Potential New Applications of Sodium–Glucose Cotransporter-2 Inhibitors Across the Continuum of Cancer-Related Cardiovascular Toxicity
Abstract
1. Introduction
2. Methodology
3. Cardiotoxicity—Definitions
3.1. Cancer Therapy-Related Cardiovascular Toxicity (CTR-CVT)
3.2. Cancer Therapy-Related Cardiac Dysfunction (CTRCD)
3.3. Current Methods of Prophylaxis for CTRCD
4. Oncological Therapies
4.1. Anthracycline-Induced Cardiotoxicity (AIC)
Molecular Mechanisms of Anthracycline-Induced Cardiotoxicity
4.2. Thoracic Radiation Therapy
4.3. Targeted Therapies
4.4. Immunotherapy
4.5. Hormone Therapy
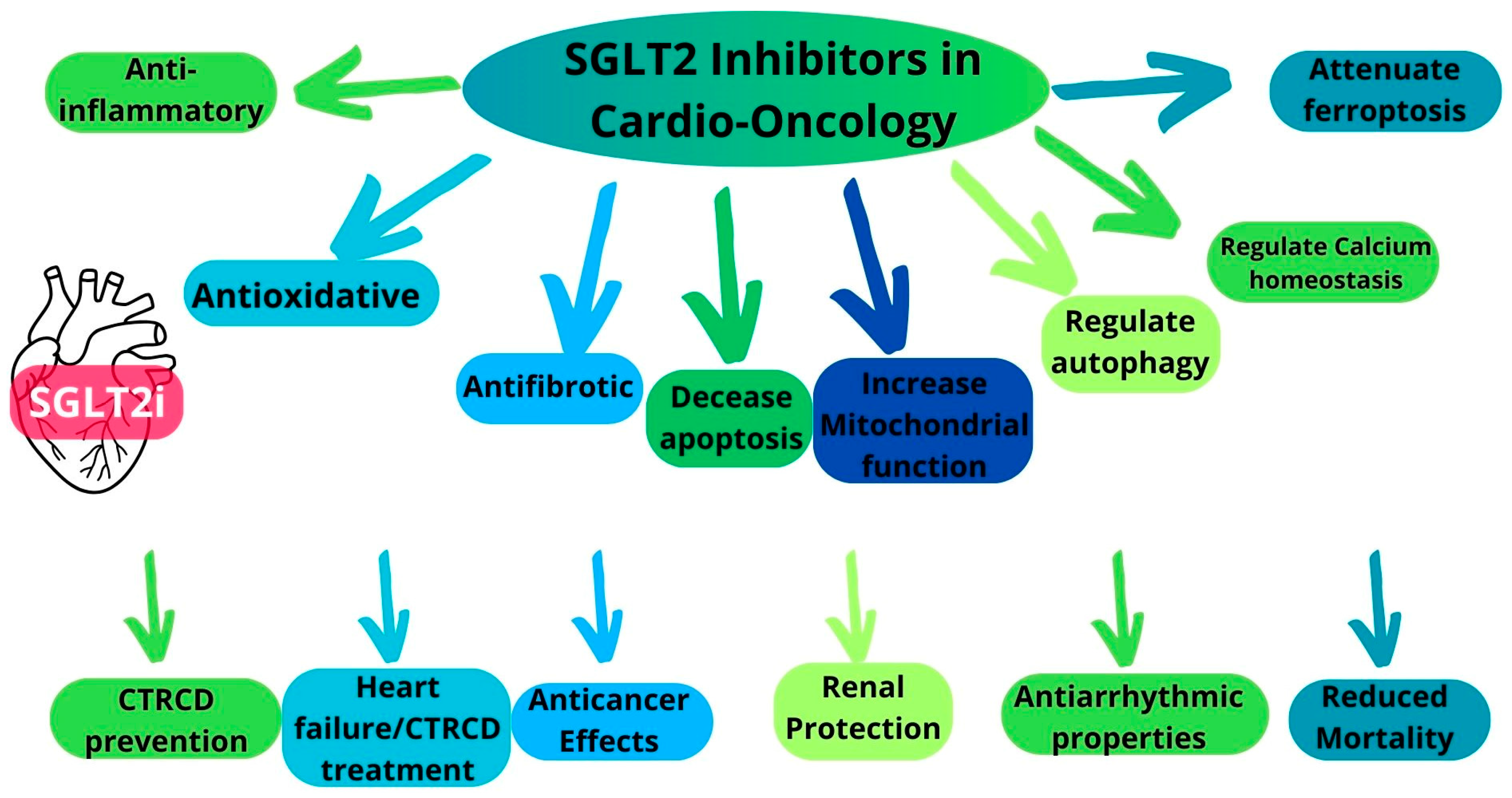
5. Multifaceted Cardioprotective Mechanisms of SGLT2i
6. Overview of the Preclinical Evidence of the Cardioprotective Effects of SGLT2i
Antifibrotic Properties of SGLT2i
7. Clinical Evidence for the Cardioprotective Properties of SGLT2i
7.1. Retrospective Studies
7.2. Case Reports
7.3. Prospective Studies
7.4. Discussion
8. Anticancer Effects of SGLT2 Inhibitors
Proposed Anticancer Mechanisms
9. SGLT2i Safety
10. All-Cause and Cancer-Specific Mortality with SGLT2 Inhibitors
11. Influence of SGLT2 Inhibitors on Heart Failure Incidence and Hospitalizations
12. Antiarrhythmic Properties of SGLT2 Inhibitors
13. SGLT2 Inhibitors as a Remedy for Anthracycline-Induced Cardiomyopathy?
14. Challenges
Current Gaps, Problems, and Future Directions
15. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Di Lisi, D.; Madaudo, C.; Macaione, F.; Galassi, A.R.; Novo, G. Cancer survivors and cardiovascular diseases: From preventive strategies to treatment. J. Cardiovasc. Med. 2025, 26, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ferrera, A.; Fiorentini, V.; Reale, S.; Solfanelli, G.; Tini, G.; Barbato, E.; Volpe, M.; Battistoni, A. Anthracyclines-Induced Cardiac Dysfunction: What Every Clinician Should Know. Rev. Cardiovasc. Med. 2023, 24, 148. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Lachin, J.M.; Inzucchi, S.E.; Fitchett, D.; Mattheus, M.; George, J.; Woerle, H.J.; Broedl, U.C.; von Eynatten, M.; Zinman, B.; et al. Empagliflozin and Clinical Outcomes in Patients with Type 2 Diabetes Mellitus, Established Cardiovascular Disease, and Chronic Kidney Disease. Circulation 2018, 137, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, P.; Bergamaschi, L.; Gragnano, F.; Gallinoro, E.; Cesaro, A.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; Sansonetti, A.; et al. Outcomes in diabetic patients treated with SGLT2-Inhibitors with acute myocardial infarction undergoing PCI: The SGLT2-I AMI PROTECT Registry. Pharmacol. Res. 2023, 187, 106597. [Google Scholar] [CrossRef]
