Impact of Age and Menopausal Status on T-DM1 (Ado-Trastuzumab Emtansine) Treatment Outcomes in HER2-Positive Breast Cancer
Abstract
1. Introduction
1.1. Background
1.2. Hormonal Influence and Menopausal Status
1.3. Clinical Relevance of Menopausal Status
1.4. Rationale and Research Gap
1.5. Objective
2. Results
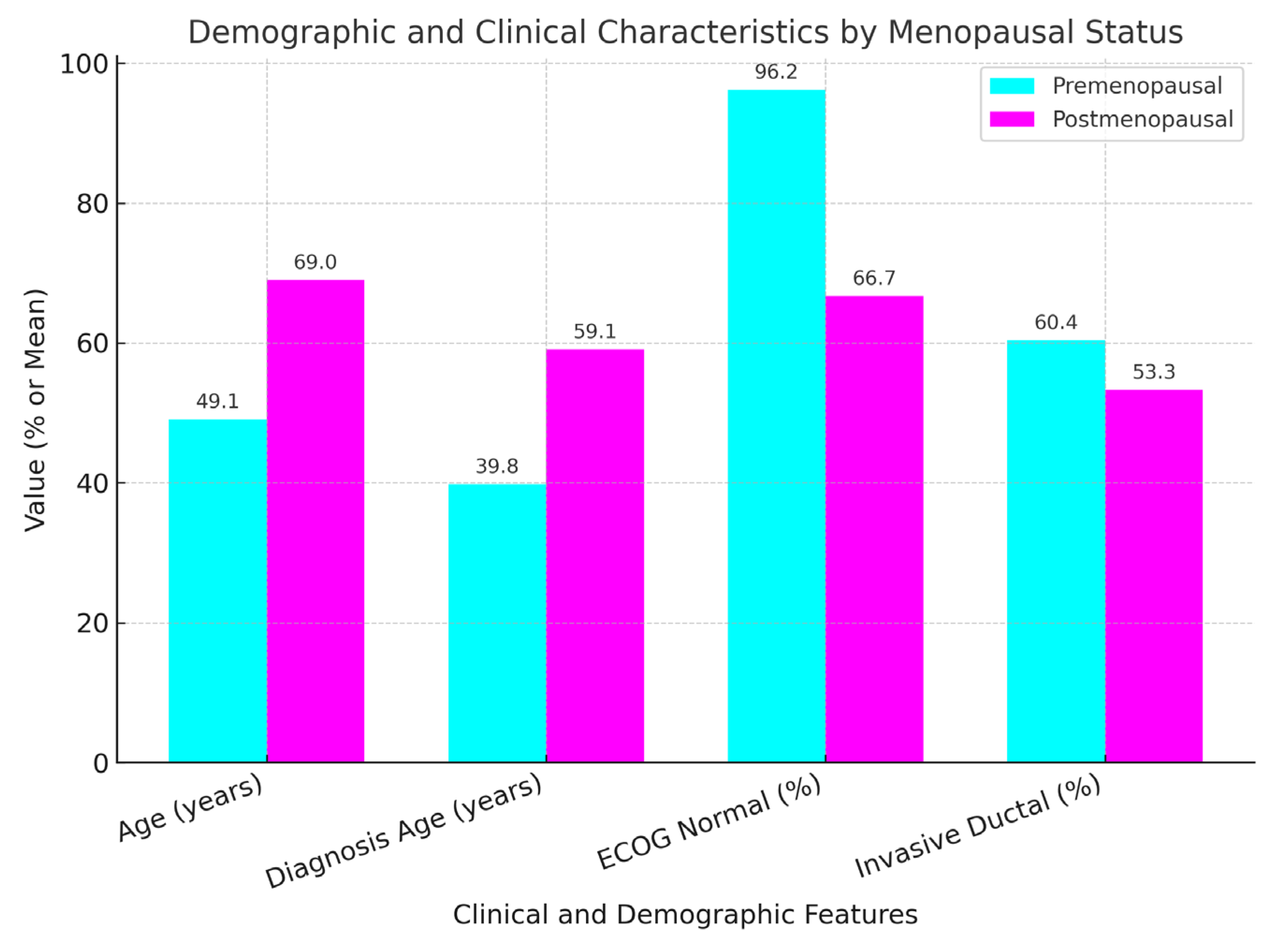
2.1. Patient and Disease Characteristics by Menopausal Status (Table 1)
| Characteristic | Premenopausal (n = 53) | Postmenopausal (n = 45) | p-Value |
|---|---|---|---|
| Age (years) | 49.07 ± 7.98 | 69 ± 8.38 | <0.01 |
| Diagnosis Age (years) | 39.81 ± 6.24 | 59.07 ± 8.01 | <0.01 |
| ECOG Normal (%) | 96.2 | 66.7 | <0.01 |
