]
- Rojas, J.; Buckner, I.; Kumar, V. Co-proccessed excipients with enhanced direct compression functionality for improved tableting performance. Drug Dev. Ind. Pharm. 2012, 38, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ballester, N.M.; Bataille, B.; Soulairol, I. Sodium alginate and alginic acid as pharmaceutical excipients for tablet formulation: Structure-function relationship. Carbohydr. Polym. 2021, 270, 118399. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Meiser, F.; Morton, D.; Larson, I. Particle Engineering of Excipients for Direct Compression: Understanding the Role of Material Properties. Curr. Pharm. Des. 2015, 21, 5877–5889. [Google Scholar] [CrossRef]
- Li, Z.; Xian, J.; Wu, F.; Lin, X.; Shen, L.; Feng, Y. Development of TCM-based composite particles for direct compaction by particle design. Powder Technol. 2018, 338, 481–492. [Google Scholar] [CrossRef]
- Al-Zoubi, N.; Odeh, F.; Nikolakakis, I. Co-spray drying of metformin hydrochloride with polymers to improve compaction behavior. Powder Technol. 2017, 307, 163–174. [Google Scholar] [CrossRef]
- Zimmermann, T.; Madubuko, N.; Groppe, P.; Raczka, T.; Dünninger, N.; Taccardi, N.; Carl, S.; Zubiri, B.A.; Spiecker, E.; Wasserscheid, P.; et al. Supraparticles on beads for supported catalytically active liquid metal solutions—The SCALMS suprabead concept. Mater. Horiz. 2023, 10, 4960–4967. [Google Scholar] [CrossRef]
- George, S.; Thomas, A.; Kumar, M.V.; Kamdod, A.S.; Rajput, A.T.; Abdullah, S. Impact of processing parameters on the quality attributes of spray-dried powders: A review. Eur. Food Res. Technol. 2023, 249, 241–257. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Gaiani, C.; Edorh, J.M.; Borges, F.; Beaupeux, E.; Maudhuit, A.; Desobry, S. Comparison of Electrostatic Spray Drying, Spray Drying, and Freeze Drying for Lacticaseibacillus rhamnosus GG Dehydration. Foods 2023, 12, 3117. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, A.F.; Duff, B.; Brennan, L.; Tajber, L. The impact of the degree of intimate mixing on the compaction properties of materials produced by crystallo-co-spray drying. Eur. J. Pharm. Sci. 2020, 154, 105505. [Google Scholar] [CrossRef]
- Party, P.; Bartos, C.; Farkas, Á.; Szabó-Révész, P.; Ambrus, R. Formulation and In Vitro and In Silico Characterization of “Nano-in-Micro” Dry Powder Inhalers Containing Meloxicam. Pharmaceutics 2021, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, L.; Chen, F.C.; Guan, Y.M.; Chen, L.H.; Zhang, J.W.; Mao, Z.X.; Ming, L.S.; Zhu, W.F. The preparation, characterization, and application of porous core-shell composite particles produced with laboratory-scale spray dryer. Drug Dev. Ind. Pharm. 2023, 49, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Eraga, S.O.; Arhewoh, M.I.; Uhumwangho, M.U.; Iwuagwu, M.A. Characterisation of a novel, multifunctional, co-processed excipient and its effect on release profile of paracetamol from tablets prepared by direct compression. Asian Pac. J. Trop. Biomed. 2015, 5, 768–772. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, M.; Wu, F.; Shen, L.; Lin, X.; Feng, Y. Direct compaction properties of Zingiberis rhizoma extracted powders coated with various shell materials: Improvements and mechanism analysis. Int. J. Pharm. 2019, 564, 10–21. [Google Scholar] [CrossRef]
- Chen, C.; Liu, M.; Lii, S.; Gao, C.; Chen, J. In vitro degradation and drug-release properties of water-soluble chitosan cross-linked oxidized sodium alginate core-shell microgels. J. Biomater. Sci. Polym. Ed. 2012, 23, 2007–2024. [Google Scholar] [CrossRef]
