A Systematic Review of Nutraceuticals from the Perspective of Life-Cycle Assessment
Abstract
1. Introduction
2. Results
2.1. Life-Cycle Assessment (LCA) Background
2.1.1. Fundamentals of LCA
2.1.2. Advanced Life-Cycle Approaches
2.2. Review Process Results
2.2.1. Bibliometric Analysis
2.2.2. Nutraceutical in Focus
| Nutraceutical Group | Sources | Source Type | Extraction Methods | References |
|---|---|---|---|---|
| Alkaloid | Crambe crambe, Rocoto chili | Marine, plant | Ethanol extraction, encapsulation | [65,66] |
| Polyphenols | Rosemary, saffron waste, mango waste, citrus waste, onion, wine lees, pomegranate peel, pine needles, moringa, oregano, Ginkgo, tomato leaf, etc. | Plant, waste, marine | Solvent extraction (ethanol, methanol), ultrasound, microwave, Soxhlet, supercritical CO2, green solvents, enzymatic, fermentation | [32,40,41,42,43,46,47,48,49,51,54,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87] |
| Carotenoids | Haematococcus pluvialis, Dunaliella salina, tomato peel, Phaffia rhodozyma, algae, carrots | Plant, waste, marine | Ultrasound, solvent-assisted, supercritical CO2, bio-based solvents, green extraction | [33,34,35,38,52,53,74,82,88,89,90,91,92,93,94,95] |
| Functional carbohydrates | Orange peels, grapefruit peels, mango waste, chicory, yacón, chickpea, onion | Waste, plant | Thermosonication, conventional heating, ultrasonication, autoclave, micronization, enzymatic synthesis | [39,44,46,68,71,96,97,98,99] |
| Protein | Fish skins, Atlantic mackerel, microalgae (Nannochloropsis, Dunaliella, etc.) | Marine | Enzymatic, NADES, conventional alkali–acid process | [36,37,93,94] |
| Omega-3 fatty acids | Fish by-products, Phaeodactylum tricornutum, Schizochytrium, microalgae | Marine | Supercritical fluid fractionation, solvent extraction | [65,74,93,94,100,101,102] |
| Terpenoids | Betulin from birch bark | Plant | Liquid CO2 with ethanol | [40] |
| Minerals and other | Salmon bones, fermentation by-products | Waste, marine | Fermentation, enzymatic hydrolysis | [103,104] |
2.2.3. Application of LCA in Nutraceutical Production
2.2.4. Key Metrics in Nutraceutical Production
2.3. Sustainability and Beyond
2.3.1. Green Metrics and Indicators for Sustainability
2.3.2. Challenges
2.3.3. Emerging Opportunities
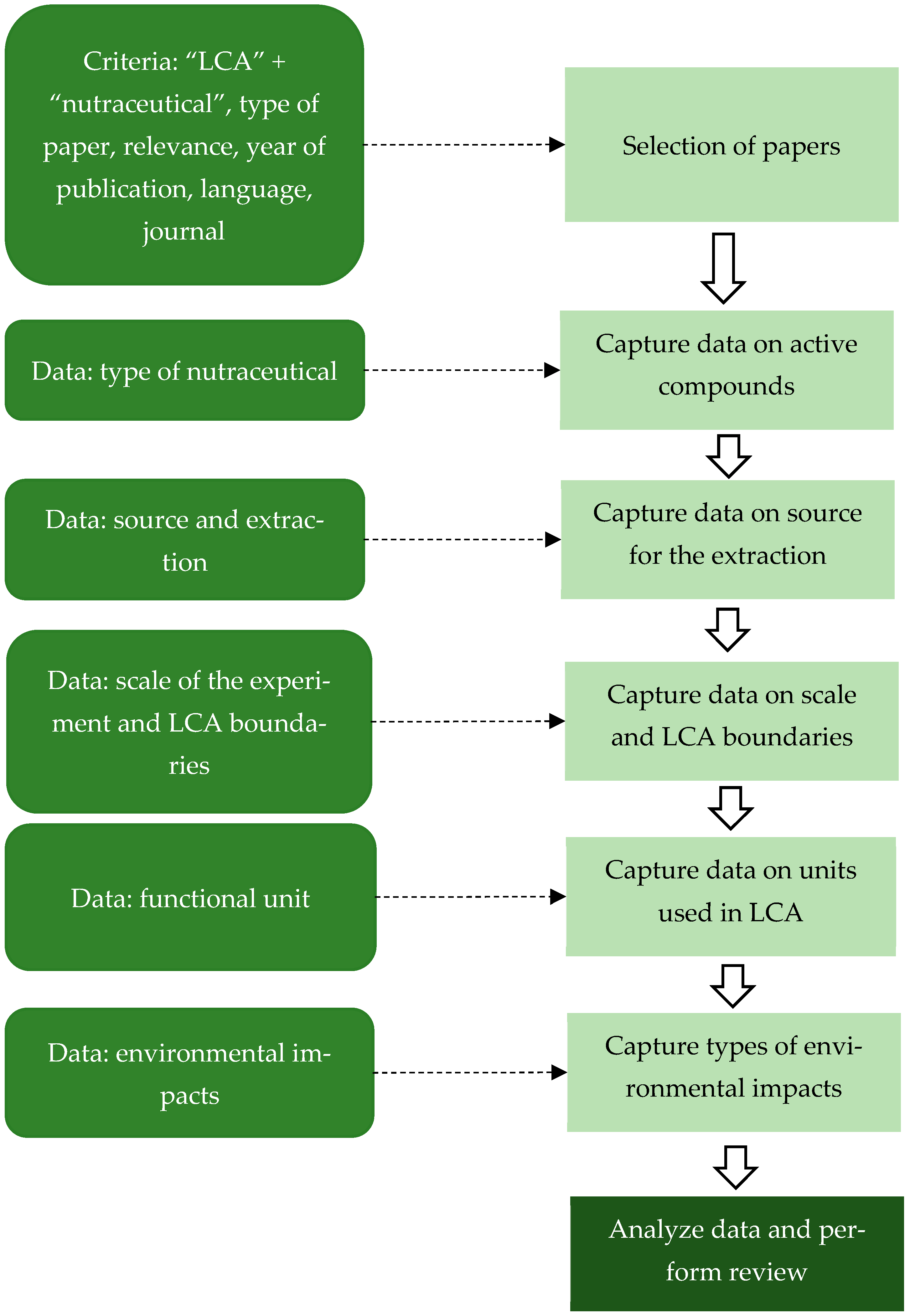
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LCA | Life-cycle assessment |
| LCSA | Life-Cycle Sustainability Assessment |
| LCC | Life-Cycle Costing |
| s-LCA | Social Life-Cycle Assessment |
| LCIA | Life-cycle impact assessment |
| TRL | Technology-readiness level |
References
- Wildman, R.E.C.; Wildman, R.; Wallace, T.C. (Eds.) Handbook of Nutraceuticals and Functional Foods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-0-429-19556-3. [Google Scholar]
- Cencic, A.; Chingwaru, W. The Role of Functional Foods, Nutraceuticals, and Food Supplements in Intestinal Health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- IMARC. Nutraceuticals Market Size, Share & Growth Report. 2033. Available online: https://www.imarcgroup.com/global-nutraceuticals-market (accessed on 30 May 2025).