| Invasive Ductal (%) | 60.4 | 53.3 | 0.235 |
| Stage, Grade, Biomarkers | No significant difference | No significant difference | >0.05 |
2.2. Metastatic Regions by Menopausal Status
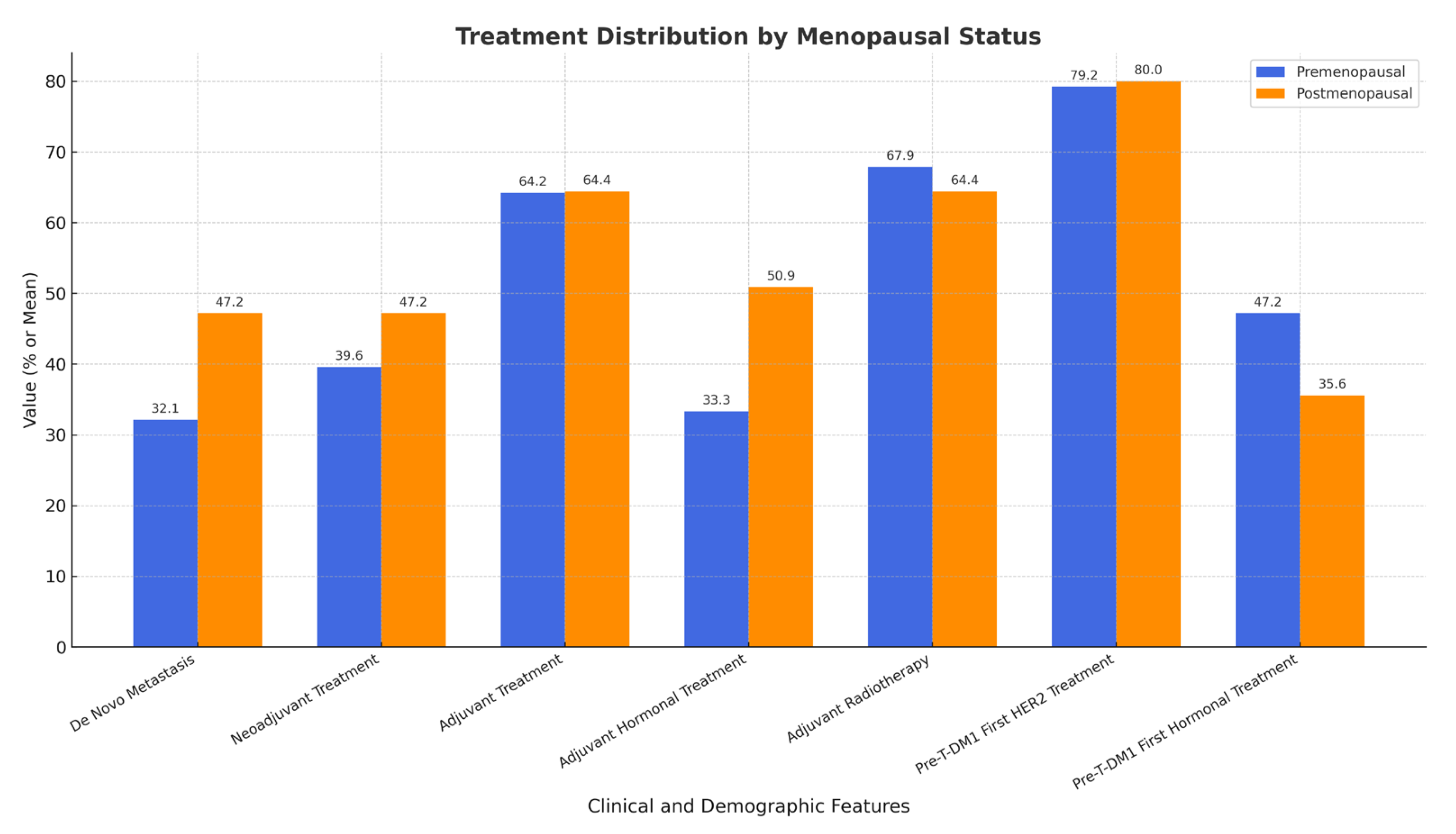
2.3. Treatment History by Menopausal Status
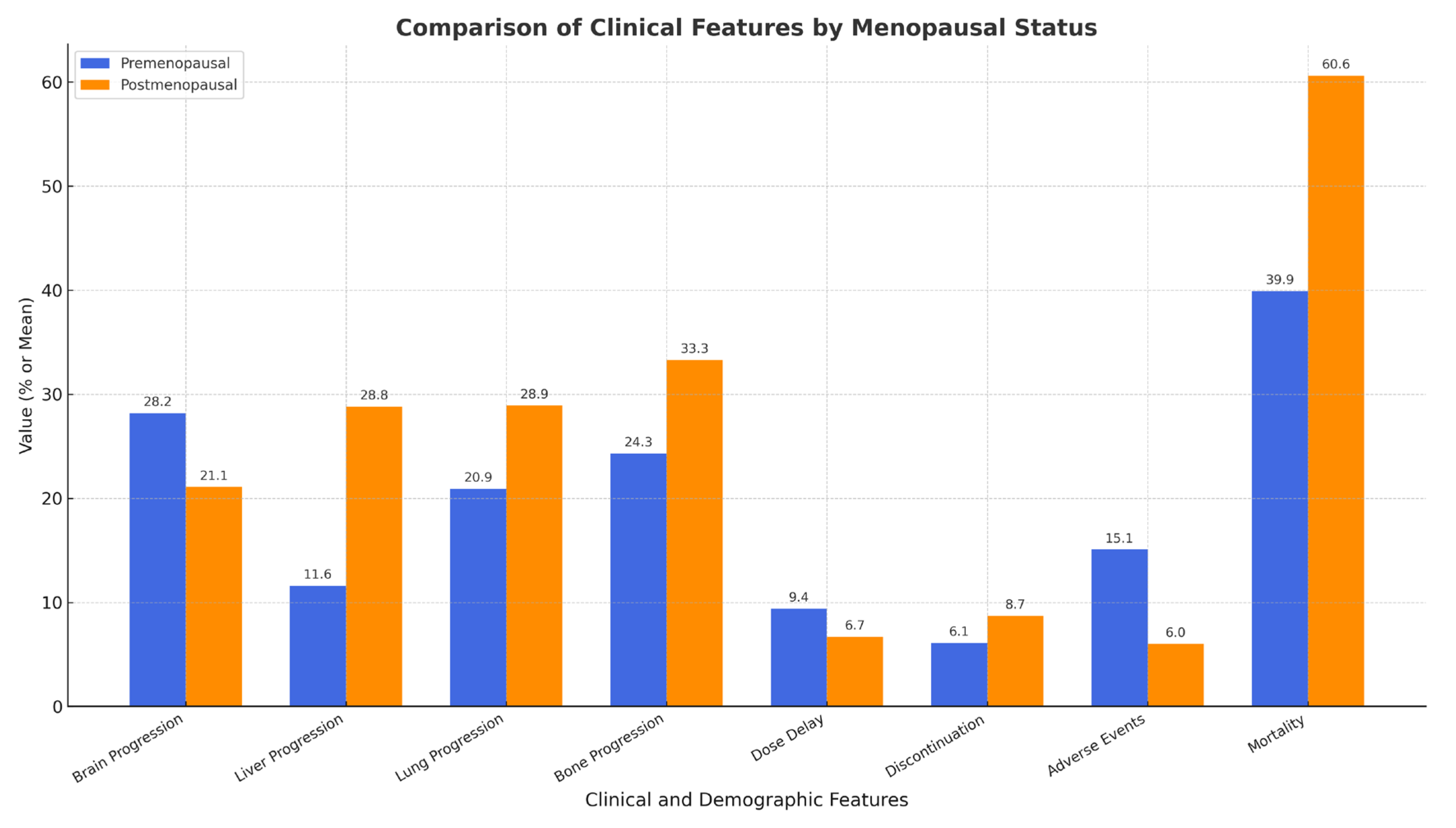
