ETAS®, a Standardized Extract of Asparagus officinalis Stem, Alleviates Sarcopenia via Regulating Protein Turnover and Mitochondrial Quality
Abstract
1. Introduction
2. Results
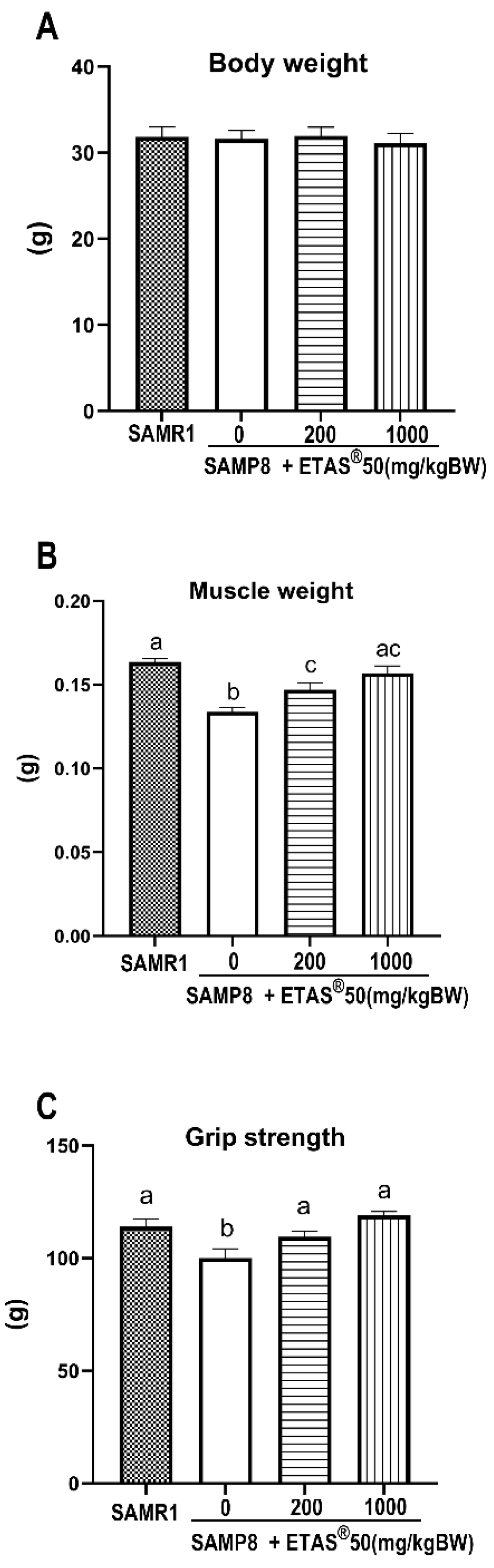
2.1. ETAS® Enhances Grip Strength and Muscle Mass in SAMP8 Mice
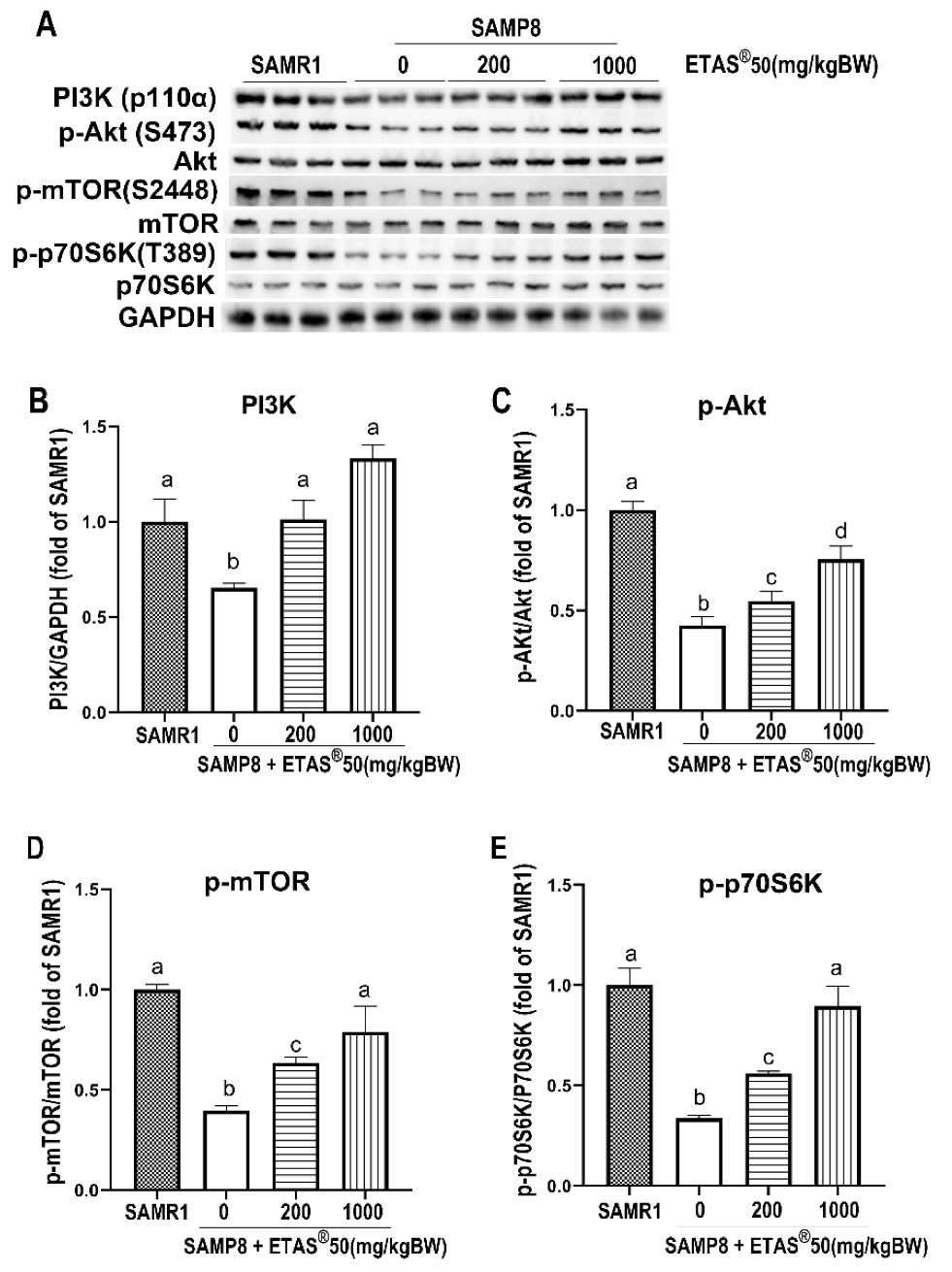
2.2. ETAS® Promotes Protein Synthesis via PI3K/Akt/mTOR/p70S6K in Skeletal Muscle of SAMP8 Mice
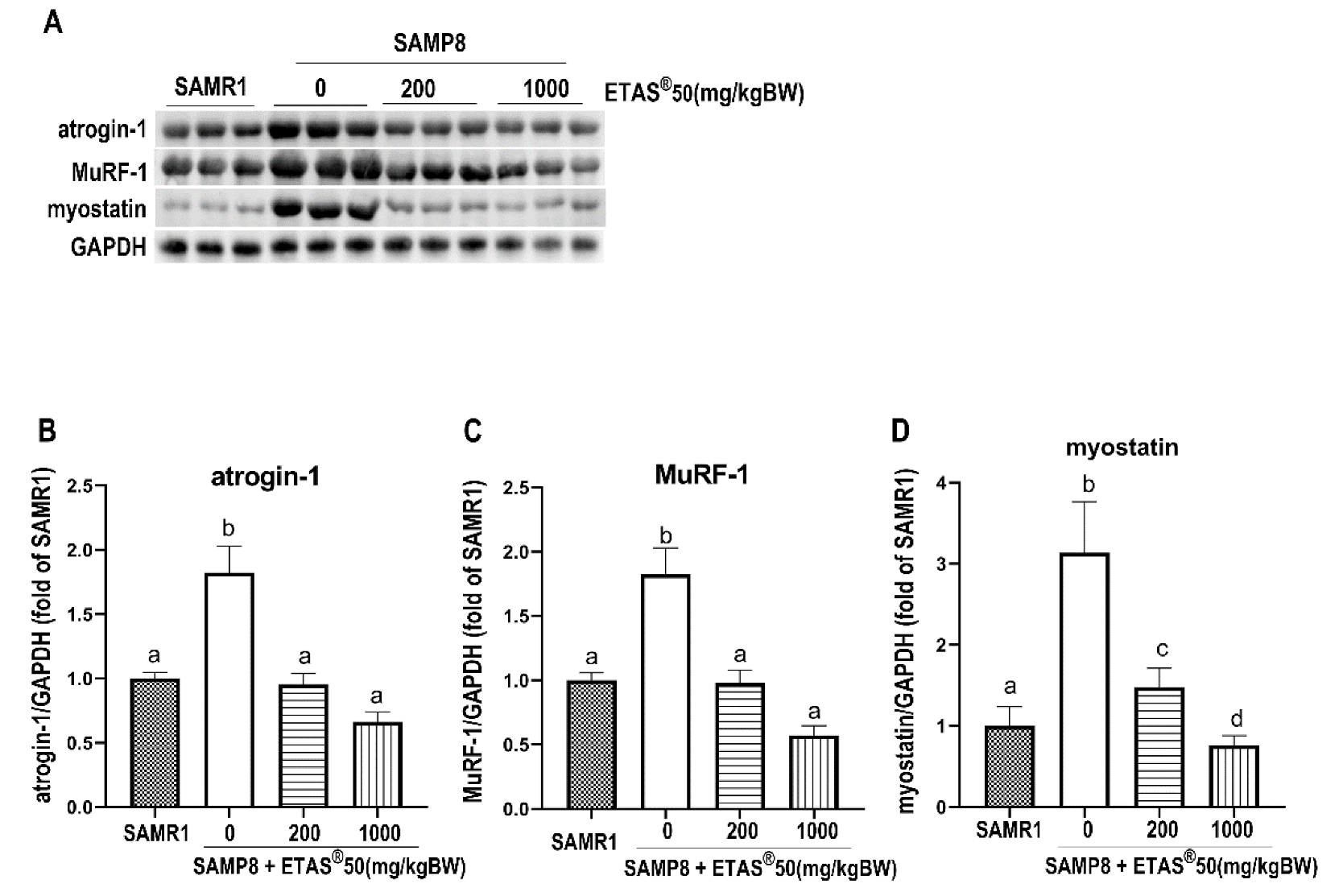
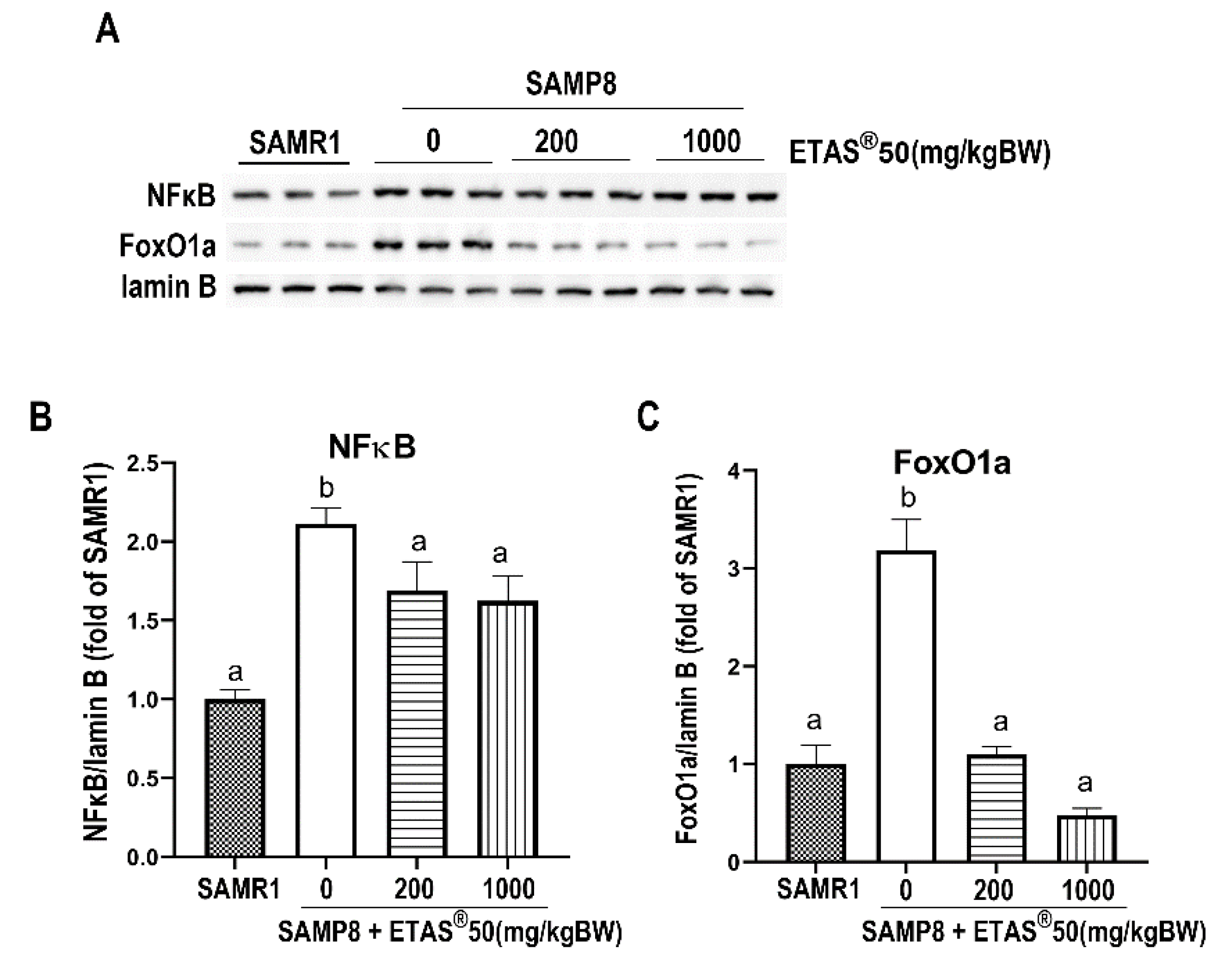
2.3. ETAS® Downregulates Protein Degradation via Ubiquitin-Proteosome System (UPS) and Myostatin in Skeletal Muscle of SAMP8 Mice
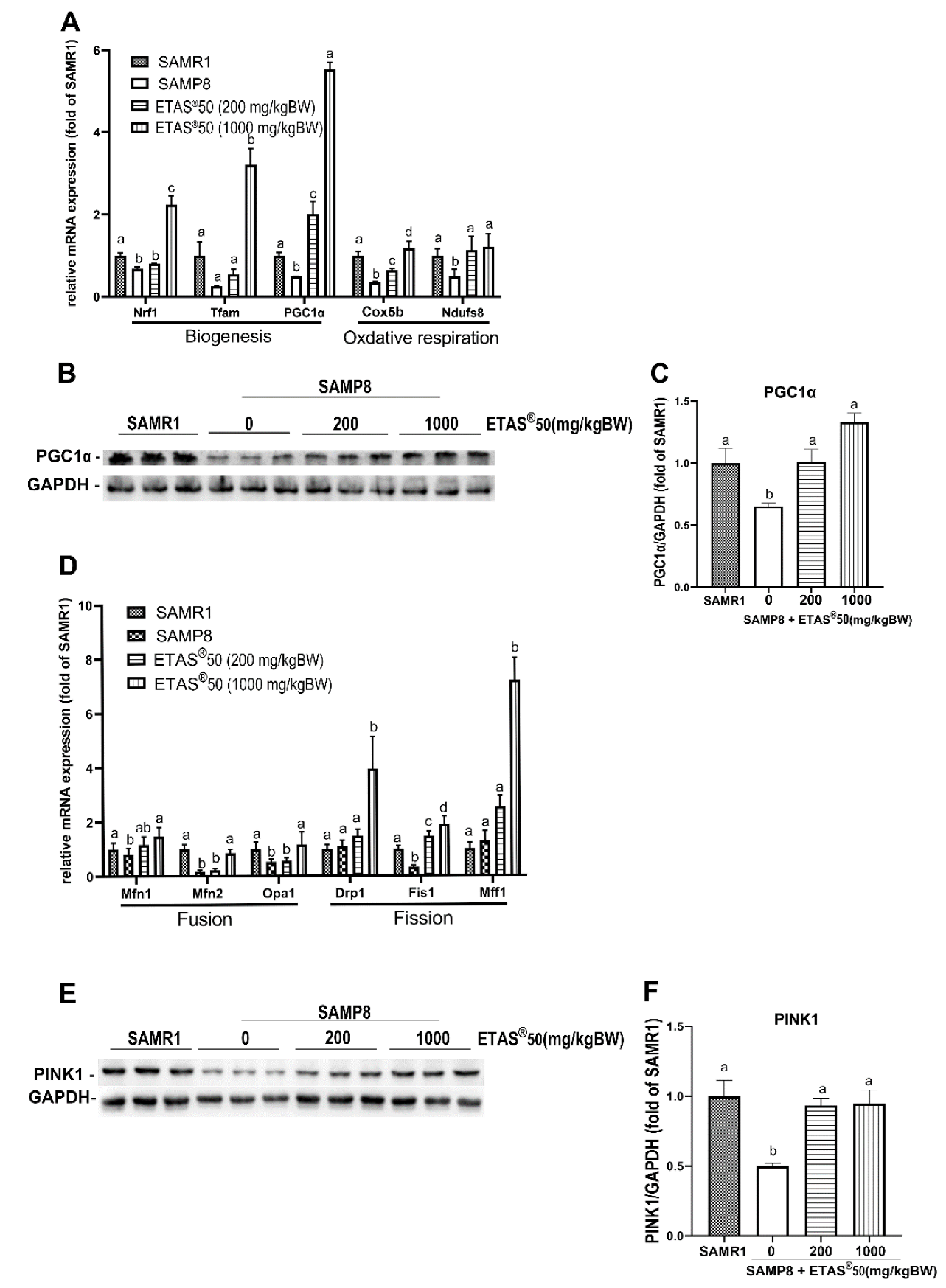
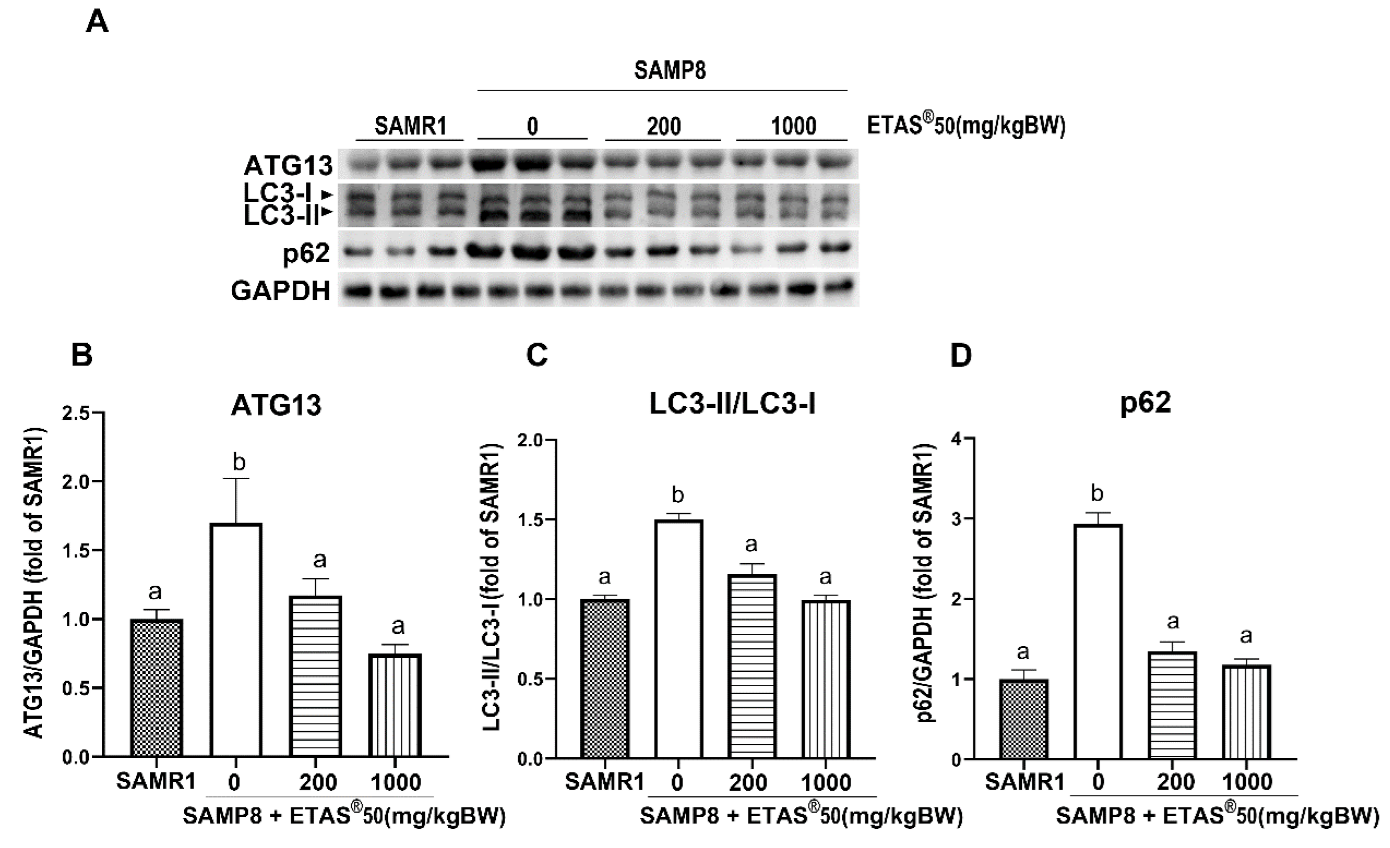
2.4. ETAS® Regulates Mitochondrial Quality Control and Autophagic Flux in Skeletal Muscle of SAMP8 Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Grip Strength Analysis
4.3. Tissue Homogenization, Nuclear Protein Extraction, and Western Blot Analysis
4.4. Real-Time Polymerase Chain Reaction (PCR)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Olas, B. A Review of the Pro-Health Activity of Asparagus officinalis L. and Its Components. Foods 2024, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.T.; Balboula, A.Z.; Homma, K.; Takanari, J.; Bai, H.; Kawahara, M.; Thi Kim Nguyen, K.; Takahashi, M. Synergistic effect of standardized extract of Asparagus officinalis stem and heat shock on progesterone synthesis with lipid droplets and mitochondrial function in bovine granulosa cells. J. Steroid Biochem. Mol. Biol. 2023, 225, 106181. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Takanari, J.; Kizaki, T. Standardized Extract of Asparagus officinalis Stem Attenuates SARS-CoV-2 Spike Protein-Induced IL-6 and IL-1beta Production by Suppressing p44/42 MAPK and Akt Phosphorylation in Murine Primary Macrophages. Molecules 2021, 26, 6189. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Wu, C.S.; Wu, T.C.; Lin, Y.H.; Chang, S.J. A Standardized Extract of Asparagus officinalis Stem (ETAS((R))) Ameliorates Cognitive Impairment, Inhibits Amyloid beta Deposition via BACE-1 and Normalizes Circadian Rhythm Signaling via MT1 and MT2. Nutrients 2019, 11, 1631. [Google Scholar] [CrossRef]
- Sakurai, T.; Ito, T.; Wakame, K.; Kitadate, K.; Arai, T.; Ogasawara, J.; Kizaki, T.; Sato, S.; Ishibashi, Y.; Fujiwara, T.; et al. Enzyme-treated Asparagus officinalis extract shows neuroprotective effects and attenuates cognitive impairment in senescence-accelerated mice. Nat. Prod. Commun. 2014, 9, 101–106. [Google Scholar] [CrossRef]
- Ito, T.; Goto, K.; Takanari, J.; Miura, T.; Wakame, K.; Nishioka, H.; Tanaka, A.; Nishihira, J. Effects of enzyme-treated asparagus extract on heat shock protein 70, stress indices, and sleep in healthy adult men. J. Nutr. Sci. Vitaminol. 2014, 60, 283–290. [Google Scholar] [CrossRef]
- Ito, T.; Ono, T.; Sato, A.; Goto, K.; Miura, T.; Wakame, K.; Nishioka, H.; Maeda, T. Toxicological assessment of enzyme-treated asparagus extract in rat acute and subchronic oral toxicity studies and genotoxicity tests. Regul. Toxicol. Pharmacol. 2014, 68, 240–249. [Google Scholar] [CrossRef]