- Vanbillemont, B.; Everaert, H.; De Beer, T. New advances in the characterization of lyophilised orally disintegrating tablets. Int. J. Pharm. 2020, 579, 119153. [Google Scholar] [CrossRef]
- Zhu, W.F.; Zhu, L.; Li, Z.; Wu, W.T.; Guan, Y.M.; Chen, L.H.; Mao, Z.X.; Ming, L.S. The Novel Use of PVP K30 as Templating Agent in Production of Porous Lactose. Pharmaceutics 2021, 13, 814. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Wu, F.; Shen, L.; Lin, X.; Feng, Y. Development on porous particles of Pueraria lobatae Radix for improving its compactibility and dissolution. RSC Adv. 2018, 8, 24250–24260. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, Y.; Gu, X.; Luan, B.; Zhu, Y.; Huang, Y.; Zhu, X. High-moisture extrusion cooking on soybean-wheat protein mixtures: Effect of sodium alginate/xanthan gum/maltodextrin on promoting a fibrous structure. Front. Nutr. 2023, 9, 1077601. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Luo, X.; Li, Q.; Jin, Z.; Naeem, A.; Zhu, W.; Chen, L.; Feng, Y.; Ming, L. The Fabrication, Drug Loading, and Release Behavior of Porous Mannitol. Molecules 2024, 29, 715. [Google Scholar] [CrossRef]
- Mustafa, W.W.; Fletcher, J.; Khoder, M.; Alany, R.G. Solid Dispersions of Gefitinib Prepared by Spray Drying with Improved Mucoadhesive and Drug Dissolution Properties. AAPS PharmSciTech 2022, 23, 48. [Google Scholar] [CrossRef]
- Grumann, H.D.; Klinken, S.; Kleinebudde, P. Influence of temperature on the compression behavior of pharmaceutical excipients. Int. J. Pharm. 2022, 628, 122305. [Google Scholar] [CrossRef]
- Partheniadis, I.; Martins, I.C.B.; Müllertz, A.; Nikolakakis, I.; Rades, T. The role of feed pretreatment and thermal stress in the development of griseofulvin/amino acid CAMS by Hot-Melt Extrusion—Structure relaxation and release/permeability evaluation. Int. J. Pharm. 2025, 679, 125765. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, L.; Lin, X.; Wang, Y.; Du, R.; Feng, Y. Research on the powder classification and the key parameters affecting tablet qualities for direct compaction based on powder functional properties. Adv. Powder Technol. 2021, 32, 565–581. [Google Scholar] [CrossRef]
- Goldenberg, M.; Vreeman, G.; Sun, D.J.; Moffit, M.; Li, M.; Zernik, M.; Ahuja, S.; Kim, Y.; Semin, D.; Sun, C.C. A material-sparing simplified buoyancy method for determining the true density of solids. Int. J. Pharm. 2023, 635, 122694. [Google Scholar] [CrossRef]
- Sadeghi, F.; Torab, M.; Khattab, M.; Homayouni, A.; Garekani, H.A. Improvement of Physico-mechanical Properties of Partially Amorphous Acetaminophen Developed from Hydroalcoholic Solution Using Spray Drying Technique. Iran. J. Basic Med. Sci. 2013, 16, 1100–1108. [Google Scholar] [PubMed]
- Khlibsuwan, R.; Pongjanyakul, T. Particle agglomeration of chitosan-magnesium aluminum silicate nanocomposites for direct compression tablets. Int. J. Pharm. 2018, 535, 410–419. [Google Scholar] [CrossRef]
- Ambrose, R.K.; Jan, S.; Siliveru, K. A review on flow characterization methods for cereal grain-based powders. J. Sci. Food Agric. 2016, 96, 359–364. [Google Scholar] [CrossRef]
- Abu-Hardan, M.; Hill, S.E. Handling properties of cereal materials in the presence of moisture and oil. Powder Technol. 2010, 198, 16–24. [Google Scholar] [CrossRef]
