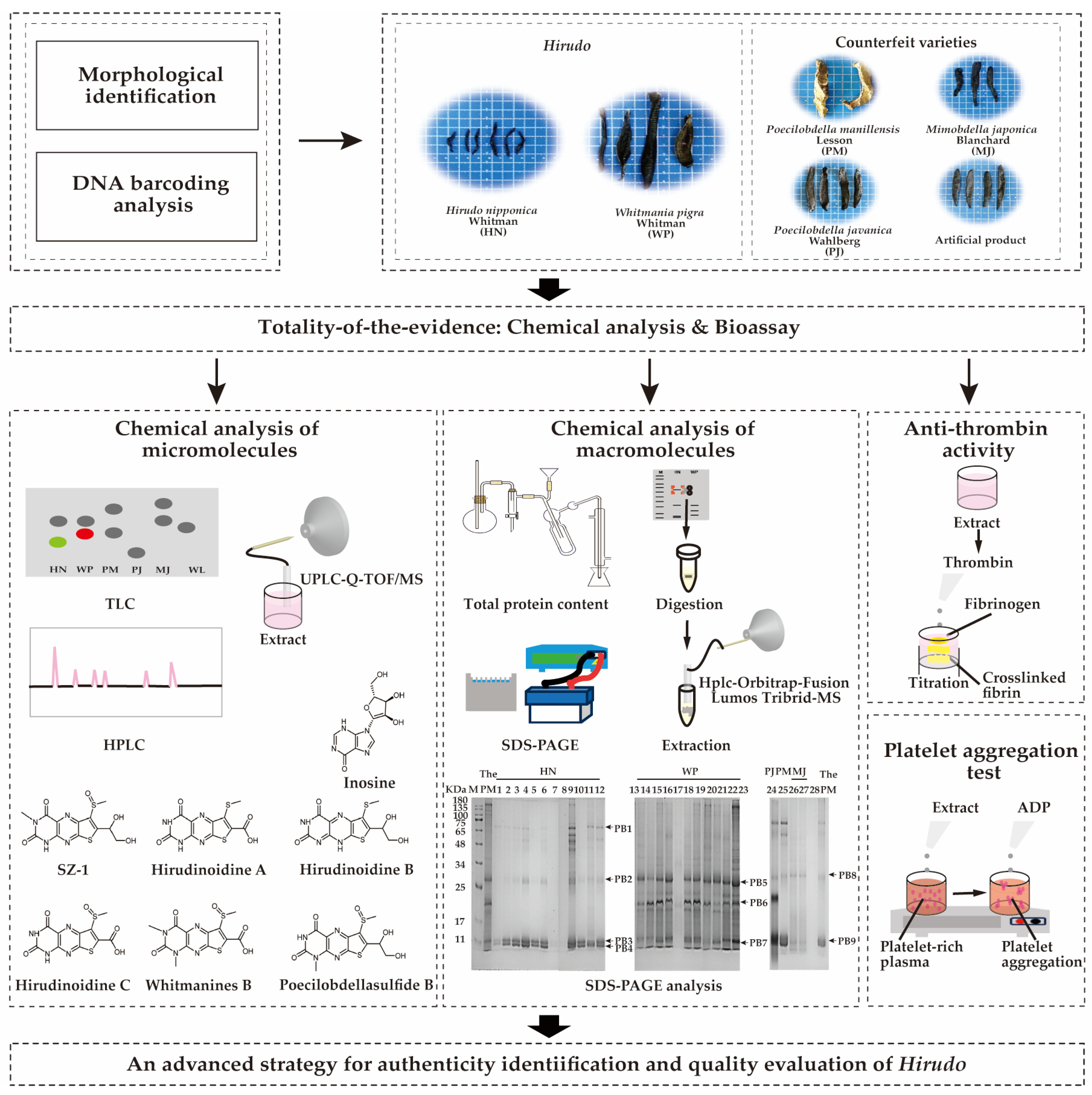
A Comprehensive Quality Evaluation System for Medicinal Leeches by Integrating Macromolecular Protein Analysis and Small-Molecule Marker Detection as Well as Quantitative Bioassays
Abstract
1. Introduction
2. Results
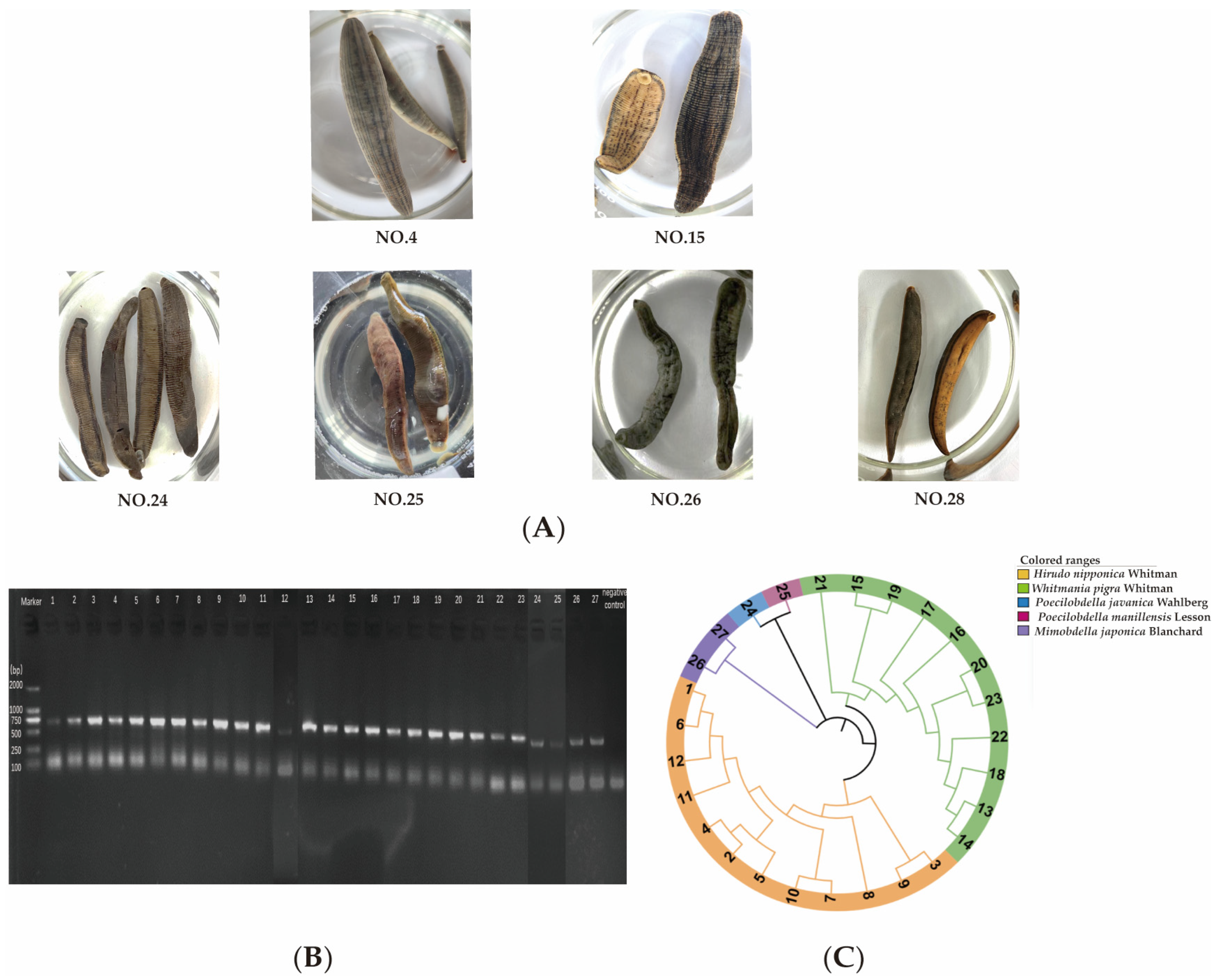
2.1. Identification of Leech Species Based on Morphological Characteristics and DNA Barcoding
2.2. Micromolecular Chemical Characterization
2.2.1. Chemical Characterization of 28 Samples by HPLC
2.2.2. Characteristic Compounds Identification in Different Leech Species
2.3. Macromolecular Chemical Characterization
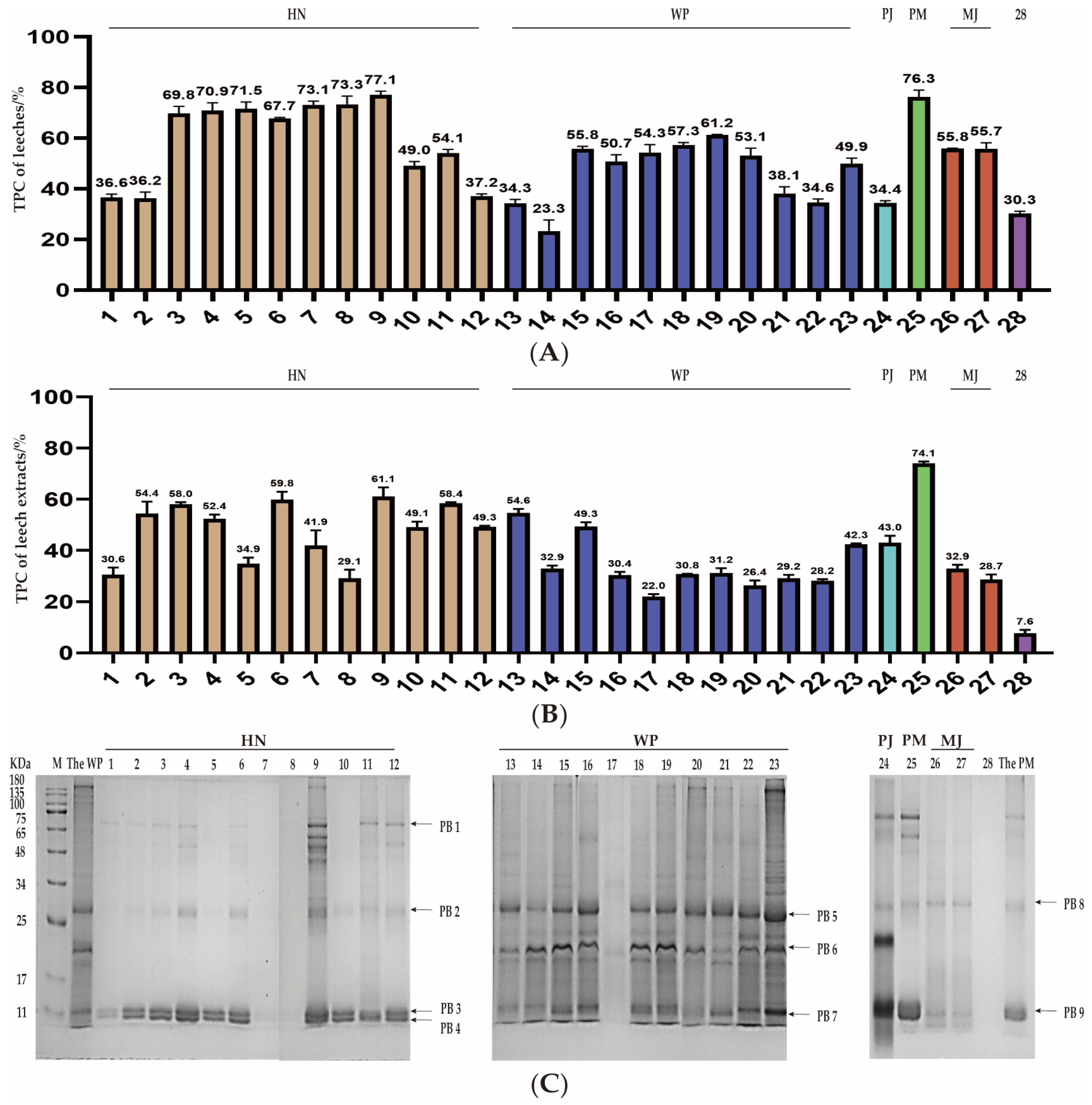
2.3.1. Total Protein Content in Dried Leech Samples and Its Extracts
2.3.2. SDS-PAGE Analysis and Distinctive Protein Identification
2.3.3. HPLC-Orbitrap Fusion Lumos Tribrid-MS Analysis of the Distinctive Proteins
2.4. Bioassays of Antithrombotic Activities
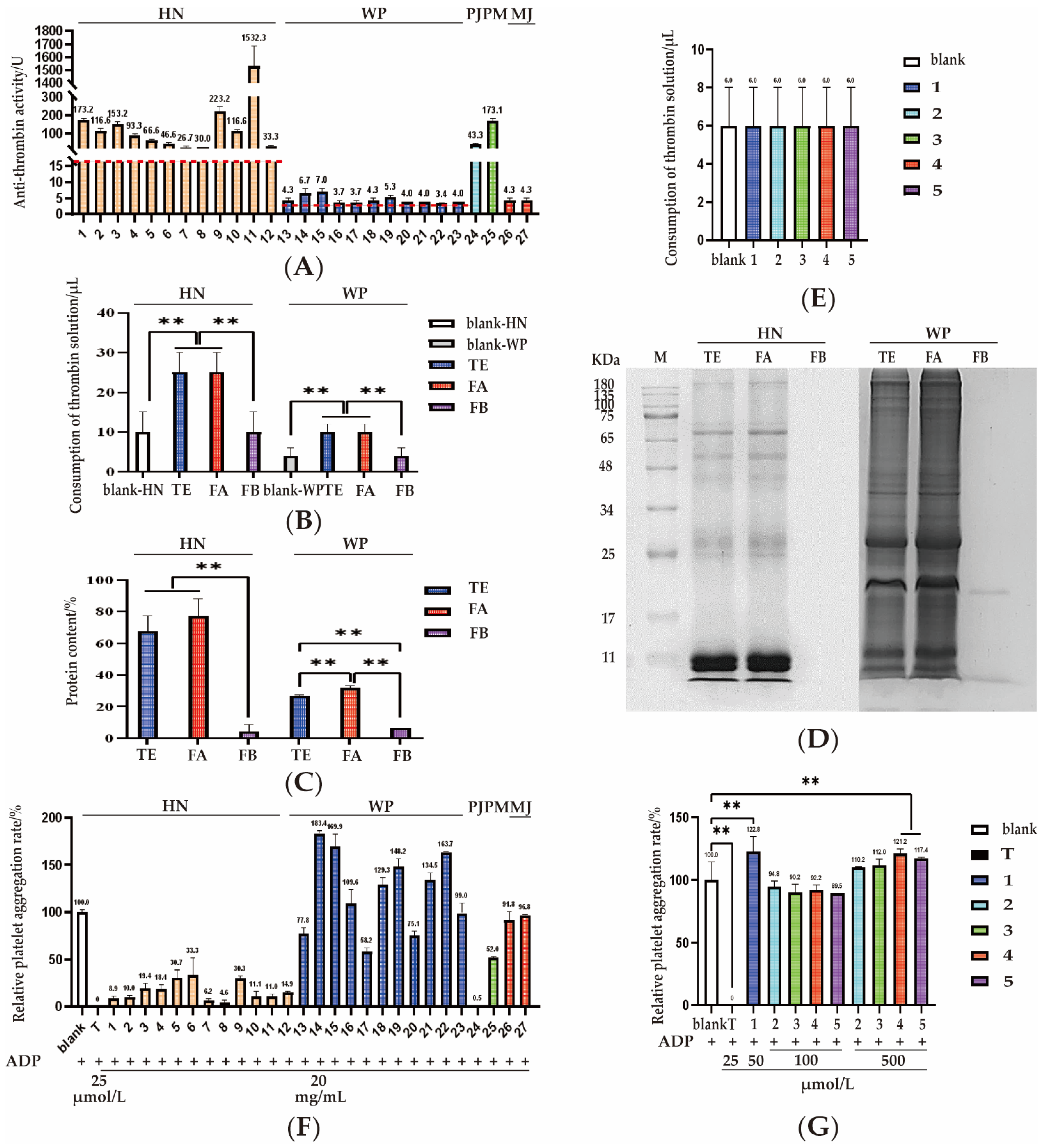
2.4.1. Anti-Thrombin Activity Assay
2.4.2. Correlation Between Composition and Activity
2.4.3. Platelet Aggregation
3. Discussion
4. Materials and Methods
4.1. Materials, Chemical Reagents
4.1.1. Chemical Reagents
4.1.2. Materials
4.2. Instruments
4.3. Samples Preparation
4.4. Morphological Characteristics and DNA Barcoding Analysis
4.4.1. Morphological Characteristics
4.4.2. DNA Barcoding Analysis
4.5. Micromolecules Analysis by HPLC and UPLC-QTOF-MS/MS
4.5.1. HPLC Analysis Condition
4.5.2. UPLC-QTOF-MS/MS Analytical Conditions
4.6. Isolation of SZ-1
4.7. TLC for Identification
4.8. Total Protein Content in Dried Leech and Total Extracts
