Discrepancies in Recommendations on Pharmacokinetic Drug Interactions for Anticancer Medications and Direct Oral Anticoagulants (DOAC): A Comparative Analysis of Different Clinical Decision Support Systems and Sources
Abstract
1. Introduction
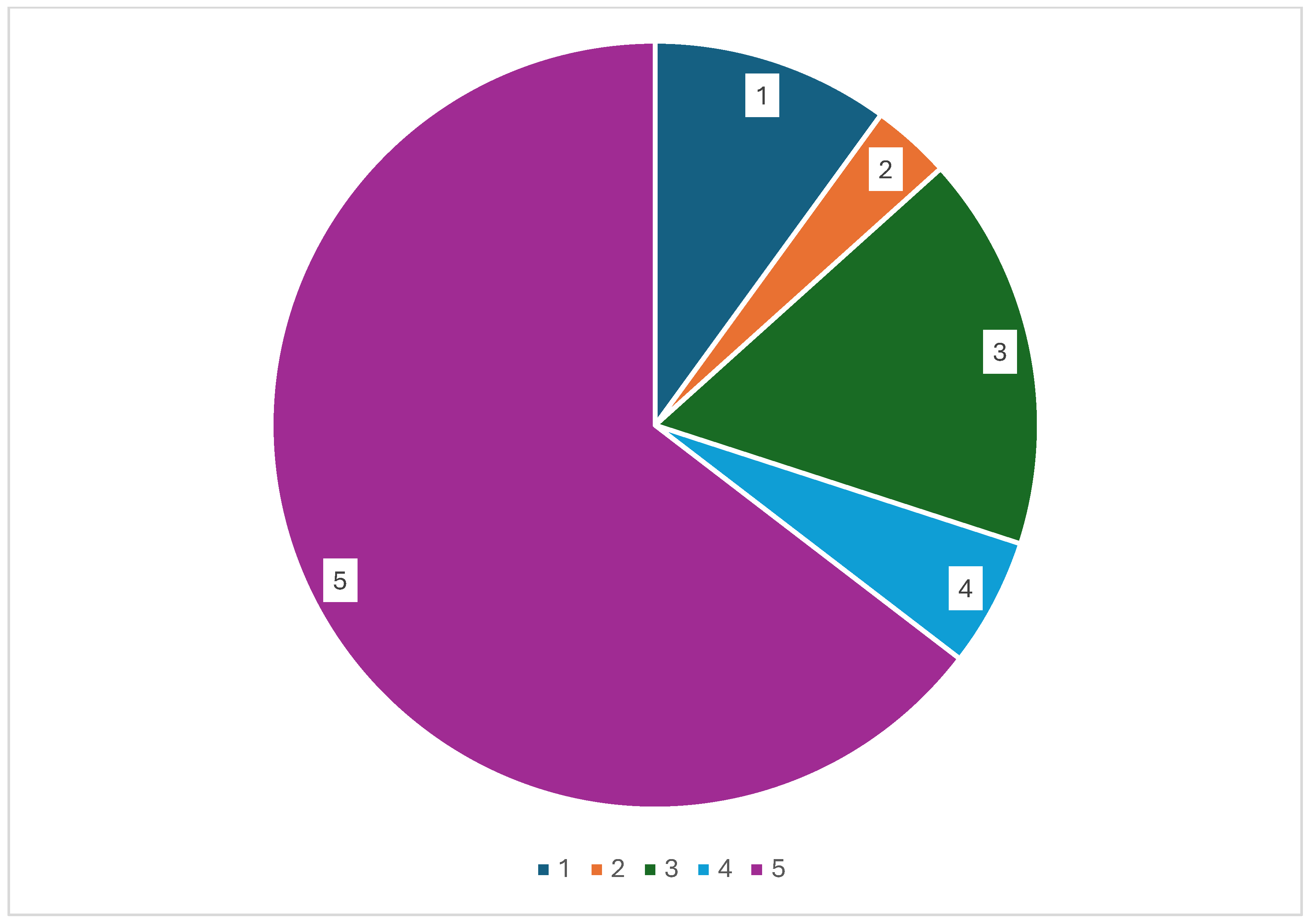
2. Results
2.1. Drug Combinations with No Pharmacokinetic Interactions
2.2. Drug Combinations to Avoid or Use with Caution
3. Discussion
4. Materials and Methods
4.1. Clinical Decision Support Systems and Recommendations
4.2. Data Collection
4.3. Management and Analysis of Data
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AHA | American Heart Association |
| CAT | Cancer-associated Thrombosis |
| CDSS | Clinical Decision Support System |
| DOAC | Direct Oral Anticoagulants |
| EHRA | European Heart Rhythm Association |
| FXa | Activated factor X |
| FDA | Food and Drug Administration |
| LMWH | Low Molecular Weight Heparin |
| P-gp | P-glycoprotein |
| SPC | Summary of Product Characteristics |
| VTE | Venous Thromboembolism |
References
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial fibrillation: Epidemiology, screening and digital health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Zakai, N.A. Epidemiology and prevention of venous thromboembolism. Nat. Rev. Cardiol. 2023, 20, 248–262. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef]
- Lee, A.Y.; Levine, M.N.; Baker, R.I.; Bowden, C.; Kakkar, A.K.; Prins, M.; Rickles, F.R.; Julian, J.A.; Haley, S.; Kovacs, M.J.; et al. Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2003, 349, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Gates, L.E.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 3063–3071. [Google Scholar] [CrossRef]
- Farge, D.; Frere, C.; Connors, J.M.; Khorana, A.A.; Kakkar, A.; Ay, C.; Muñoz, A.; Brenner, B.; Prata, P.H.; Brilhante, D.; et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022, 23, e334–e347. