Negative Impact of Olanzapine on ICU Delirium Resolution: An Emulated Clinical Trial
Abstract
1. Introduction
2. Results
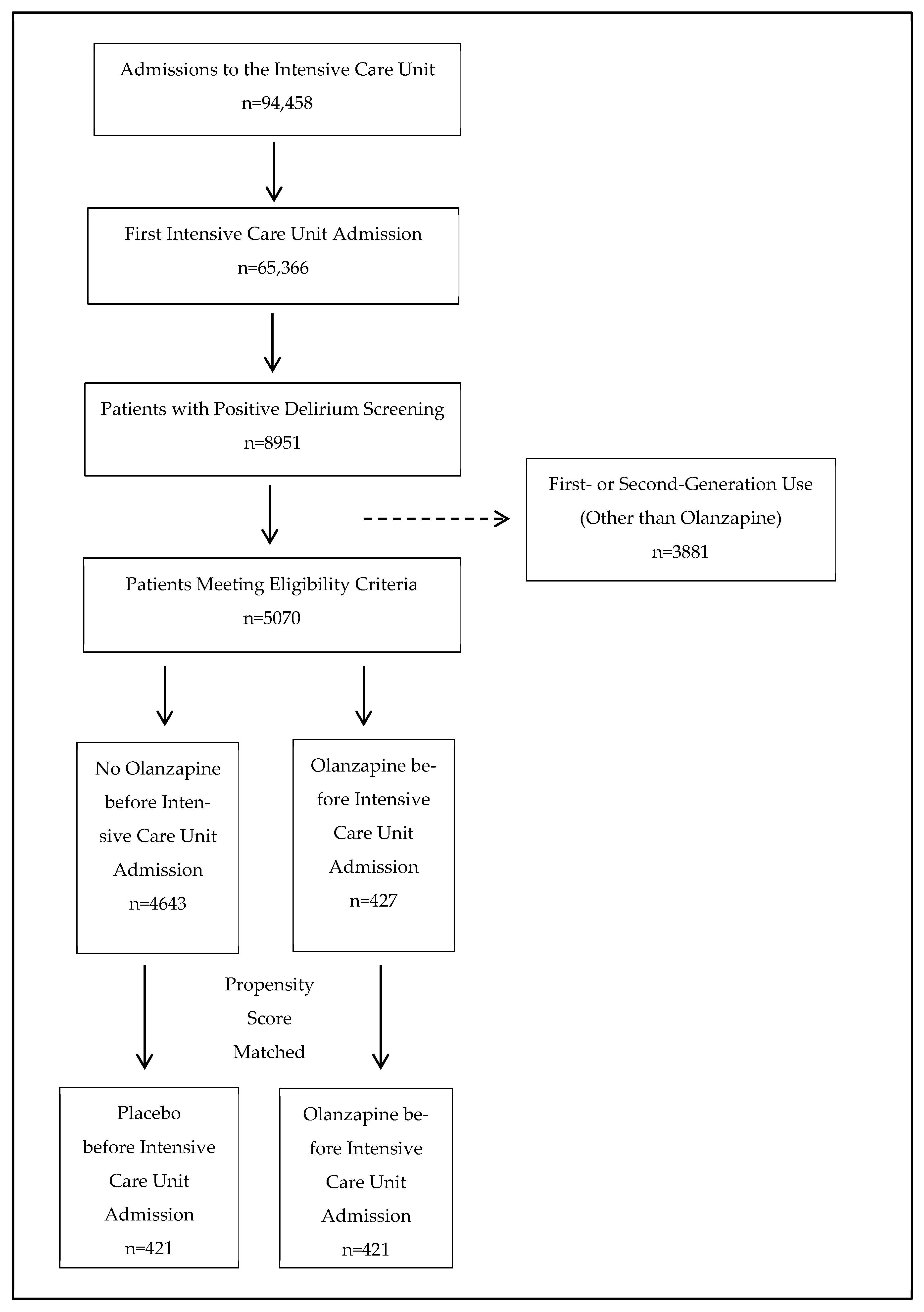
2.1. Participant Flow
2.2. Propensity Score Matching
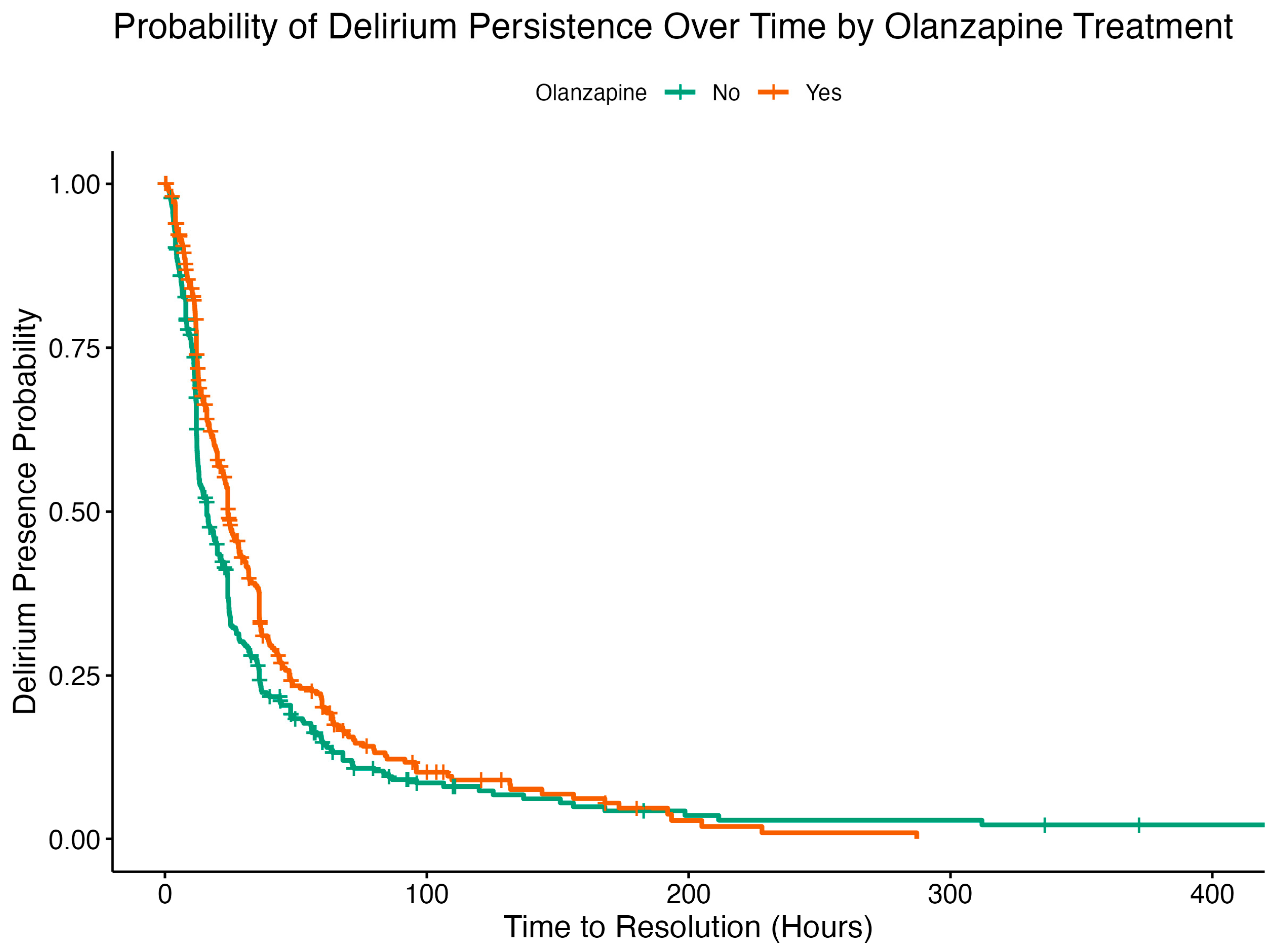
2.3. Cox Proportional Hazard Analysis
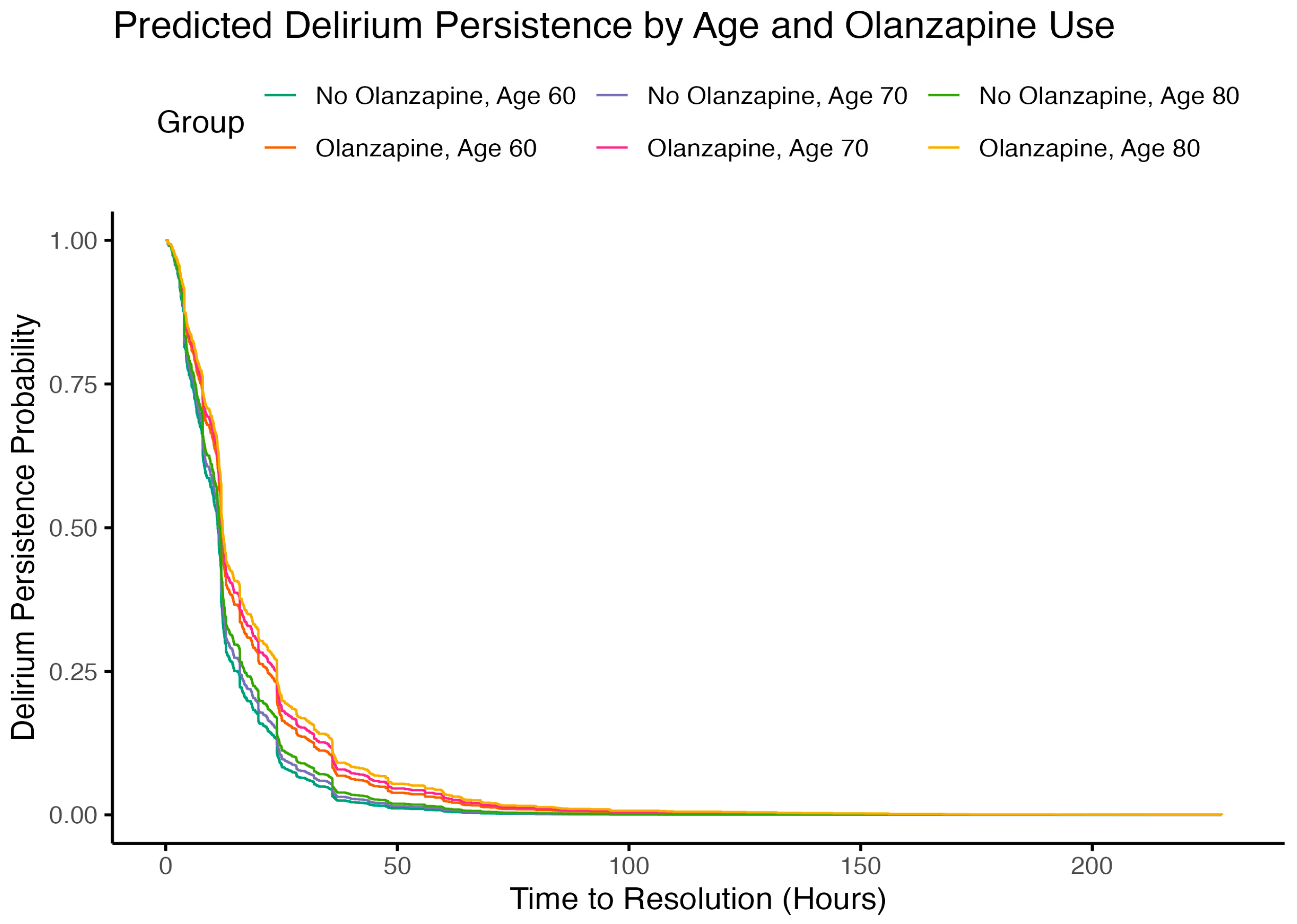
2.4. Subgroup Analysis
3. Discussion
4. Materials and Methods
4.1. Data Source
4.2. Study Design
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brennan, K.; Sanchez, D.; Hedges, S.; Lynch, J.; Hou, Y.C.; Al Sayfe, M.; Shunker, S.-A.; Bogdanoski, T.; Hunt, L.; Alexandrou, E.; et al. A Nurse-Led Intervention to Reduce the Incidence and Duration of Delirium among Adults Admitted to Intensive Care: A Stepped-Wedge Cluster Randomised Trial. Aust. Crit. Care 2023, 36, 441–448. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Desai, S.V.; Law, T.J.; Needham, D.M. Long-Term Complications of Critical Care. Crit. Care Med. 2011, 39, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. Long-Term Cognitive Impairment after Critical Illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-Y.; Luo, R.-Y.; Wang, M.-T.; Yuan, C.-Y.; Sun, Y.-Y.; Jing, J.-Y. Mechanisms Underlying Delirium in Patients with Critical Illness. Front. Aging Neurosci. 2024, 16, 1446523. [Google Scholar] [CrossRef]
