Accelerating Effects of Poloxamer and Its Structural Analogs on the Crystallization of Nitrendipine Polymorphs
Abstract
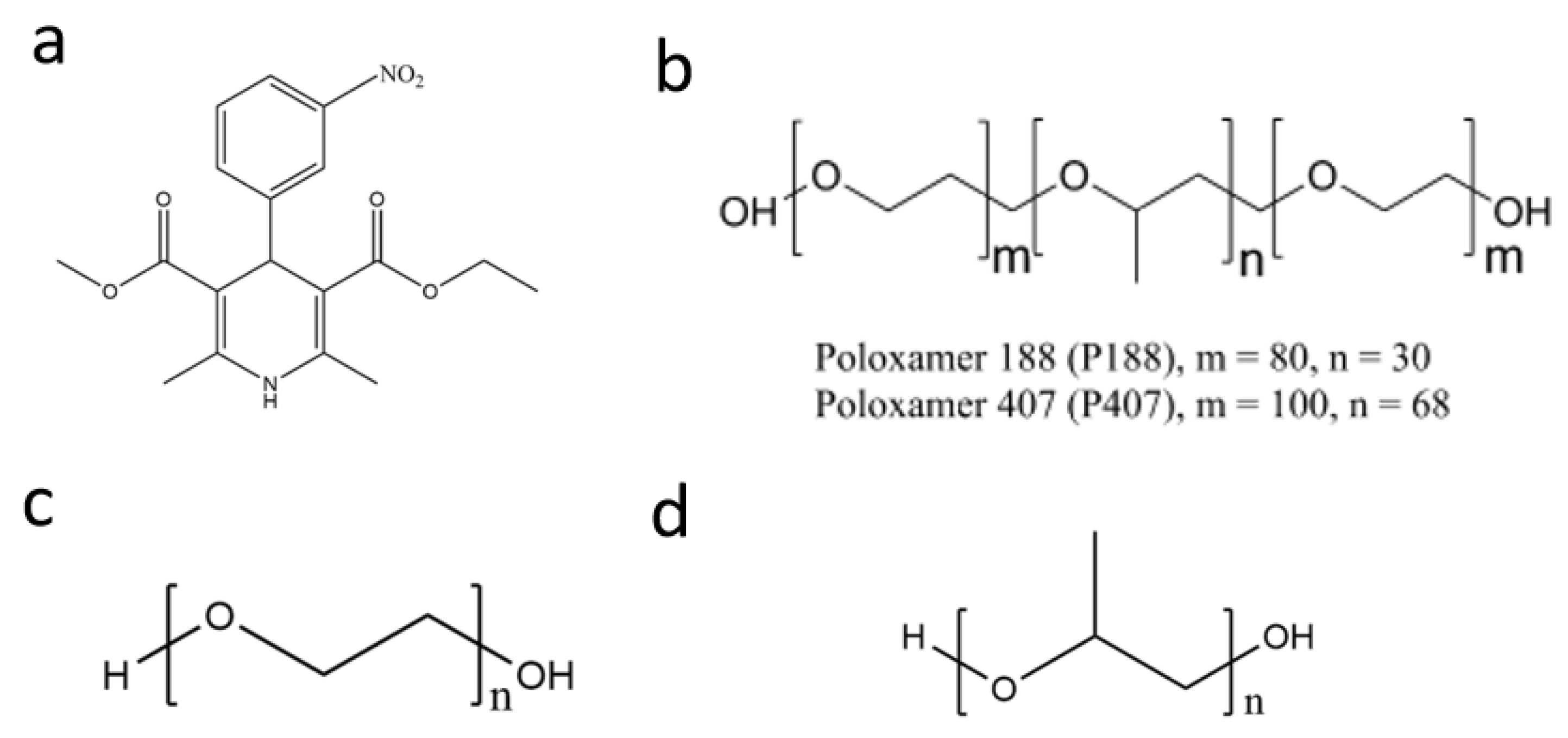
1. Introduction
2. Results and Discussion
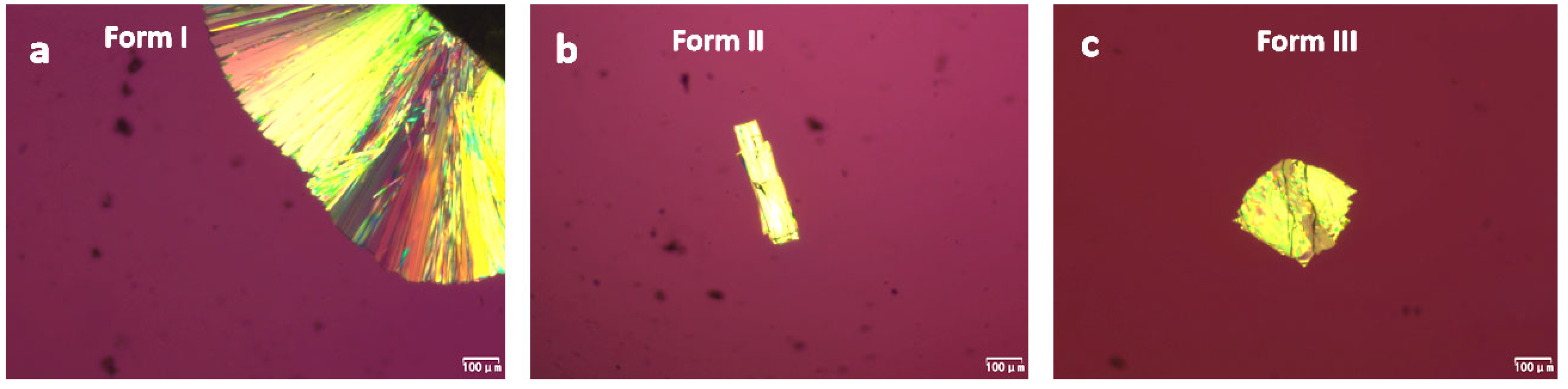
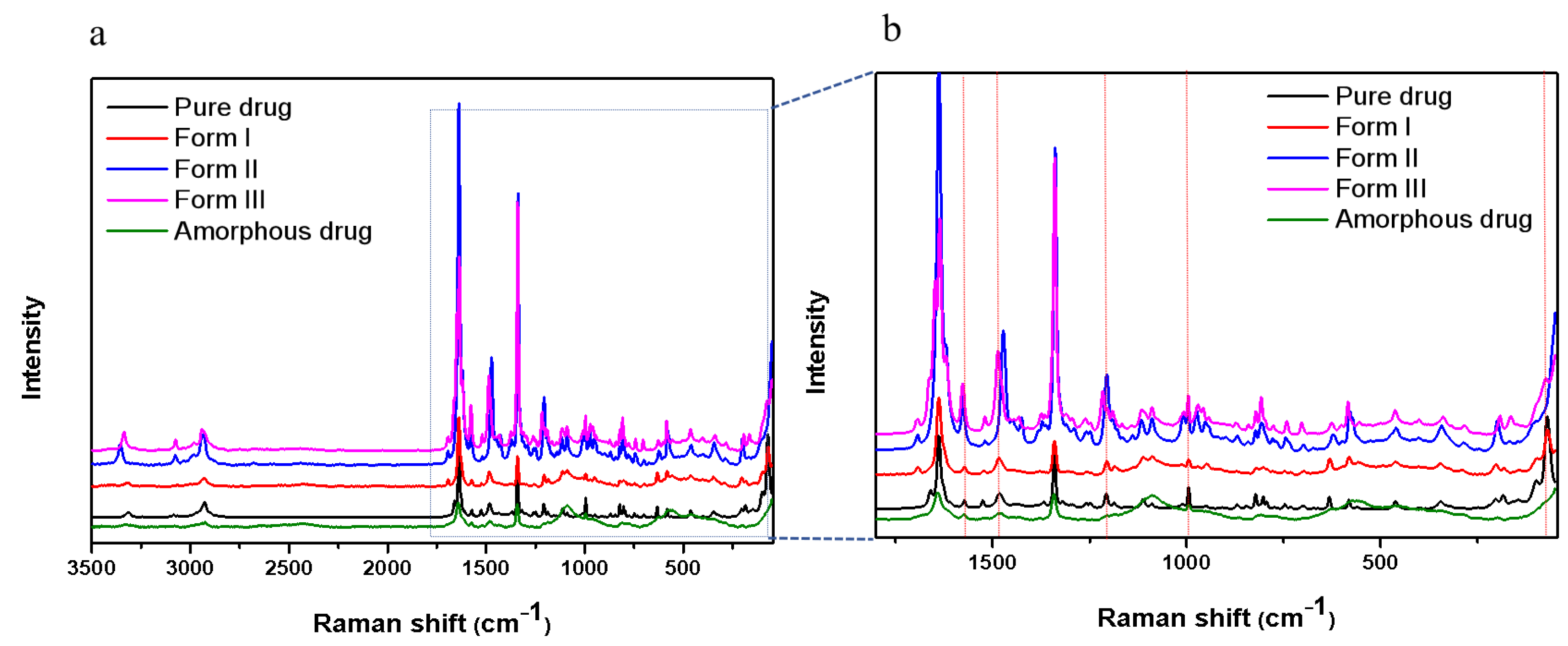
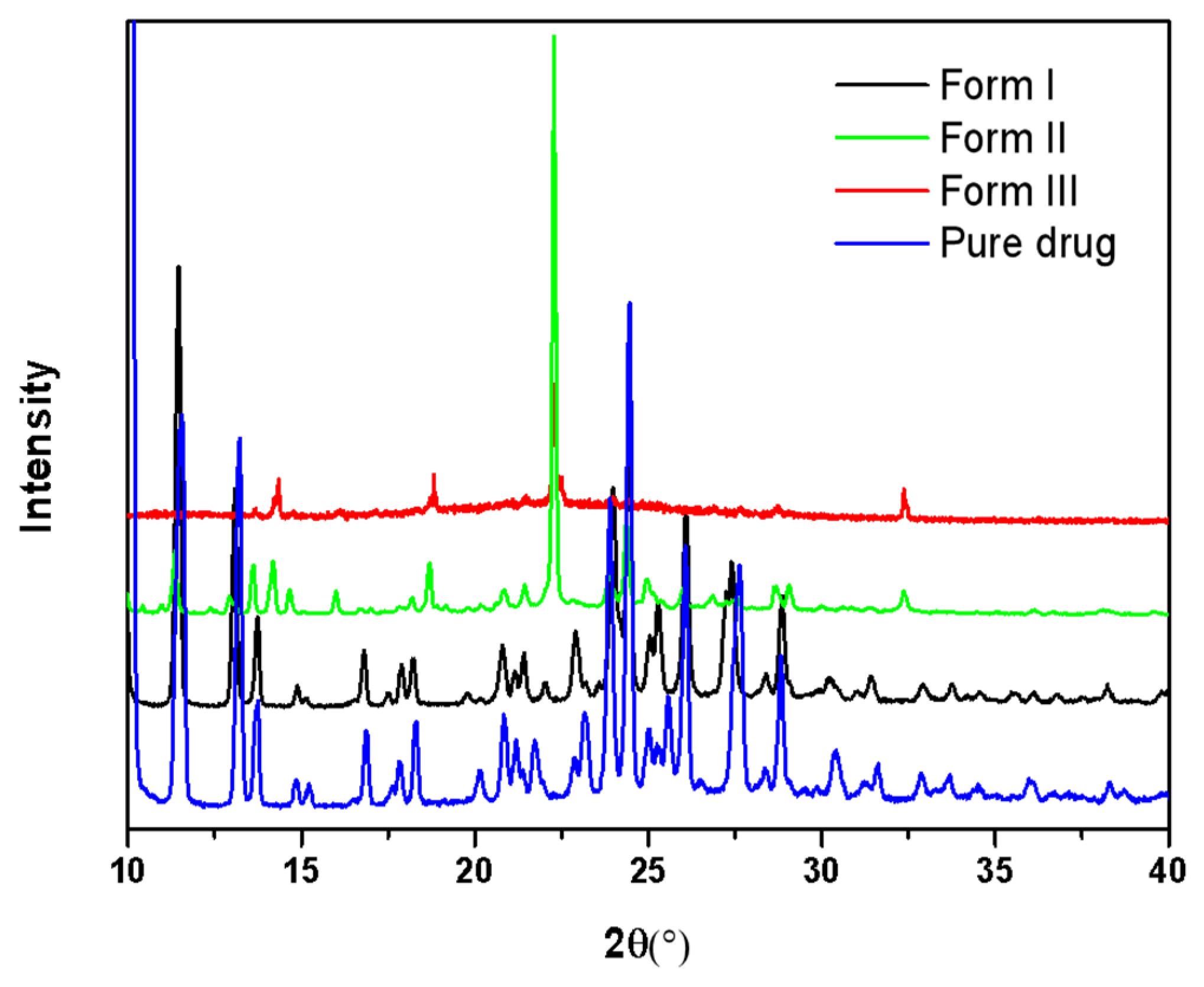
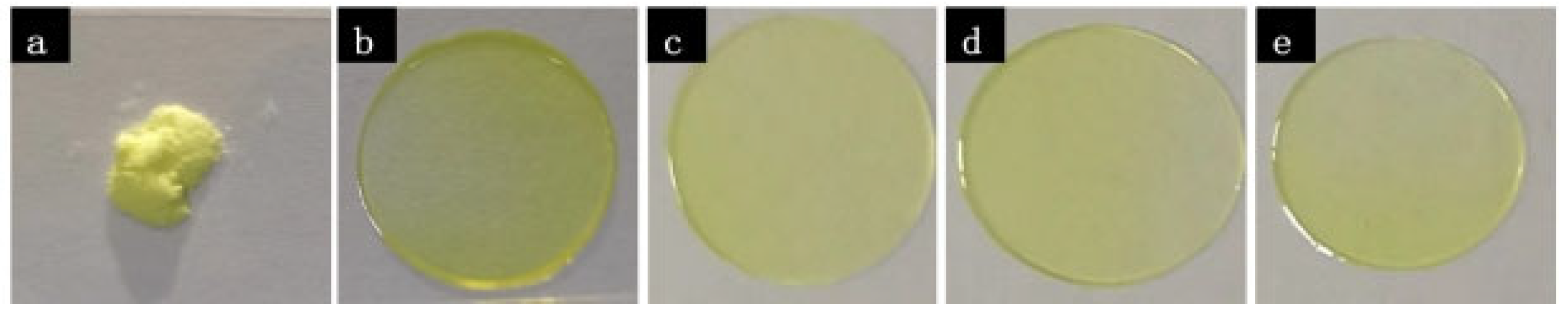
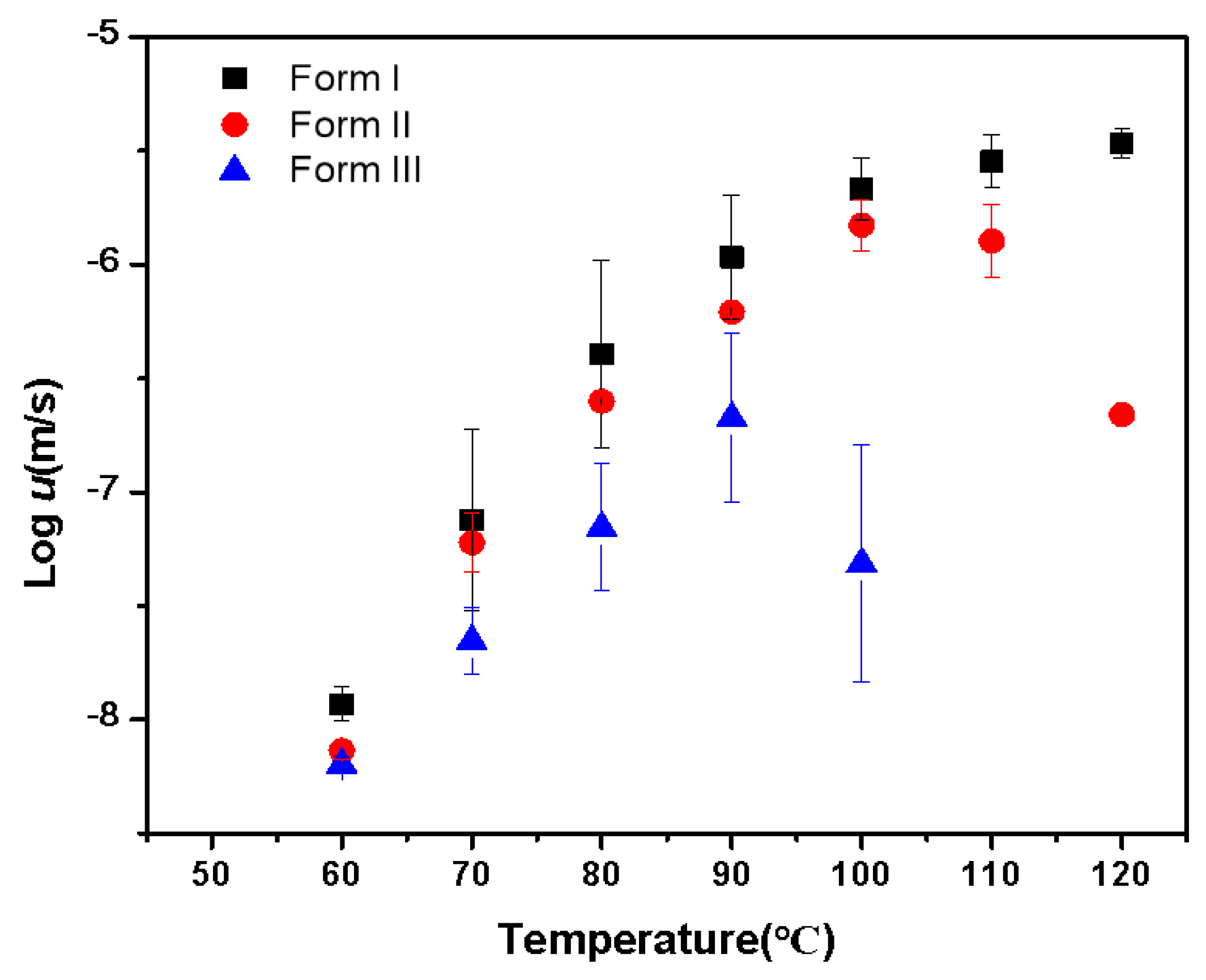
2.1. Crystallization of Pure NTP from the Melt
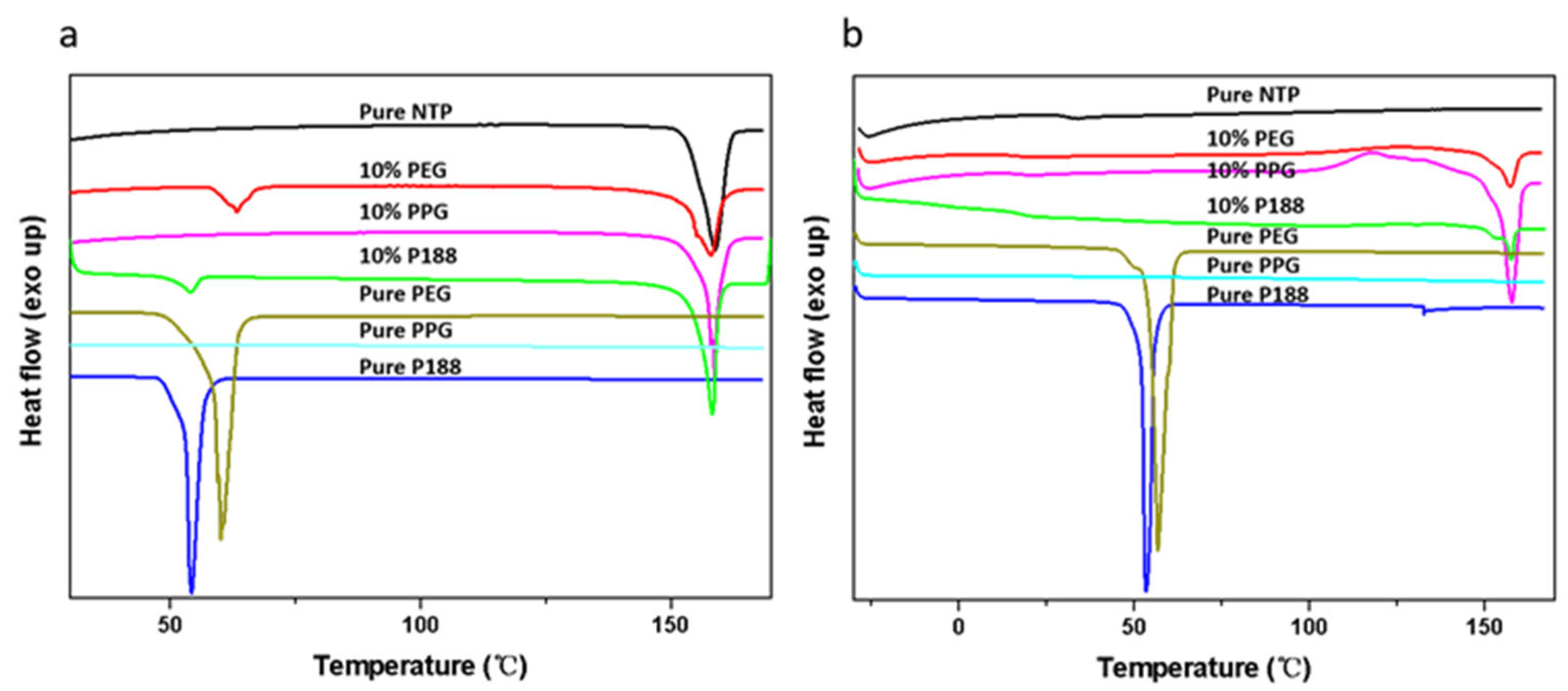
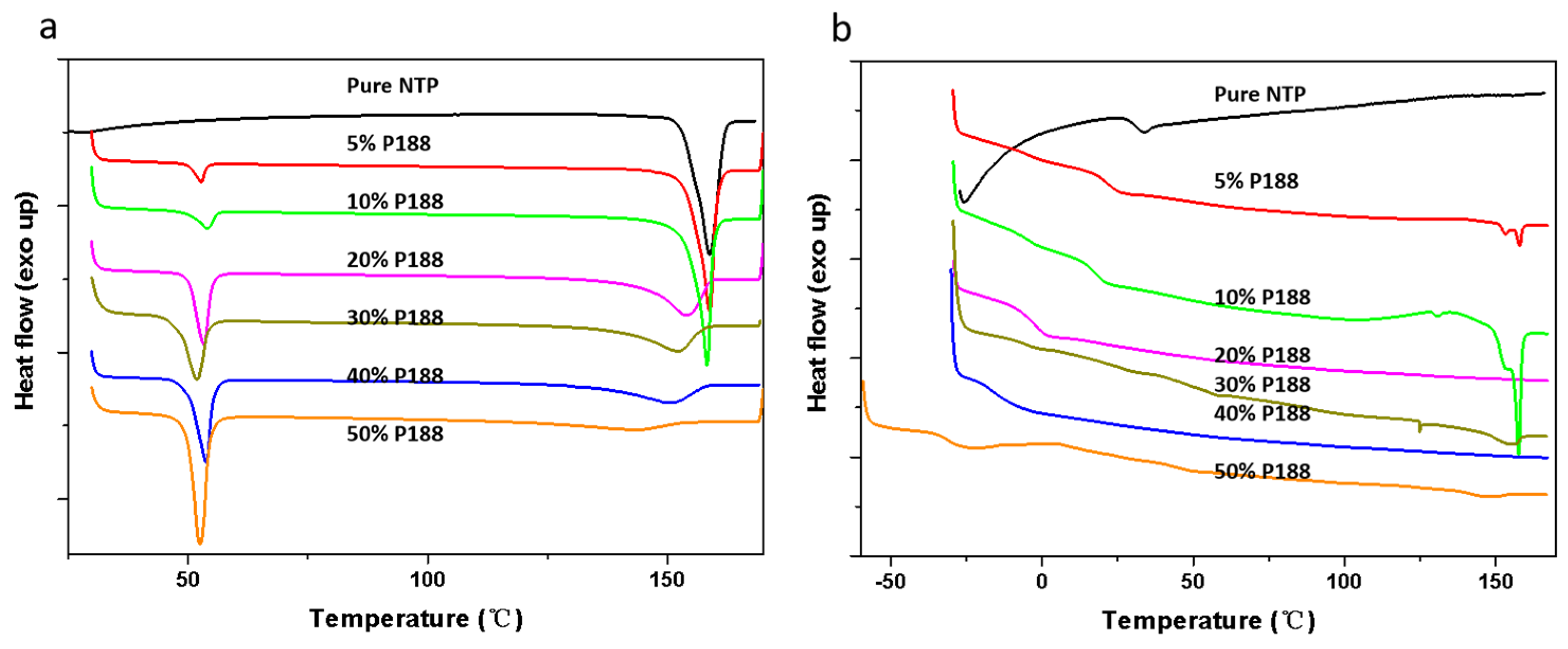
2.2. State of Mixing Between NTP and Additives
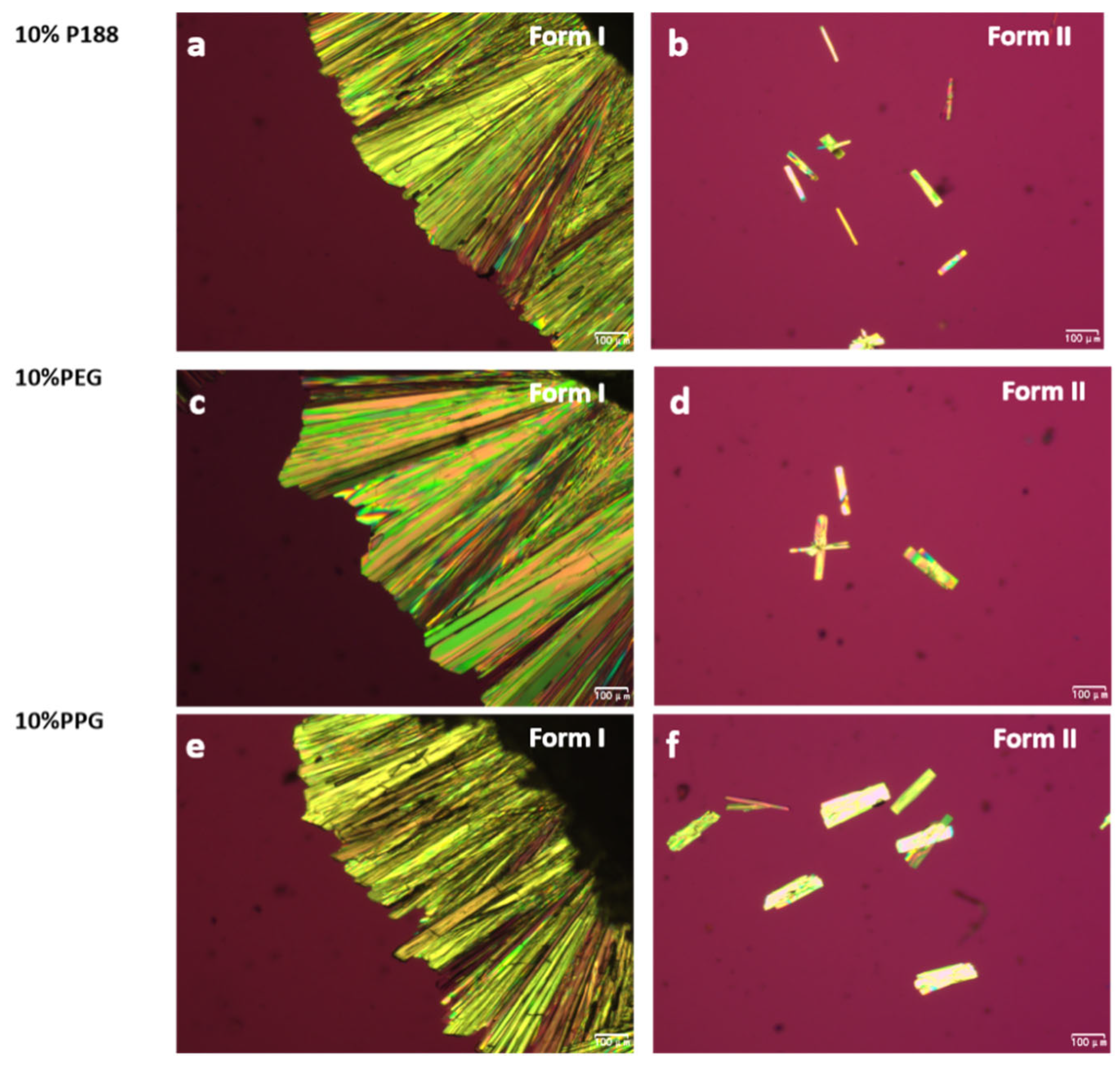
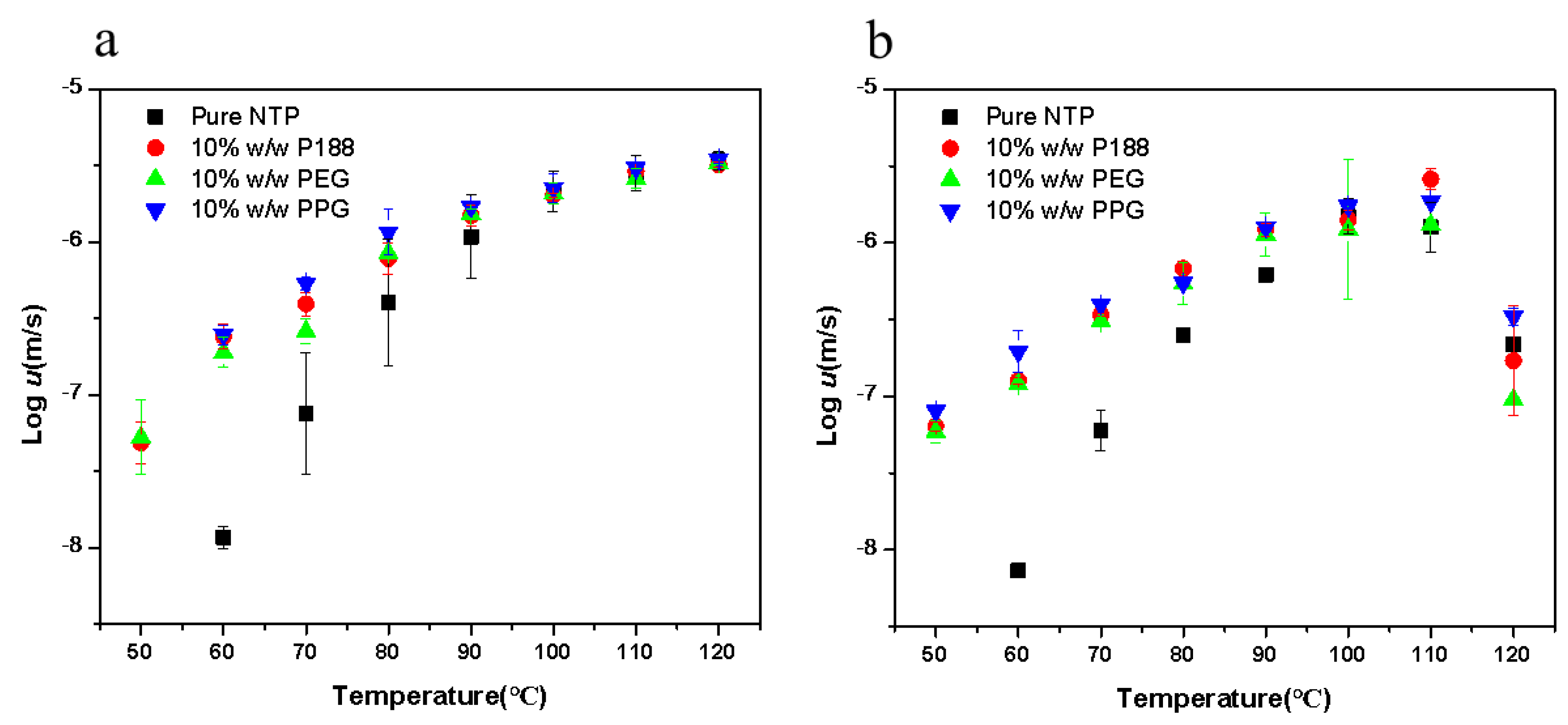
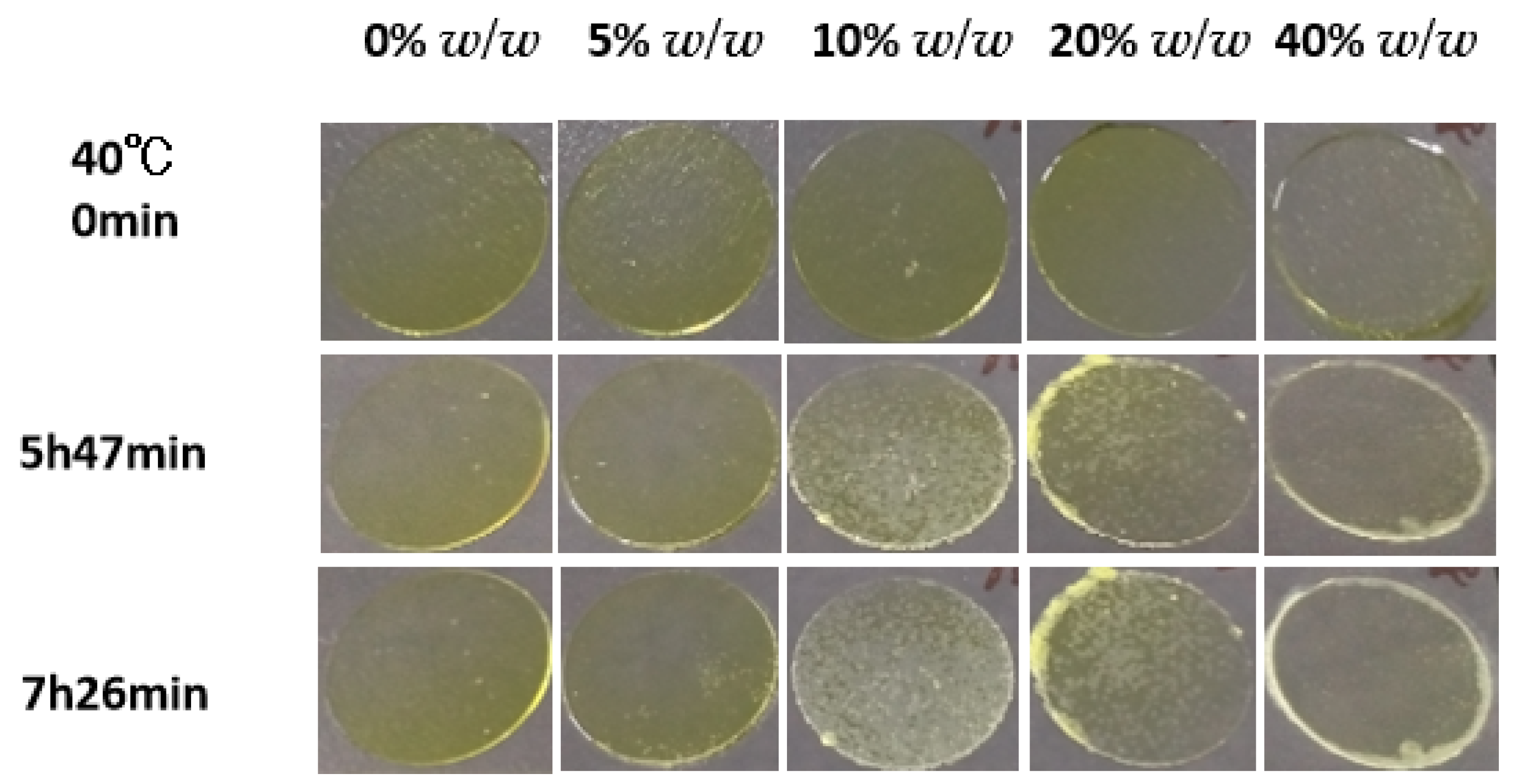
2.3. Crystallizations of NTP Doped with Additives
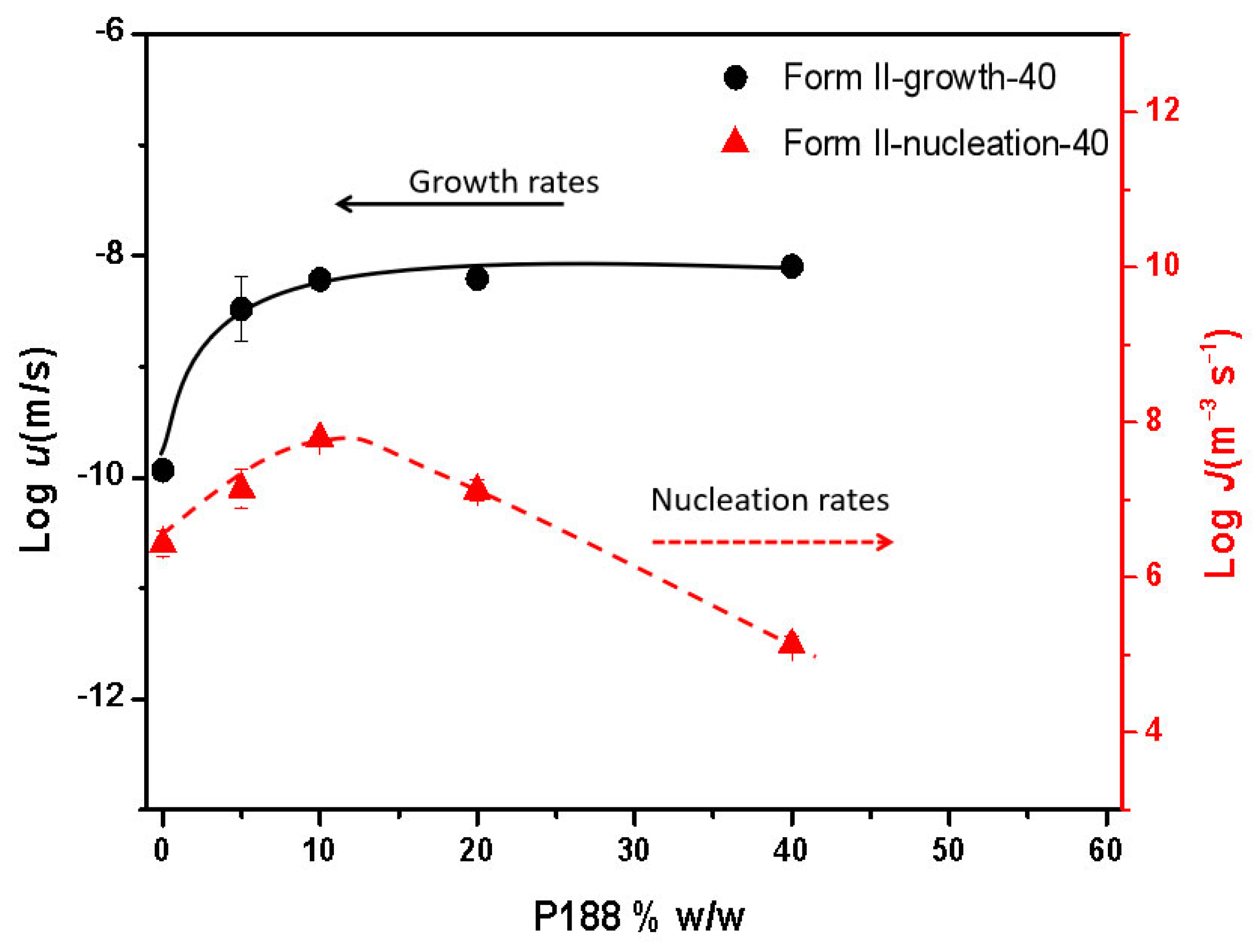
2.4. Different Effects on Nucleation and Growth Rates with Increasing Poloxamer Content
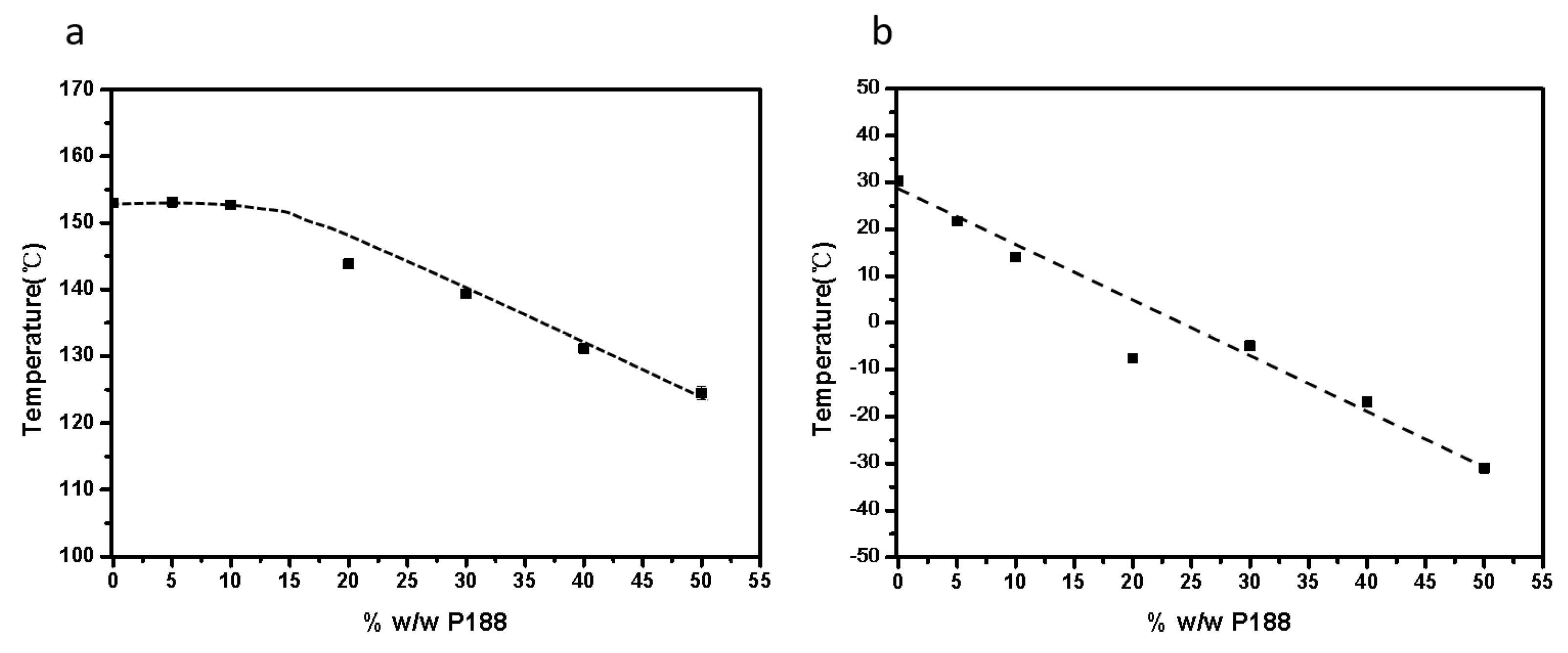
2.5. Effects of Tg and Tm on Crystallization Kinetics
3. Materials and Methods
3.1. Materials
3.2. Preparation of Drug/Additive Physical Mixtures and ASDs
3.3. Polarized Light Microscopy (PLM)
3.4. Powder X-Ray Diffraction (PXRD)
3.5. Raman Microscopy
3.6. Differential Scanning Calorimetry (DSC)
3.7. Crystal Growth Rates of NTP