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and cancer risk: A potentially modifiable relationship. Am. J. Prev. Med. 2014, 46 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, L.; Vanassche, T.; Van Cutsem, E.; Van Aelst, L.; Verhamme, P. Pharmacokinetic drug–drug interactions with direct anticoagulants in the management of cancer-associated thrombosis. Br. J. Clin. Pharmacol. 2023, 89, 2369–2376. [Google Scholar] [CrossRef]
- Sorigue, M.; Sarrate, E.; Miljkovic, M.D. Interactions between direct anticoagulants and chemotherapy. Ann. Oncol. 2019, 30, 1170. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.R.; Mohile, S.G.; Juba, K.M.; Awad, H.; Wells, M.; Poh Loh, K.; Flannery, M.; Culakova, E.; Tylock, R.T.; Ramsdale, E.A. Association of polypharmacy and potential drug-drug interactions with adverse treatment outcomes in older adults with advanced cancer. Cancer 2023, 129, 1096–1104. [Google Scholar] [CrossRef]
- Beavers, C.J.; Rodgers, J.E.; Bagnola, A.J.; Beckie, T.M.; Campia, U.; Di Palo, K.E.; Okwuosa, T.M.; Przespolewski, E.R.; Dent, S. Cardio-Oncology Drug Interactions: A Scientific Statement From the American Heart Association. Circulation 2022, 145, e811–e838. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Rienecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Adams, J. Pharmacogenomics and personalized medicine. Nat. Educ. 2008, 1, 194. [Google Scholar]
- Janusmed Interaktioner Och Riskprofil. Available online: https://janusmed.se/interaktioner (accessed on 12 May 2025). (In Swedish).
- FASS. Available online: www.fass.se (accessed on 12 May 2025). (In Swedish).
- Tukukino, C.; Eriksson, A.L.; Hamdan, W.; Wallin Hybelius, F.; Wallerstedt, S.M. Interaction alerts: A comparison of classifications and recommendations for clinical management between Janusmed and three other knowledge resources. Basic. Clin. Pharmacol. Toxicol. 2023, 132, 403–415. [Google Scholar] [CrossRef]
- FDA. Available online: https://www.fda.gov/drugs/drug-interactions-labeling/healthcare-professionals-fdas-examples-drugs-interact-cyp-enzymes-and-transporter-systems (accessed on 12 May 2025).