- Lewis, K.; Balas, M.C.; Stollings, J.L.; McNett, M.; Girard, T.D.; Chanques, G.; Kho, M.E.; Pandharipande, P.P.; Weinhouse, G.L.; Brummel, N.E.; et al. A Focused Update to the Clinical Practice Guidelines for the Prevention and Management of Pain, Anxiety, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2025, 53, e711–e727. [Google Scholar] [CrossRef]
- Austin, P.C. Balance Diagnostics for Comparing the Distribution of Baseline Covariates between Treatment Groups in Propensity-score Matched Samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Richelson, E. Receptor Pharmacology of Neuroleptics: Relation to Clinical Effects. J. Clin. Psychiatry 1999, 60 (Suppl. 10), 5–14. [Google Scholar]
- Bymaster, F.P.; Calligaro, D.O.; Falcone, J.F.; Marsh, R.D.; Moore, N.A.; Tye, N.C.; Seeman, P.; Wong, D.T. Radioreceptor Binding Profile of the Atypical Antipsychotic Olanzapine. Neuropsychopharmacology 1996, 14, 87–96. [Google Scholar] [CrossRef]
- Levey, A.I.; Edmunds, S.M.; Koliatsos, V.; Wiley, R.G.; Heilman, C.J. Expression of M1-M4 Muscarinic Acetylcholine Receptor Proteins in Rat Hippocampus and Regulation by Cholinergic Innervation. J. Neurosci. 1995, 15, 4077–4092. [Google Scholar] [CrossRef]