- Crouter, A.; Briens, L. The Effect of Moisture on the Flowability of Pharmaceutical Excipients. AAPS PharmSciTech 2014, 15, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sonnergaard, J.M. Quantification of the compactibility of pharmaceutical powders. Eur. J. Pharm. Biopharm. 2006, 63, 270–277. [Google Scholar] [CrossRef]
- Peeters, E.; Silva, A.F.; Fonteyne, M.; De Beer, T.; Vervaet, C.; Remon, J.P. Influence of extended dwell time during pre- and main compression on the properties of ibuprofen tablets. Eur. J. Pharm. Biopharm. 2018, 128, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Etzler, F.M.; Bramante, T.; Deanne, R.; Sienkiewicz, S.; Chen, F.J. Tablet Tensile Strength: An Adhesion Science Perspective. J. Adhes. Sci. Technol. 2011, 25, 501–519. [Google Scholar] [CrossRef]
- Li, J.; Wu, F.; Lin, X.; Shen, L.; Wang, Y.; Feng, Y. Novel application of hydroxypropyl methylcellulose to improving direct compaction properties of tablet fillers by co-spray drying. RSC Adv. 2015, 5, 69289–69298. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Wu, F.; Shen, L.; Wang, Y.; Feng, Y. Application of the central composite design to optimize the calcium carbonate-HPMC co-processed excipient prepared by co-spray drying. RSC Adv. 2015, 5, 94105–94114. [Google Scholar] [CrossRef]
- Sun, C.C. Dependence of ejection force on tableting speed-A compaction simulation study. Powder Technol. 2015, 279, 123–126. [Google Scholar] [CrossRef]
- Mirani, A.G.; Patankar, S.P.; Borole, V.S.; Pawar, A.S.; Kadam, V.J. Direct Compression High Functionality Excipient Using Coprocessing Technique: A Brief Review. Curr. Drug Deliv. 2011, 8, 426–435. [Google Scholar] [CrossRef]
- Fredholt, F.; Di Meo, C.; Sloth, S.; Müllertz, A.; Berthelsen, R. Direct visualizing of paracetamol immediate release tablet disintegration in vivo and in vitro. Eur. J. Pharm. Biopharm. 2022, 180, 63–70. [Google Scholar] [CrossRef]
- Rahim, S.A.; Al-Zoubi, N.; Khader, H.; Alwaraydat, R.; Al-Akayleh, F. Ethanol-induced dose dumping from sodium alginate matrix tablets: Investigation of the effects of medium viscosity and pH. Int. J. Pharm. 2023, 632, 122568. [Google Scholar] [CrossRef]
- Dhondt, J.; Bertels, J.; Kumar, A.; Van Hauwermeiren, D.; Ryckaert, A.; Van Snick, B.; Klingeleers, D.; Vervaet, C.; De Beer, T. A multivariate formulation and process development platform for direct compression. Int. J. Pharm. 2022, 623, 121962. [Google Scholar] [CrossRef]
- Yankin, A.; Murtaza, H.A.; Ospanov, A.; Zharkynbekova, G.; Yuldasheva, D.; Perveen, A.; Talamona, D. Comprehensive analysis of ultrasonically atomized 316L stainless steel powder using adjusted additive manufacturing suitability factor. Powder Technol. 2024, 444, 120004. [Google Scholar] [CrossRef]
- Li, Z.; Peng, W.; Zhu, L.; Liu, W.; Yang, L.; Chen, L.; Naeem, A.; Zhu, W.; Feng, Y.; Ming, L. Study on Improving the Performance of Traditional Medicine Extracts with High Drug Loading Based on Co-spray Drying Technology. AAPS PharmSciTech 2023, 24, 247. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Chen, H.; Aburub, A.; Sun, C.C. A platform direct compression formulation for low dose sustained-release tablets enabled by a dual particle engineering approach. Powder Technol. 2019, 342, 856–863. [Google Scholar] [CrossRef]
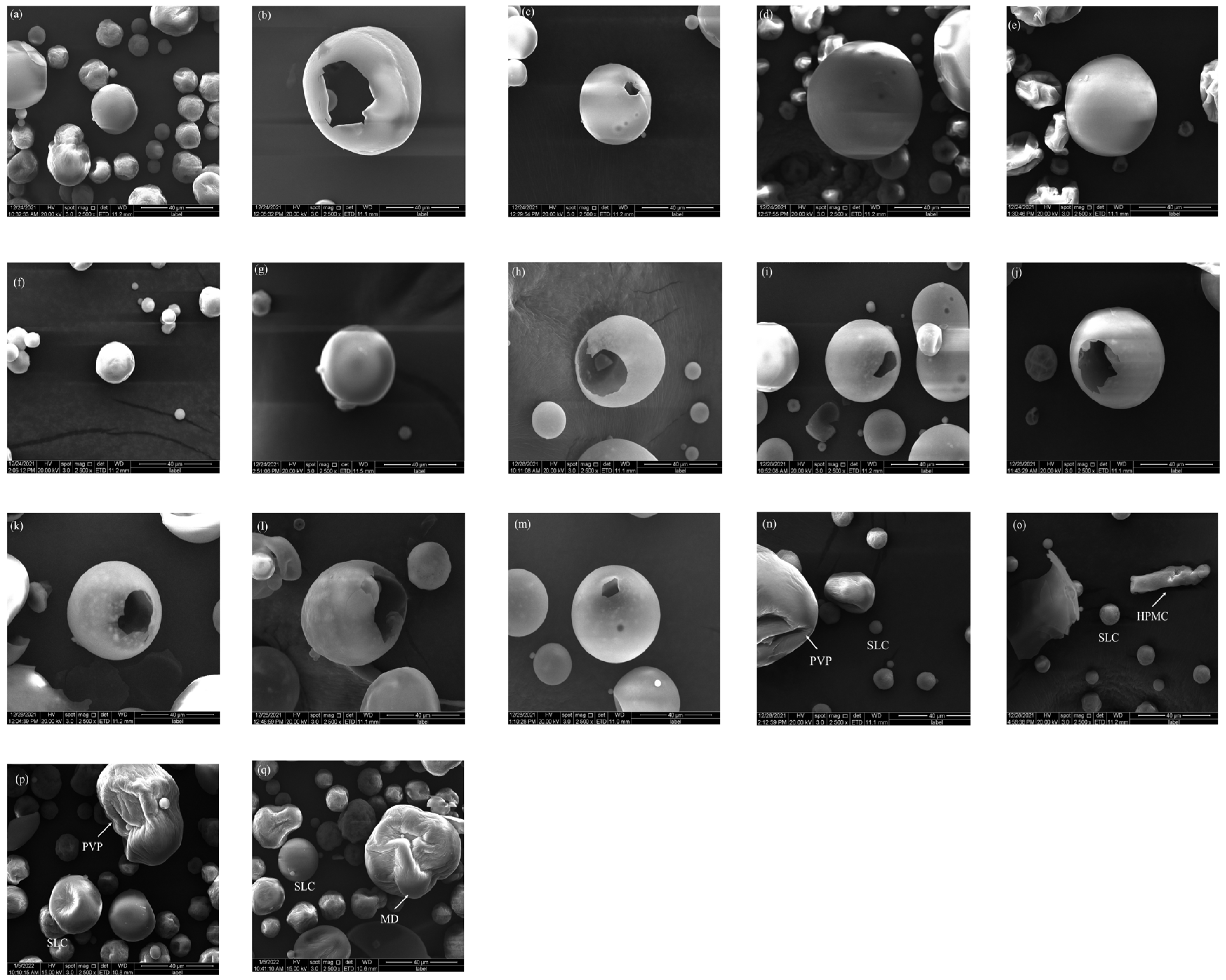
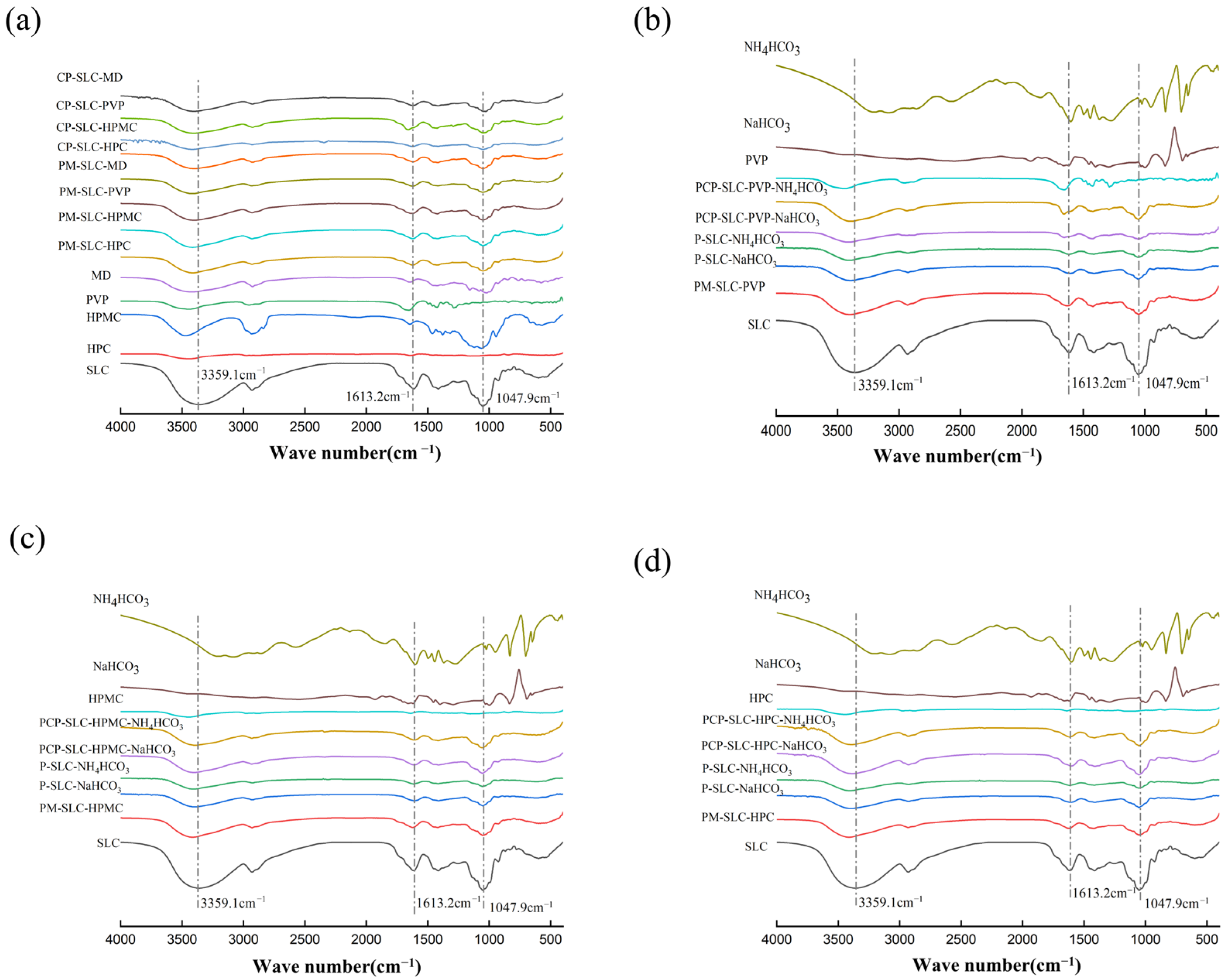
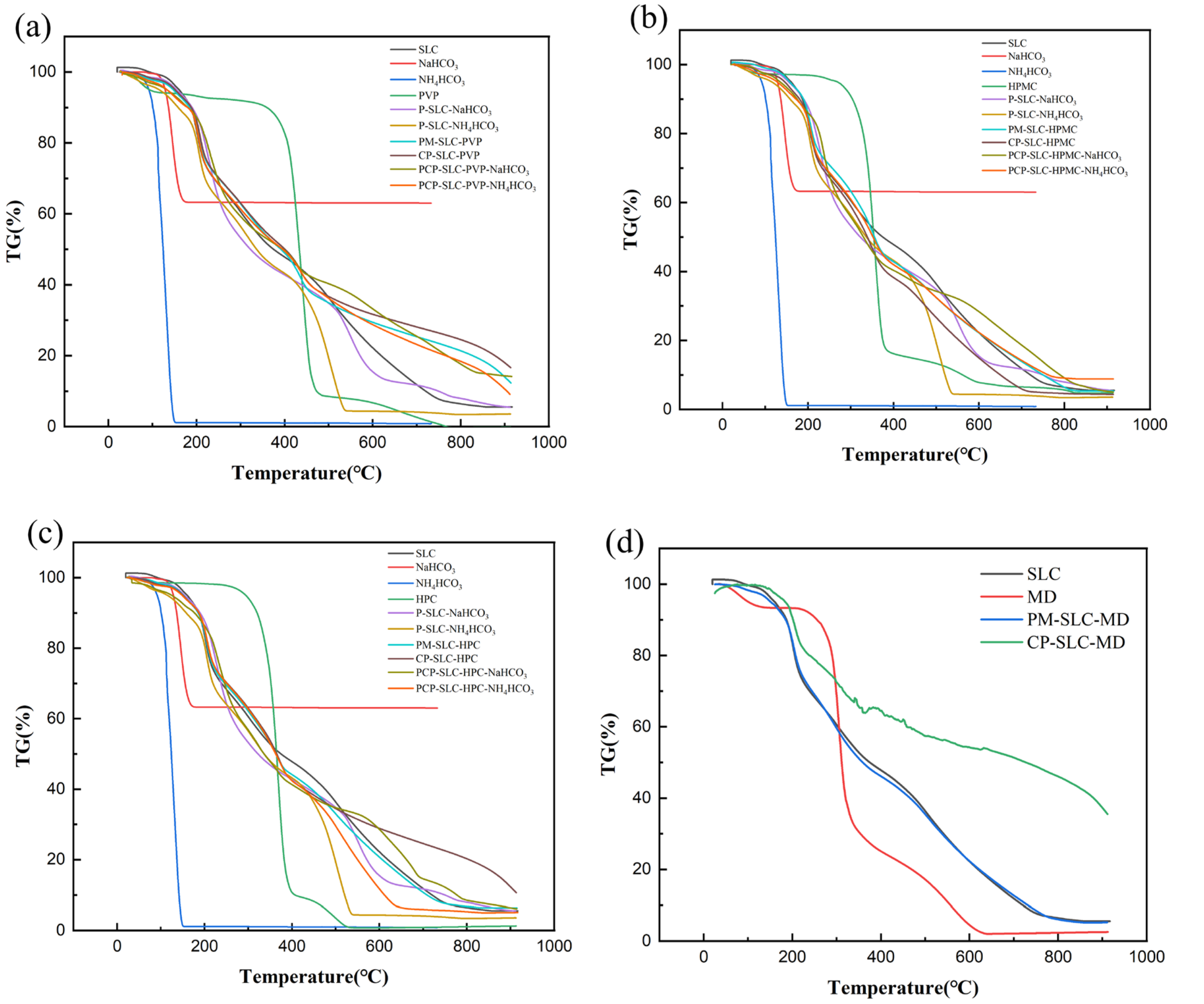
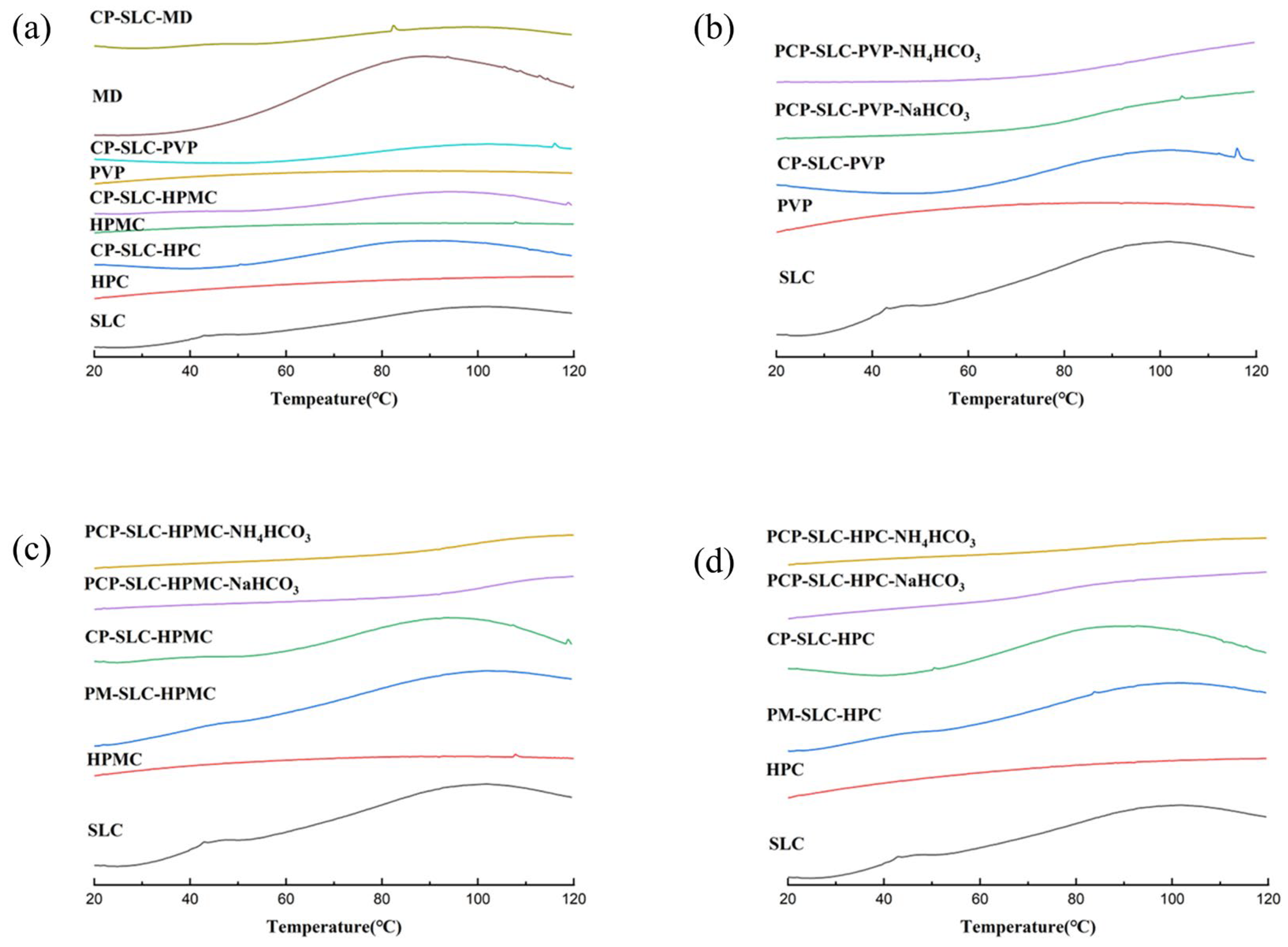
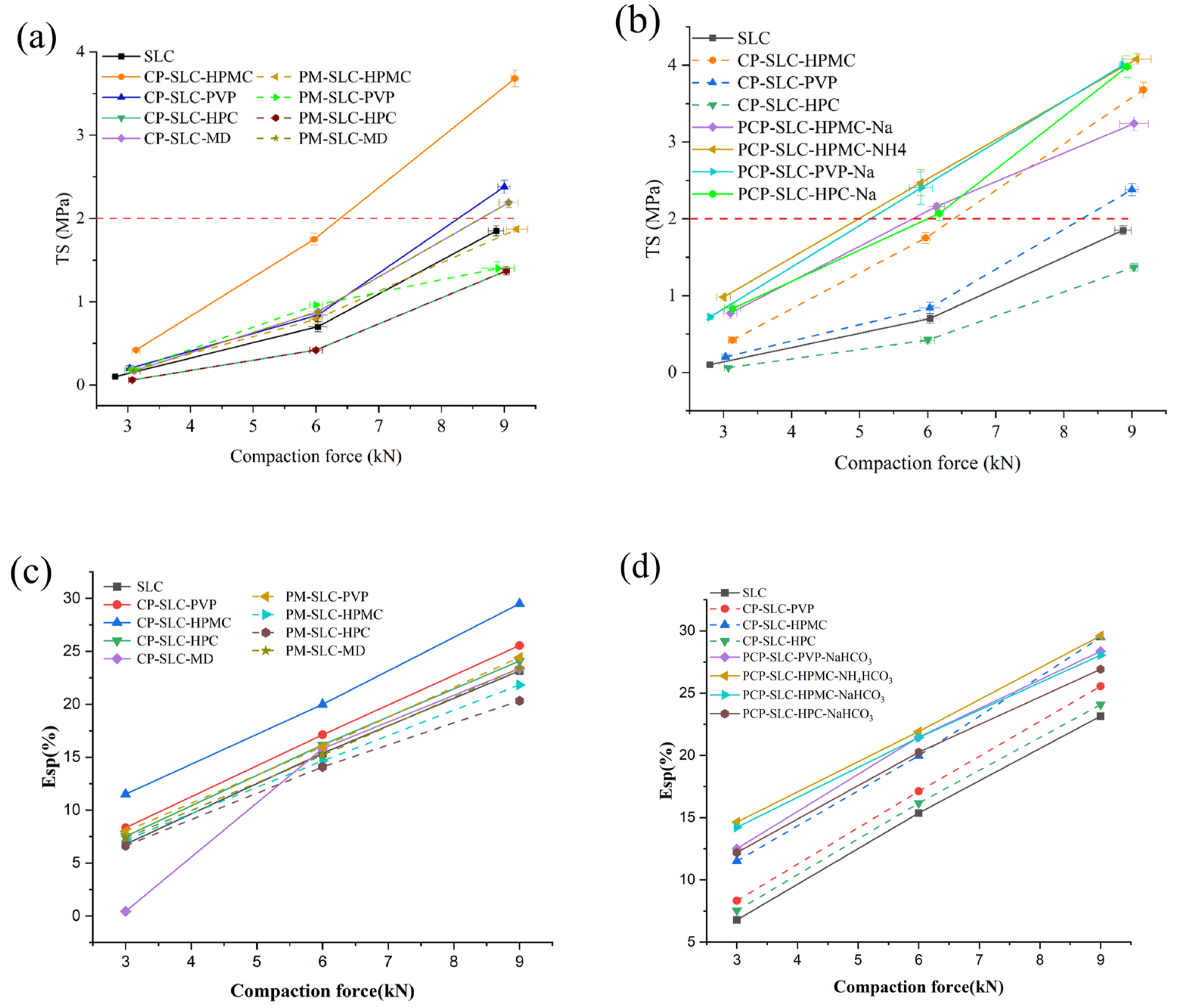
