Influence of Puncture Devices on the Accuracy of Cyclophosphamide Dosing for Chemotherapy Administration
Abstract
1. Introduction
2. Results
2.1. Chromatographic Analysis
2.2. Method Validation
2.2.1. Linearity
2.2.2. Analytical Thresholds
2.2.3. Specificity
2.2.4. Precision and Accuracy
2.2.5. Robustness
2.2.6. Stability
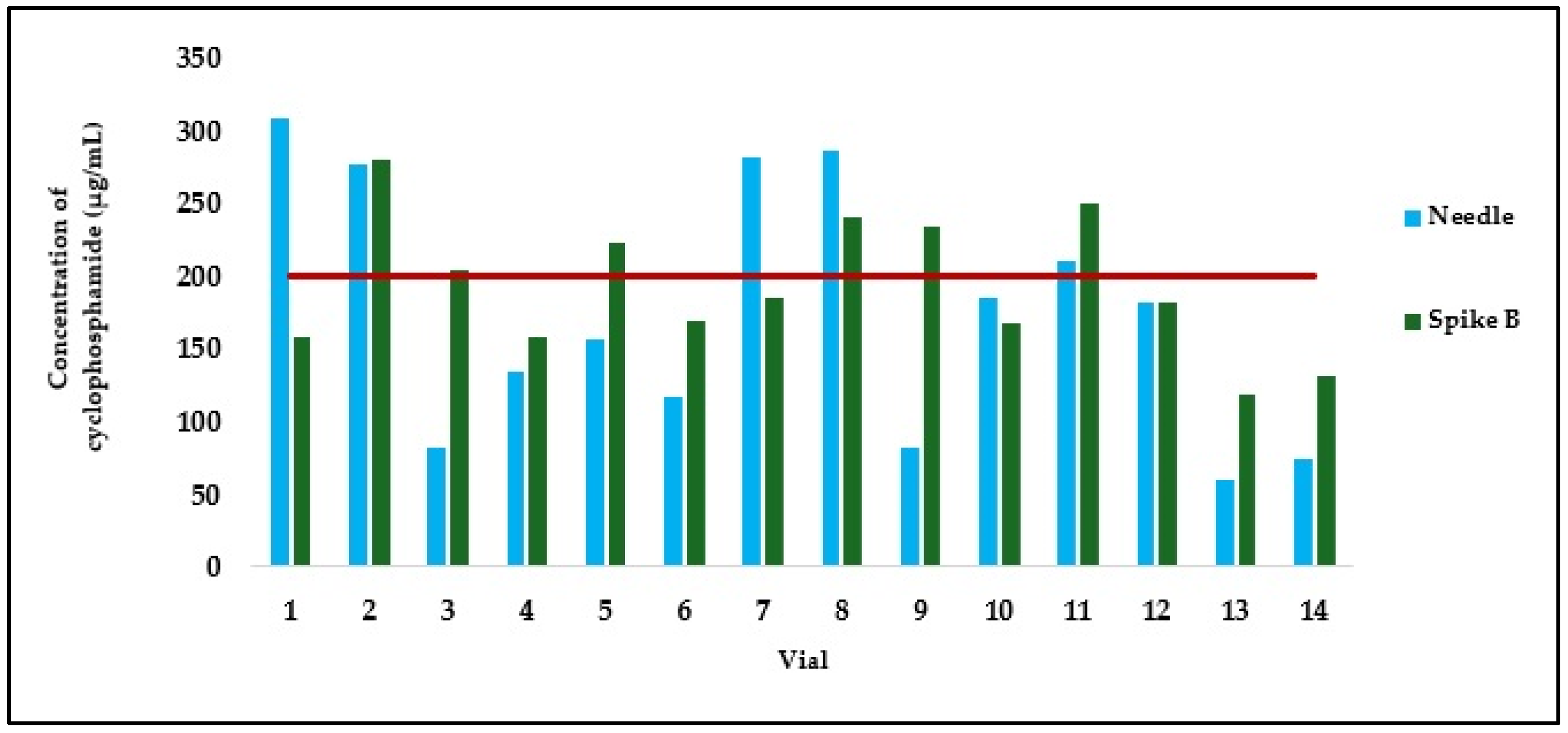
2.3. Quality Control of Cyclophosphamide Solutions Aspirated with Different Devices
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Calibrators and Quality Control Samples
4.3. HPLC-DAD Analytical Settings
4.4. Method Validation and Acceptance Criteria
4.4.1. Linearity
4.4.2. Limits of Detection and Quantification
4.4.3. Specificity
4.4.4. Precision
4.4.5. Accuracy
4.4.6. Robustness
4.4.7. Stability
4.5. Proof of Applicability
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| ASHP | Association of Health-System Pharmacists |
| CSTD | Closed system transfer device |
| CV | Coefficient of variation |
| GC-MS | Gas chromatography coupled with mass spectrometry |
| HPLC-DAD | High-performance liquid chromatography coupled to a diode array detector |
| ICH | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
| ISOPP | International Society of Oncology Pharmacy Practitioners |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| LC-MS/MS | Liquid chromatography coupled with tandem mass spectrometry |
| NIOSH | National Institute for Occupational Safety and Health |
| NCD | Noncommunicable diseases |
| RSD | Relative standard deviation |
| SmPC | Summary of product characteristics |
| UPLC-MS/MS | Ultra-performance liquid chromatography coupled with tandem mass spectrometry |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A Double-Edged Sword in Cancer Treatment. Cancer Immunol. Immunother. 2021, 71, 507–526. [Google Scholar] [CrossRef]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bharti, A.C.; Vishnoi, K.; Singh, S.M.; Aggarwal, B.B. Chapter 1—Pathways Linked to Cancer Chemoresistance and Their Targeting by Nutraceuticals. In Role of Nutraceuticals in Cancer Chemosensitization; Bharti, A.C., Aggarwal, B.B., Eds.; Cancer Sensitizing Agents for Chemotherapy; Academic Press: Cambridge, MA, USA, 2018; Volume 2, pp. 1–30. [Google Scholar]