- Shinoe, T.; Matsui, M.; Taketo, M.M.; Manabe, T. Modulation of Synaptic Plasticity by Physiological Activation of M1 Muscarinic Acetylcholine Receptors in the Mouse Hippocampus. J. Neurosci. 2005, 25, 11194–11200. [Google Scholar] [CrossRef]
- Wess, J. Muscarinic Acetylcholine Receptor Knockout Mice: Novel Phenotypes and Clinical Implications. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 423–450. [Google Scholar] [CrossRef] [PubMed]
- Anagnostaras, S.G.; Murphy, G.G.; Hamilton, S.E.; Mitchell, S.L.; Rahnama, N.P.; Nathanson, N.M.; Silva, A.J. Selective Cognitive Dysfunction in Acetylcholine M1 Muscarinic Receptor Mutant Mice. Nat. Neurosci. 2003, 6, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Langmead, C.J.; Watson, J.; Reavill, C. Muscarinic Acetylcholine Receptors as CNS Drug Targets. Pharmacol. Ther. 2008, 117, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Lamping, K.G.; Duttaroy, A.; Zhang, W.; Cui, Y.; Bymaster, F.P.; McKinzie, D.L.; Felder, C.C.; Deng, C.X.; Faraci, F.M.; et al. Cholinergic Dilation of Cerebral Blood Vessels Is Abolished in M5 Muscarinic Acetylcholine Receptor Knockout Mice. Proc. Natl. Acad. Sci. USA 2001, 98, 14096–14101. [Google Scholar] [CrossRef]
- Hamidovic, A. Quetiapine Use Is Associated with Longer ICU Stay Compared to Control and Haloperidol: A Propensity Score–Matched Analysis Using the MIMIC-IV Database. J. Clin. Med. 2025, 14, 4438. [Google Scholar] [CrossRef]