2.4. TDM-1 Treatment Response and Side Effects
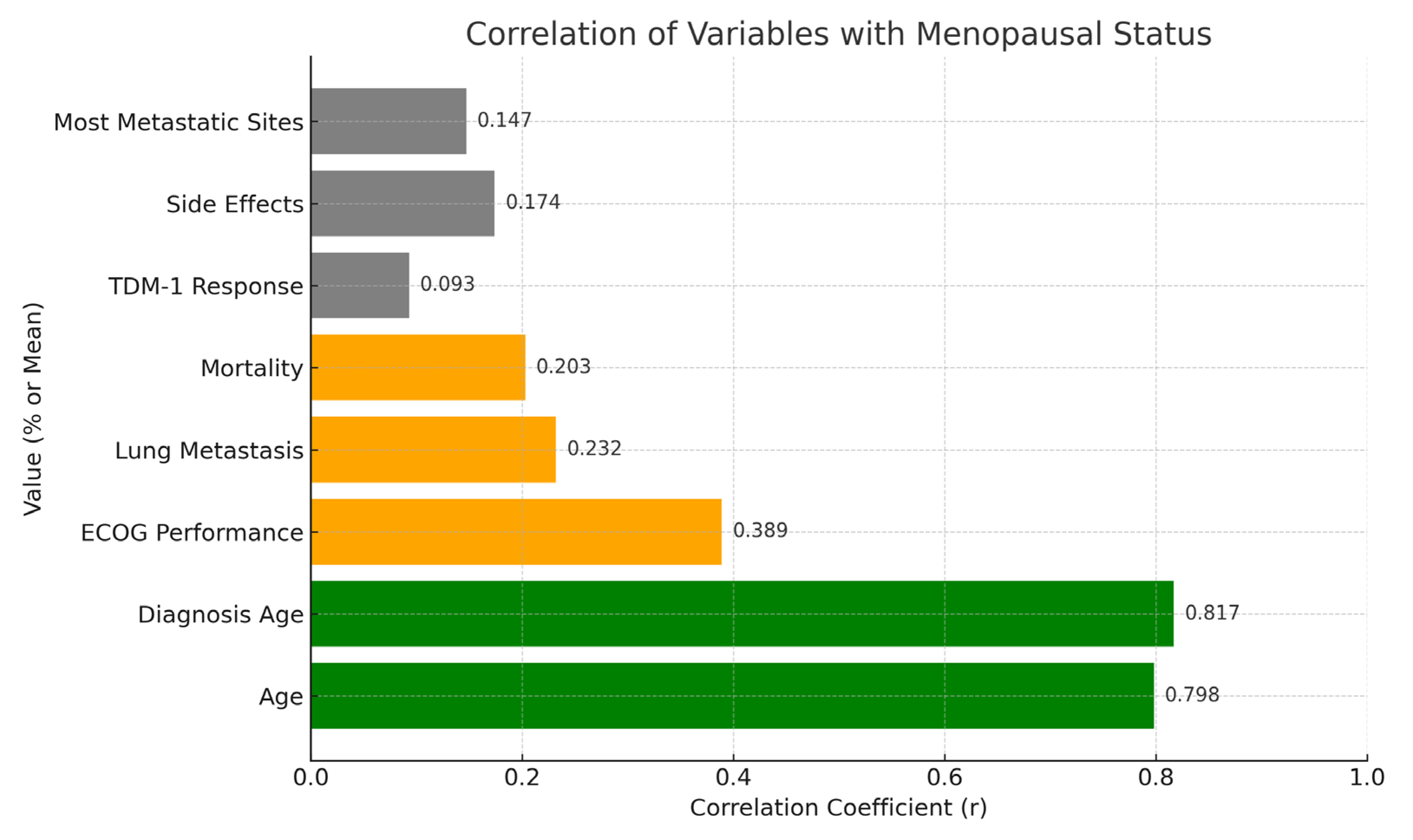
2.5. Correlation Analysis
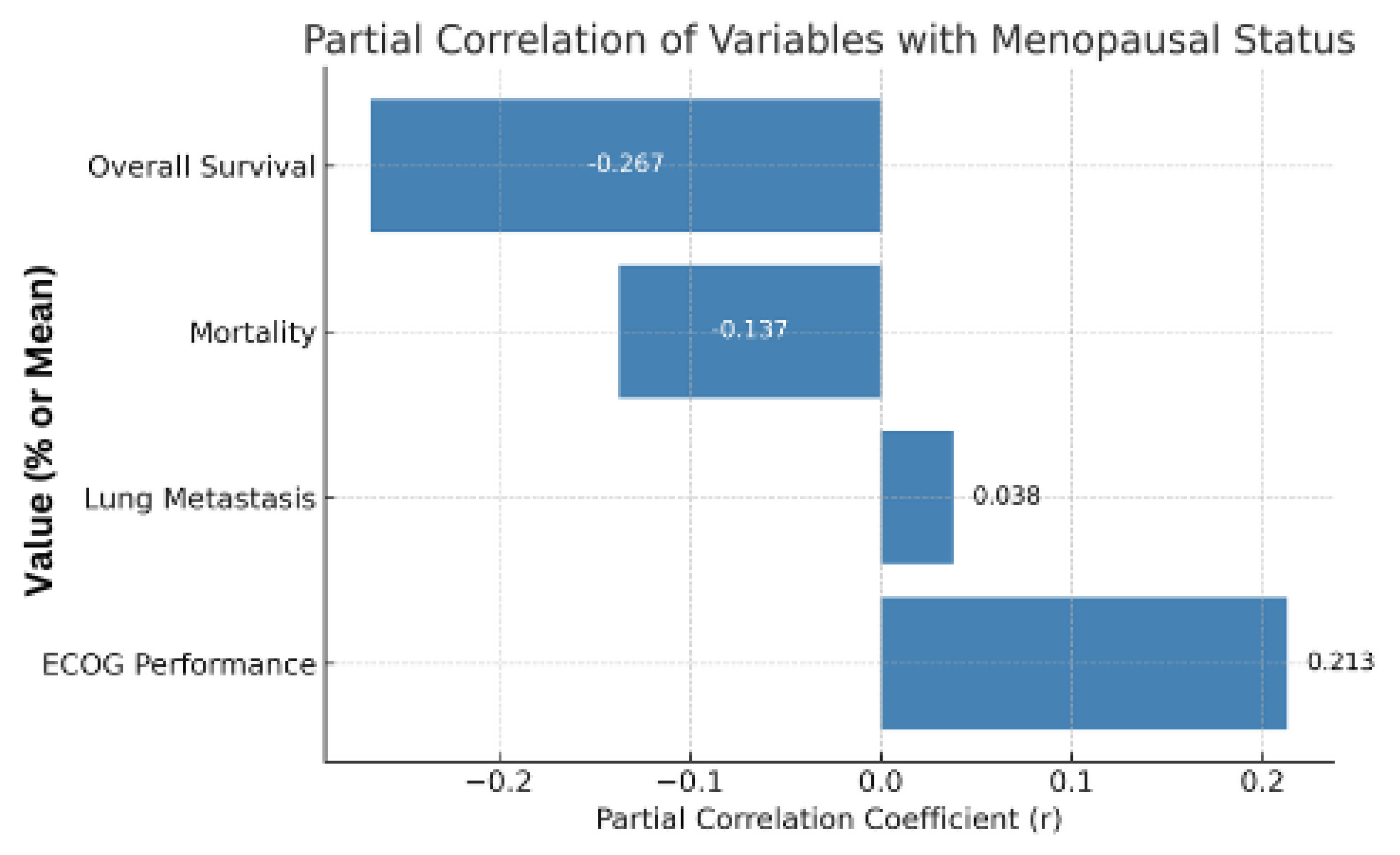
2.6. Partial Correlation Controlling for Age
3. Discussion
3.1. Interpretation of Findings
3.2. Comparison with the Literature
3.3. Clinical Implications
3.4. Future Directions
3.5. Strengths and Limitations
4. Materials and Methods
4.1. Study Design
4.2. Patient Population
4.3. Data Collection
4.4. Statistical Analysis
5. Conclusions
5.1. Summary
5.2. Significance
5.3. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Breast Cancer Fact Sheet. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 4 May 2025).