- The National Institute of Diabetes and Digestive and Kidney Diseases. Cyclophosphamide. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Mills, K.A.; Chess-Williams, R.; McDermott, C. Novel Insights into the Mechanism of Cyclophosphamide-Induced Bladder Toxicity: Chloroacetaldehyde’s Contribution to Urothelial Dysfunction in Vitro. Arch. Toxicol. 2019, 93, 3291–3303. [Google Scholar] [CrossRef]
- Korkmaz, A.; Topal, T.; Oter, S. Pathophysiological Aspects of Cyclophosphamide and Ifosfamide Induced Hemorrhagic Cystitis; Implication of Reactive Oxygen and Nitrogen Species as Well as PARP Activation. Cell Biol. Toxicol. 2007, 23, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Dan, D.; Fischer, R.; Adler, S.; Förger, F.; Villiger, P.M. Cyclophosphamide: As Bad as Its Reputation? Long-Term Single Centre Experience of Cyclophosphamide Side Effects in the Treatment of Systemic Autoimmune Diseases. Swiss Med. Wkly. 2014, 144, w14030. [Google Scholar] [CrossRef]
- Martin, F.; Lauwerys, B.; Lefèbvre, C.; Devogelaer, J.P.; Houssiau, F.A. Side-Effects of Intravenous Cyclophosphamide Pulse Therapy. Lupus 1997, 6, 254–257. [Google Scholar] [CrossRef]
- Ogino, M.H.; Tadi, P. Cyclophosphamide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- ISOPP. ISOPP Standards for the Safe Handling of Cytotoxics. J. Oncol. Pharm. Pract. 2022, 28, S1–S126. [Google Scholar] [CrossRef]
- Power, L.A.; Coyne, J.W. ASHP Guidelines on Handling Hazardous Drugs. Am. J. Health Syst. Pharm. 2018, 75, 1996–2031. [Google Scholar] [CrossRef]
- NIOSH. Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings; U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health: Washington, DC, USA, 2004.