- Olson, J.C.; Jacoby, J. Cue Utilization in the Quality Perception Process. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 1972. [Google Scholar]
- Rajic, S.; Simunovic, S.; Djordjevic, V.; Raseta, M.; Tomasevic, I.; Djekic, I. Quality Multiverse of Beef and Pork Meat in a Single Score. Foods 2022, 11, 1154. [Google Scholar] [CrossRef]
- Apaolaza, V.; Hartmann, P.; López, C.; Barrutia, J.M.; Echebarria, C. Natural Ingredients Claim’s Halo Effect on Hedonic Sensory Experiences of Perfumes. Food Qual. Prefer. 2014, 36, 81–86. [Google Scholar] [CrossRef]
- Lee, W.J.; Shimizu, M.; Kniffin, K.M.; Wansink, B. You Taste What You See: Do Organic Labels Bias Taste Perceptions? Food Qual. Prefer. 2013, 29, 33–39. [Google Scholar] [CrossRef]
- Djekic, I.; Rajic, S.; Dordevic, V.; Lazic, I.; Jovanovic, J.; Simunovic, S.; Terjung, N.; Volker, H.; Tomasevic, I. Halo Effect of Persuasive Messages towards Pork. Fleischwirtschaft 2024, 104, 58–65. [Google Scholar]
- Burlingame, B.; Dernini, S. Sustainable Diets and Biodiversity: Directions and Solutions for Policy, Research and Action. In Proceedings of the International Scientific Symposium Biodiversity and Sustainable Diets United Against Hunger, Rome, Italy, 3–5 November 2010; FAO: Rome, Italy, 2012. ISBN 978-92-5-107288-2. [Google Scholar]
- Chen, Y.-S.; Chang, C.-H. Greenwash and Green Trust: The Mediation Effects of Green Consumer Confusion and Green Perceived Risk. J. Bus. Ethics 2013, 114, 489–500. [Google Scholar] [CrossRef]
- European Commission. Proposal for a Directive of the European Parliament and of the Council Amending Directives 2006/43/EC, 2013/34/EU, (EU) 2022/2464 and (EU) 2024/1760 as Regards Certain Corporate Sustainability Reporting and Due Diligence Requirements; European Union: Brussels, Belgium, 2025. [Google Scholar]
- European Commission. Directive 2022/2464 of the European Parliament and of the Council of 14 December 2022 Amending Regulation (EU) No 537/2014, Directive 2004/109/EC, Directive 2006/43/EC and Directive 2013/34/EU, as Regards Corporate Sustainability Reporting; European Union: Brussels, Belgium, 2022; Volume L322, pp. 15–80. [Google Scholar]
- European Commission. Regulation 2019/2088 of the European Parliament and of the Council of 27 November 2019 on Sustainability-related Disclosures in the Financial Services Sector; European Union: Brussels, Belgium, 2019; pp. 1–16. [Google Scholar]
- European Commission. Regulation 2020/852 of the European Parliament and of the Council of 18 June 2020 on the Establishment of a Framework to Facilitate Sustainable Investment, and Amending Regulation (EU) 2019/2088; European Union: Brussels, Belgium, 2020; Volume L198, pp. 13–43. [Google Scholar]
- European Commission. Directive 2024/1760 of the European Parliament and of the Council of 13 June 2024 on Corporate Sustainability Due Diligence and Amending Directive (EU) 2019/1937 and Regulation (EU) 2023/2859; European Union: Brussels, Belgium, 2024; Volume L, pp. 1–58. [Google Scholar]
- Sala, S.; Amadei, A.M.; Beylot, A.; Ardente, F. The Evolution of Life Cycle Assessment in European Policies over Three Decades. Int. J. Life Cycle Assess. 2021, 26, 2295–2314. [Google Scholar] [CrossRef]
- UN Sustainable Development Goals: 17 Goals to Transform Our World. Available online: https://www.un.org/en/exhibits/page/sdgs-17-goals-transform-world (accessed on 30 May 2025).
- European Commission. Regulation No 880/92 of 23 March 1992 on a Community Eco-Label Award Scheme; European Union: Brussels, Belgium, 1992; pp. 1–7. [Google Scholar]
- EU The European Green Deal—European Commission. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 30 May 2025).
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO International Organization for Standardization: Geneva, Switzerland, 2006.