- Members, W.C.; Members, A.A.J.C. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J. Card. Fail. 2022, 28, e1–e167. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2024, 26, 5–17. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Chertow, G.M.; Correa-Rotter, R.; Greene, T.; Hou, F.F.; Lindberg, M.; McMurray, J.; Rossing, P.; Toto, R.; et al. Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol. Dial. Transplant. 2020, 35, 274–282. [Google Scholar] [CrossRef]
- Duan, H.Y.; Barajas-Martinez, H.; Antzelevitch, C.; Hu, D. The potential anti-arrhythmic effect of SGLT2 inhibitors. Cardiovasc. Diabetol. 2024, 23, 252. [Google Scholar] [CrossRef]
- Jaiswal, V.; Ang, S.P.; Kumar, D.; Deb, N.; Jaiswal, A.; Joshi, A.; Nasir, Y.M.; Bandyopadhyay, D.; Michos, E.D.; Benjamin, E.J.; et al. Sodium-Glucose Cotransporter-2 Inhibitors and Arrhythmias: A Meta-Analysis of 38 Randomized Controlled Trials. JACC Adv. 2025, 4, 101615. [Google Scholar] [CrossRef]
- Heck, S.L.; Mecinaj, A.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; Røsjø, H.; Steine, K.; Geisler, J.; et al. Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA): Extended Follow-Up of a 2×2 Factorial, Randomized, Placebo-Controlled, Double-Blind Clinical Trial of Candesartan and Metoprolol. Circulation 2021, 143, 2431–2440. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.M.; Garcia Arango, M.; Dasari, H.; Arciniegas Calle, M.; Adjei, E.; Rico Mesa, J.; Scott, C.G.; Thompson, C.A.; Cerhan, J.R.; Haddad, T.C.; et al. Association of Anthracycline with Heart Failure in Patients Treated for Breast Cancer or Lymphoma, 1985-2010. JAMA Netw. Open 2023, 6, e2254669. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.B.; Bongiovanni, C.; Da Pra, S.; Miano, C.; Sacchi, F.; Lauriola, M.; D’Uva, G. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms and Strategies for Cardioprotection. Front. Cardiovasc. Med. 2022, 9, 847012. [Google Scholar] [CrossRef]
- Guerra, C.C.D.S.; Sant’Ana, G.; Almeida, O.L.R. Incidence of Cardiovascular Complications in Pediatric Patients Treated with Anthracyclines at a Brazilian Cancer Center. Arq. Bras. Cardiol. 2024, 121, e20210352. [Google Scholar] [CrossRef]
- Xie, S.; Sun, Y.; Zhao, X.; Xiao, Y.; Zhou, F.; Lin, L.; Wang, W.; Lin, B.; Wang, Z.; Fang, Z.; et al. An update of the molecular mechanisms underlying anthracycline induced cardiotoxicity. Front. Pharmacol. 2024, 15, 1406247. [Google Scholar] [CrossRef]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.G.; Carbone, A.; Paccone, A.; Altucci, L.; Conte, M.; Canale, M.L.; et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol. 2021, 20, 150. [Google Scholar] [CrossRef]
- Quagliariello, V.; Canale, M.L.; Bisceglia, I.; Iovine, M.; Paccone, A.; Maurea, C.; Scherillo, M.; Merola, A.; Giordano, V.; Palma, G.; et al. Sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents ejection fraction reduction, reduces myocardial and renal NF-κB expression and systemic pro-inflammatory biomarkers in models of short-term doxorubicin cardiotoxicity. Front. Cardiovasc. Med. 2024, 11, 1289663. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef]