- Bugiani, O. Why Is Delirium More Frequent in the Elderly? Neurol. Sci. 2021, 42, 3491–3503. [Google Scholar] [CrossRef]
- Hughes, C.G.; Boncyk, C.S.; Fedeles, B.; Pandharipande, P.P.; Chen, W.; Patel, M.B.; Brummel, N.E.; Jackson, J.C.; Raman, R.; Ely, E.W.; et al. Association between Cholinesterase Activity and Critical Illness Brain Dysfunction. Crit. Care 2022, 26, 377. [Google Scholar] [CrossRef]
- Maldonado, J.R. Delirium Pathophysiology: An Updated Hypothesis of the Etiology of Acute Brain Failure. Int. J. Geriatr. Psychiatry 2018, 33, 1428–1457. [Google Scholar] [CrossRef]
- Liu, S.B.; Liu, S.; Gao, K.; Wu, G.Z.; Zu, G.; Jie Liu, J. Olanzapine for the Treatment of ICU Delirium: A Systematic Review and Meta-Analysis. Ther. Adv. Psychopharmacol. 2023, 13, 20451253231152113. [Google Scholar] [CrossRef]
- Ely, E.W.; Margolin, R.; Francis, J.; May, L.; Truman, B.; Dittus, R.; Speroff, T.; Gautam, S.; Bernard, G.R.; Inouye, S.K. Evaluation of Delirium in Critically Ill Patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit. Care Med. 2001, 29, 1370–1379. [Google Scholar] [CrossRef]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Soft. 2011, 42, 1–28. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
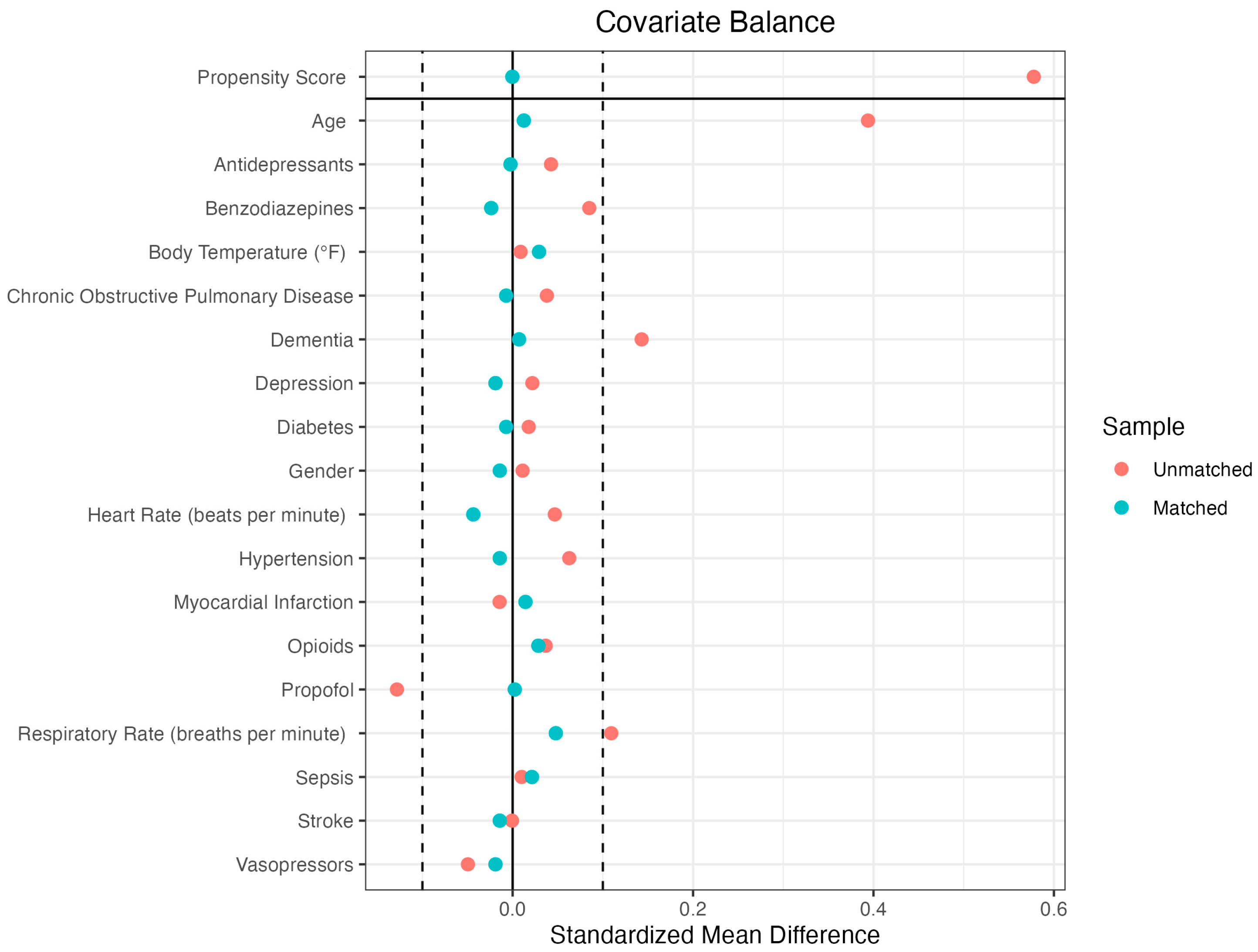