- Djekic, I.; Sanjuán, N.; Clemente, G.; Jambrak, A.R.; Djukić-Vuković, A.; Brodnjak, U.V.; Pop, E.; Thomopoulos, R.; Tonda, A. Review on Environmental Models in the Food Chain—Current Status and Future Perspectives. J. Clean. Prod. 2018, 176, 1012–1025. [Google Scholar] [CrossRef]
- Djekic, I.; Tomasevic, I. Environmental Indicators in the Meat Chain. In Quantification of Sustainability Indicators in the Food Sector; Muthu, S.S., Ed.; Springer: Singapore, 2019; pp. 55–82. ISBN 978-981-13-2408-6. [Google Scholar]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The Ecoinvent Database Version 3 (Part I): Overview and Methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Djekic, I.; Pojić, M.; Tonda, A.; Putnik, P.; Bursać Kovačević, D.; Režek-Jambrak, A.; Tomasevic, I. Scientific Challenges in Performing Life-Cycle Assessment in the Food Supply Chain. Foods 2019, 8, 301. [Google Scholar] [CrossRef]
- Jolliet, O.; Müller-Wenk, R.; Bare, J.; Brent, A.; Goedkoop, M.; Heijungs, R.; Itsubo, N.; Peña, C.; Pennington, D.; Potting, J.; et al. The LCIA Midpoint-Damage Framework of the UNEP/SETAC Life Cycle Initiative. Int. J. LCA 2004, 9, 394–404. [Google Scholar] [CrossRef]
- Alejandrino, C.; Mercante, I.; Bovea, M.D. Life Cycle Sustainability Assessment: Lessons Learned from Case Studies. Environ. Impact Assess. Rev. 2021, 87, 106517. [Google Scholar] [CrossRef]
- Finkbeiner, M.; Schau, E.M.; Lehmann, A.; Traverso, M. Towards Life Cycle Sustainability Assessment. Sustainability 2010, 2, 3309–3322. [Google Scholar] [CrossRef]
- Jasinski, D.; Djekic, I.; Dobrovic, L. Sustainability Assessment of Employing Chemical Recycling Technologies on Multilayer Packaging Waste. Sustainability 2025, 17, 556. [Google Scholar] [CrossRef]
- UNEP. Guidelines for Social Life Cycle Assessment of Products and Organizations; UNEP: Nairobi, Kenya, 2020. [Google Scholar]
- Rivela, B.; Kuczenski, B.; Sucozhañay, D. Chapter 6—Life Cycle Sustainability Assessment-Based Tools. In Assessing Progress Towards Sustainability; Teodosiu, C., Fiore, S., Hospido, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 93–118. ISBN 978-0-323-85851-9. [Google Scholar]
- Swarr, T.E.; Hunkeler, D.; Klöpffer, W.; Pesonen, H.-L.; Ciroth, A.; Brent, A.C.; Pagan, R. Environmental Life-Cycle Costing: A Code of Practice. Int. J. Life Cycle Assess. 2011, 16, 389–391. [Google Scholar] [CrossRef]
- Arias, A.; Torres, E.; Feijoo, G.; Moreira, M.T. Delving the Potential of Quercetin-Grafted Chitosan from a Technological and Environmental Perspective. Environ. Impact Assess. Rev. 2025, 112, 107754. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M.T. Life Cycle Assessment of the Production of the Red Antioxidant Carotenoid Astaxanthin by Microalgae: From Lab to Pilot Scale. J. Clean. Prod. 2014, 64, 332–344. [Google Scholar] [CrossRef]
- Ye, C.; Mu, D.; Horowitz, N.; Xue, Z.; Chen, J.; Xue, M.; Zhou, Y.; Klutts, M.; Zhou, W. Life Cycle Assessment of Industrial Scale Production of Spirulina Tablets. Algal Res. 2018, 34, 154–163. [Google Scholar] [CrossRef]
- Onorato, C.; Rösch, C. Comparative Life Cycle Assessment of Astaxanthin Production with Haematococcus pluvialis in Different Photobioreactor Technologies. Algal Res. 2020, 50, 102005. [Google Scholar] [CrossRef]
- Bashiri, B.; Cropotova, J.; Kvangarsnes, K.; Gavrilova, O.; Vilu, R. Environmental and Economic Life Cycle Assessment of Enzymatic Hydrolysis-Based Fish Protein and Oil Extraction. Resources 2024, 13, 61. [Google Scholar] [CrossRef]
- Batista, M.P.; Ortigueira, J.; Fernández, N.; Gaspar, F.B.; do Rosário Bronze, M.; Duarte, A.R.C.; Lopes, T.F. Sustainability Assessment of Collagen Extraction from Fish Skins: A Comparative Life Cycle Assessment of Conventional and NADES-Enhanced Processes. J. Environ. Chem. Eng. 2025, 13, 115911. [Google Scholar] [CrossRef]
- Yadav, R.D.; Khare, T.; Dhamole, P.B. Process Development and Techno-Economic Assessment of Lycopene Extraction from Tomatoes Using Surfactant. Biomass Conv. Bioref. 2024, 14, 19947–19960. [Google Scholar] [CrossRef]
- Gerbino, E.; Quentier, C.; Pénicaud, C. Dataset on the Life Cycle Assessment of Fructo- and Galacto-Oligosaccharides (FOS and GOS) Produced by Synthesis or Hydrolysis. Data Brief 2022, 43, 108478. [Google Scholar] [CrossRef]
- Ekman, A.; Campos, M.; Lindahl, S.; Co, M.; Börjesson, P.; Karlsson, E.N.; Turner, C. Bioresource Utilisation by Sustainable Technologies in New Value-Added Biorefinery Concepts—Two Case Studies from Food and Forest Industry. J. Clean. Prod. 2013, 57, 46–58. [Google Scholar] [CrossRef]