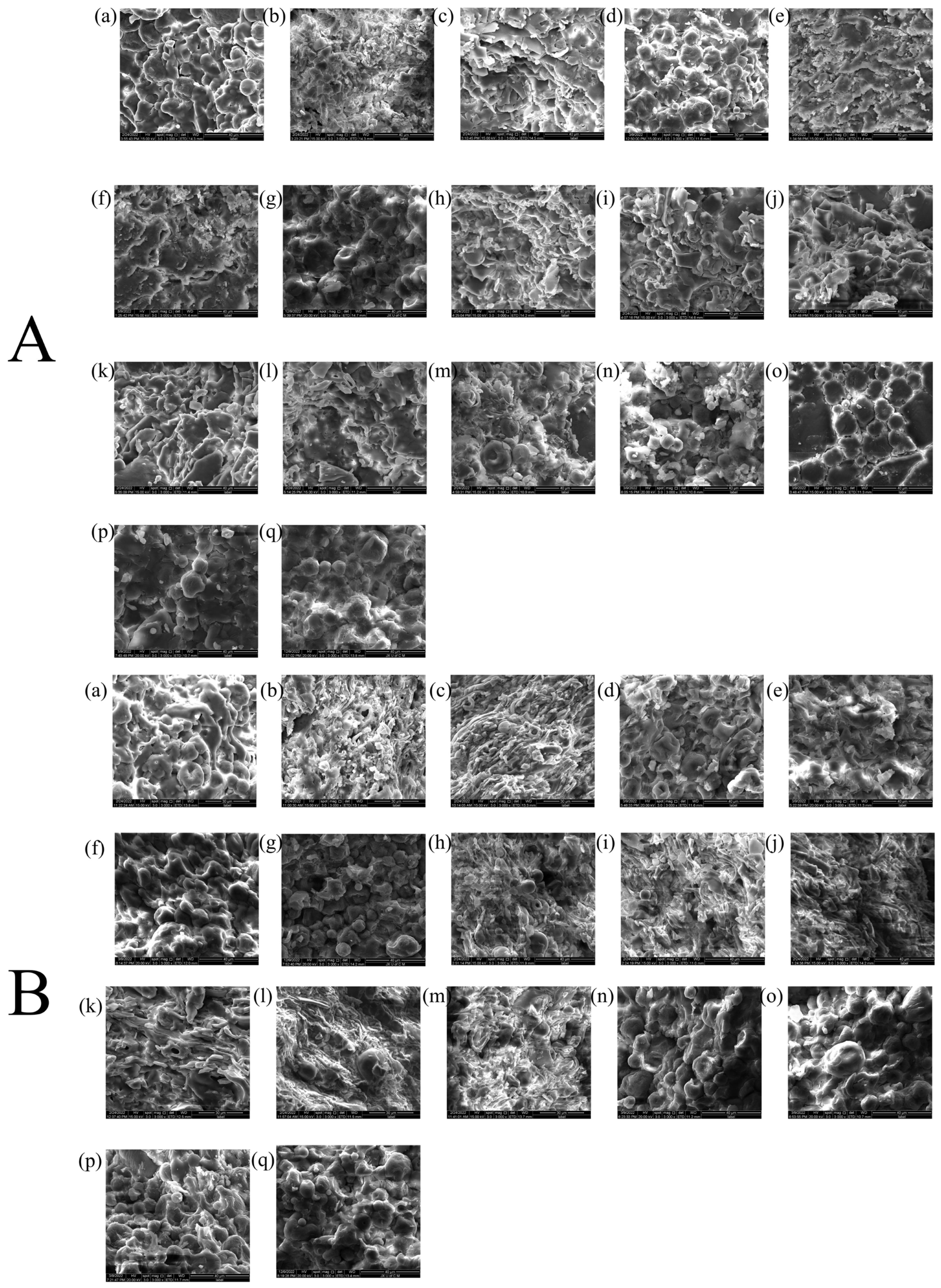
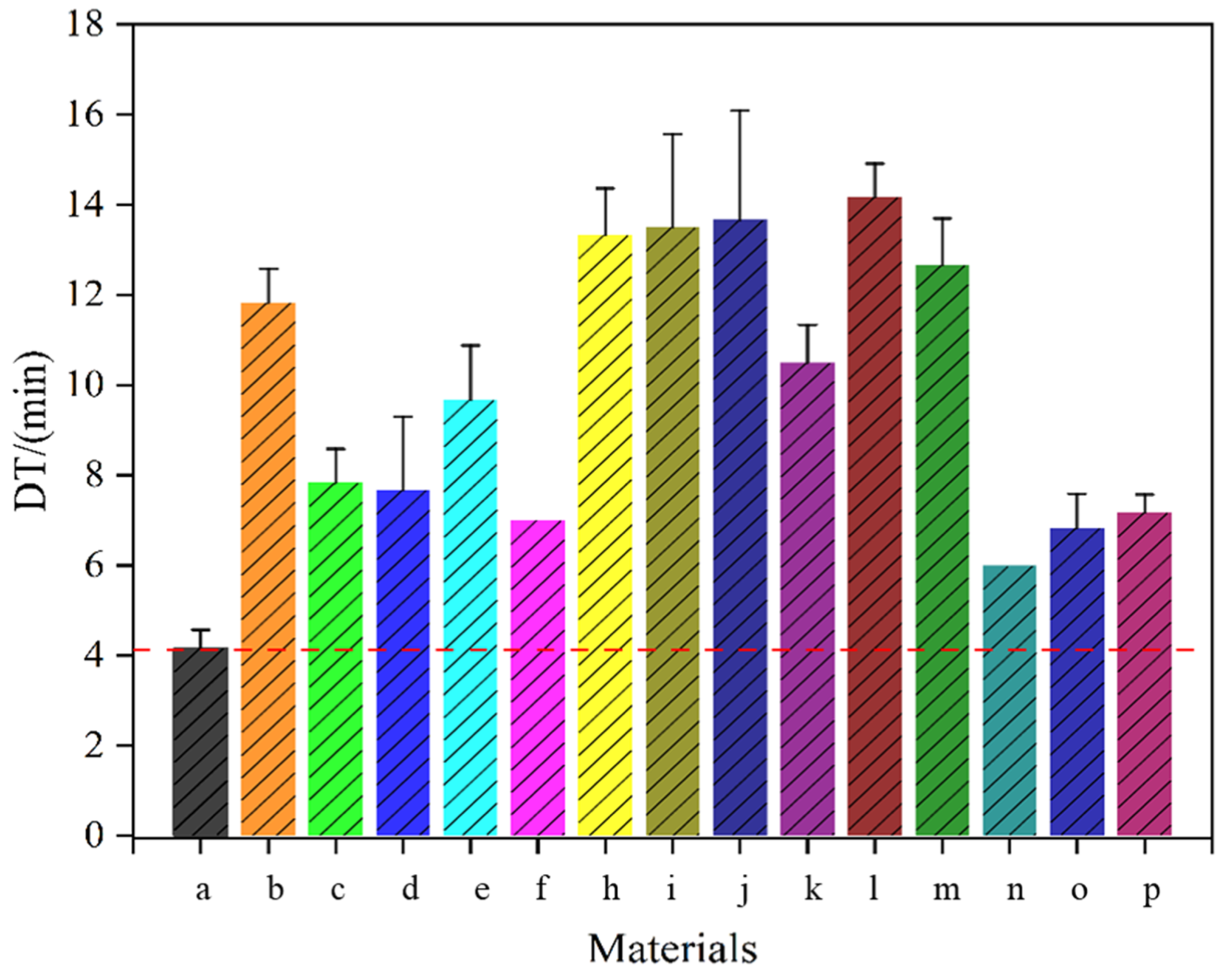
| Sample | MC (%) | ρb (g/mL) | ρt (g/mL) | ρtrue (g/mL) | D0.5 (μm) | Un | Span | BET SSA (m2/g) | Ad PV (cm2/g) | De PV (cm2/g) | Ad PD (nm) | De PD (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 2.13 ± 0.03 | 0.3593 ± 0.0006 | 0.5924 ± 0.0102 | 1.2913 ± 0.0007 | 13.4823 ± 0.0321 | 0.5643 ± 0.0101 | 1.8263 ± 0.0201 | 0.3787 ± 0.0017 | 0.000646 | 0.000638 | 87.509 | 80.362 |
| b | 4.21 ± 0.03 | 0.0627 ± 0.0021 | 0.0955 ± 0.0040 | 0.4695 ± 0.0004 | 10.5050 ± 0.1032 | 0.6533 ± 0.0025 | 2.1227 ± 0.0074 | 1.2402 ± 0.0180 | 0.002137 | 0.002187 | 139.985 | 116.822 |
| c | 3.52 ± 0.03 | 0.1060 ± 0.0000 | 0.1614 ± 0.0014 | 0.5209 ± 0.0004 | 9.7910 ± 0.0601 | 0.7103 ± 0.0050 | 2.2970 ± 0.0125 | 0.8784 ± 0.0084 | 0.001395 | 0.001442 | 115.137 | 92.913 |
| d | 2.27 ± 0.03 | 0.2540 ± 0.0021 | 0.4097 ± 0.0154 | 1.2411 ± 0.0004 | 13.8463 ± 0.1276 | 0.5283 ± 0.0084 | 1.7200 ± 0.0225 | 0.2397 ± 0.0090 | 0.000391 | 0.000434 | 311.343 | 293.510 |
| e | 2.43 ± 0.03 | 0.2487 ± 0.0021 | 0.4060 ± 0.0215 | 1.1049 ± 0.0008 | 24.5400 ± 0.0390 | 0.9643 ± 0.0206 | 2.1557 ± 0.0290 | 0.3558 ± 0.0035 | 0.000644 | 0.000655 | 113.780 | 107.683 |
| f | 2.28 ± 0.03 | 0.3893 ± 0.0102 | 0.6420 ± 0.0261 | 1.2678 ± 0.0010 | 14.9400 ± 0.0269 | 0.5300 ± 0.0087 | 1.7093 ± 0.0193 | 0.2134 ± 0.0042 | 0.000380 | 0.000387 | 204.497 | 155.211 |
| g | 2.28 ± 0.03 | 0.3503 ± 0.0091 | 0.5531 ± 0.0098 | 1.2795 ± 0.0007 | 13.4347 ± 0.0343 | 0.5653 ± 0.0118 | 1.8277 ± 0.0327 | 0.3686 ± 0.0029 | 0.000674 | 0.000679 | 108.541 | 101.777 |
| h | 3.45 ± 0.01 | 0.1177 ± 0.0012 | 0.1919 ± 0.0056 | 0.6073 ± 0.0004 | 10.7367 ± 0.1069 | 0.6987 ± 0.0049 | 2.2733 ± 0.017 | 0.4460 ± 0.0210 | 0.000884 | 0.000885 | 381.459 | 314.406 |
| i | 3.03 ± 0.06 | 0.1427 ± 0.0060 | 0.2278 ± 0.0132 | 0.7217 ± 0.0008 | 10.7863 ± 0.0536 | 0.7100 ± 0.0148 | 2.3117 ± 0.0384 | 0.5263 ± 0.0157 | 0.000761 | 0.000789 | 248.231 | 277.170 |
| j | 3.62 ± 0.03 | 0.1360 ± 0.0020 | 0.2267 ± 0.0033 | 0.9326 ± 0.0011 | 20.7403 ± 0.0299 | 1.0767 ± 0.0462 | 2.8067 ± 0.0319 | 0.6037 ± 0.0281 | 0.000773 | 0.000824 | 223.533 | 118.470 |
| k | 3.28 ± 0.03 | 0.1463 ± 0.0021 | 0.2386 ± 0.0015 | 1.0731 ± 0.0016 | 20.7137 ± 0.0279 | 0.7593 ± 0.0057 | 2.4423 ± 0.0193 | 0.5476 ± 0.0125 | 0.000770 | 0.000783 | 156.582 | 126.144 |
| l | 2.82 ± 0.03 | 0.1057 ± 0.0032 | 0.1585 ± 0.0055 | 0.5156 ± 0.0004 | 13.0220 ± 0.0285 | 0.7210 ± 0.0151 | 2.3127 ± 0.0402 | 0.3854 ± 0.0225 | 0.000779 | 0.000780 | 372.150 | 339.027 |
| m | 2.80 ± 0.05 | 0.1450 ± 0.0010 | 0.2132 ± 0.0015 | 0.6114 ± 0.0004 | 13.8087 ± 0.0375 | 0.8493 ± 0.1166 | 2.5083 ± 0.0847 | 0.4423 ± 0.0129 | 0.000736 | 0.000730 | 200.719 | 160.132 |
| n | 1.93 ± 0.03 | 0.4207 ± 0.0021 | 0.6164 ± 0.0226 | 1.2894 ± 0.0006 | 15.1680 ± 0.0795 | 0.9513 ± 0.0087 | 2.7570 ± 0.0286 | 0.2918 ± 0.0026 | 0.000504 | 0.000528 | 110.382 | 95.244 |
| o | 1.92 ± 0.03 | 0.4350 ± 0.0044 | 0.6275 ± 0.0084 | 1.3079 ± 0.0004 | 14.8217 ± 0.0656 | 0.9900 ± 0.0260 | 2.8560 ± 0.0387 | 0.3381 ± 0.0023 | 0.000496 | 0.000502 | 82.100 | 77.795 |
| p | 1.78 ± 0.03 | 0.4670 ± 0.0108 | 0.6579 ± 0.0201 | 1.3190 ± 0.0008 | 16.3103 ± 0.2599 | 3.7100 ± 0.3822 | 17.8927 ± 1.9880 | 0.3147 ± 0.0044 | 0.000379 | 0.000413 | 90.643 | 80.956 |
| q | 2.03 ± 0.03 | 0.4247 ± 0.0057 | 0.6067 ± 0.0081 | 1.3137 ± 0.0002 | 14.2833 ± 0.1553 | 0.6590 ± 0.0340 | 2.0530 ± 0.0544 | 0.3287 ± 0.0026 | 0.000457 | 0.000457 | 81.255 | 75.557 |