4.9. Protein Profiles by SDS-PAGE and LC-MS/MS
4.9.1. SDS-PAGE Analysis
4.9.2. LC-MS/MS Acquisition
4.10. Anti-Thrombin Activity
4.11. Adenosine Diphosphate (ADP)-Induced Platelet Aggregation Test
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.H.; Zhou, Y.Y.; Wang, Y.; Zhang, Z.P. Therapeutic Potentials of Medicinal Leech in Chinese Medicine. Am. J. Chin. Med. 2024, 52, 1027–1051. [Google Scholar] [CrossRef]
- Li, F.G.; Shi, X.Y.; Yang, L.; Lu, X.; Qi, Y.; Li, P.; Yang, H.; Gao, W. Quantitative proteomics based bioactive proteins discovery and quality control of medicinal leeches. J. Ethnopharmacol. 2024, 319, 117117. [Google Scholar] [CrossRef]
- Meng, F.M.; Liu, Z.C.; Sun, J.W.; Kong, D.J.; Wang, Y.X.; Tong, X.R.; Cao, Y.R.; Bi, X.X. Insights into gut microbiota communities of Poecilobdella manillensis, a prevalent Asian medicinal leech. J. Appl. Microbiol. 2022, 133, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.F.; Guo, Y.H.; Yao, L.J.; Xu, Y.H.; Zhou, J.; Hua, M.L. Development of a mitochondrial mini-barcode and its application in metabarcoding for identification of leech in traditional Chinese medicine. Sci. Rep. 2025, 15, 1698. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.C.; Wei, F. Handbook of Traditional Identification of Chinese Medicinal Materials, 1st ed.; People’s Medical Publishing House: Beijing, China, 2019; pp. 128–134. [Google Scholar]
- Jiang, Q.; Wang, L.N.; Liu, Q.; Yang, C.M.; Zhang, Y.Q. Research progress on processing history evolution, chemical constituents and pharmacological action of Hirudo. China J. Chin. Mater. Med. 2022, 47, 5806–5816. [Google Scholar]