3.8. Nucleation Rates of NTP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iyer, R.; Petrovska, J.V.; Berginc, K.; Jaklic, M.; Fabiani, F.; Harlacher, C. Amorphous Solid Dispersions (ASDs): The Influence of Material Properties, Manufacturing Processes and Analytical Technologies in Drug Product Development. Pharmaceutics 2021, 13, 1682. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Stacy, Y.; Wang, Y.N.; Xin, J.B. Physical stability of amorphous pharmaceutical solids: Nucleation, crystal growth, phase separation and effects of the polymers. Int. J. Pharm. 2020, 590, 119925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Wu, H.; Cai, T. Effect of polymeric excipients on nucleation and crystal growth kinetics of amorphous fluconazole. Biomater. Sci. 2021, 9, 4308–4316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, M.S.; Luo, M.Q.; Cai, T. Advances in the Development of Amorphous Solid Dispersions the Role of Polymeric Carriers. Asian J. Pharm. Sci. 2023, 18, 100834. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Q.; Xu, Z.; Yang, Q.; Hao, G.; Liu, M.; Zeng, Z. Recent Progress on Crystal Nucleation of Amorphous Solid Dispersion. Cryst. Growth Des. 2024, 24, 8655–8666. [Google Scholar] [CrossRef]
- Correa Soto, C.E.; Gao, Y.; Indulkar, A.S.; Ueda, K.; Zhang, G.G.Z.; Taylor, L. Impact of Surfactants on the Performance of Clopidogrel-Copovidone Amorphous Solid Dispersions: Increased Drug Loading and Stabilization of Nanodroplets. Pharm. Res. 2022, 39, 167–188. [Google Scholar] [CrossRef]