- Clark, B.A.; Sessink, P.J.M. Use of a Closed System Drug-Transfer Device Eliminates Surface Contamination with Antineoplastic Agents. J. Oncol. Pharm. Pract. 2013, 19, 99–104. [Google Scholar] [CrossRef]
- Simon, N.; Vasseur, M.; Pinturaud, M.; Soichot, M.; Richeval, C.; Humbert, L.; Lebecque, M.; Sidikou, O.; Barthelemy, C.; Bonnabry, P.; et al. Effectiveness of a Closed-System Transfer Device in Reducing Surface Contamination in a New Antineoplastic Drug-Compounding Unit: A Prospective, Controlled, Parallel Study. PLoS ONE 2016, 11, e0159052. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Odagiri, N.; Terui, K.; Iwasaki, Y.; Hosoya, E.; Hayakari, M. Availability evaluation of closed systems by using practical training kits for preparation of antitumor drugs. Gan To Kagaku Ryoho 2010, 37, 1753–1757. [Google Scholar]
- Ikeno, Y.; Arii, D.; Nakajima, H.; Murooka, K.; Nojima, M.; Kidokoro, A. Verification of reduction in preparation time and cost of cyclophosphamide when using the closed-system drug transfer device. Gan To Kagaku Ryoho 2014, 41, 611–615. [Google Scholar]
- Jorgenson, J.; Spivey, S.; Au, C.; Canann, D.; Ritter, H.; Smith, B. Contamination Comparison of Transfer Devices Intended for Handling Hazardous Drugs. Hosp. Pharm. 2008, 43, 718–722. [Google Scholar] [CrossRef]
- Yoshida, J.; Tei, G.; Mochizuki, C.; Masu, Y.; Koda, S.; Kumagai, S. Use of a Closed System Device to Reduce Occupational Contamination and Exposure to Antineoplastic Drugs in the Hospital Work Environment. Ann. Occup. Hyg. 2009, 53, 153–160. [Google Scholar] [CrossRef]
- Vyas, N.; Turner, A.; Clark, J.M.; Sewell, G.J. Evaluation of a Closed-System Cytotoxic Transfer Device in a Pharmaceutical Isolator. J. Oncol. Pharm. Pract. 2016, 22, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Boiano, J.M.; Steege, A.L.; Sweeney, M.H. Adherence to Safe Handling Guidelines by Health Care Workers Who Administer Antineoplastic Drugs. J. Occup. Environ. Hyg. 2014, 11, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Silva, I.; Rego, M.; Correia, P.; Moreira, F. Characterization of Education, Technical Practices and Attitudes of Portuguese Pharmacy Technicians towards Manipulation of Cytotoxic Drugs. J. Oncol. Pharm. Pract. 2024, 30, 893–901. [Google Scholar] [CrossRef]
- Viegas, S.; Oliveira, d.A.C.; Carolino, E.; Pádua, M. Occupational Exposure to Cytotoxic Drugs: The Importance of Surface Cleaning to Prevent or Minimise Exposure. Arh. Hig. Rada Toksikol. 2018, 69, 238–249. [Google Scholar] [CrossRef]