- Carrasco, R.; Castillo, R.L.; Gormaz, J.G.; Carrillo, M.; Thavendiranathan, P. Role of Oxidative Stress in the Mechanisms of Anthracycline-Induced Cardiotoxicity: Effects of Preventive Strategies. Oxid. Med. Cell Longev. 2021, 2021, 8863789. [Google Scholar] [CrossRef]
- Narezkina, A.; Narayan, H.K.; Zemljic-Harpf, A.E. Molecular mechanisms of anthracycline cardiovascular toxicity. Clin. Sci. 2021, 135, 1311–1332. [Google Scholar] [CrossRef] [PubMed]
- Osataphan, N.; Abdel-Qadir, H.; Zebrowska, A.M.; Borowiec, A. Sodium-Glucose Cotransporter 2 Inhibitors During Cancer Therapy: Benefits, Risks, and Ongoing Clinical Trials. Curr. Oncol. Rep. 2024, 26, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Zaghlol, R.; Pedersen, L.; Qamer, S.; Yoo, S.G.K.; Ladin, D.A.; Parvathaneni, A.; Bergom, C.; Mitchell, J.D. Cardiac Complications of Radiation Therapy. Cardiol. Clin. 2025, 43, 129–149. [Google Scholar] [CrossRef]
- Dreyfuss, A.D.; Velalopoulou, A.; Avgousti, H.; Bell, B.I.; Verginadis, I.I. Preclinical models of radiation-induced cardiac toxicity: Potential mechanisms and biomarkers. Front. Oncol. 2022, 12, 920867. [Google Scholar] [CrossRef]
- Rolski, F.; Mączewski, M. Cardiac Fibrosis: Mechanistic Discoveries Linked to SGLT2 Inhibitors. Pharmaceuticals 2025, 18, 313. [Google Scholar] [CrossRef]
- Kitani, T.; Ong, S.G.; Lam, C.K.; Rhee, J.W.; Zhang, J.Z.; Oikonomopoulos, A.; Ma, N.; Tian, L.; Lee, J.; Telli, M.L.; et al. Human-Induced Pluripotent Stem Cell Model of Trastuzumab-Induced Cardiac Dysfunction in Patients with Breast Cancer. Circulation 2019, 139, 2451–2465. [Google Scholar] [CrossRef]
- Gordon, L.I.; Burke, M.A.; Singh, A.T.; Prachand, S.; Lieberman, E.D.; Sun, L.; Naik, T.J.; Prasad, S.V.; Ardehali, H. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J. Biol. Chem. 2009, 284, 2080–2087. [Google Scholar] [CrossRef]
- Sharma, A.; Fierro, M.E.; Governor, S.; Kothare, A.; Pak, S.; Liu, K.; Alam, Z.; Otchere, P. Incidence and risk factors of trastuzumab-induced cardiac dysfunction in a predominantly Hispanic South Texas population: A descriptive study. Cardiooncology 2025, 11, 23. [Google Scholar] [CrossRef]
- Yu, A.F.; Lin, I.H.; Jorgensen, J.; Copeland-Halperin, R.; Feldman, S.; Ibtida, I.; Assefa, A.; Johnson, M.N.; Dang, C.T.; Liu, J.E.; et al. Nomogram for Predicting Risk of Cancer Therapy-Related Cardiac Dysfunction in Patients with Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer. J. Am. Heart Assoc. 2023, 12, e029465. [Google Scholar] [CrossRef]
- Zheng, H.; Mahmood, S.S.; Khalique, O.K.; Zhan, H. Trastuzumab-Induced Cardiotoxicity: When and How Much Should We Worry? JCO Oncol. Pract. 2024, 20, 1055–1063. [Google Scholar] [CrossRef]