- Yao, X.; Benson, E.G.; Gui, Y.; Stelzer, T.; Zhang, G.G.Z.; Yu, L. Surfactants Accelerate Crystallization of Amorphous Nifedipine by Similar Enhancement of Nucleation and Growth Independent of Hydrophilic-Lipophilic Balance. Mol. Pharm. 2022, 19, 2343–2350. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.Q.; Luo, L.Q.; Li, K.; Zi, T.T.; Ren, J.J.; Pan, L.; Wang, Z.Y.; Wang, Z.H.; Liu, M.Z.; et al. Impact of Poloxamer on Crystal Nucleation and Growth of Amorphous Clotrimazole. Pharmaceutics 2023, 15, 2164. [Google Scholar] [CrossRef]
- Karolewicz, B.; Gajda, M.; Owczarek, A.; Pluta, J.; Górniak, A. Physicochemical Characterization and Dissolution Studies of Solid Dispersions of Clotrimazole with Pluronic F127. Trop. J. Pharm. Res. 2014, 13, 1225–1232. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Prezotti, F.; Araujo, F.; Lopes, C.; Loureiro, A.; Marques, S.; Sarmento, B. Third-generation solid dispersion combining Soluplus and poloxamer 407 enhances the oral bioavailability of resveratrol. Int. J. Pharm. 2021, 595, 120245. [Google Scholar] [CrossRef]
- Real, D.A.; Gagliano, A.; Orzan, L.; Leonardi, D.; Salomon, C.J. Amorphous solid dispersions of triclabendazole Keeping the supersaturated drug solution using poloxamers. J. Drug Deliv. Sci. Technol. 2024, 91, 105223. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yan, Q.L.; Zhong, X.P.; Hu, C.H. Evaluation of microstructure, dissolution rate, and oral bioavailability of paclitaxel poloxamer 188 solid dispersion. Drug Deliv. Transl. Res. 2024, 14, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, Y.; Liu, Y.; Guo, Y.F.; Hu, C.H. Influence of Intermolecular Interactions on Crystallite Size in Crystalline Solid Dispersions. Pharmaceutics 2023, 15, 2493. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Z.; Qian, F. Crystallization of bifonazole and acetaminophen within the matrix of semicrystalline, PEO-PPO-PEO triblock copolymers. Mol. Pharm. 2015, 12, 590–599. [Google Scholar] [CrossRef]
- Miyazaki, T.; Aso, Y.; Yoshioka, S.; Kawanishi, T. Differences in crystallization rate of nitrendipine enantiomers in amorphous solid dispersions with HPMC and HPMCP. Int. J. Pharm. 2011, 407, 111–118. [Google Scholar] [CrossRef]
- Burger, A.; Rollinger, J.M.; Brüggeller, P. Binary System of (R)- and (S)-Nitrendipines Polymorphism and Structure. J. Drug Deliv. Sci. Technol. 1997, 86, 674–679. [Google Scholar] [CrossRef]
- Li, J.; Fan, N.; Li, C.; Wang, J.; Li, S.; He, Z. The tracking of interfacial interaction of amorphous solid dispersions formed by water-soluble polymer and nitrendipine. Appl. Surf. Sci. 2017, 420, 136–144. [Google Scholar] [CrossRef]