- Ren, L.; Xue, X.; Zhang, F.; Xu, Q.; Liang, X. High Performance Liquid Chromatography-Mass Spectrometry Analysis of Protoberberine Alkaloids in Medicine Herbs. J. Sep. Sci. 2007, 30, 833–842. [Google Scholar] [CrossRef]
- Martins, I.; Souza, J.; Sanson, A.; Vieira, E.; Giusti-Paiva, A. Simultaneous Determination of Cyclophosphamide and Ifosfamide in Plasma Using SPE-HPLC-UV Method. Lat. Am. J. Pharm. 2009, 28, 41–46. [Google Scholar]
- Kennedy, R.; Groepper, D.; Tagen, M.; Christensen, R.; Navid, F.; Gajjar, A.; Stewart, C.F. Stability of Cyclophosphamide in Extemporaneous Oral Suspensions. Ann. Pharmacother. 2010, 44, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Gustavo González, A.; Ángeles Herrador, M. A Practical Guide to Analytical Method Validation, Including Measurement Uncertainty and Accuracy Profiles. TrAC Trends Anal. Chem. 2007, 26, 227–238. [Google Scholar] [CrossRef]
- Ismail, R.; Lee, H.Y.; Mahyudin, N.A.; Bakar, F.A. Linearity Study on Detection and Quantification Limits for the Determination of Avermectins Using Linear Regression. J. Food Drug Anal. 2014, 22, 407–412. [Google Scholar] [CrossRef]
- Wakui, N.; Ookubo, T.; Iwasaki, Y.; Ito, R.; Mitui, M.; Yano, Y.; Saito, K.; Nakazawa, H. Determination of Exposure of Dispensary Drug Preparers to Cyclophosphamide by Passive Sampling and Liquid Chromatography with Tandem Mass Spectrometry. J. Oncol. Pharm. Pract. 2013, 19, 31–37. [Google Scholar] [CrossRef]
- da Silva, C.B.P.; Julio, I.P.; Donadel, G.E.; Martins, I. UPLC-MS/MS Method for Simultaneous Determination of Cyclophosphamide, Docetaxel, Doxorubicin and 5-Fluorouracil in Surface Samples. J. Pharmacol. Toxicol. Methods 2016, 82, 68–73. [Google Scholar] [CrossRef]
- Česen, M.; Kosjek, T.; Busetti, F.; Kompare, B.; Heath, E. Human Metabolites and Transformation Products of Cyclophosphamide and Ifosfamide: Analysis, Occurrence and Formation during Abiotic Treatments. Environ. Sci. Pollut. Res. Int. 2016, 23, 11209–11223. [Google Scholar] [CrossRef]
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of New Methods. Forensic Sci. Int. 2007, 165, 216–224. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis; European Medicines Agency: Amsterdam, The Netherlands, 2022.
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Validation of Analytical Procedures: Q2(R2); ICH: Geneva, Switzerland, 2024. [Google Scholar]