- Min, J.; Wu, L.; Liu, Y.; Song, G.; Deng, Q.; Jin, W.; Yu, W.; Abudureyimu, M.; Pei, Z.; Ren, J. Empagliflozin attenuates trastuzumab-induced cardiotoxicity through suppression of DNA damage and ferroptosis. Life Sci. 2023, 312, 121207. [Google Scholar] [CrossRef] [PubMed]
- Zito, C.; Manganaro, R.; Ciappina, G.; Spagnolo, C.C.; Racanelli, V.; Santarpia, M.; Silvestris, N.; Carerj, S. Cardiotoxicity Induced by Immune Checkpoint Inhibitors: What a Cardio-Oncology Team Should Know and Do. Cancers 2022, 14, 5403. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, V.; Suero-Abreu, G.A.; Neilan, T.G. Immune checkpoint inhibitors and myocardial infarction. J. Thromb. Thrombolysis 2025. [Google Scholar] [CrossRef] [PubMed]
- Perelman, M.G.; Brzezinski, R.Y.; Waissengrin, B.; Leshem, Y.; Bainhoren, O.; Rubinstein, T.A.; Perelman, M.; Rozenbaum, Z.; Havakuk, O.; Topilsky, Y.; et al. Sodium-glucose co-transporter-2 inhibitors in patients treated with immune checkpoint inhibitors. Cardiooncology 2024, 10, 2. [Google Scholar] [CrossRef]
- Challa, A.A.; Calaway, A.C.; Cullen, J.; Garcia, J.; Desai, N.; Weintraub, N.L.; Deswal, A.; Kutty, S.; Vallakati, A.; Addison, D.; et al. Cardiovascular Toxicities of Androgen Deprivation Therapy. Curr. Treat. Options Oncol. 2021, 22, 47. [Google Scholar] [CrossRef]
- Chen, K.; Wong, T.H.; Tan, Y.G.; Tay, K.J.; Tan, W.C.; Chan, J.; Ho, H.; Cheng, C.; Teoh, J.Y.; Chiu, P.K.; et al. Cardio-oncology in advanced prostate cancer. Front. Oncol. 2024, 14, 1386597. [Google Scholar] [CrossRef]
- Koutroumpakis, E.; Patel, R.; Khadke, S.; Bedrosian, A.; Kumar, A.; Kong, Y.; Connell, B.; Upadhyay, J.; Dani, S.S.; Hahn, A.W.; et al. Associations of SGLT2i with Cardiorenal Outcomes Among Diabetics with Prostate Cancer on Hormone Therapy. Cardiovasc. Drugs Ther. 2024. [Google Scholar] [CrossRef]
- Tang, Z.; Chang, Y.C.; Chi, K.Y.; Chang, Y.; Lee, T.N.; Hsiao, C.L.; Chiang, C.H.; Wang, Q.; Jang, A. Impact of Sodium-glucose cotransporter-2 inhibitors on Cardiovascular Outcomes of Prostate Cancer Patients Receiving Gonadotropin-Releasing Hormone Agonists. Eur. J. Prev. Cardiol. 2024, zwae267. [Google Scholar] [CrossRef]
- Hsieh, P.L.; Chu, P.M.; Cheng, H.C.; Huang, Y.T.; Chou, W.C.; Tsai, K.L.; Chan, S.H. Dapagliflozin Mitigates Doxorubicin-Caused Myocardium Damage by Regulating Akt-Mediated Oxidative Stress, Cardiac Remodeling, and Inflammation. Int. J. Mol. Sci. 2022, 23, 10146. [Google Scholar] [CrossRef]
- El-Sawy, W.S.M.; El-Bahrawy, A.H.; Messiha, B.A.S.; Hemeida, R.A.M.; Khalaf, M.M. The impact of PPAR-γ/Nrf-2/HO-1, NF-κB/IL-6/ Keap-1, and Bcl-2/caspase-3/ATG-5 pathways in mitigation of DOX-induced cardiotoxicity in an animal model: The potential cardioprotective role of oxyresveratrol and/or dapagliflozin. Food Chem. Toxicol. 2024, 191, 114863. [Google Scholar] [CrossRef]