- Carlqvist, K.; Wallberg, O.; Lidén, G.; Börjesson, P. Life Cycle Assessment for Identification of Critical Aspects in Emerging Technologies for the Extraction of Phenolic Compounds from Spruce Bark. J. Clean. Prod. 2022, 333, 130093. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Castro-Puyana, M.; Börjesson, P.; Mendiola, J.A.; Turner, C.; Ibáñez, E. Life Cycle Assessment of Green Pilot-Scale Extraction Processes to Obtain Potent Antioxidants from Rosemary Leaves. J. Supercrit. Fluids 2012, 72, 205–212. [Google Scholar] [CrossRef]
- Nutrizio, M.; Jambrak, A.; Rezic, T.; Djekic, I. Extraction of Phenolic Compounds from Oregano Using High Voltage Electrical Discharges-Sustainable Perspective. Int. J. Food Sci. Technol. 2022, 57, 1104–1113. [Google Scholar] [CrossRef]
- Di Clemente, N.A.; La Cava, E.; Sgroppo, S.; Gomez-Zavaglia, A.; Gerbino, E. Sustainability Assessment of Pectin Extraction from Citrus paradisi Peel to Support the Encapsulation of Lactic Acid Bacteria. Appl. Food Res. 2025, 5, 100716. [Google Scholar] [CrossRef]
- Nadar, C.G.; Arora, A.; Shastri, Y. Sustainability Challenges and Opportunities in Pectin Extraction from Fruit Waste. ACS Eng. Au 2022, 2, 61–74. [Google Scholar] [CrossRef]
- Shinde, P.N.; Mandavgane, S.A.; Karadbhajane, V. Process Development and Life Cycle Assessment of Pomegranate Biorefinery. Environ. Sci. Pollut. Res. 2020, 27, 25785–25793. [Google Scholar] [CrossRef]
- Frascari, D.; Molina Bacca, A.E.; Wardenaar, T.; Oertlé, E.; Pinelli, D. Continuous Flow Adsorption of Phenolic Compounds from Olive Mill Wastewater with Resin XAD16N: Life Cycle Assessment, Cost–Benefit Analysis and Process Optimization. J. Chem. Technol. Biotechnol. 2019, 94, 1968–1981. [Google Scholar] [CrossRef]
- Agraso-Otero, A.; Rebolledo-Leiva, R.; Entrena-Barbero, E.; González-García, S. Integrated Process Design, Techno-Economic and Environmental Analysis of Chokeberry Pomace Biorefineries: Phenolic Compounds Extraction with Ethanol or Energy Production? Environ. Technol. Innov. 2025, 38, 104165. [Google Scholar] [CrossRef]
- Khalaf, D.; Pradal, D.; Dimitrov, K. Multi-Criteria Optimization Including Environmental Impacts of Ultrasound-Assisted Extraction of Phenolic Antioxidants from Blackcurrant Pomace by-Product. Chem. Eng. Process. Process Intensif. 2024, 204, 109935. [Google Scholar] [CrossRef]
- Pérez-López, P.; Ternon, E.; González-García, S.; Genta-Jouve, G.; Feijoo, G.; Thomas, O.P.; Moreira, M.T. Environmental Solutions for the Sustainable Production of Bioactive Natural Products from the Marine Sponge Crambe crambe. Sci. Total Environ. 2014, 475, 71–82. [Google Scholar] [CrossRef]
- Frusciante, L.; Geminiani, M.; Shabab, B.; Olmastroni, T.; Scavello, G.; Rossi, M.; Mastroeni, P.; Nyong’a, C.N.; Salvini, L.; Lamponi, S.; et al. Exploring the Antioxidant and Anti-Inflammatory Potential of Saffron (Crocus sativus) Tepals Extract within the Circular Bioeconomy. Antioxidants 2024, 13, 1082. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Dias, A.C.R.V.; Santos-Ebinuma, V.C.; Shaaban Sadek, M.; Ahmad, M.; de Andrade, C.R.; Haddad, F.F.; dos Santos, J.L.; Scarim, C.B.; Pereira, J.F.B.; et al. Is the Carotenoid Production from Phaffia rhodozyma Yeast Genuinely Sustainable? A Comprehensive Analysis of Biocompatibility, Environmental Assessment, and Techno-Economic Constraints. Bioresour. Technol. 2024, 397, 130456. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Kurnia, K.A.; Dias, A.C.R.V.; Raghavan, V.; Santos-Ebinuma, V.C.; Pessoa Jr, A. An Eco-Friendly Approach for the Recovery of Astaxanthin and β-Carotene from Phaffia rhodozyma Biomass Using Bio-Based Solvents. Bioresour. Technol. 2022, 345, 126555. [Google Scholar] [CrossRef]
- Vinci, G.; Maddaloni, L.; Prencipe, S.A.; Orlandini, E.; Sambucci, M. Simple and Reliable Eco-Extraction of Bioactive Compounds from Dark Chocolate by Deep Eutectic Solvents. A Sustainable Study. Int. J. Food Sci. Technol. 2023, 58, 4051–4065. [Google Scholar] [CrossRef]
- Jambrak, A.; Nutrizio, M.; Dukic, J.; Djekic, I.; Vincekovic, M.; Juric, S.; Pataro, G.; Tiwari, B.; Goksen, G.; Semencic, M.; et al. Digitalisation, Bioinformatics, and Delivery Systems in Sustainable Nonthermal Extraction of Proteins. Int. J. Food Sci. Technol. 2025, 60, vvae038. [Google Scholar] [CrossRef]
- Nutrizio, M.; Dukic, J.; Sabljak, I.; Samardzija, A.; Fuckar, V.; Djekic, I.; Jambrak, A. Upcycling of Food By-Products and Waste: Nonthermal Green Extractions and Life Cycle Assessment Approach. Sustainability 2024, 16, 9143. [Google Scholar] [CrossRef]
- Fuckar, V.; Nutrizio, M.; Grudenic, A.; Djekic, I.; Jambrak, A. Sustainable Ultrasound Assisted Extractions and Valorization of Coffee Silver Skin (CS). Sustainability 2023, 15, 8198. [Google Scholar] [CrossRef]
- What Is Sustainable Technology?: Perceptions, Paradoxes and Possibility. Available online: https://www.routledge.com/What-is-Sustainable-Technology-Perceptions-Paradoxes-and-Possibilities/Mulder-Ferrer-Lente/p/book/9781906093501?srsltid=AfmBOorXM2QtGSFIwC9xm875M0VTPLUkuMfvlzO1L5JfqBcKMJGl4vfk (accessed on 11 June 2025).