- Chen, H.; Pui, Y.; Liu, C.; Chen, Z.; Su, C.C.; Hageman, M.; Hussain, M.; Haskell, R.; Stefanski, K.; Foster, K.; et al. Moisture-Induced Amorphous Phase Separation of Amorphous Solid Dispersions: Molecular Mechanism, Microstructure, and Its Impact on Dissolution Performance. J. Pharm. Sci. 2018, 107, 317–326. [Google Scholar] [CrossRef]
- Gorajana, A.; Rajendran, A.; Dua, K.; Pabreja, K.; Hoon, T.P. Preparation, Characterization, and In Vitro Evaluation of Nitrendipine Solid Dispersions. J. Dispers. Sci. Technol. 2012, 33, 676–684. [Google Scholar] [CrossRef]
- Xia, D.; Wu, J.X.; Cui, F.; Qu, H.; Rades, T.; Rantanen, J.; Yang, M. Solvent-mediated amorphous-to-crystalline transformation of nitrendipine in amorphous particle suspensions containing polymers. Eur. J. Pharm. Sci. 2012, 46, 446–454. [Google Scholar] [CrossRef]
- Tammann, G. Ueber die Abhängigkeit der Zahl der Kerne, welche sich in verschiedenen unterkühlten Flüssigkeiten bilden, von der Temperatur. Z. Phys. Chem. 1898, 25, 441–479. [Google Scholar] [CrossRef]
- Tammann, G.; Jenckel, E. Die kristallisationsgeschwindigkeit und die kernzahl des glycerins in abhangigkeit von der temperatur. Z. Anorg. Allg. Chem. 1930, 193, 76–80. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Z.; Gui, Y.; Shi, C.; Zhang, G.G.Z.; Yu, L. Crystal nucleation rates in glass-forming molecular liquids: D-sorbitol, D-arabitol, D-xylitol, and glycerol. J. Chem. Phys. 2018, 149, 054503. [Google Scholar] [CrossRef]