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Means Treated | Means Control | Standardized Mean Difference | Means Treated | Means Control | Standardized Mean Difference | |
| Propensity Score | 0.1145 | 0.0827 | 0.5036 | 0.1145 | 0.1144 | 0.0008 |
| Demographics | ||||||
| Age | 72.9739 | 66.5803 | 0.4264 | 72.9739 | 72.8195 | 0.0103 |
| Gender | 0.2138 | 0.1713 | 0.1037 | 0.2138 | 0.2043 | 0.0232 |
| Medical Conditions | ||||||
| Chronic Obstructive Pulmonary Disease | 0.1306 | 0.0927 | 0.1125 | 0.1306 | 0.152 | −0.0634 |
| Dementia | 0.247 | 0.104 | 0.3316 | 0.247 | 0.247 | 0 |
| Depression | 0.2067 | 0.1848 | 0.054 | 0.2067 | 0.1876 | 0.0469 |
| Diabetes | 0.3302 | 0.3123 | 0.0379 | 0.3302 | 0.3325 | −0.0051 |
| Hypertension | 0.5463 | 0.4836 | 0.126 | 0.5463 | 0.5487 | −0.0048 |
| Myocardial Infarction | 0.133 | 0.1475 | −0.0427 | 0.133 | 0.1686 | −0.1049 |
| Sepsis | 0.2518 | 0.2418 | 0.023 | 0.2518 | 0.2755 | −0.0547 |
| Stroke | 0.2043 | 0.205 | −0.0017 | 0.2043 | 0.1781 | 0.0648 |
| Medications | ||||||
| Antidepressants | 0.2138 | 0.1713 | 0.1037 | 0.2138 | 0.2043 | 0.0232 |
| Benzodiazepines | 90.304 | 89.2533 | 0.0499 | 90.304 | 92.3539 | −0.0973 |
| Propofol | 20.3183 | 19.6346 | 0.1105 | 20.3183 | 20.5701 | −0.0407 |
| Vasopressors | 98.2389 | 98.2224 | 0.011 | 98.2389 | 98.1964 | 0.0284 |
| Vitals | ||||||
| Heart Rate | 90.304 | 89.2533 | 0.0499 | 90.304 | 92.3539 | −0.0973 |