- Djekic, I.; Tomasević, I. Analysis and Comparison of Environmental Impacts of Nonthermal Food Technologies. In Nonthermal Processing in Agri-Food-Bio Sciences: Sustainability and Future Goals; Režek Jambrak, A., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 671–685. ISBN 978-3-030-92415-7. [Google Scholar]
- Salvador-Carulla, L.; Woods, C.; de Miquel, C.; Lukersmith, S. Adaptation of the Technology Readiness Levels for Impact Assessment in Implementation Sciences: The TRL-IS Checklist. Heliyon 2024, 10, e29930. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Lowe, G. Carotenoids—Antioxidant Properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef]
- Lv, Q.; Long, J.; Gong, Z.; Nong, K.; Liang, X.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1934578x211027745. [Google Scholar] [CrossRef]
- Rodrigues, M.; Rosa, A.; Almeida, A.; Martins, R.; Ânia Ribeiro, T.; Pintado, M.; Gonçalves, R.F.S.; Pinheiro, A.C.; Fonseca, A.J.M.; Maia, M.R.G.; et al. Omega-3 Fatty Acids from Fish by-Products: Innovative Extraction and Application in Food and Feed. Food Bioprod. Process. 2024, 145, 32–41. [Google Scholar] [CrossRef]
- Buljeta, I.; Šubarić, D.; Babić, J.; Pichler, A.; Šimunović, J.; Kopjar, M. Extraction of Dietary Fibers from Plant-Based Industry Waste: A Comprehensive Review. Appl. Sci. 2023, 13, 9309. [Google Scholar] [CrossRef]
- Porcelli, R.; Dotto, F.; Pezzolesi, L.; Marazza, D.; Greggio, N.; Righi, S. Comparative Life Cycle Assessment of Microalgae Cultivation for Non-Energy Purposes Using Different Carbon Dioxide Sources. Sci. Total Environ. 2020, 721, 137714. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Aristizabal, N.; Galvis-Nieto, J.D.; Narvaez-Perez, J.M.; Jurado-Erazo, D.K.; Rodriguez, L.J.; Orrego, C.E. Evaluation of a Sustainable Production of Encapsulated Chili Pepper Powder (Capsicum pubescens) through Convective and Vacuum Drying. Processes 2024, 12, 2154. [Google Scholar] [CrossRef]
- Cortés, A.; Moreira, M.T.; Feijoo, G. Integrated Evaluation of Wine Lees Valorization to Produce Value-Added Products. Waste Manag. 2019, 95, 70–77. [Google Scholar] [CrossRef]
- Manhongo, T.T.; Chimphango, A.; Thornley, P.; Röder, M. An Economic Viability and Environmental Impact Assessment of Mango Processing Waste-Based Biorefineries for Co-Producing Bioenergy and Bioactive Compounds. Renew. Sustain. Energy Rev. 2021, 148, 111216. [Google Scholar] [CrossRef]
- Joglekar, S.N.; Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Process of Fruit Peel Waste Biorefinery: A Case Study of Citrus Waste Biorefinery, Its Environmental Impacts and Recommendations. Environ. Sci. Pollut. Res. 2019, 26, 34713–34722. [Google Scholar] [CrossRef] [PubMed]
- Salzano De Luna, M.; Vetrone, G.; Viggiano, S.; Panzella, L.; Marotta, A.; Filippone, G.; Ambrogi, V. Pine Needles as a Biomass Resource for Phenolic Compounds: Trade-Off between Efficiency and Sustainability of the Extraction Methods by Life Cycle Assessment. ACS Sustain. Chem. Eng. 2023, 11, 4670–4677. [Google Scholar] [CrossRef]
- Santiago, B.; Arias Calvo, A.; Gullón, B.; Feijoo, G.; Moreira, M.T.; González-García, S. Production of Flavonol Quercetin and Fructooligosaccharides from Onion (Allium cepa L.) Waste: An Environmental Life Cycle Approach. Chem. Eng. J. 2020, 392, 123772. [Google Scholar] [CrossRef]
- Vauchel, P.; Colli, C.; Pradal, D.; Philippot, M.; Decossin, S.; Dhulster, P.; Dimitrov, K. Comparative LCA of Ultrasound-Assisted Extraction of Polyphenols from Chicory Grounds under Different Operational Conditions. J. Clean. Prod. 2018, 196, 1116–1123. [Google Scholar] [CrossRef]
- Pappas, V.M.; Samanidis, I.; Stavropoulos, G.; Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Analysis of Five-Extraction Technologies’ Environmental Impact on the Polyphenols Production from Moringa Oleifera Leaves Using the Life Cycle Assessment Tool Based on ISO 14040. Sustainability 2023, 15, 2328. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Ulloa, R.G.; Sineiro, J.; Feijoo, G.; Moreira, M.T. Life Cycle Assessment of the Production of Bioactive Compounds from Tetraselmis Suecica at Pilot Scale. J. Clean. Prod. 2014, 64, 323–331. [Google Scholar] [CrossRef]
- Barjoveanu, G.; Pătrăuțanu, O.-A.; Teodosiu, C.; Volf, I. Life Cycle Assessment of Polyphenols Extraction Processes from Waste Biomass. Sci. Rep. 2020, 10, 13632. [Google Scholar] [CrossRef]
- Arias, A.; Costa, C.E.; Feijoo, G.; Moreira, M.T.; Domingues, L. Process Modeling, Environmental and Economic Sustainability of the Valorization of Whey and Eucalyptus Residues for Resveratrol Biosynthesis. Waste Manag. 2023, 172, 226–234. [Google Scholar] [CrossRef]
- Li, X.; Yuan, Y.; Li, F.; Shimizu, N. Early-Stage Life Cycle Assessment and Optimization of Aqueous Crude Glycerol Extraction and Nanofiltration Concentration of Tomato Leaf Residue. ACS Sustain. Chem. Eng. 2024, 12, 2646–2655. [Google Scholar] [CrossRef]
- Da Silva, E.S.; Nunes, A.O.; Hoskin, R.T. Ultrasound-Assisted Polyphenol Extraction of Acerola and Jambolan Pomaces: Comparison of Extraction Protocols, Kinetic Modeling, and Life Cycle Assessment. Chem. Eng. Process. Process Intensif. 2023, 191, 109443. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Y.; Fan, Z.; Chen, Y.; Cui, Z. Life Cycle Assessment for Evaluation of Novel Solvents and Technologies: A Case Study of Flavonoids Extraction from Ginkgo Biloba Leaves. Sci. Total Environ. 2024, 922, 171319. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; Feijoo, G.; Moreira, M.T. Agri-Food Waste to Phenolic Compounds: Life Cycle and Eco-Efficiency Assessments. J. Environ. Manag. 2025, 380, 124935. [Google Scholar] [CrossRef] [PubMed]
- Hren, R.; Naumoska, K.; Jug, U.; Čuček, L.; Likozar, B.; Novak, U.; Vujanović, A. Life Cycle Assessment of Pilot-Scale Bio-Refining of Invasive Japanese Knotweed Alien Plant towards Bio-Based Bioactive Compounds. Processes 2023, 11, 1393. [Google Scholar] [CrossRef]
- Pérez-López, P.; Balboa, E.M.; González-García, S.; Domínguez, H.; Feijoo, G.; Moreira, M.T. Comparative Environmental Assessment of Valorization Strategies of the Invasive Macroalgae Sargassum Muticum. Bioresour. Technol. 2014, 161, 137–148. [Google Scholar] [CrossRef]
- Bouchez, A.; Vauchel, P.; D’Alessandro, L.G.; Dimitrov, K. Multi-Objective Optimization Tool for Ultrasound-Assisted Extraction Including Environmental Impacts. Chem. Eng. Res. Des. 2020, 164, 324–337. [Google Scholar] [CrossRef]