- Dong, H.; Ren, J.X.; Wang, J.J.; Ding, L.S.; Zhao, J.J.; Liu, S.Y.; Gao, H.M. Chinese Medicinal Leech: Ethnopharmacology, Phytochemistry, and Pharmacological Activities. Evid.-Based Complement. Altern. Med. 2016, 2016, 7895935. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Gao, Q.; Chai, X.X.; Ren, W.; Zhang, G.F.; Kong, Y.J.; Zhang, Y.; Gao, J.P.; Lei, X.X.; Ma, L. A novel thrombin inhibitory peptide discovered from leech using affinity chromatography combined with ultra-high performance liquid chromatography-high resolution mass spectroscopy. J. Chromatogr. B 2020, 1151, 122153. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Ji, X.R.; Liu, Y.Y.; Jiang, S.; Zhang, J.Q.; Zhang, J.L.; Kong, Y. WPK5, a novel kunitz-type peptide from the leech Whitmania pigra inhibiting factor XIa, and its loop-replaced mutant to improve potency. Biomedicines 2021, 9, 1745. [Google Scholar] [CrossRef]
- Liao, J.M.; Gao, M.; Ding, Y.L.; Bi, Q.R.; Huang, D.D.; Luo, X.X.; Yang, P.L.; Li, Y.; Huang, Y.; Yao, C.L.; et al. Characterization of the natural peptidome of four leeches by integrated proteogenomics and pseudotargeted peptidomics. Anal. Bioanal. Chem. 2023, 415, 2795–2807. [Google Scholar] [CrossRef]
- Ren, S.H.; Liu, Z.J.; Cao, Y.; Hua, Y.; Chen, C.; Guo, W.; Kong, Y. A novel protease-activated receptor 1 inhibitor from the leech Whitmania pigra. Chin. J. Nat. Med. 2019, 17, 591–599. [Google Scholar] [CrossRef]