| Respiratory Rate | 20.3183 | 19.6346 | 0.1105 | 20.3183 | 20.5701 | −0.0407 |
| Body Temperature | 98.2389 | 98.2224 | 0.011 | 98.2389 | 98.1964 | 0.0284 |
| Term | Estimate | SE | Robust SE | Statistic | p Value | Confidence-Low | Confidence-High |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age | 0.9985 | 0.0011 | 0.0011 | −1.4209 | 0.1553 | 0.9963 | 1.0006 |
| Gender (F) | 0.8086 | 0.0865 | 0.0881 | −2.4111 | 0.0159 | 0.6803 | 0.9610 |
| Medications | |||||||
| Olanzapine | 0.7342 | 0.0832 | 0.0792 | −3.9005 | 0.0001 | 0.6287 | 0.8575 |
| Antidepressants | 0.8813 | 0.1086 | 0.0934 | −1.3525 | 0.1762 | 0.7338 | 1.0584 |
| Benzodiazepines | 0.9023 | 0.0888 | 0.0871 | −1.1802 | 0.2379 | 0.7606 | 1.0703 |
| Propofol | 0.6030 | 0.0962 | 0.0909 | −5.5614 | 0.0000 | 0.5046 | 0.7207 |
| Vasopressors | 0.7571 | 0.1020 | 0.0964 | −2.8872 | 0.0039 | 0.6268 | 0.9145 |
| Vitals | |||||||
| Heart Rate | 0.9971 | 0.0021 | 0.0020 | −1.4751 | 0.1402 | 0.9933 | 1.0010 |
| Respiratory Rate | 0.9882 | 0.0075 | 0.0078 | −1.5135 | 0.1301 | 0.9732 | 1.0035 |
| Body Temperature | 0.9978 | 0.0317 | 0.0312 | −0.0693 | 0.9447 | 0.9387 | 1.0607 |
| Medical Conditions | |||||||
| Chronic Obstructive Pulmonary Disease | 0.9984 | 0.1230 | 0.1278 | −0.0122 | 0.9903 | 0.7772 | 1.2827 |