| Sample | AR (°) | CI | HR | CoI | Cs | PFSD |
|---|---|---|---|---|---|---|
| a | 44.61 ± 0.68 | 39.33 ± 1.15 | 1.65 ± 0.03 | 27.05 ± 1.55 | 174.48 ± 8.31 | 0.79 ± 0.05 |
| b | 46.12 ± 0.00 | 34.33 ± 0.58 | 1.52 ± 0.01 | 13.34 ± 0.89 | 118.27 ± 7.61 | 1.43 ± 0.09 |
| c | 45.37 ± 0.65 | 34.33 ± 0.58 | 1.52 ± 0.01 | 18.51 ± 0.36 | 72.67 ± 2.75 | 1.20 ± 0.01 |
| d | 40.91 ± 0.76 | 38.00 ± 0.00 | 1.61 ± 0.00 | 17.77 ± 0.32 | 164.69 ± 4.91 | 0.93 ± 0.05 |
| e | 40.91 ± 0.76 | 38.67 ± 2.31 | 1.63 ± 0.06 | 28.16 ± 0.42 | 179.79 ± 6.58 | 0.94 ± 0.07 |
| f | 42.61 ± 0.00 | 39.33 ± 1.15 | 1.65 ± 0.03 | 31.26 ± 0.69 | 175.8 ± 5.15 | 0.96 ± 0.09 |
| g | 40.91 ± 0.76 | 36.67 ± 0.58 | 1.58 ± 0.01 | 19.01 ± 1.23 | 167.03 ± 1.63 | 0.93 ± 0.07 |
| h | 43.02 ± 0.70 | 38.67 ± 1.15 | 1.63 ± 0.03 | 12.47 ± 0.97 | 92.73 ± 6.35 | 1.28 ± 0.08 |
| i | 42.61 ± 0.00 | 37.33 ± 1.15 | 1.60 ± 0.03 | 15.87 ± 0.47 | 78.17 ± 0.83 | 1.13 ± 0.09 |
| j | 40.03 ± 0.00 | 40.00 ± 0.00 | 1.67 ± 0.00 | 22.68 ± 0.89 | 64.44 ± 2.37 | 1.05 ± 0.07 |
| k | 37.23 ± 0.00 | 38.67 ± 1.15 | 1.63 ± 0.03 | 21.17 ± 0.70 | 67.40 ± 4.60 | 0.99 ± 0.05 |
| l | 42.61 ± 0.00 | 33.33 ± 0.58 | 1.50 ± 0.01 | 13.39 ± 1.07 | 82.61 ± 11.09 | 1.04 ± 0.07 |
| m | 41.35 ± 0.00 | 32.00 ± 0.00 | 1.47 ± 0.00 | 12.70 ± 0.70 | 59.76 ± 4.29 | 1.06 ± 0.06 |
| n | 44.61 ± 0.68 | 31.67 ± 3.79 | 1.47 ± 0.08 | 30.36 ± 0.90 | 200.97 ± 6.86 | 0.97 ± 0.08 |
| o | 43.83 ± 0.00 | 30.67 ± 1.15 | 1.44 ± 0.02 | 36.33 ± 0.76 | 183.11 ± 5.74 | 1.04 ± 0.06 |
| p | 39.57 ± 0.79 | 29.00 ± 1.00 | 1.41 ± 0.02 | 19.09 ± 0.79 | 188.21 ± 9.41 | 1.07 ± 0.04 |
| q | 43.83 ± 0.00 | 30.00 ± 0.00 | 1.43 ± 0.00 | 35.25 ± 1.54 | 158.43 ± 3.84 | 1.13 ± 0.05 |
| Materials | CF (kN) | CR (%) | FES (%) | EF (N) | UDWF (N) |
|---|---|---|---|---|---|
| a | 2.80 ± 0.00 | 29.771 ± 0.010 | 5.104 ± 0.101 | 13.517 ± 0.557 | 0.307 ± 0.008 |
| 6.03 ± 0.15 | 25.625 ± 0.030 | 6.981 ± 0.125 | 86.589 ± 3.729 | 0.638 ± 0.015 | |
| 8.87 ± 0.12 | 23.208 ± 0.062 | 9.594 ± 0.400 | 126.302 ± 3.139 | 0.976 ± 0.022 | |
| d | 3.03 ± 0.06 | 30.025 ± 0.033 | 5.138 ± 0.332 | 31.413 ± 1.715 | 0.328 ± 0.008 |
| 6.03 ± 0.15 | 26.039 ± 0.030 | 7.616 ± 0.240 | 87.240 ± 2.033 | 0.648 ± 0.010 | |
| 9.00 ± 0.10 | 23.322 ± 0.025 | 9.629 ± 0.618 | 117.350 ± 2.689 | 1.005 ± 0.016 | |
| e | 3.13 ± 0.06 | 26.739 ± 0.000 | 5.391 ± 0.112 | 16.276 ± 1.849 | 0.545 ± 0.013 |
| 5.97 ± 0.06 | 23.067 ± 0.045 | 7.623 ± 0.202 | 72.428 ± 5.175 | 1.120 ± 0.032 | |
| 9.17 ± 0.06 | 20.605 ± 0.058 | 10.207 ± 0.429 | 107.910 ± 1.292 | 2.104 ± 0.074 | |
| f | 3.07 ± 0.06 | 29.225 ± 0.033 | 5.478 ± 0.330 | 3.287 ± 0.536 | 0.332 ± 0.006 |
| 6.00 ± 0.10 | 25.263 ± 0.025 | 7.447 ± 0.234 | 46.224 ± 0.746 | 0.670 ± 0.013 | |
| 9.03 ± 0.06 | 22.923 ± 0.025 | 10.295 ± 0.367 | 63.965 ± 1.292 | 1.050 ± 0.009 | |
| g | 3.10 ± 0.10 | 29.149 ± 0.057 | 4.966 ± 0.235 | 0.336 ± 0.004 | 52.450 ± 0.620 |
| 6.03 ± 0.15 | 25.154 ± 0.033 | 7.167 ± 0.327 | 90.332 ± 1.953 | 0.633 ± 0.022 | |
| 9.07 ± 0.15 | 22.891 ± 0.008 | 9.739 ± 0.499 | 123.861 ± 5.221 | 0.962 ± 0.039 | |
| i | 2.80 ± 0.00 | 15.516 ± 0.072 | 7.171 ± 0.511 | 74.577 ± 1.605 | 0.516 ± 0.002 |
| 5.90 ± 0.17 | 13.178 ± 0.050 | 11.231 ± 0.417 | 155.111 ± 40.316 | 1.024 ± 0.020 | |
| 8.87 ± 0.06 | 11.559 ± 0.025 | 15.354 ± 0.459 | 253.223 ± 9.601 | 1.129 ± 0.029 | |
| j | 3.00 ± 0.10 | 15.250 ± 0.042 | 7.439 ± 0.300 | 71.289 ± 4.709 | 0.574 ± 0.026 |