- Belen, E.; Canbolat, I.P.; Yigittürk, G.; Cetinarslan, Ö.; Akdeniz, C.S.; Karaca, M.; Sönmez, M.; Erbas, O. Cardio-protective effect of dapagliflozin against doxorubicin induced cardiomyopathy in rats. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4403–4408. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Lin, Y.W.; Ho, C.H.; Chen, Z.C.; Liu, P.Y.; Shih, J.Y. Dapagliflozin suppresses ER stress and protects doxorubicin-induced cardiotoxicity in breast cancer patients. Arch. Toxicol. 2021, 95, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Kounatidis, D.; Vallianou, N.G.; Karampela, I.; Rebelos, E.; Kouveletsou, M.; Dalopoulos, V.; Koufopoulos, P.; Diakoumopoulou, E.; Tentolouris, N.; Dalamaga, M. Anti-Diabetic Therapies and Cancer: From Bench to Bedside. Biomolecules 2024, 14, 1479. [Google Scholar] [CrossRef]
- Oh, C.M.; Cho, S.; Jang, J.Y.; Kim, H.; Chun, S.; Choi, M.; Park, S.; Ko, Y.G. Cardioprotective Potential of an SGLT2 Inhibitor Against Doxorubicin-Induced Heart Failure. Korean Circ. J. 2019, 49, 1183–1195. [Google Scholar] [CrossRef]
- Chang, H.Y.; Hsu, H.C.; Fang, Y.H.; Liu, P.Y.; Liu, Y.W. Empagliflozin attenuates doxorubicin-induced cardiotoxicity by inhibiting the JNK signaling pathway. Biomed. Pharmacother. 2024, 176, 116759. [Google Scholar] [CrossRef]
- Yang, C.C.; Chen, Y.T.; Wallace, C.G.; Chen, K.H.; Cheng, B.C.; Sung, P.H.; Li, Y.C.; Ko, S.F.; Chang, H.W.; Yip, H.K. Early administration of empagliflozin preserved heart function in cardiorenal syndrome in rat. Biomed. Pharmacother. 2019, 109, 658–670. [Google Scholar] [CrossRef]
- Chen, M. Empagliflozin attenuates doxorubicin-induced cardiotoxicity by activating AMPK/SIRT-1/PGC-1α-mediated mitochondrial biogenesis. Toxicol. Res. 2023, 12, 216–223. [Google Scholar] [CrossRef]
- Sabatino, J.; De Rosa, S.; Tammè, L.; Iaconetti, C.; Sorrentino, S.; Polimeni, A.; Mignogna, C.; Amorosi, A.; Spaccarotella, C.; Yasuda, M.; et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc. Diabetol. 2020, 19, 66. [Google Scholar] [CrossRef]
- Vaziri, Z.; Saleki, K.; Aram, C.; Alijanizadeh, P.; Pourahmad, R.; Azadmehr, A.; Ziaei, N. Empagliflozin treatment of cardiotoxicity: A comprehensive review of clinical, immunobiological, neuroimmune, and therapeutic implications. Biomed. Pharmacother. 2023, 168, 115686. [Google Scholar] [CrossRef]
- Barış, V.; Dinçsoy, A.B.; Gedikli, E.; Zırh, S.; Müftüoğlu, S.; Erdem, A. Empagliflozin Significantly Prevents the Doxorubicin-induced Acute Cardiotoxicity via Non-antioxidant Pathways. Cardiovasc. Toxicol. 2021, 21, 747–758. [Google Scholar] [CrossRef]
- Lin, R.; Peng, X.; Li, Y.; Wang, X.; Liu, X.; Jia, X.; Zhang, C.; Liu, N.; Dong, J. Empagliflozin attenuates doxorubicin-impaired cardiac contractility by suppressing reactive oxygen species in isolated myocytes. Mol. Cell. Biochem. 2024, 479, 2105–2118. [Google Scholar] [CrossRef] [PubMed]