| Dementia | 0.8108 | 0.1122 | 0.1096 | −1.9137 | 0.0557 | 0.6541 | 1.0051 |
| Depression | 1.0512 | 0.1085 | 0.0949 | 0.5257 | 0.5991 | 0.8727 | 1.2662 |
| Diabetes | 0.8728 | 0.0903 | 0.0837 | −1.6259 | 0.1040 | 0.7407 | 1.0284 |
| Hypertension | 0.9752 | 0.0846 | 0.0812 | −0.3089 | 0.7574 | 0.8317 | 1.1435 |
| Myocardial Infarction | 1.0104 | 0.1379 | 0.1351 | 0.0765 | 0.9390 | 0.7754 | 1.3166 |
| Sepsis | 0.7070 | 0.1115 | 0.1112 | −3.1188 | 0.0018 | 0.5686 | 0.8791 |
| Stroke | 0.7668 | 0.1045 | 0.1015 | −2.6148 | 0.0089 | 0.6284 | 0.9357 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidovic, A.; Davis, J. Negative Impact of Olanzapine on ICU Delirium Resolution: An Emulated Clinical Trial. Pharmaceuticals 2025, 18, 1019. https://doi.org/10.3390/ph18071019
Hamidovic A, Davis J. Negative Impact of Olanzapine on ICU Delirium Resolution: An Emulated Clinical Trial. Pharmaceuticals. 2025; 18(7):1019. https://doi.org/10.3390/ph18071019
Chicago/Turabian StyleHamidovic, Ajna, and John Davis. 2025. "Negative Impact of Olanzapine on ICU Delirium Resolution: An Emulated Clinical Trial" Pharmaceuticals 18, no. 7: 1019. https://doi.org/10.3390/ph18071019
APA StyleHamidovic, A., & Davis, J. (2025). Negative Impact of Olanzapine on ICU Delirium Resolution: An Emulated Clinical Trial. Pharmaceuticals, 18(7), 1019. https://doi.org/10.3390/ph18071019