- Anderson, W.F.; Chu, K.C.; Chatterjee, N. Tumor variants by hormone receptor expression in White and Black women with breast cancer. J. Natl. Cancer Inst. 2002, 94, 364–371. [Google Scholar]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Kim, S.B.; González-Martín, A.; LoRusso, P.M.; Ferrero, J.-M.; Smitt, M.; Yu, R.; Leung, A.C.F.; Wildiers, H. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Benz, C.C.; Scott, G.K.; Sarup, J.C.; Johnson, R.M.; Tripathy, D.; Coronado, E.; Shepard, H.M.; Osborne, C.K. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res. Treat. 1992, 24, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Yancik, R.; Wesley, M.N.; Ries, L.A.G.; Havlik, R.J.; Edwards, B.K.; Yates, J.W. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA 2001, 285, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S. Neoadjuvant treatment of breast cancer: Maximizing pathologic complete response rates to improve prognosis. Curr. Opin. Obstet. Gynecol. 2015, 27, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.U.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Schiff, R. Mechanisms of endocrine resistance in breast cancer. Annu. Rev. Med. 2011, 62, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Pondé, N.; Gelber, R.D.; Piccart, M. Menopause and breast cancer: Does menopausal status impact adjuvant trastuzumab outcome? Breast Cancer Res. Treat. 2018, 171, 265–272. [Google Scholar]
- Hurvitz, S.A.; Andre, F.; Jiang, Z.; Shao, Z.; Mano, M.S.; Neciosup, S.P.; Tseng, L.-M.; Zhang, Q.; Shen, K.; Liu, D.; et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015, 16, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, S.; Osborne, C.K.; Creighton, C.J.; Qin, L.; Tsimelzon, A.; Huang, S.; Weiss, H.; Rimawi, M.; Schiff, R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008, 68, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, J.; Earl, H.M. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 2019, 393, 2599–2612. [Google Scholar] [CrossRef]
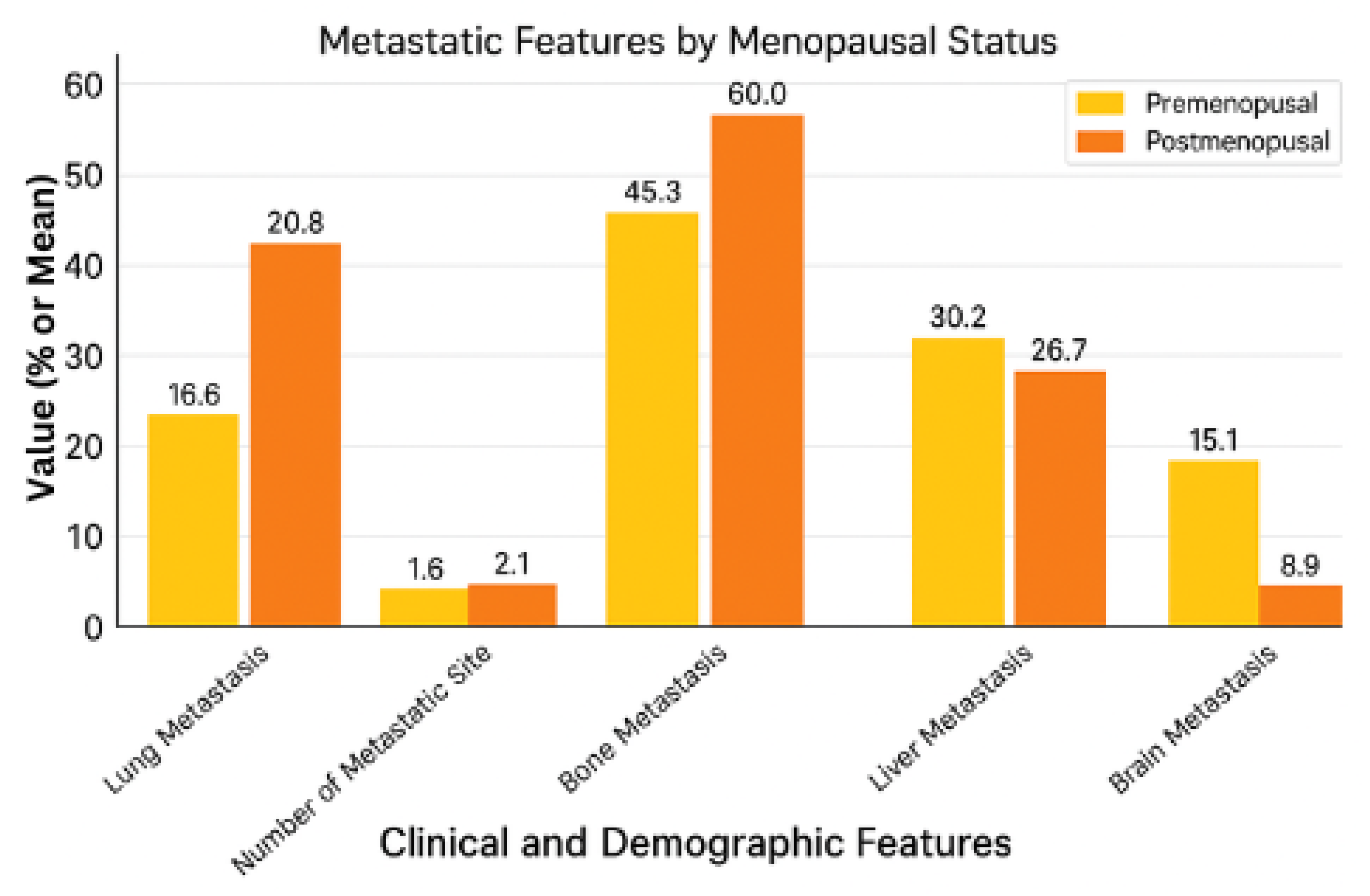