- Medina-Hernández, D.; Cádiz, L.; Mastrangelo, A.; Moreno-Arciniegas, A.; Fernández Tocino, M.; Cueto Becerra, A.A.; Díaz-Guerra Priego, A.; Skoza, W.A.; Higuero-Verdejo, M.I.; López-Martín, G.J.; et al. SGLT2i Therapy Prevents Anthracycline-Induced Cardiotoxicity in a Large Animal Model by Preserving Myocardial Energetics. JACC CardioOncol. 2025, 7, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Guo, D.L. The effect of sodium-glucose cotransporter-2 inhibitors on cardiac structure remodeling and function: A meta-analysis of randomized controlled trials. Eur. J. Intern. Med. 2023, 114, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Gongora, C.A.; Drobni, Z.D.; Quinaglia Araujo Costa Silva, T.; Zafar, A.; Gong, J.; Zlotoff, D.A.; Gilman, H.K.; Hartmann, S.E.; Sama, S.; Nikolaidou, S.; et al. Sodium-Glucose Co-Transporter-2 Inhibitors and Cardiac Outcomes Among Patients Treated with Anthracyclines. JACC Heart Fail. 2022, 10, 559–567. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Carrasco, R.; Austin, P.C.; Chen, Y.; Zhou, L.; Fang, J.; Su, H.M.H.; Lega, I.C.; Kaul, P.; Neilan, T.G.; et al. The Association of Sodium-Glucose Cotransporter 2 Inhibitors with Cardiovascular Outcomes in Anthracycline-Treated Patients with Cancer. JACC CardioOncol. 2023, 5, 318–328. [Google Scholar] [CrossRef]
- Chiang, C.H.; Ma, K.S.; Peng, C.Y.; Hsia, Y.P.; Horng, C.S.; Chen, C.Y.; Chang, Y.C.; See, X.Y.; Chen, Y.J.; Wang, S.S.; et al. Impact of sodium-glucose cotransporter-2 inhibitors on heart failure and mortality in patients with cancer. Heart 2023, 109, 470–477. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, M.; Jun, J.E.; Yon, D.K. Sodium-glucose cotransporter-2 inhibitors improve clinical outcomes in patients with type 2 diabetes mellitus undergoing anthracycline-containing chemotherapy: An emulated target trial using nationwide cohort data in South Korea. Sci. Rep. 2023, 13, 21756. [Google Scholar] [CrossRef]
- Avula, V.; Sharma, G.; Kosiborod, M.N.; Vaduganathan, M.; Neilan, T.G.; Lopez, T.; Dent, S.; Baldassarre, L.; Scherrer-Crosbie, M.; Barac, A.; et al. SGLT2 Inhibitor Use and Risk of Clinical Events in Patients with Cancer Therapy-Related Cardiac Dysfunction. JACC Heart Fail. 2024, 12, 67–78. [Google Scholar] [CrossRef]
- Bhatti, A.W.; Patel, R.; Dani, S.S.; Khadke, S.; Makwana, B.; Lessey, C.; Shah, J.; Al-Husami, Z.; Yang, E.H.; Thavendiranathan, P.; et al. SGLT2i and Primary Prevention of Cancer Therapy-Related Cardiac Dysfunction in Patients with Diabetes. JACC CardioOncol. 2024, 6, 863–875. [Google Scholar] [CrossRef]
- Fath, A.R.; Aglan, M.; Aglan, A.; Chilton, R.J.; Trakhtenbroit, A.; Al-Shammary, O.A.; Oppong-Nkrumah, O.; Lenihan, D.J.; Dent, S.F.; Otchere, P. Cardioprotective Potential of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Cancer Treated with Anthracyclines: An Observational Study. Am. J. Cardiol. 2024, 222, 175–182. [Google Scholar] [CrossRef]