- Bogaard, L.; Tsoi, K.; van de Steeg, B.; Brandon, E.F.A.; Geers, L.; van Herwaarden, M.; Jansman, F.; Maas, D.; Monster-Simons, M.; Ong, D.S.Y.; et al. A practical assessment protocol for clinically relevant P-glycoprotein-mediated drug-drug interactions. Front. Pharmacol. 2024, 15, 1412692. [Google Scholar] [CrossRef]
- Fung, K.W.; Kapusnik-Uner, J.; Cunningham, J.; Higby-Baker, S.; Bodenreider, O. Comparison of three commercial knowledge bases for detection of drug-drug interactions in clinical decision support. J. Am. Med. Inform. Assoc. 2017, 24, 806–812. [Google Scholar] [CrossRef]
- Smithburger, P.L.; Kane-Gill, S.L.; Seybert, A.L. Drug-drug interactions in cardiac and cardiothoracic intensive care units. Drug Saf. 2010, 33, 879–888. [Google Scholar] [CrossRef]
- Scott, M.; Glenn, T. A comparison of potential psychiatric drug interactions from six drug interaction database programs. Psychiatry Res. 2019, 275, 366–372. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Lafaie, L.; Hodin, S.; Saïb, S.; Bertoletti, L.; Delavenne, X. Tyrosine kinase inhibitors and direct oral anticoagulants: In vitro evaluation of drug–drug interaction mediated by P-glycoprotein. Fundam. Clin. Pharmacol. 2022, 36, 860–868. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Tosetto, A.; Abshire, T.; Arnold, D.M.; Coller, B.; James, P.; Neunert, C.; Lillicrap, D.; on behalf of the ISTH/SSC Joint VWF and Perinatal/Pediatric Hemostasis Subcommittees Working Group. ISTH/SSC bleeding assessment tool: A standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J. Thromb. Haemost. 2010, 8, 2063–2065. [Google Scholar] [CrossRef]
- Maggiore, R.J.; Gross, C.P.; Hurria, A. Polypharmacy in older adults with cancer. Oncologist 2010, 15, 507–522. [Google Scholar] [CrossRef]
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [CrossRef] [PubMed]
- Oliveira, R.F.; Oliveira, A.I.; Cruz, A.S.; Ribeiro, O.; Afreixo, V.; Pimentel, F. Polypharmacy and drug interactions in older patients with cancer receiving chemotherapy: Associated factors. BMC Geriatr. 2024, 24, 557. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, T.W.; McNeil, M.J.; Kamal, A.H.; Currow, D.C.; Abernethy, A.P. Polypharmacy in patients with advanced cancer and the role of medication discontinuation. Lancet Oncol. 2015, 16, e333–e341. [Google Scholar] [CrossRef] [PubMed]
- Balducci, L.; Goetz-Parten, D.; Steinman, M.A. Polypharmacy and the management of the older cancer patient. Ann. Oncol. 2013, 23 (Suppl. S7), vii36–vii40. [Google Scholar] [CrossRef]
- Hauptmann, J.; Stürzebecher, J. Synthetic Inhibitors of Thrombin and Factor Xa. Thromb. Res. 1999, 93, 203–241. [Google Scholar] [CrossRef]
- Hammar, T.; Jonsén, E.; Björneld, O.; Askfors, Y.; Andersson, M.L.; Lincke, A. Potential Adverse Drug Events Identified with Decision Support Algorithms from Janusmed Risk Profile-A Retrospective Population-Based Study in a Swedish Region. Pharmacy 2024, 12, 168. [Google Scholar] [CrossRef]
- Janusmed Interaktioner Och Riskprofil. Available online: https://janusinfo.se/inenglish.4.7e3d365215ec82458644daab.html (accessed on 12 May 2025). (In Swedish).