- Priyadarshini, A.; Tiwari, B.K.; Rajauria, G. Assessing the Environmental and Economic Sustainability of Functional Food Ingredient Production Process. Processes 2022, 10, 445. [Google Scholar] [CrossRef]
- Manríquez-Zúñiga, A.N.; De La Torre, A.R.; Valdés-Santiago, L.; Hernández-Bustos, D.A.; Cuéllar-Sojo, S.; Hernández-Rayas, A.; Perez-Vega, S.; Molina-Guerrero, C.E. Broccoli Leaves (Brassica oleracea var. italica) as a Source of Bioactive Compounds and Chemical Building Blocks: Optimal Extraction Using Dynamic Maceration and Life Cycle Assessment. Sustainability 2023, 15, 16616. [Google Scholar] [CrossRef]
- Bouchez, A.; Vauchel, P.; Périno, S.; Dimitrov, K. Multi-Criteria Optimization Including Environmental Impacts of a Microwave-Assisted Extraction of Polyphenols and Comparison with an Ultrasound-Assisted Extraction Process. Foods 2023, 12, 1750. [Google Scholar] [CrossRef]
- Jara-Quijada, E.; Pérez-Won, M.; Tabilo-Munizaga, G.; González-Cavieres, L.; Palma-Acevedo, A.; Herrera-Lavados, C.; Lemus-Mondaca, R. Polyphenol Extraction of Green Tea Through Pulsed Electric Field: Yield Improvement and Environmental Assessment. Food Bioprocess Technol. 2024, 17, 2718–2734. [Google Scholar] [CrossRef]
- Espada, J.J.; Pérez-Antolín, D.; Vicente, G.; Bautista, L.F.; Morales, V.; Rodríguez, R. Environmental and Techno-Economic Evaluation of β-Carotene Production from Dunaliella Salina. A Biorefinery Approach. Biofuels Bioprod. Biorefin. 2020, 14, 43–54. [Google Scholar] [CrossRef]
- Rabbani, M.; Hosseini, A.; Karim, M.A.; Fahimi, A.; Karimi, K.; Vahidi, E. Environmental Impact Assessment of a Novel Third-Generation Biorefinery Approach for Astaxanthin and Biofuel Production. Sci. Total Environ. 2024, 912, 168733. [Google Scholar] [CrossRef] [PubMed]
- Aldaghi, S.A.; Ubais, R.; Schmitt, I.; Wendisch, V.F.; Costamagna, M.; Perucca, M. Life Cycle Assessment of Bacterial, Algal, and Synthetic Approaches for Astaxanthin Production at a Laboratory Scale: Comparative Environmental Analysis and Sensitivity of Energy Sources. Processes 2023, 11, 2911. [Google Scholar] [CrossRef]
- Yadav, R.D.; Dhamole, P.B. Techno Economic and Life Cycle Assessment of Lycopene Production from Tomato Peels Using Different Extraction Methods. Biomass Conv. Bioref. 2024, 14, 25495–25511. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Papadaki, S.; Krokida, M. Life Cycle Analysis of β-Carotene Extraction Techniques. J. Food Eng. 2015, 167, 51–58. [Google Scholar] [CrossRef]
- Deprá, M.C.; Severo, I.A.; Dos Santos, A.M.; Zepka, L.Q.; Jacob-Lopes, E. Environmental Impacts on Commercial Microalgae-Based Products: Sustainability Metrics and Indicators. Algal Res. 2020, 51, 102056. [Google Scholar] [CrossRef]
- Dias, R.R.; Deprá, M.C.; Zepka, L.Q.; Jacob-Lopes, E. Roadmap to Net-Zero Carbon Emissions in Commercial Microalgae-Based Products: Environmental Sustainability and Carbon Offset Costs. J. Appl. Phycol. 2022, 34, 1255–1268. [Google Scholar] [CrossRef]
- De Souza Celente, G.; De Cassia De Souza Schneider, R.; Julich, J.; Rizzetti, T.M.; Lobo, E.A.; Sui, Y. Life Cycle Assessment of Microalgal Cultivation Medium: Biomass, Glycerol, and Beta-Carotene Production by Dunaliella salina and Dunaliella tertiolecta. Int. J. Life Cycle Assess. 2024, 29, 2269–2282. [Google Scholar] [CrossRef]
- Khanpit, V.V.; Tajane, S.P.; Mandavgane, S.A. Technoeconomic and Life Cycle Analysis of Soluble Dietary Fiber Concentrate Production from Waste Orange Peels. Waste Manag. 2023, 155, 29–39. [Google Scholar] [CrossRef]
- Khanpit, V.V.; Tajane, S.P.; Mandavgane, S.A. Production of Soluble Dietary Fiber Concentrate from Waste Orange Peels: Study of Nutritional and Physicochemical Properties and Life Cycle Assessment. Biomass Conv. Bioref. 2023, 13, 14615–14627. [Google Scholar] [CrossRef]
- Khanpit, V.V.; Tajane, S.P.; Mandavgane, S.A. Orange Waste Peel to High Value Soluble Dietary Fiber Concentrate: Comparison of Conversion Methods and Their Environmental Impact. Biomass Conv. Bioref. 2023, 13, 14413–14423. [Google Scholar] [CrossRef]
- Hingsamer, M.; Kulmer, V.; De Roode, M.; Kernitzkyi, M. Environmental and Socio-Economic Impacts of New Plant Breeding Technologies: A Case Study of Root Chicory for Inulin Production. Front. Genome Ed. 2022, 4, 919392. [Google Scholar] [CrossRef]
- Qin, Z.-H.; Hu, X.; Mou, J.-H.; He, G.-H.; Ye, G.-B.; Li, H.-Y.; Chopra, S.S.; Dong, L.; Lin, C.S.K.; Wang, X. Environmental Profiling Microalgae-Based Eicosapentaenoic Acid Production along the Technical Advancement via Life Cycle Assessment. J. Clean. Prod. 2023, 397, 136477. [Google Scholar] [CrossRef]
- Hublin, A.; Malbaša, H.; Stanec Svedrović, D.; Jerman Vranić, M. Using Life Cycle Assessment to Achieve a Circular Economy of Fish Waste. Waste Biomass Valorization 2024, 15, 4487–4499. [Google Scholar] [CrossRef]
- Togarcheti, S.C.; Padamati, R.B. Comparative Life Cycle Assessment of EPA and DHA Production from Microalgae and Farmed Fish. Clean Technol. 2021, 3, 699–710. [Google Scholar] [CrossRef]
- Boudreau, S.; Hrapovic, S.; Liu, Y.; Leung, A.C.W.; Lam, E.; Kerton, F.M. Isolation of Hydroxyapatite from Atlantic Salmon Processing Waste Using a Protease and Lipase Mixture. RSC Sustain. 2023, 1, 1554–1564. [Google Scholar] [CrossRef]
- Cooreman-Algoed, M.; Boone, L.; Uitterhaegen, E.; Taelman, S.E.; De Soete, W.; Dewulf, J. Environmental Life Cycle Assessment of Nutraceuticals: A Case Study on Methylcobalamin in Different Packaging Types. Sci. Total Environ. 2023, 893, 164780. [Google Scholar] [CrossRef]
- Ubando, A.T.; Anderson, S.; Ng, E.; Chen, W.-H.; Culaba, A.B.; Kwon, E.E. Life Cycle Assessment of Microalgal Biorefinery: A State-of-the-Art Review. Bioresour. Technol. 2022, 360, 127615. [Google Scholar] [CrossRef]
- openLCA. openLCA 2 Manual; openLCA: Berlin, Germany, 2025. [Google Scholar]
- SimaPro. SimaPro Database Manual; SimaPro: Amersfoort, The Netherlands, 2020. [Google Scholar]
- Martin-Gorriz, B.; Gallego-Elvira, B.; Martínez-Alvarez, V.; Maestre-Valero, J.F. Life Cycle Assessment of Fruit and Vegetable Production in the Region of Murcia (South-East Spain) and Evaluation of Impact Mitigation Practices. J. Clean. Prod. 2020, 265, 121656. [Google Scholar] [CrossRef]
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO International Organization for Standardization: Geneva, Switzerland, 2006.