- Viegas, S.; Pádua, M.; Veiga, A.C.; Carolino, E.; Gomes, M. Antineoplastic Drugs Contamination of Workplace Surfaces in Two Portuguese Hospitals. Environ. Monit. Assess. 2014, 186, 7807–7818. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Ghassabian, S. Linearity of Calibration Curves for Analytical Methods: A Review of Criteria for Assessment of Method Reliability. In Calibration and Validation of Analytical Methods; Stauffer, M.T., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 6; ISBN 978-1-78923-085-7. [Google Scholar]
- Constantinidis, T.C.; Vagka, E.; Dallidou, P.; Basta, P.; Drakopoulos, V.; Kakolyris, S.; Chatzaki, E. Occupational Health and Safety of Personnel Handling Chemotherapeutic Agents in Greek Hospitals. Eur. J. Cancer Care 2011, 20, 123–131. [Google Scholar] [CrossRef]
- Fahrenbruch, R.; Kintzel, P.; Bott, A.M.; Gilmore, S.; Markham, R. Dose Rounding of Biologic and Cytotoxic Anticancer Agents: A Position Statement of the Hematology/Oncology Pharmacy Association. J. Oncol. Pract. 2018, 14, e130–e136. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Woelich, S.K.; Mattila, M.; McCleary, S.; Thomas, J.; Wellner, D. Cost Avoidance from Automated Dose Rounding of Biological and Cytotoxic Anticancer Drugs in an Integrated Delivery Network. Blood 2020, 136, 10–11. [Google Scholar] [CrossRef]
- Buckle, G.; Maranda, L.; Skiles, J.; Ong’echa, J.M.; Foley, J.; Epstein, M.; Vik, T.A.; Schroeder, A.; Lemberger, J.; Rosmarin, A.; et al. Factors Influencing Survival among Kenyan Children Diagnosed with Endemic Burkitt Lymphoma between 2003 and 2011: A Historical Cohort Study. Int. J. Cancer 2016, 139, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.; Bradley, R.; Braybrooke, J.; Liu, Z.; Peto, R.; Davies, L.; Dodwell, D.; McGale, P.; Pan, H.; Taylor, C.; et al. Increasing the Dose Intensity of Chemotherapy by More Frequent Administration or Sequential Scheduling: A Patient-Level Meta-Analysis of 37 298 Women with Early Breast Cancer in 26 Randomised Trials. Lancet 2019, 393, 1440–1452. [Google Scholar] [CrossRef]
- Nielson, C.M.; Bylsma, L.C.; Fryzek, J.P.; Saad, H.A.; Crawford, J. Relative Dose Intensity of Chemotherapy and Survival in Patients with Advanced Stage Solid Tumor Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1609–e1618. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Brown, J.S. The Evolution and Ecology of Resistance in Cancer Therapy. Cold Spring Harb. Perspect. Med. 2020, 10, a040972. [Google Scholar] [CrossRef]