- Ou, X.; Li, X.; Rong, H.; Yu, L.; Lu, M. A general method for cultivating single crystals from melt microdroplets. Chem. Commun. 2020, 56, 9950–9953. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Q.; Guo, M.; Liu, Z.; Cai, T. Melt Crystallization of Indomethacin Polymorphs in the Presence of Poly(ethylene oxide): Selective Enrichment of the Polymer at the Crystal-Liquid Interface. Mol. Pharm. 2020, 17, 2064–2071. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Q.; Tao, J.; Peng, Y.Y.; Cai, T. Impact of Polymer Enrichment at the Crystal−Liquid Interface on Crystallization Kinetics of Amorphous Solid Dispersions. Mol. Pharm. 2019, 16, 1385–1396. [Google Scholar]
- Shi, Q.; Cai, T. Fast Crystal Growth of Amorphous Griseofulvin: Relations between Bulk and Surface Growth Modes. Cryst. Growth Des. 2016, 16, 3279–3286. [Google Scholar] [CrossRef]
- Musumeci, D.; Hasebe, M.; Yu, L. Crystallization of Organic Glasses: How Does Liquid Flow Damage Surface Crystal Growth? Cryst. Growth Des. 2016, 16, 2931–2936. [Google Scholar] [CrossRef]
- Kestur, U.S.; Taylor, L.S. Role of polymer chemistry in influencing crystal growth rates from amorphous felodipine. Crystengcomm 2010, 12, 2390–2397. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Xu, M.; Chen, Z.; Peng, X.; Yang, Q.; Cai, T.; Zeng, Z. Discovery of a new polymorph of clotrimazole through melt crystallization: Understanding nucleation and growth kinetics. J. Chem. Phys. 2022, 158, 034503. [Google Scholar] [CrossRef]