- Hu, B.; Xu, L.X.; Li, Y.; Bai, X.; Xing, M.C.; Cao, Q.; Liang, H.; Song, S.L.; Ji, A.G. A peptide inhibitor of macrophage migration in atherosclerosis purified from the leech Whitmania pigra. J. Ethnopharmacol. 2020, 254, 112723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Yang, R.; Wang, L.W.; Li, Y.; Han, J.; Yang, Y.Y.; Zheng, H.X.; Lu, M.Y.; Shen, Y.P.; Yang, H. Purification and characterization of a novel thermostable anticoagulant protein from medicinal leech Whitmania pigra Whitman. J. Ethnopharmacol. 2022, 288, 114990. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.S.; Xie, F.F.; Wu, M.L.; Qiu, Y.J.; Li, G.W.; Tong, P.Z.; Luo, W.H. Quality Evaluation of Leeches from Different Sources Based on Characteristic Mapping and Biological Activity. J. Chin. Mater. Med. 2024, 47, 2017–2021. [Google Scholar]
- Li, G.W.; Qiu, Y.J.; Tong, P.Z.; Hu, Q.P.; Deng, L.P.; Yang, L.; Sun, D.M. Quality analysis of Hirudo with different origins and its adulterants. Nat. Prod. Res. Dev. 2023, 35, 997–1006. [Google Scholar]
- Liu, W.Z.; Fan, J.W.; Li, Y.F.; Qiu, X.J.; Liu, X.D.; Su, R.Q.; Zhao, Z.Q. Simultaneous determination of seven components in dried Whitmania pigra by HPLC. Chin. J. Pharm. Anal. 2014, 34, 1417–1421. [Google Scholar]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/botanical-drug-development-guidance-industry (accessed on 1 May 2025).
- Zhang, F.Y.; Li, B.C.; Wen, Y.; Liu, Y.Y.; Liu, R.; Liu, J.; Liu, S.; Jiang, Y.P. An integrated strategy for the comprehensive pro-filing of the chemical constituents of Aspongopus chinensis using UPLC-QTOF-MS combined with molecular networking. Pharm. Biol. 2022, 60, 1349–1364. [Google Scholar] [CrossRef]