| Metastatic Feature | Premenopausal (n = 53) | Postmenopausal (n = 45) | p-Value |
|---|---|---|---|
| Lung Metastasis | 20.8% (11/53) | 42.2% (19/45) | 0.038 * |
| Number of Metastatic Sites | 1.58 ± 1.05 (Mean ± SD) | 2.07 ± 1.21 (Mean ± SD) | 0.030 * |
| Bone Metastasis | 45.3% (24/53) | 60.0% (27/45) | 0.211 |
| Liver Metastasis | 30.2% (16/53) | 26.7% (12/45) | 0.873 |
| Brain Metastasis | 15.1% (8/53) | 8.9% (4/45) | 0.532 |
| Treatment Feature | Premenopausal (n = 53) | Postmenopausal (n = 45) | p-Value |
|---|---|---|---|
| De Novo Metastasis | 32.1% (17/53) | 47.2% (25/53) | 1.000 |
| Neoadjuvant Treatment | 39.6% (21/53) | 47.2% (25/53) | 0.665 |
| Adjuvant Treatment | 64.2% (34/53) | 64.4% (29/45) | 1.000 |
| Adjuvant Hormonal Treatment | 50.9% (27/53) | 33.3% (15/45) | 0.121 |
| Adjuvant Radiotherapy | 67.9% (36/53) | 64.4% (29/45) | 0.882 |
| Pre-TDM-1 First HER2 Treatment | 79.2% (42/53) | 80.0% (36/45) | 1.000 |
| Pre-TDM-1 First Hormonal Treatment | 47.2% (25/53) | 35.6% (16/45) | 0.339 |
| Feature | Premenopausal (n = 53) | Postmenopausal (n = 45) | p-Value |
|---|---|---|---|
| Brain Progression | 28.3% (15/53) | 31.1% (14/45) | 0.935 |
| Liver Progression | 13.2% (7/53) | 28.9% (13/45) | 0.095 |
| Lung Progression | 20.8% (11/53) | 33.3% (15/45) | 0.240 |
| Bone Progression | 24.5% (13/53) | 33.3% (15/45) | 0.461 |
| Dose Delay | 9.4% (5/53) | 6.7% (3/45) | 0.898 |
| Discontinuation | 5.7% (3/53) | 6.7% (3/45) | 1.000 |
| Adverse Events | 15.1% (8/53) | 8.9% (4/45) | 0.532 |
| Mortality | 39.6% (21/53) | 60.0% (27/45) | 0.071 |
| Overall Survival (years) | 6.23 ± 4.23 | 5.63 ± 4.13 | 0.477 |
| Variable | Correlation with Menopause (r) | p-Value |
|---|---|---|
| Age | 0.798 | <0.01 |
| Diagnosis Age | 0.837 | <0.01 |
| ECOG Performance | 0.389 | <0.01 |
| Lung Metastasis | 0.232 | <0.05 |
| Mortality | 0.203 | <0.05 |
| TDM-1 Response (e.g., Brain Progression) | 0.031 | 0.935 |
| Side Effects (e.g., Adverse Events) | −0.094 | 0.532 |
| Most Metastatic Sites (e.g., Bone) | 0.147 | 0.147 |
| Variable | Partial Correlation with Menopause (r) | p-Value |