- Yasueda, A.; Sakaue, M.; Maeda, K.; Hayashi, N.; Ito, T. Safety Evaluation of a Standardised Extract of Asparagus officinalis Stem in Healthy Volunteers: A Double-Blind and Randomised Controlled Trial. J. Herb. Herbal. Med. 2023, 42, 100789. [Google Scholar] [CrossRef]
- Shirato, K.; Koda, T.; Takanari, J.; Sakurai, T.; Ogasawara, J.; Imaizumi, K.; Ohno, H.; Kizaki, T. Anti-Inflammatory Effect of ETAS(R)50 by Inhibiting Nuclear Factor-kappaB p65 Nuclear Import in Ultraviolet-B-Irradiated Normal Human Dermal Fibroblasts. Evid. Based Complement. Altern. Med. 2018, 2018, 5072986. [Google Scholar] [CrossRef]
- Shirato, K.; Takanari, J.; Sakurai, T.; Ogasawara, J.; Imaizumi, K.; Ohno, H. Enzyme-Treated Asparagus Extract Prevents’Hydrogen Peroxide-Induced Pro-Inflammatory Responses by Suppressing p65 Nuclear Translocation in Skin L929 Fibroblasts. Nat. Prod. Commun. 2016, 11, 1883–1888. [Google Scholar]
- Shirato, K.; Koda, T.; Takanari, J.; Ogasawara, J.; Sakurai, T.; Ohno, H.; Kizaki, T. ETAS(R)50 Attenuates Ultraviolet-B-Induced Interleukin-6 Expression by Suppressing Akt Phosphorylation in Normal Human Dermal Fibroblasts. Evid. Based Complement. Altern. Med. 2018, 2018, 1547120. [Google Scholar] [CrossRef]