- Liang, C.R.; Yao, Y.Q.; Ding, H.R.; Li, X.M.; Li, Y.B.; Cai, T. Rapid classification and identification of chemical components of Astragali radix by UPLC-Q-TOF-MS. Phytochem. Anal. 2022, 33, 943–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.C.; Fei, Q.Q.; Huang, X.J.; Yu, S.; Qiu, R.L.; Guan, L.; Wu, B.X.; Shan, M.Q. LC-MS based strategy for chemical profiling and quantification of dispensing granules of Ginkgo biloba seeds. Heliyon 2024, 10, e36909. [Google Scholar] [CrossRef]
- Feng, Y.J.; You, X.J.; Ding, J.H.; Zhang, Y.F.; Yuan, B.F.; Feng, Y.Q. Identification of Inosine and 2′-O-Methylinosine Modifications in Yeast Messenger RNA by Liquid Chromatography-Tandem Mass Spectrometry Analysis. Anal. Chem. 2022, 94, 4747–4755. [Google Scholar] [CrossRef]
- Martinez, V.C.; Alcaraz, A.J.R.; Vera, M.; Guirado, A.; Esparza, M.M.; Penarrubia, P.G. Therapeutic potential of pteridine derivatives: A comprehensive review. Med. Res. Rev. 2019, 39, 461–516. [Google Scholar] [CrossRef]
- Li, T.; Wang, G.C.; Wang, C.H.; Ye, W.C. Three New Pteridines from the Leech Whitmania pigra. Chem. Lett. 2013, 42, 983–985. [Google Scholar] [CrossRef]
- Li, T. Chemical Constituents from Whitmania pigra. Master’s Thesis, Jinan University, Guangzhou, China, 2013. [Google Scholar]
- Milani, A.; Basirnejad, M.; Bolhassani, A. Heat-shock proteins in diagnosis and treatment: An overview of different biochemical and immunological functions. Immuntherapy 2019, 11, 215–239. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Liao, L.S.; Wan, X.D.; Zhang, F.F.; Zhang, T.; Luo, X.M.; Zhao, S.; Feng, J.X. PoxCbh, a novel CENPB-type HTH domain protein, regulates cellulase and xylanase gene expression in Penicillium oxalicum. Mol. Microbiol. 2021, 116, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China Volume 1; China Medical Science and Technology Press: Beijing, China, 2020; pp. 85–86.