| 5.90 ± 0.10 | 12.845 ± 0.053 | 12.286 ± 0.693 | 92.285 ± 1.692 | 0.967 ± 0.066 | |
| 9.07 ± 0.21 | 11.576 ± 0.027 | 15.189 ± 0.209 | 116.374 ± 3.251 | 1.351 ± 0.029 | |
| k | 3.10 ± 0.10 | 15.230 ± 0.027 | 7.748 ± 0.203 | 30.468 ± 1.947 | 0.621 ± 0.013 |
| 6.13 ± 0.12 | 12.934 ± 0.057 | 12.205 ± 0.501 | 72.103 ± 4.793 | 1.305 ± 0.048 | |
| 9.03 ± 0.21 | 11.669 ± 0.003 | 16.371 ± 0.652 | 97.819 ± 1.492 | 1.830 ± 0.070 | |
| m | 3.13 ± 0.12 | 15.118 ± 0.000 | 6.920 ± 0.013 | 37.936 ± 3.968 | 0.509 ± 0.012 |
| 6.17 ± 0.06 | 13.502 ± 0.073 | 9.355 ± 0.209 | 75.988 ± 1.773 | 0.975 ± 0.010 | |
| 8.93 ± 0.06 | 11.669 ± 0.003 | 15.539 ± 0.230 | 87.24 ± 5.356 | 1.653 ± 0.028 | |
| n | 3.07 ± 0.12 | 34.583 ± 0.124 | 5.694 ± 0.190 | 19.857 ± 1.715 | 0.117 ± 0.002 |
| 6.00 ± 0.10 | 29.868 ± 0.094 | 7.527 ± 0.329 | 68.522 ± 1.491 | 0.217 ± 0.010 | |
| 8.90 ± 0.26 | 27.107 ± 0.113 | 10.187 ± 0.512 | 106.283 ± 5.378 | 0.321 ± 0.006 | |
| o | 3.07 ± 0.06 | 33.565 ± 0.045 | 5.286 ± 0.125 | 24.251 ± 1.848 | 0.116 ± 0.001 |
| 6.00 ± 0.10 | 29.390 ± 0.039 | 7.859 ± 0.330 | 69.820 ± 1.459 | 0.218 ± 0.006 | |
| 9.20 ± 0.17 | 26.511 ± 0.130 | 10.960 ± 0.667 | 97.332 ± 1.972 | 0.327 ± 0.010 | |
| p | 3.00 ± 0.00 | 33.587 ± 0.039 | 5.617 ± 0.195 | 18.858 ± 0.807 | 0.122 ± 0.001 |
| 6.00 ± 0.10 | 29.435 ± 0.000 | 8.235 ± 0.000 | 54.525 ± 1.715 | 0.235 ± 0.007 | |
| 8.97 ± 0.21 | 26.713 ± 0.035 | 10.65 ± 0.513 | 78.125 ± 3.686 | 0.334 ± 0.003 | |
| q | 3.03 ± 0.12 | 34.007 ± 0.248 | 5.749 ± 0.362 | 23.866 ± 1.861 | 0.122 ± 0.000 |
| 5.90 ± 0.10 | 28.249 ± 2.305 | 7.894 ± 0.583 | 69.823 ± 5.755 | 0.215 ± 0.002 | |
| 8.97 ± 0.21 | 26.455 ± 0.029 | 10.438 ± 0.449 | 108.399 ± 4.257 | 0.311 ± 0.006 |
| Materials | CF (kN) | E1 (Nm) | E2 (Nm) | E3 (Nm) | ET (Nm) | R1 (%) | R2 (%) | R3 (%) | PL (%) |
|---|---|---|---|---|---|---|---|---|---|
| a | 2.80 ± 0.00 | 1.417 ± 0.067 | 1.357 ± 0.032 | 0.077 ± 0.006 | 2.850 ± 0.104 | 49.695 ± 0.529 | 47.618 ± 0.636 | 2.687 ± 0.107 | 0.947 ± 0.003 |
| 6.03 ± 0.15 | 3.087 ± 0.196 | 3.073 ± 0.090 | 0.267 ± 0.032 | 6.427 ± 0.290 | 48.003 ± 0.957 | 47.844 ± 0.764 | 4.153 ± 0.509 | 0.920 ± 0.009 | |
| 8.87 ± 0.12 | 4.503 ± 0.071 | 4.630 ± 0.087 | 0.553 ± 0.081 | 9.687 ± 0.181 | 46.495 ± 0.670 | 47.802 ± 0.762 | 5.703 ± 0.737 | 0.893 ± 0.013 | |
| d | 3.03 ± 0.06 | 1.700 ± 0.030 | 1.667 ± 0.047 | 0.107 ± 0.031 | 3.473 ± 0.067 | 48.947 ± 0.541 | 47.980 ± 0.544 | 3.073 ± 0.897 | 0.940 ± 0.017 |
| 6.03 ± 0.15 | 3.500 ± 0.082 | 3.423 ± 0.035 | 0.297 ± 0.006 | 7.220 ± 0.118 | 48.473 ± 0.340 | 47.418 ± 0.298 | 4.109 ± 0.093 | 0.920 ± 0.002 | |
| 9.00 ± 0.10 | 5.177 ± 0.146 | 5.110 ± 0.062 | 0.573 ± 0.081 | 10.860 ± 0.288 | 47.666 ± 0.090 | 47.065 ± 0.670 | 5.269 ± 0.601 | 0.899 ± 0.012 | |
| e | 3.13 ± 0.06 | 3.550 ± 0.123 | 2.303 ± 0.060 | 0.073 ± 0.006 | 5.927 ± 0.145 | 59.891 ± 0.885 | 38.869 ± 0.876 | 1.239 ± 0.126 | 0.969 ± 0.003 |
| 5.97 ± 0.06 | 6.003 ± 0.055 | 3.997 ± 0.067 | 0.303 ± 0.080 | 10.303 ± 0.091 | 58.266 ± 0.135 | 38.794 ± 0.850 | 2.940 ± 0.760 | 0.930 ± 0.018 | |
| 9.17 ± 0.06 | 8.633 ± 0.135 | 5.900 ± 0.010 | 0.493 ± 0.015 | 15.027 ± 0.14 | 57.451 ± 0.363 | 39.266 ± 0.405 | 3.283 ± 0.083 | 0.923 ± 0.002 | |
| f | 3.07 ± 0.06 | 1.193 ± 0.058 | 1.507 ± 0.035 | 0.120 ± 0.017 | 2.820 ± 0.085 | 42.310 ± 1.239 | 53.444 ± 1.225 | 4.246 ± 0.508 | 0.926 ± 0.009 |
| 6.00 ± 0.10 | 2.547 ± 0.051 | 3.233 ± 0.071 | 0.290 ± 0.010 | 6.070 ± 0.115 | 41.955 ± 0.253 | 53.266 ± 0.182 | 4.780 ± 0.222 | 0.918 ± 0.004 | |