- Ito, T.; Maeda, T.; Goto, K.; Miura, T.; Wakame, K.; Nishioka, H.; Sato, A. Enzyme-treated asparagus extract promotes expression of heat shock protein and exerts antistress effects. J. Food Sci. 2014, 79, H413–H419. [Google Scholar] [CrossRef]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gao, P.; Li, Z.; Dai, A.; Yang, M.; Chen, S.; Su, J.; Deng, Z.; Li, L. Forkhead Box O Signaling Pathway in Skeletal Muscle Atrophy. Am. J. Pathol. 2022, 192, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Jin, H.; Mikhail, H.; Pavel, V.; Yang, G.; Ji, B.; Lu, B.; Li, Y. Autophagy in sarcopenia: Possible mechanisms and novel therapies. Biomed. Pharmacother. 2023, 165, 115147. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yadav, A.; Phogat, J.; Dabur, R. Dynamics and Interplay between Autophagy and Ubiquitin-proteasome system Coordination in Skeletal Muscle Atrophy. Curr. Mol. Pharmacol. 2022, 15, 475–486. [Google Scholar] [CrossRef]
- Affourtit, C.; Carre, J.E. Mitochondrial involvement in sarcopenia. Acta Physiol. 2024, 240, e14107. [Google Scholar] [CrossRef]
- Dantas, W.S.; Zunica, E.R.M.; Heintz, E.C.; Vandanmagsar, B.; Floyd, Z.E.; Yu, Y.; Fujioka, H.; Hoppel, C.L.; Belmont, K.P.; Axelrod, C.L.; et al. Mitochondrial uncoupling attenuates sarcopenic obesity by enhancing skeletal muscle mitophagy and quality control. J. Cachexia Sarcopenia Muscle 2022, 13, 1821–1836. [Google Scholar] [CrossRef]
- Liu, H.W.; Chang, Y.C.; Chan, Y.C.; Hu, S.H.; Liu, M.Y.; Chang, S.J. Dysregulations of mitochondrial quality control and autophagic flux at an early age lead to progression of sarcopenia in SAMP8 mice. Biogerontology 2020, 21, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.H.; U, K.P.; Yiu, T.; Ong, M.T.; Lee, W.Y. Sarcopenia: Current treatments and new regenerative therapeutic approaches. J. Orthop. Transl. 2020, 23, 38–52. [Google Scholar] [CrossRef]