- Huang, R.H.; Shu, Q.; Liang, S.P. Purification and Identification of Native Hirudin From the Digestive Juice of Leeches. Acta Sci. Nat. Univ. Norm. Hunan 1998, 1, 57–60+66. [Google Scholar]
- Zhang, W.; Zhang, R.X.; Li, J.; Liang, F.; Qian, Z.Z. Species study on Chinese medicine leech and discussion on its resource sustainable utilization. Chin. J. Chin. Mater. Med. 2013, 38, 914–918. [Google Scholar]
- Kui, H.Q.; Lei, Y.; Jia, C.X.; Xin, Q.C.; Tursun, R.; Zhong, M.; Liu, C.X.; Yuan, R.J. Antithrombotic pharmacodynamics and metabolomics study in raw and processed products of Whitmania pigra Whitman. Heliyon 2024, 10, e27828. [Google Scholar] [CrossRef]
- Chen, S.L. Standard DNA Barcodes of Chinese Matera Medica in Chinese Pharmacopoeia; Science Press: Beijing, China, 2015; pp. 39–40. [Google Scholar]
- Chinese Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China Volume 4; China Medical Science and Technology Press: Beijing, China, 2020; pp. 95–96.
- Li, X.X.; Chu, Z.; Feng, C.R.; Song, P.; Yang, T.; Zhou, L.R.; Zhao, X.; Chai, X.; Xing, J.L.; Chen, S.; et al. Unveiling the molecular mechanisms of size-dependent effect of polystyrene micro/nano-plastics on Chlamydomonas reinhardtii through proteomic profiling. Chemosphere 2024, 358, 142220. [Google Scholar] [CrossRef]

| Name | RT (min) | [M + H]+ Detected | [M + H]+ Expected | Error (ppm) | Formula | Fragments | Leech Species | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HN | WP | PJ | PM | MJ | |||||||