| 9.03 ± 0.06 | 4.010 ± 0.111 | 4.817 ± 0.067 | 0.613 ± 0.040 | 9.440 ± 0.130 | 42.477 ± 0.933 | 51.026 ± 0.570 | 6.497 ± 0.403 | 0.887 ± 0.005 | |
| g | 3.10 ± 0.10 | 1.750 ± 0.075 | 0.087 ± 0.006 | 2.836 ± 0.065 | 4.673 ± 0.113 | 37.445 ± 0.995 | 1.854 ± 0.107 | 60.701 ± 1.099 | 0.030 ± 0.002 |
| 6.03 ± 0.15 | 3.253 ± 0.198 | 3.160 ± 0.092 | 0.353 ± 0.025 | 6.767 ± 0.264 | 48.051 ± 1.056 | 46.712 ± 0.474 | 5.237 ± 0.582 | 0.899 ± 0.009 | |
| 9.07 ± 0.15 | 4.723 ± 0.035 | 4.677 ± 0.190 | 0.627 ± 0.050 | 10.027 ± 0.145 | 47.116 ± 0.880 | 46.630 ± 1.227 | 6.254 ± 0.567 | 0.882 ± 0.012 | |
| i | 2.80 ± 0.00 | 5.347 ± 0.295 | 1.500 ± 0.010 | 0.053 ± 0.012 | 6.900 ± 0.276 | 77.457 ± 1.155 | 21.765 ± 0.962 | 0.778 ± 0.194 | 0.966 ± 0.007 |
| 5.90 ± 0.17 | 6.647 ± 0.170 | 2.577 ± 0.051 | 0.290 ± 0.052 | 9.513 ± 0.180 | 69.863 ± 0.852 | 27.094 ± 0.865 | 3.043 ± 0.493 | 0.899 ± 0.016 | |
| 8.87 ± 0.06 | 7.287 ± 0.115 | 3.403 ± 0.055 | 0.707 ± 0.050 | 11.397 ± 0.116 | 63.934 ± 0.368 | 29.867 ± 0.743 | 6.198 ± 0.386 | 0.828 ± 0.012 | |
| j | 3.00 ± 0.10 | 5.637 ± 0.442 | 1.757 ± 0.061 | 0.190 ± 0.147 | 7.583 ± 0.639 | 74.353 ± 0.415 | 23.233 ± 1.271 | 2.415 ± 1.663 | 0.907 ± 0.063 |
| 5.90 ± 0.10 | 7.097 ± 0.076 | 2.630 ± 0.072 | 0.290 ± 0.061 | 10.017 ± 0.18 | 70.857 ± 0.832 | 26.253 ± 0.274 | 2.890 ± 0.559 | 0.901 ± 0.016 | |
| 9.07 ± 0.21 | 8.447 ± 0.194 | 3.553 ± 0.076 | 0.583 ± 0.110 | 12.583 ± 0.11 | 67.120 ± 0.963 | 28.241 ± 0.750 | 4.638 ± 0.891 | 0.859 ± 0.024 | |
| k | 3.10 ± 0.10 | 5.070 ± 0.231 | 1.707 ± 0.061 | 0.120 ± 0.010 | 6.897 ± 0.251 | 73.500 ± 0.968 | 24.755 ± 0.822 | 1.745 ± 0.205 | 0.934 ± 0.006 |
| 6.13 ± 0.12 | 6.647 ± 0.215 | 2.573 ± 0.055 | 0.347 ± 0.045 | 9.567 ± 0.244 | 69.471 ± 0.691 | 26.901 ± 0.186 | 3.628 ± 0.507 | 0.881 ± 0.014 | |
| 9.03 ± 0.21 | 7.943 ± 0.228 | 3.367 ± 0.095 | 0.637 ± 0.081 | 11.947 ± 0.235 | 66.483 ± 0.657 | 28.178 ± 0.311 | 5.339 ± 0.779 | 0.841 ± 0.021 | |
| l | 3.13 ± 0.12 | 5.580 ± 0.190 | 1.463 ± 0.015 | 0.083 ± 0.032 | 7.127 ± 0.214 | 78.292 ± 0.459 | 20.547 ± 0.713 | 1.162 ± 0.411 | 0.946 ± 0.020 |
| 6.17 ± 0.06 | 6.397 ± 0.567 | 2.430 ± 0.229 | 0.230 ± 0.061 | 9.057 ± 0.723 | 70.606 ± 1.650 | 26.866 ± 2.239 | 2.528 ± 0.591 | 0.913 ± 0.024 | |
| 8.93 ± 0.06 | 6.377 ± 0.220 | 3.230 ± 0.026 | 0.557 ± 0.049 | 10.163 ± 0.248 | 62.732 ± 0.638 | 31.792 ± 0.738 | 5.476 ± 0.448 | 0.853 ± 0.012 | |
| n | 3.07 ± 0.12 | 1.307 ± 0.015 | 1.600 ± 0.082 | 0.117 ± 0.015 | 3.023 ± 0.076 | 43.237 ± 1.153 | 52.898 ± 1.384 | 3.865 ± 0.553 | 0.932 ± 0.010 |
| 6.00 ± 0.10 | 2.490 ± 0.061 | 3.193 ± 0.070 | 0.283 ± 0.023 | 5.967 ± 0.078 | 41.730 ± 0.695 | 53.518 ± 0.785 | 4.752 ± 0.450 | 0.918 ± 0.008 | |
| 8.90 ± 0.26 | 4.030 ± 0.139 | 4.887 ± 0.021 | 0.550 ± 0.066 | 9.467 ± 0.08 | 42.566 ± 1.188 | 51.623 ± 0.608 | 5.811 ± 0.713 | 0.899 ± 0.011 | |
| o | 3.07 ± 0.06 | 1.360 ± 0.165 | 1.437 ± 0.023 | 0.127 ± 0.006 | 2.923 ± 0.179 | 46.414 ± 2.778 | 49.247 ± 2.584 | 4.339 ± 0.228 | 0.919 ± 0.002 |
| 6.00 ± 0.10 | 2.707 ± 0.071 | 2.930 ± 0.082 | 0.360 ± 0.017 | 5.997 ± 0.135 | 45.134 ± 0.231 | 48.857 ± 0.386 | 6.009 ± 0.407 | 0.89 ± 0.007 | |
| 9.20 ± 0.17 | 4.037 ± 0.211 | 4.367 ± 0.175 | 0.680 ± 0.050 | 9.083 ± 0.355 | 44.427 ± 0.724 | 48.072 ± 0.069 | 7.501 ± 0.727 | 0.865 ± 0.011 | |
| p | 3.00 ± 0.00 | 1.140 ± 0.036 | 1.327 ± 0.006 | 0.127 ± 0.012 | 2.593 ± 0.042 | 43.952 ± 0.682 | 51.168 ± 1.031 | 4.881 ± 0.366 | 0.913 ± 0.008 |