- Chienwichai, K.; Choomnirat, A.; Sangkaew, S.; Sunanthamethee, N.; Chang, A. Impact of Dosing Strategy on Clinical Outcomes of Patients with Lupus Nephritis Initially Treated with Lower-than-Recommended-Dose Cyclophosphamide. Heliyon 2024, 10, e37359. [Google Scholar] [CrossRef]
- Harper, L.; Morgan, M.D.; Walsh, M.; Hoglund, P.; Westman, K.; Flossmann, O.; Tesar, V.; Vanhille, P.; Groot, K.d.; Luqmani, R.; et al. Pulse versus Daily Oral Cyclophosphamide for Induction of Remission in ANCA-Associated Vasculitis: Long-Term Follow-Up. Ann. Rheum. Dis. 2012, 71, 955–960. [Google Scholar] [CrossRef]
- Tolar, J.; Deeg, H.J.; Arai, S.; Horwitz, M.; Antin, J.H.; McCarty, J.M.; Adams, R.H.; Ewell, M.; Leifer, E.S.; Gersten, I.D.; et al. Fludarabine-Based Conditioning for Marrow Transplantation from Unrelated Donors in Severe Aplastic Anemia: Early Results of a Cyclophosphamide Dose Deescalation Study Show Life-Threatening Adverse Events at Predefined Cyclophosphamide Dose Levels. Biol. Blood Marrow Transplant. 2012, 18, 1007–1011. [Google Scholar] [CrossRef]
- Gagelmann, N.; Kröger, N. Dose Intensity for Conditioning in Allogeneic Hematopoietic Cell Transplantation: Can We Recommend “When and for Whom” in 2021? Haematologica 2021, 106, 1794–1804. [Google Scholar] [CrossRef]
- Schraa, S.J.; Frerichs, K.A.; Agterof, M.J.; Hunting, J.C.B.; Los, M.; Jong, P.C. de Relative Dose Intensity as a Proxy Measure of Quality and Prognosis in Adjuvant Chemotherapy for Breast Cancer in Daily Clinical Practice. Eur. J. Cancer 2017, 79, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Długosz-Danecka, M.; Szmit, S.; Ogórka, T.; Skotnicki, A.B.; Jurczak, W. The Average Relative Dose Intensity of R-CHOP Is an Independent Factor Determining Favorable Overall Survival in Diffuse Large B-Cell Lymphoma Patients. Cancer Med. 2019, 8, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmadi, M.; Lazo-Langner, A.; Mangel, J.; Phm, A.D.; Liu, K.; Minuk, L. Effect of Unintentional Cyclophosphamide Underdosing on Diffuse Large B-Cell Lymphoma Response to Chemotherapy: A Retrospective Review. CMAJ Open 2016, 4, E236–E239. [Google Scholar] [CrossRef] [PubMed]
- Shabir, G.A. Validation of High-Performance Liquid Chromatography Methods for Pharmaceutical Analysis: Understanding the Differences and Similarities between Validation Requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. J. Chromatogr. A 2003, 987, 57–66. [Google Scholar] [CrossRef]

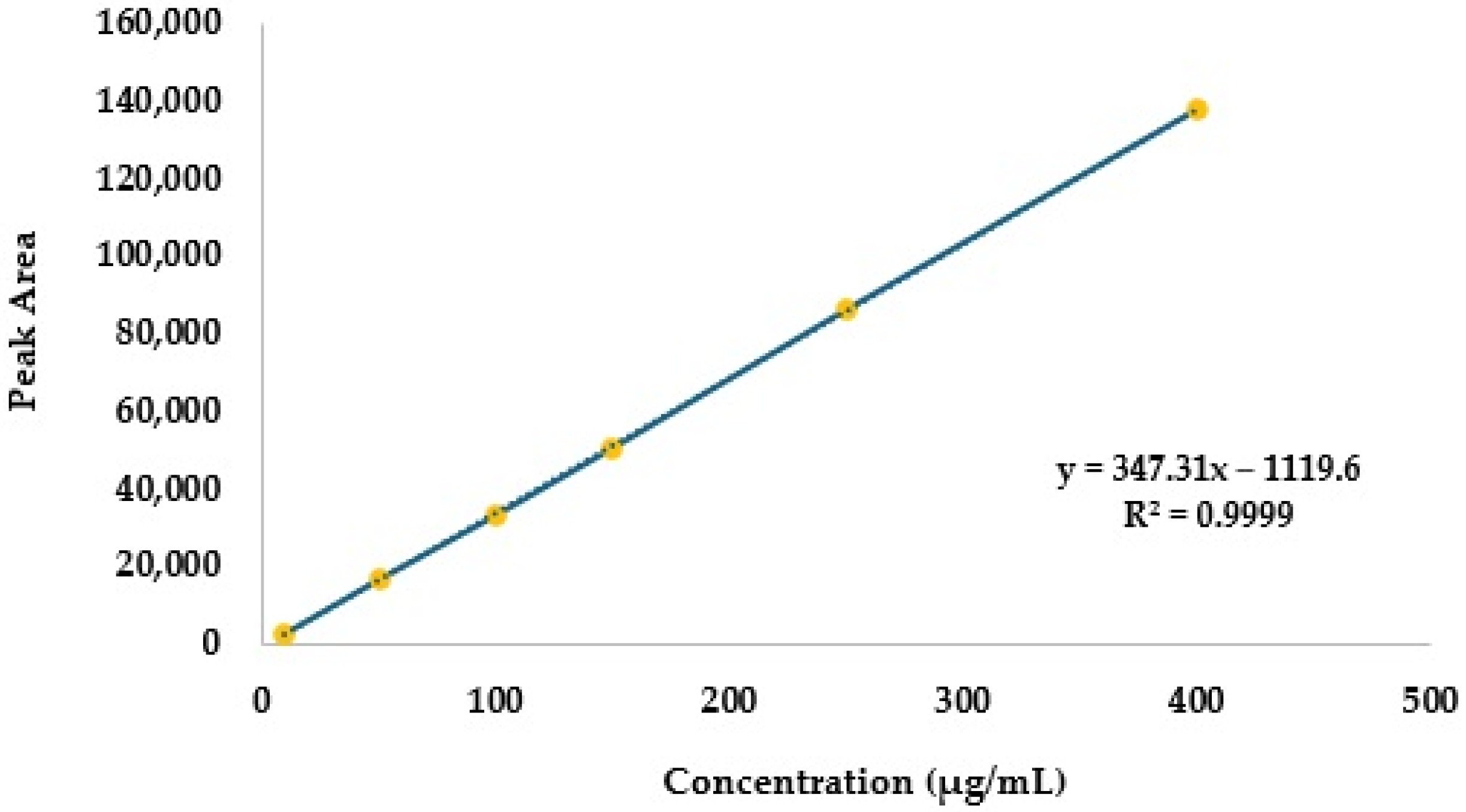


| Concentration (µg/mL) | Mean Peak Area | Standard Deviation | Relative Standard Deviation (%) | Back-Calculated Concentration (µg/mL) | Difference (%) |