| hypoxanthine (9) | 1.68 | 137.0459 | 137.0463 | −2.9 | C5H4N4O | 119.0358, 110.0345 | + | + | + | + | + |
| xanthine (10) | 1.86 | 153.0408 | 153.0413 | −3.3 | C5H4N4O2 | 110.0340 | + | + | + | + | + |
| leucine (11) | 2.47 | 132.1019 | 132.1025 | −4.5 | C6H13NO2 | 113.9624, 86.0943 | + | + | + | + | + |
| inosine (4) | 2.73 | 269.0862 | 269.0886 | −8.9 | C10H12N4O5 | 137.0445, 110.0673 | + | + | + | + | + |
| 2-piperidone (12) | 4.10 | 100.0755 | 100.0762 | −7.0 | C5H9NO | 82.0647, 72.0768, | + | + | + | + | + |
| phenylalanine (13) | 4.41 | 166.0862 | 166.0868 | −3.6 | C9H11NO2 | 149.0216, 120.0800, 103.0536, 91.0529 | + | + | + | + | + |
| hirudinoidine C (5) | 5.02 | 326.9858 | 326.9858 | 0 | C10H6N4O5S2 | 264.9786, 219.9649 | + | + | + | + | − |
| tryptophan (14) | 7.89 | 205.0962 | 205.0977 | −7.3 | C11H12N2O2 | 188.0751, 146.0580, 118.0643, 91.0531 | + | + | + | + | + |
| unknown (1) | 10.18 | 357.0330 | 357.0327 | 0.8 | C12H12N4O5S2 | 295.0060, 234.9884 | + | − | − | − | − |
| hirudinoidine B (3) | 11.97 | 327.0209 | 327.0222 | −4.0 | C11H10N4O4S2 | 281.0528, 221.9807 | + | + | + | + | − |
| poecilobdellasulfide B (7) | 13.71 | 357.0320 | 357.0327 | −2.0 | C12H12N4O5S2 | 294.0190, 235.0243 | − | − | + | + | − |
| hirudinoidine A (2) | 17.32 | 310.9910 | 310.9909 | 0.3 | C10H6N4O4S2 | 264.9838, 219.9634 | + | + | + | + | − |
| whitmanine B (6) | 20.44 | 355.0169 | 355.0171 | −0.6 | C12H10N4O5S2 | 293.0145, 249.0433 | + | + | + | + | − |
| Position | δH | δC | Position | δH | δC |
|---|---|---|---|---|---|
| 2 | - | 161.9 | 9a | - | 157.7 |
| 4 | - | 153.7 | 13 | 5.68, t (5.8) | 67.1 |