- Nandagopal, N.; Roux, P.P. Regulation of global and specific mRNA translation by the mTOR signaling pathway. Translation 2015, 3, e983402. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Abati, E.; Manini, A.; Comi, G.P.; Corti, S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell Mol. Life Sci. 2022, 79, 374. [Google Scholar] [CrossRef]
- Sasako, T.; Umehara, T.; Soeda, K.; Kaneko, K.; Suzuki, M.; Kobayashi, N.; Okazaki, Y.; Tamura-Nakano, M.; Chiba, T.; Accili, D.; et al. Deletion of skeletal muscle Akt1/2 causes osteosarcopenia and reduces lifespan in mice. Nat. Commun. 2022, 13, 5655. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Q.; Zhou, X.; Mitch, W.E.; Goldberg, A.L. Myostatin/activin pathway antagonism: Molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 2013, 45, 2333–2347. [Google Scholar] [CrossRef]
- Goodman, C.A.; Mayhew, D.L.; Hornberger, T.A. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal 2011, 23, 1896–1906. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. The connection between the dynamic remodeling of the mitochondrial network and the regulation of muscle mass. Cell Mol. Life Sci. 2021, 78, 1305–1328. [Google Scholar] [CrossRef] [PubMed]
- LeBrasseur, N.K.; Schelhorn, T.M.; Bernardo, B.L.; Cosgrove, P.G.; Loria, P.M.; Brown, T.A. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J. Gerontol. A 2009, 64, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.; Wilhelm, L.; Prescott, A.R.; Ostacolo, K.; Zhao, J.F.; Ogmundsdottir, M.H.; Ganley, I.G. PINK1 regulated mitophagy is evident in skeletal muscles. Autophagy Rep. 2024, 3, 2326402. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, Y.T.; Liu, H.W.; Chan, Y.C.; Liu, M.Y.; Hu, S.H.; Tseng, W.T.; Wu, H.L.; Wang, M.F.; Chang, S.J. Oligonol Alleviates Sarcopenia by Regulation of Signaling Pathways Involved in Protein Turnover and Mitochondrial Quality. Mol. Nutr. Food Res. 2019, 63, e1801102. [Google Scholar] [CrossRef]
- Liu, H.W.; Chan, Y.C.; Wei, C.C.; Chen, Y.A.; Wang, M.F.; Chang, S.J. An alternative model for studying age-associated metabolic complications: Senescence-accelerated mouse prone 8. Exp. Gerontol. 2017, 99, 61–68. [Google Scholar] [CrossRef]
- Thoma, A.; Lightfoot, A.P. NF-kB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Takanari, J.; Abe, K.; Nagayama, A.; Ikeya, Y.; Kohda, N. Isolation and Structure Determination of a Heat Shock Protein Inducer, Asparagus-Derived Proline-Containing 3-Alkyldiketopiperazines (Asparaprolines), From a Standardized Extract of Asparagus officinalis Stem. Nat. Prod. Commun. 2020, 15, 1934578X20914681. [Google Scholar] [CrossRef]
- Senf, S.M. Skeletal muscle heat shock protein 70: Diverse functions and therapeutic potential for wasting disorders. Front. Physiol. 2013, 4, 330. [Google Scholar] [CrossRef]