- Wolff, D.; Duffy, A. Development and Demonstration of an Uncertainty Management Methodology for Life Cycle Assessment in a Tiered-Hybrid Case Study of an Irish Apartment Development. Int. J. Life Cycle Assess. 2021, 26, 989–1007. [Google Scholar] [CrossRef]
- Sonderegger, T. Implementation of Life Cycle Impact Assessment Methods in the Ecoinvent Database v3. 11; Ecoinvent Association: Zurich, Switzerland, 2024; p. 68. [Google Scholar]
- Bruijn, H.; Duin, R.; Huijbregts, M.A.J.; Guinee, J.B.; Gorree, M.; Heijungs, R.; Huppes, G.; Kleijn, R.; Koning, A.; Oers, L.; et al. Handbook on Life Cycle Assessment: Operational Guide to the ISO Standards; Eco-Efficiency in Industry and Science; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; ISBN 978-0-306-48055-3. [Google Scholar]
- EPLCA. EC European Platform on LCA. Available online: https://eplca.jrc.ec.europa.eu/LCDN/developerEF.html (accessed on 24 August 2025).
- International Reference Life Cycle Data System (ILCD). Handbook: General Guide for Life Cycle Assessment: Provisions and Action Steps, 1st ed.; European Commission: Luxembourg, 2011; ISBN 978-92-79-17451-3. [Google Scholar]
- EPA. Tool for the Reduction and Assessment of Chemical and Other Environmental Impacts (TRACI) TRACI Version 2.1; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2012; p. 24. [Google Scholar]
- Jolliet, O.; Margni, M.; Charles, R.; Humbert, S.; Payet, J.; Rebitzer, G.; Rosenbaum, R. IMPACT 2002+: A New Life Cycle Impact Assessment Methodology. Int. J. LCA 2003, 8, 324–330. [Google Scholar] [CrossRef]
- Rybaczewska-Błażejowska, M.; Jezierski, D. Comparison of ReCiPe 2016, ILCD 2011, CML-IA Baseline and IMPACT 2002+ LCIA Methods: A Case Study Based on the Electricity Consumption Mix in Europe. Int. J. Life Cycle Assess. 2024, 29, 1799–1817. [Google Scholar] [CrossRef]
- Djekic, I.; Bozickovic, I.; Djordjevic, V.; Smetana, S.; Terjung, N.; Ilic, J.; Doroski, A.; Tomasevic, I. Can We Associate Environmental Footprints with Production and Consumption Using Monte Carlo Simulation? Case Study with Pork Meat. J. Sci. Food Agric. 2021, 101, 960–969. [Google Scholar] [CrossRef]
- Čuček, L.; Klemeš, J.J.; Kravanja, Z. Overview of Environmental Footprints. In Assessing and Measuring Environmental Impact and Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 131–193. ISBN 978-0-12-799968-5. [Google Scholar]
- Hertwich, E.G.; Mateles, S.F.; Pease, W.S.; McKone, T.E. Human Toxicity Potentials for Life-Cycle Assessment and Toxics Release Inventory Risk Screening. Environ. Toxicol. Chem. 2001, 20, 928–939. [Google Scholar] [CrossRef] [PubMed]
- van der Giesen, C.; Cucurachi, S.; Guinée, J.; Kramer, G.J.; Tukker, A. A Critical View on the Current Application of LCA for New Technologies and Recommendations for Improved Practice. J. Clean. Prod. 2020, 259, 120904. [Google Scholar] [CrossRef]
- Eltohamy, H.; Cecere, G.; Rigamonti, L. Ex-Ante Life Cycle Assessment of FineFuture Flotation Technology: Case Study of Grecian Magnesite. Int. J. Life Cycle Assess. 2023, 28, 1348–1365. [Google Scholar] [CrossRef]
- Costa, D.; Quinteiro, P.; Dias, A.C. A Systematic Review of Life Cycle Sustainability Assessment: Current State, Methodological Challenges, and Implementation Issues. Sci. Total Environ. 2019, 686, 774–787. [Google Scholar] [CrossRef]
- Adrianto, L.R.; van der Hulst, M.K.; Tokaya, J.P.; Arvidsson, R.; Blanco, C.F.; Caldeira, C.; Guillén-Gonsálbez, G.; Sala, S.; Steubing, B.; Buyle, M.; et al. How Can LCA Include Prospective Elements to Assess Emerging Technologies and System Transitions? The 76th LCA Discussion Forum on Life Cycle Assessment, 19 November 2020. Int. J. Life Cycle Assess. 2021, 26, 1541–1544. [Google Scholar] [CrossRef]