|---|---|---|---|---|---|
| 10 | 2506 | 172 | 6.86 | 10.48 | 4.8 |
| 50 | 16,442 | 238 | 1.45 | 50.75 | 1.5 |
| 100 | 33,125 | 356 | 1.07 | 98.97 | −1.0 |
| 150 | 50,648 | 312 | 0.62 | 149.61 | −0.3 |
| 250 | 86,372 | 232 | 0.27 | 252.85 | 1.1 |
| 400 | 137,604 | 237 | 0.17 | 400.91 | 0.2 |
| Concentration (µg/mL) | Repeatability | Intermediate Precision | Accuracy | ||||
|---|---|---|---|---|---|---|---|
| Mean Area ± Standard Deviation | Relative Standard Deviation (%) | Mean Area ± Standard Deviation | Relative Standard Deviation (%) | Mean Area | Back-calculated Concentration (µg/mL) | Accuracy (%) | |
| 20 | 5220 ± 139 | 2.66 | 5119 ± 235 | 4.59 | 5118 ± 234 | 17.96 | 89.8 |
| 50 | 16,107 ± 280 | 1.74 | 16,442 ± 380 | 2.31 | 16,002 ± 335 | 49.30 | 98.6 |
| 150 | 51,769 ± 781 | 1.51 | 51,648 ± 115 | 0.22 | 50,648 ± 481 | 149.05 | 99.4 |
| Parameter | Parameter Setting | Peak Area (Mean ± Standard Deviation) | % of Peak Area in Relation to Optimised Method | Retention Time (Minutes) | Difference of Retention Time in Relation to Optimised Method (Minutes) |
|---|---|---|---|---|---|
| pH | 6.6 * | 15,883 ± 137 | - | 8.08 | - |
| 6.4 | 15,660 ± 207 | 98.6 | 8.08 | 0.0 | |
| Flow rate | 0.8 mL/min * | 15,883 ± 137 | - | 8.08 | - |
| 1.0 mL/min | 15,152 ± 208 | 95.4 | 7.65 | −0.43 |
| T0 | T24 | ||||||
|---|---|---|---|---|---|---|---|
| Concentration (µg/mL) | Room Temperature (≈25 °C) | Refrigerator (≈4 °C) | Freezer (≈−20 °C) | ||||
| Mean Peak Area | Mean Peak Area | Stability (%) | Mean Peak Area | Stability (%) | Mean Peak Area | Stability (%) | |
| 30 | 7525 | 7348 | 97.65 | 7526 | 100.01 | 7762 | 103.16 |
| 60 | 20,237 | 20,769 | 102.63 | 22,065 | 109.04 | 20,444 | 101.03 |
| 185 | 63,649 | 63,077 | 99.10 | 64,356 | 101.11 | 60,118 | 94.45 |
| Vial Number | Concentration of Solution Withdrawn with Needle (µg/mL) * | Concentration of Solution Withdrawn with Spike A (µg/mL) * | Difference Between Withdrawal with Needle and Vial |
|---|---|---|---|
| 1 | 308.84 | 112.23 | 196.61 |
| 2 | 333.78 | 193.11 | 140.67 |
| 3 | 336.66 | 128.23 | 208.43 |
| 4 | 326.06 | 105.38 | 220.68 |
| 5 | 318.16 | 120.23 | 197.93 |
| 6 | 327.36 | 192.44 | 134.92 |
| 7 | 323.46 | 164.44 | 159.02 |
| 8 | 334.37 | 146.87 | 187.50 |
| 9 | 503.20 | 234.39 | 268.81 |
| 10 | 445.76 | 278.91 | 166.85 |
| 11 | 412.45 | 243.70 | 168.75 |
| 12 | 454.12 | 194.77 | 259.35 |
| 13 | 446.24 | 278.91 | 167.33 |