- Pomella, S.; Cassandri, M.; Antoniani, F.; Crotti, S.; Mediani, L.; Silvestri, B.; Medici, M.; Rota, R.; Rosa, A.; Carra, S. Heat Shock Proteins: Important Helpers for the Development, Maintenance and Regeneration of Skeletal Muscles. Muscles 2023, 2, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Gwag, T.; Park, K.; Kim, E.; Son, C.; Park, J.; Nikawa, T.; Choi, I. Inhibition of C2C12 myotube atrophy by a novel HSP70 inducer, celastrol, via activation of Akt1 and ERK1/2 pathways. Arch. Biochem. Biophys. 2013, 537, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sato, A.; Ono, T.; Goto, K.; Maeda, T.; Takanari, J.; Nishioka, H.; Komatsu, K.; Matsuura, H. Isolation, structural elucidation, and biological evaluation of a 5-hydroxymethyl-2-furfural derivative, asfural, from enzyme-treated asparagus extract. J. Agric. Food Chem. 2013, 61, 9155–9159. [Google Scholar] [CrossRef]
- Ciarlone, G.E.; Swift, J.M.; Williams, B.T.; Mahon, R.T.; Roney, N.G.; Yu, T.; Gasier, H.G. 5-Hydroxymethylfurfural reduces skeletal muscle superoxide production and modifies force production in rats exposed to hypobaric hypoxia. Physiol. Rep. 2023, 11, e15743. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef]
- ISO 9001:2015; Quality Management Systems—Requirements. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 22000:2018; Food Safety Management Systems—Requirements for Any Organization in the Food Chain. International Organization for Standardization: Geneva, Switzerland, 2018.