| 6.00 ± 0.10 | 2.567 ± 0.074 | 2.817 ± 0.047 | 0.357 ± 0.059 | 5.740 ± 0.142 | 44.714 ± 0.531 | 49.078 ± 0.577 | 6.207 ± 0.961 | 0.888 ± 0.016 | |
| 8.97 ± 0.21 | 3.817 ± 0.084 | 4.067 ± 0.093 | 0.587 ± 0.060 | 8.470 ± 0.061 | 45.067 ± 1.306 | 48.009 ± 0.759 | 6.924 ± 0.667 | 0.874 ± 0.009 | |
| q | 3.03 ± 0.12 | 1.293 ± 0.029 | 1.477 ± 0.015 | 0.090 ± 0.040 | 2.860 ± 0.026 | 45.222 ± 0.963 | 51.633 ± 0.447 | 3.145 ± 1.401 | 0.943 ± 0.025 |
| 5.90 ± 0.10 | 2.500 ± 0.020 | 3.037 ± 0.081 | 0.317 ± 0.064 | 5.853 ± 0.156 | 42.725 ± 0.798 | 51.880 ± 0.377 | 5.395 ± 0.940 | 0.906 ± 0.015 | |
| 8.97 ± 0.21 | 3.893 ± 0.064 | 4.683 ± 0.211 | 0.617 ± 0.038 | 9.193 ± 0.293 | 42.365 ± 0.825 | 50.928 ± 0.681 | 6.706 ± 0.299 | 0.884 ± 0.004 |
| Material | Sample | SLC (w/w) | Modifiers (w/w) | Pore-Forming Agent (w/w) | Viscosity (mPa·s) | Surface Tension (mN/m) | Solids Content (%) |
|---|---|---|---|---|---|---|---|
| SLC | a | 100 | / | / | 21.4 ± 0.173 | 40.7 ± 0.1 | 0.2378 ± 0.0001 |
| P-SLC-NH4HCO3 | b | 100 | / | NH4HCO3-7 | 14.7 ± 0.252 | 40.1 ± 0.2 | 0.1691 ± 0.0042 |
| P-SLC-NaHCO3 | c | 100 | / | NaHCO3-7 | 11.7 ± 0.000 | 40.5 ± 0.1 | 0.1710 ± 0.0002 |
| CP-SLC-PVP | d | 90 | PVP-10 | / | 11.5 ± 0.458 | 38.1 ± 0.2 | 0.1488 ± 0.0013 |
| CP-SLC-HPMC | e | 90 | HPMC-10 | / | 15.8 ± 0.173 | 37.6 ± 0.1 | 0.1096 ± 0.0003 |
| CP-SLC-HPC EF | f | 90 | HPC EF-10 | / | 16.2 ± 0.300 | 38.7 ± 0.1 | 0.0876 ± 0.0007 |
| CP-SLC-MD | g | 90 | MD-10 | / | 13.5 ± 0.000 | 37.4 ± 0.1 | 0.1803 ± 0.0003 |
| PCP-SLC-PVP-NH4HCO3 | h | 90 | PVP-10 | NH4HCO3-7 | 18.8 ± 0.173 | 40.1 ± 0.1 | 0.0833 ± 0.0011 |
| PCP-SLC-PVP-NaHCO3 | i | 90 | PVP-10 | NaHCO3-7 | 21.4 ± 0.173 | 39.4 ± 0.1 | 0.0823 ± 0.0005 |
| PCP-SLC-HPMC-NH4HCO3 | j | 90 | HPMC-10 | NH4HCO3-7 | 17.5 ± 0.173 | 40.0 ± 0.1 | 0.0717 ± 0.0008 |
| PCP-SLC-HPMC-NaHCO3 | k | 90 | HPMC-10 | NaHCO3-7 | 21.2 ± 0.173 | 37.6 ± 0.2 | 0.0727 ± 0.0003 |
| PCP-SLC-HPC EF-NH4HCO3 | l | 90 | HPC EF-10 | NH4HCO3-7 | 22.6 ± 0.173 | 35.7 ± 0.1 | 0.0667 ± 0.0002 |
| PCP-SLC-HPC EF-NaHCO3 | m | 90 | HPC EF-10 | NaHCO3-7 | 15.7 ± 0.173 | 39.3 ± 0.2 | 0.0756 ± 0.0003 |
| PM-SLC-PVP | n | 90 | PVP-10 | / | / | / | / |
| PM-SLC-HPMC | o | 90 | HPMC-10 | / | / | / | / |
| PM-SLC-HPC EF | p | 90 | HPC EF-10 | / | / | / | / |
| PM-SLC-MD | q | 90 | MD-10 | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Sun, C.; Sun, P.; Yang, L.; Yang, Q.; Zhu, W.; Guan, Y.; Liu, W.; Ming, L. Preparation and Application of Shen Ling Cao Composite Particles with Different Structures Based on Co-Spray Drying. Pharmaceuticals 2025, 18, 1369. https://doi.org/10.3390/ph18091369
Li Z, Sun C, Sun P, Yang L, Yang Q, Zhu W, Guan Y, Liu W, Ming L. Preparation and Application of Shen Ling Cao Composite Particles with Different Structures Based on Co-Spray Drying. Pharmaceuticals. 2025; 18(9):1369. https://doi.org/10.3390/ph18091369
Chicago/Turabian StyleLi, Zhe, Caiyun Sun, Ping Sun, Lingyu Yang, Qi Yang, Weifeng Zhu, Yongmei Guan, Wenjun Liu, and Liangshan Ming. 2025. "Preparation and Application of Shen Ling Cao Composite Particles with Different Structures Based on Co-Spray Drying" Pharmaceuticals 18, no. 9: 1369. https://doi.org/10.3390/ph18091369
APA StyleLi, Z., Sun, C., Sun, P., Yang, L., Yang, Q., Zhu, W., Guan, Y., Liu, W., & Ming, L. (2025). Preparation and Application of Shen Ling Cao Composite Particles with Different Structures Based on Co-Spray Drying. Pharmaceuticals, 18(9), 1369. https://doi.org/10.3390/ph18091369