- Zah, R.; Faist, M.; Reinhard, J.; Birchmeier, D. Standardized and Simplified Life-Cycle Assessment (LCA) as a Driver for More Sustainable Biofuels. J. Clean. Prod. 2009, 17, S102–S105. [Google Scholar] [CrossRef]
- Gradin, K.T.; Björklund, A. The Common Understanding of Simplification Approaches in Published LCA Studies—A Review and Mapping. Int. J. Life Cycle Assess. 2021, 26, 50–63. [Google Scholar] [CrossRef]
- Beemsterboer, S.; Baumann, H.; Wallbaum, H. Ways to Get Work Done: A Review and Systematisation of Simplification Practices in the LCA Literature. Int. J. Life Cycle Assess. 2020, 25, 2154–2168. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Filho, W.L.; Setti, A.F.F.; Azeiteiro, U.M.; Lokupitiya, E.; Donkor, F.K.; Etim, N.N.; Matandirotya, N.; Olooto, F.M.; Sharifi, A.; Nagy, G.J.; et al. An Overview of the Interactions between Food Production and Climate Change. Sci. Total Environ. 2022, 838, 156438. [Google Scholar] [CrossRef] [PubMed]
- Pantović, A.; Tomić, N.; Petrović, T.; Djekić, I. Sustainable Perspectives of Disposable Tableware: A Systematic Review. Sustainability 2025, 17, 1434. [Google Scholar] [CrossRef]
- Djekic, I.; Lorenzo, J.; Munekata, P.; Gagaoua, M.; Tomasevic, I. Review on Characteristics of Trained Sensory Panels in Food Science. J. Texture Stud. 2021, 52, 501–509. [Google Scholar] [CrossRef]


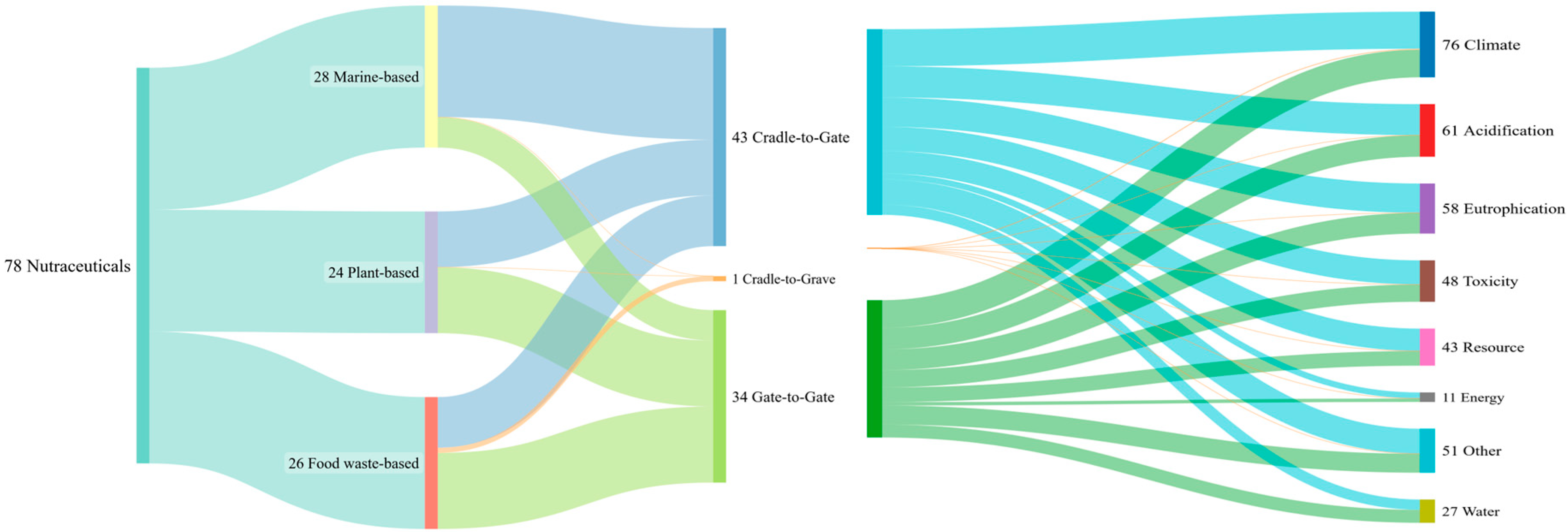
| Polyphenols | Carotenoids | Functional Carbohydrates | Omega-3 Fatty Acids | Protein | Minerals and Other | Alkaloid | Terpenoids | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 34 (43.6%) | 17 (21.8%) | 10 (12.8%) | 8 (10.3%) | 4 (5.1%) | 2 (2.6%) | 2 (2.6%) | 1 (1.3%) | 78 (100%) |
| Origin of materials used to extract bioactive compounds for nutraceuticals | |||||||||
| Marine-based | 3 | 11 | 0 | 8 | 4 | 1 | 1 | 0 | 28 (35.9%) |
| Plant-based | 18 | 2 | 2 | 0 | 0 | 0 | 1 | 1 | 24 (30.8%) |
| Food waste-based | 13 | 4 | 8 | 0 | 0 | 1 | 0 | 0 | 26 (33.3%) |
| Scale of applications | |||||||||
| Laboratory | 18 | 6 | 4 | 3 | 1 | 0 | 1 | 0 | 33 (42.3%) |
| Lab and pilot | 7 | 2 | 2 | 1 | 0 | 1 | 1 | 0 | 14 (17.9%) |
| Pilot | 2 | 6 | 1 | 2 | 1 | 0 | 0 | 1 | 13 (16.7%) |
| Industrial | 4 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 9 (11.5%) |
| Other | 3 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 9 (11.5%) |
| Boundaries used in LCA studies | |||||||||
| Cradle to gate | 16 | 11 | 4 | 7 | 3 | 0 | 2 | 0 | 43 (55.1%) |
| Gate to gate | 18 | 6 | 6 | 1 | 1 | 1 | 0 | 1 | 34 (43.6%) |
| Cradle to grave | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 (1.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djekic, I.; Smigic, N.; Vitali Čepo, D. A Systematic Review of Nutraceuticals from the Perspective of Life-Cycle Assessment. Pharmaceuticals 2025, 18, 1278. https://doi.org/10.3390/ph18091278
Djekic I, Smigic N, Vitali Čepo D. A Systematic Review of Nutraceuticals from the Perspective of Life-Cycle Assessment. Pharmaceuticals. 2025; 18(9):1278. https://doi.org/10.3390/ph18091278
Chicago/Turabian StyleDjekic, Ilija, Nada Smigic, and Dubravka Vitali Čepo. 2025. "A Systematic Review of Nutraceuticals from the Perspective of Life-Cycle Assessment" Pharmaceuticals 18, no. 9: 1278. https://doi.org/10.3390/ph18091278
APA StyleDjekic, I., Smigic, N., & Vitali Čepo, D. (2025). A Systematic Review of Nutraceuticals from the Perspective of Life-Cycle Assessment. Pharmaceuticals, 18(9), 1278. https://doi.org/10.3390/ph18091278