- Petersson, L.; Schörgenhofer, C.; Askfors, Y.; Justad, H.; Dahl, M.-L.; Andersson, M.L. Pharmacological Risk Assessment Among Older Patients with Polypharmacy Using the Clinical Decision Support System Janusmed Risk Profile: A Cross-Sectional Register Study. Drugs Aging 2023, 40, 369–376. [Google Scholar] [CrossRef]
| Drug Group | Medications |
|---|---|
| Antimitotic agents | docetaxel, paclitaxel, vinblastine, vincristine, vinorelbine |
| Antimetabolites | azacitidine, azathioprine, capecitabine, cladribine, clofarabine cytarabine, decitabine, fludarabine, fluorouracil, gemcitabine, mercaptopurine, methotrexate, nelarabine, pemetrexed, tegafur, thioguanine |
| Topoisomeras inhibitors | etoposide, irinotecan, topotecan |
| Anthracyclines | daunorubicin, doxorubicin, idarubicin, mitoxantrone |
| Alkylating agents | bendamustine, busulfan, carmustine, cyclophosphamide, dacarbazine, iphosphamide, klorambucile, lomustine, melphalan, temozolomide |
| Platinum-based agents | bleomycin, carboplatin, cisplatin, mitomycin C, oxaliplatin |
| Tyrosine kinase inhibitors | crizotinib, dasatinib, erlotinib, gefitinib, imatinib, ibrutinib, lapatinib, nilotinib, sunitinib, vandetanib, vemurafenib |
| Monoclonal antibodies | alemtuzumab, bevacizumab, brentuximab, cetuximab, rituximab, trastuzumab |
| Hormone treatment | abiraterone, anastrozole, bicalutamide, enzalutamide, flutamide, fulvestrant, letrozole, leuprorelin, mitotane, raloxifene, tamoxifen |
| Immunomodulating agents | cyclosporine, dexamethasone, everolimus, sirolimus, tacrolimus, temsirolimus |
| Proteasome inhibitors | bortezomib, ixazomib, karfilzomib |
| Mechanism According to EHRA | Mechanism According to Janusmed | EHRA-Recommendation | Janusmed-Recommendation | |
|---|---|---|---|---|
| Antimitotic agent | ||||
| Vinblastine | Strong P-gp induction | N/A | Contraindicated/not advisable | No DDI |
| Anthracyclines | ||||
| Doxorubicin | Strong P-gp induction, mild CYP3A4 inhibition | N/A | Contraindicated/not advisable | No DDI |
| Tyrosine kinase inhibitors | ||||
| Imatinib | Moderate CYP3A4 inhibition, strong P-gp inhibition | Moderate CYP3A4 inhibition and/or P-gp inhibition | Contraindicated/not advisable | Caution Grade C |
| Crizotinib | Moderate CYP3A4 inhibition, strong P-gp inhibition | Moderate CYP3A4 inhibition | Contraindicated/not advisable | Caution for apixaban and rivaroxaban (Grade B), no DDI for edoxaban |
| Vandetanib | Strong P-gp inhibition | N/A | Contraindicated/not advisable | No DDI |
| Sunitinib | Strong P-gp inhibitor | N/A | Contraindicated/not advisable | Caution (Grade C) Additive effect on haemostasis but no DDI |
| Hormone treatment | ||||
| Abiraterone | Moderate CYP3A4 inhibition, strong P-gp inhibition | N/A | Contraindicated/not advisable | No DDI |
| Enzalutamide | Strong CYP3A4 induction, strong P-gp inhibition | Strong CYP3A4 induction | Contraindicated/not advisable | Caution (Grade C) for apixaban and rivaroxaban, Grade B for edoxaban |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowinski, K.; Chaireti, R. Discrepancies in Recommendations on Pharmacokinetic Drug Interactions for Anticancer Medications and Direct Oral Anticoagulants (DOAC): A Comparative Analysis of Different Clinical Decision Support Systems and Sources. Pharmaceuticals 2025, 18, 1044. https://doi.org/10.3390/ph18071044
Nowinski K, Chaireti R. Discrepancies in Recommendations on Pharmacokinetic Drug Interactions for Anticancer Medications and Direct Oral Anticoagulants (DOAC): A Comparative Analysis of Different Clinical Decision Support Systems and Sources. Pharmaceuticals. 2025; 18(7):1044. https://doi.org/10.3390/ph18071044
Chicago/Turabian StyleNowinski, Karolina, and Roza Chaireti. 2025. "Discrepancies in Recommendations on Pharmacokinetic Drug Interactions for Anticancer Medications and Direct Oral Anticoagulants (DOAC): A Comparative Analysis of Different Clinical Decision Support Systems and Sources" Pharmaceuticals 18, no. 7: 1044. https://doi.org/10.3390/ph18071044
APA StyleNowinski, K., & Chaireti, R. (2025). Discrepancies in Recommendations on Pharmacokinetic Drug Interactions for Anticancer Medications and Direct Oral Anticoagulants (DOAC): A Comparative Analysis of Different Clinical Decision Support Systems and Sources. Pharmaceuticals, 18(7), 1044. https://doi.org/10.3390/ph18071044