- Giangiacomi, F.; Faggiano, A.; Cardinale, D.; Rossi, F.G.; Pollina, A.; Gherbesi, E.; Gnan, E.; Carugo, S.; Vicenzi, M. Case report: Sodium-glucose cotransporter 2 inhibitors induce left ventricular reverse remodeling in anthracycline-related cardiac dysfunction-a case series. Front. Cardiovasc. Med. 2023, 10, 1250185. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Hayashi, Y.; Oyanagi, N.; Tanaka, K.; Ozaki, K.; Kashiwa, A.; Hosaka, Y.; Tsuchida, K.; Takahashi, K. Add-on multidrug treatment based on quadruple therapy successfully treated worsening heart failure caused by anthracycline-induced cardiomyopathy in a survivor of cancer as a young adult: A case report. BMC Cardiovasc. Disord. 2024, 24, 505. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.J.; Gregorietti, V.; Costa, D.; López-Fernández, T. Use of EMPAgliflozin in the prevention of CARDiotoxicity: The EMPACARD—PILOT trial. Cardiooncology 2024, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Henson, B.D.; Bale-Neary, C.A.; Mecaskey, R.; Gbujie, O.; Zhan, M.; Rao, K.; Carbone, S. Sodium-Glucose Cotransporter 2 Inhibitors, Malnutrition, Cachexia, and Survival in Patients with Heart Failure with a History of Anthracycline Treatment. J. Cardiovasc. Pharmacol. 2024, 84, 486–489. [Google Scholar] [CrossRef]
- Tabowei, G.; Dadzie, S.K.; Perswani, P.; Nawaz, S.; Kaur, M.; Moqattash, M.; Wei, C.R.; Hirani, S. Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors in Preventing Heart Failure in Patients Receiving Anthracycline-Based Cancer Therapy: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e60086. [Google Scholar] [CrossRef]
- Bhalraam, U.; Veerni, R.B.; Paddock, S.; Meng, J.; Piepoli, M.; López-Fernández, T.; Tsampasian, V.; Vassiliou, V.S. Impact of sodium-glucose cotransporter-2 inhibitors on heart failure outcomes in cancer patients and survivors: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2025, zwaf026. [Google Scholar] [CrossRef]
- Xu, B.; Kang, B.; Li, S.; Fan, S.; Zhou, J. Sodium-glucose cotransporter 2 inhibitors and cancer: A systematic review and meta-analysis. J. Endocrinol. Investig. 2024, 47, 2421–2436. [Google Scholar] [CrossRef]
- Pandey, A.; Alcaraz, M.; Saggese, P.; Soto, A.; Gomez, E.; Jaldu, S.; Yanagawa, J.; Scafoglio, C. Exploring the Role of SGLT2 Inhibitors in Cancer: Mechanisms of Action and Therapeutic Opportunities. Cancers 2025, 17, 466. [Google Scholar] [CrossRef]
- Nevola, R.; Villani, A.; Imbriani, S.; Alfano, M.; Criscuolo, L.; Beccia, D.; Ruocco, R.; Femine, A.D.; Gragnano, F.; Cozzolino, D.; et al. Sodium-Glucose Co-Transporters Family: Current Evidence, Clinical Applications and Perspectives. Front. Biosci. 2023, 28, 103. [Google Scholar] [CrossRef]