- Wu, H.; Yao, X.; Gui, Y.; Hao, H.; Yu, L. Surface Enhancement of Crystal Nucleation in Amorphous Acetaminophen. Cryst. Growth Des. 2022, 22, 5598–5606. [Google Scholar] [CrossRef]
- Yao, X.; Huang, C.; Benson, E.G.; Shi, C.; Zhang, G.G.Z.; Yu, L. Effect of polymers on crystallization in glass-forming molecular liquids: Equal suppression of nucleation and growth and master curve for prediction. Cryst. Growth Des. 2019, 20, 237–244. [Google Scholar] [CrossRef]
- Karthika, S.; Radhakrishnan, T.K.; Kalaichelvi, P. A Review of Classical and Nonclassical Nucleation Theories. Cryst. Growth Des. 2016, 16, 6663–6681. [Google Scholar] [CrossRef]
- Davey, R.J.; Schroeder, S.L.; ter Horst, J.H. Nucleation of organic crystals--a molecular perspective. Angew. Chem. Int. Ed. 2013, 52, 2166–2179. [Google Scholar] [CrossRef]
- Sato, T.; Taylor, L.S. Acceleration of the crystal growth rate of low molecular weight organic compounds in supercooled liquids in the presence of polyhydroxybutyrate. CrystEngComm 2017, 19, 80–87. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, J.; Zhang, G.G.; Yu, L. Solubilities of crystalline drugs in polymers: An improved analytical method and comparison of solubilities of indomethacin and nifedipine in PVP, PVP/VA, and PVAc. J. Pharm. Sci. 2010, 99, 4023–4031. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, Q.; Xu, M.; Tan, X.; Peng, X.; Yang, Z.; Li, K.; Yang, J.; Chen, J.; Xun, X.; et al. Accelerating Effects of Poloxamer and Its Structural Analogs on the Crystallization of Nitrendipine Polymorphs. Pharmaceuticals 2025, 18, 1000. https://doi.org/10.3390/ph18071000
Zhang J, Yang Q, Xu M, Tan X, Peng X, Yang Z, Li K, Yang J, Chen J, Xun X, et al. Accelerating Effects of Poloxamer and Its Structural Analogs on the Crystallization of Nitrendipine Polymorphs. Pharmaceuticals. 2025; 18(7):1000. https://doi.org/10.3390/ph18071000
Chicago/Turabian StyleZhang, Jie, Qiusheng Yang, Meixia Xu, Xinqiang Tan, Xucong Peng, Ziqing Yang, Kang Li, Jia Yang, Jie Chen, Xuan Xun, and et al. 2025. "Accelerating Effects of Poloxamer and Its Structural Analogs on the Crystallization of Nitrendipine Polymorphs" Pharmaceuticals 18, no. 7: 1000. https://doi.org/10.3390/ph18071000
APA StyleZhang, J., Yang, Q., Xu, M., Tan, X., Peng, X., Yang, Z., Li, K., Yang, J., Chen, J., Xun, X., Xiao, S., Zhou, L., Liu, M., & Zeng, Z. (2025). Accelerating Effects of Poloxamer and Its Structural Analogs on the Crystallization of Nitrendipine Polymorphs. Pharmaceuticals, 18(7), 1000. https://doi.org/10.3390/ph18071000