|---|---|---|
| ECOG Performance | 0.213 | >0.05 |
| Lung Metastasis | 0.038 | >0.05 |
| Mortality | −0.137 | >0.05 |
| Overall Survival | −0.287 | >0.05 |
| Feature | Premenopausal | Postmenopausal | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 49.07 ± 7.98 | 69.00 ± 8.38 | <0.01 |
| ECOG Normal (%) | 96.2% | 66.7% | <0.01 |
| Lung Metastasis (%) | 20.8% | 42.2% | 0.038 |
| Number of Metastatic Sites (mean) | 1.58 | 0.07 | 0.030 |
| Mortality (%) | 39.6% | 60.0% | 0.071 |
| T-DM1 Response/Side Effects | No significant difference | No significant difference | >0.05 |
| Correlation with Menopause (Mortality) | r = 0.203 | <0.05 | |
| Independent Effect After Controlling for Age | Not significant | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surmeli, H.; Isik, D.; Kinikoglu, O.; Altintas, Y.E.; Ozkerim, U.; Oksuz, S.; Basoglu, T.; Odabas, H.; Turan, N. Impact of Age and Menopausal Status on T-DM1 (Ado-Trastuzumab Emtansine) Treatment Outcomes in HER2-Positive Breast Cancer. Pharmaceuticals 2025, 18, 931. https://doi.org/10.3390/ph18060931
Surmeli H, Isik D, Kinikoglu O, Altintas YE, Ozkerim U, Oksuz S, Basoglu T, Odabas H, Turan N. Impact of Age and Menopausal Status on T-DM1 (Ado-Trastuzumab Emtansine) Treatment Outcomes in HER2-Positive Breast Cancer. Pharmaceuticals. 2025; 18(6):931. https://doi.org/10.3390/ph18060931
Chicago/Turabian StyleSurmeli, Heves, Deniz Isik, Oguzcan Kinikoglu, Yunus Emre Altintas, Ugur Ozkerim, Sıla Oksuz, Tugba Basoglu, Hatice Odabas, and Nedim Turan. 2025. "Impact of Age and Menopausal Status on T-DM1 (Ado-Trastuzumab Emtansine) Treatment Outcomes in HER2-Positive Breast Cancer" Pharmaceuticals 18, no. 6: 931. https://doi.org/10.3390/ph18060931
APA StyleSurmeli, H., Isik, D., Kinikoglu, O., Altintas, Y. E., Ozkerim, U., Oksuz, S., Basoglu, T., Odabas, H., & Turan, N. (2025). Impact of Age and Menopausal Status on T-DM1 (Ado-Trastuzumab Emtansine) Treatment Outcomes in HER2-Positive Breast Cancer. Pharmaceuticals, 18(6), 931. https://doi.org/10.3390/ph18060931