| 4a | - | 125.3 | 14 | 3.65, d (5.7) | 66.9 |
| 5a | - | 137.5 | N3-CH3 | 3.26 | 27.8 |
| 6 | - | 128.8 | -CH3 | 3.15 | 40.2 |
| 7 | - | 153.2 | 13-OH | 6.27, brs | - |
| 8a | - | 141.6 | 14-OH | 5.11, brs | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liu, Y.; Lou, W.; Chen, L.; Xie, T.; Wang, Z.; Ma, Y.; Gao, H. A Comprehensive Quality Evaluation System for Medicinal Leeches by Integrating Macromolecular Protein Analysis and Small-Molecule Marker Detection as Well as Quantitative Bioassays. Pharmaceuticals 2025, 18, 887. https://doi.org/10.3390/ph18060887
Wang W, Liu Y, Lou W, Chen L, Xie T, Wang Z, Ma Y, Gao H. A Comprehensive Quality Evaluation System for Medicinal Leeches by Integrating Macromolecular Protein Analysis and Small-Molecule Marker Detection as Well as Quantitative Bioassays. Pharmaceuticals. 2025; 18(6):887. https://doi.org/10.3390/ph18060887
Chicago/Turabian StyleWang, Wenduan, Yufei Liu, Wenjiao Lou, Liangmian Chen, Tianze Xie, Zhimin Wang, Yue Ma, and Huimin Gao. 2025. "A Comprehensive Quality Evaluation System for Medicinal Leeches by Integrating Macromolecular Protein Analysis and Small-Molecule Marker Detection as Well as Quantitative Bioassays" Pharmaceuticals 18, no. 6: 887. https://doi.org/10.3390/ph18060887
APA StyleWang, W., Liu, Y., Lou, W., Chen, L., Xie, T., Wang, Z., Ma, Y., & Gao, H. (2025). A Comprehensive Quality Evaluation System for Medicinal Leeches by Integrating Macromolecular Protein Analysis and Small-Molecule Marker Detection as Well as Quantitative Bioassays. Pharmaceuticals, 18(6), 887. https://doi.org/10.3390/ph18060887