| 14 | 482.78 | 289.13 | 193.65 |
| 15 | 393.13 | 111.56 | 281.57 |
| 16 | 391.87 | 104.14 | 287.73 |
| 17 | 415.37 | 119.93 | 295.44 |
| Mean | 385.51 | 177.55 | 207.96 (p < 0.001) |
| Standard Deviation | 64.18 | 66.49 | 52.40 |
| Coefficient of Variation (%) | 16.65 | 37.45 | 25.20 |
| Vial Number | Concentration of Solution Withdrawn with Needle (µg/mL) * | Concentration of Solution Withdrawn with Spike B (µg/mL) * | Difference Between Withdrawing with Needle and Vial |
|---|---|---|---|
| 18 | 308.46 | 157.93 | 150.53 |
| 19 | 277.13 | 280.77 | −3.64 |
| 20 | 82.52 | 204.18 | −121.66 |
| 21 | 134.88 | 158.19 | −23.31 |
| 22 | 156.21 | 223.05 | −66.84 |
| 23 | 117.23 | 169.70 | −52.47 |
| 24 | 281.35 | 185.02 | 96.33 |
| 25 | 286.3 | 240.60 | 45.7 |
| 26 | 82.63 | 234.35 | −151.72 |
| 27 | 185.15 | 167.70 | 17.45 |
| 28 | 210.80 | 250.33 | −39.53 |
| 29 | 182.29 | 182.74 | −0.45 |
| 30 | 60.56 | 118.92 | −58.36 |
| 31 | 74.23 | 131.59 | −57.36 |
| Mean | 174.27 | 193.22 | −18.95 |
| Standard Deviation | 81.45 | 48.05 | 65.97 |
| Coefficient of Variation (%) | 46.74 | 24.87 | −348.09 |
| Paired Samples Test | |||||||
|---|---|---|---|---|---|---|---|
| Paired Differences | t | dF | Sig. (2-tailed) | ||||
| Mean | Standard Deviation | Standard. Error Mean | 95% Confidence Interval of the Difference | ||||
| Lower | Upper | ||||||
| 207.96 | 52.40 | 12.71 | 181.01 | 234.90 | −16.36 | 16 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, S.; Cardoso, A.; Ferreira, D.; Dias da Silva, D.; Moreira, F. Influence of Puncture Devices on the Accuracy of Cyclophosphamide Dosing for Chemotherapy Administration. Pharmaceuticals 2025, 18, 879. https://doi.org/10.3390/ph18060879
Carvalho S, Cardoso A, Ferreira D, Dias da Silva D, Moreira F. Influence of Puncture Devices on the Accuracy of Cyclophosphamide Dosing for Chemotherapy Administration. Pharmaceuticals. 2025; 18(6):879. https://doi.org/10.3390/ph18060879
Chicago/Turabian StyleCarvalho, Susana, Andreia Cardoso, Débora Ferreira, Diana Dias da Silva, and Fernando Moreira. 2025. "Influence of Puncture Devices on the Accuracy of Cyclophosphamide Dosing for Chemotherapy Administration" Pharmaceuticals 18, no. 6: 879. https://doi.org/10.3390/ph18060879
APA StyleCarvalho, S., Cardoso, A., Ferreira, D., Dias da Silva, D., & Moreira, F. (2025). Influence of Puncture Devices on the Accuracy of Cyclophosphamide Dosing for Chemotherapy Administration. Pharmaceuticals, 18(6), 879. https://doi.org/10.3390/ph18060879