- Kerr, H.L.; Krumm, K.; Anderson, B.; Christiani, A.; Strait, L.; Li, T.; Irwin, B.; Jiang, S.; Rybachok, A.; Chen, A.; et al. Mouse sarcopenia model reveals sex- and age-specific differences in phenotypic and molecular characteristics. J. Clin. Investig. 2024, 134, e172890. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Dilution | Brand |
|---|---|---|
| PI3K (p110a, #4249), p-Akt (Thr308, #4056), Akt (#9272), p-mTOR (Ser2448, #5536), mTOR (#2983), p-p70S6K (Thr389, #9205), p70S6K (#2708), FoxO1a (#2880) | 1:1000 | Cell Signaling Technology (Danvers, MA, USA) |
| GAPDH (GTX100118), NFκB (GTX102090), MuRF-1 (GTX110475), and p62 (GTX100685) | 1:2000 1:1000 | GeneTex Inc. (Irvine, CA, USA) |
| Atrogin-1 (ab168372), Myostatin (ab203076), and lamin B (ab16048) | 1:1000 | Abcam (Cambridge, MA, USA) |
| PGC1α (sc-13067) | 1:1000 | Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) |
| LC3B (14600-1-AP), ATG13 (18258-1-AP) | 1:1000 | proteintech ® (Proteintech Group Inc., Rosemont, IL, USA) |
| PINK1 (A7131) β-actin (AC006) | 1:1000 1:10,000 | Abclonal®, BioAb Co., Ltd., New Taipei City, Taiwan |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Mfn1 | AGTCAGCGGTGAAAGCAAAGT | GGTCTTCCCTCTCTTCCATTGAAT |
| Mfn2 | ATATAGAGGAAGGTCTGGGCCG | CCGCATAGATACAGGAAGAAGGG |
| OPA1 | TGACAAACTTAAGGAGGCTGTG | CATTGTGCTGAATAACCCTCAA |
| Drp1 | CGGTTCCCTAAACTTCACGA | GCACCATTTCATTTGTCACG |
| Fis1 | AGCTGGTTCTGTGTCCAAG | TGTTCCTCTTTGCTCCCTTTG |
| Mff1 | CTAATCTTTCCTCTGCCCGT | GATGAGGATTAGAAGTGGCGG |
| PGC1α | ACTATGAATCAAGCCACTACAGAC | TTCATCCCTCTTGAGCCTTTCG |
| Nrf1 | ACAGATAGTCCTGTCTGGG | TGGTACATGCTCACAGGGA |
| Tfam | AAGACCTCGTTCAGCATAT | TTTTCCAAGCCTCATTTACAAGC |
| COX5b | TTCAAGGTTACTTCGCGGAGT | CGGGACTAGATAGGGTCTTCC |
| Ndufs8 | AGTGGCGGCAACGTACAAG | TCGAAAGAGGTAACTTAGGGTCA |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-J.; Chen, Y.-C.; Chang, Y.-C.; Cheng, C.-C.; Chan, Y.-C. ETAS®, a Standardized Extract of Asparagus officinalis Stem, Alleviates Sarcopenia via Regulating Protein Turnover and Mitochondrial Quality. Pharmaceuticals 2025, 18, 1243. https://doi.org/10.3390/ph18091243
Chang S-J, Chen Y-C, Chang Y-C, Cheng C-C, Chan Y-C. ETAS®, a Standardized Extract of Asparagus officinalis Stem, Alleviates Sarcopenia via Regulating Protein Turnover and Mitochondrial Quality. Pharmaceuticals. 2025; 18(9):1243. https://doi.org/10.3390/ph18091243
Chicago/Turabian StyleChang, Sue-Joan, Yung-Chia Chen, Yun-Ching Chang, Chung-Che Cheng, and Yin-Ching Chan. 2025. "ETAS®, a Standardized Extract of Asparagus officinalis Stem, Alleviates Sarcopenia via Regulating Protein Turnover and Mitochondrial Quality" Pharmaceuticals 18, no. 9: 1243. https://doi.org/10.3390/ph18091243
APA StyleChang, S.-J., Chen, Y.-C., Chang, Y.-C., Cheng, C.-C., & Chan, Y.-C. (2025). ETAS®, a Standardized Extract of Asparagus officinalis Stem, Alleviates Sarcopenia via Regulating Protein Turnover and Mitochondrial Quality. Pharmaceuticals, 18(9), 1243. https://doi.org/10.3390/ph18091243