- Kabel, A.M.; Arab, H.H.; Abd Elmaaboud, M.A. Effect of dapagliflozin and/or L-arginine on solid tumor model in mice: The interaction between nitric oxide, transforming growth factor-beta 1, autophagy, and apoptosis. Fundam. Clin. Pharmacol. 2021, 35, 968–978. [Google Scholar] [CrossRef]
- Benedetti, R.; Benincasa, G.; Glass, K.; Chianese, U.; Vietri, M.T.; Congi, R.; Altucci, L.; Napoli, C. Effects of novel SGLT2 inhibitors on cancer incidence in hyperglycemic patients: A meta-analysis of randomized clinical trials. Pharmacol. Res. 2022, 175, 106039. [Google Scholar] [CrossRef] [PubMed]
- Dabour, M.S.; George, M.Y.; Daniel, M.R.; Blaes, A.H.; Zordoky, B.N. The Cardioprotective and Anticancer Effects of SGLT2 Inhibitors: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Mukai, J.; Kanno, S.; Kubota, R. A literature review and meta-analysis of safety profiles of SGLT2 inhibitors in Japanese patients with diabetes mellitus. Sci. Rep. 2021, 11, 13472. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Qamar, U.; Fujiwara, Y.; Guha, A.; Naqash, A.R.; Yang, E.H.; Addison, D.; Barac, A.; Asad, Z.U.A. The Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Cardiovascular Outcomes in Patients with Cancer: A Systematic Review and Meta-Analysis. Am. J. Cardiol. 2024, 216, 87–90. [Google Scholar] [CrossRef]
- Kuo, H.H.; Wang, K.T.; Chen, H.H.; Lai, Z.Y.; Lin, P.L.; Chuang, Y.J.; Liu, L.Y. Cardiovascular outcomes associated with SGLT2 inhibitor therapy in patients with type 2 diabetes mellitus and cancer: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2024, 16, 108. [Google Scholar] [CrossRef]
- Huang, Y.M.; Chen, W.M.; Jao, A.T.; Chen, M.; Shia, B.C.; Wu, S.Y. Effects of SGLT2 inhibitors on clinical cancer survival in patients with type 2 diabetes. Diabetes Metab. 2024, 50, 101500. [Google Scholar] [CrossRef]
- Chiang, C.H.; Hsia, Y.P.; Jaroenlapnopparat, A.; Horng, C.S.; Wong, K.Y.; Wang, S.S.; Chang, Y.C.; Chen, B.S.; Luan, Y.Z.; Wang, C.H.; et al. The impact of sodium-glucose cotransporter-2 inhibitors on outcome of patients with diabetes mellitus and colorectal cancer. J. Gastroenterol. Hepatol. 2024, 39, 902–907. [Google Scholar] [CrossRef]
- Hendryx, M.; Dong, Y.; Ndeke, J.M.; Luo, J. Sodium-glucose cotransporter 2 (SGLT2) inhibitor initiation and hepatocellular carcinoma prognosis. PLoS ONE 2022, 17, e0274519. [Google Scholar] [CrossRef]
- Luo, J.; Hendryx, M.; Dong, Y. Sodium-glucose cotransporter 2 (SGLT2) inhibitors and non-small cell lung cancer survival. Br. J. Cancer 2023, 128, 1541–1547. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Piccoli, G.F.; Mesquita, L.A.; Stein, C.; Aziz, M.; Zoldan, M.; Degobi, N.A.H.; Spiazzi, B.F.; Lopes Junior, G.L.; Colpani, V.; Gerchman, F. Do GLP-1 Receptor Agonists Increase the Risk of Breast Cancer? A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2021, 106, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.H.; Song, J.; Chi, K.Y.; Chang, Y.C.; Xanthavanij, N.; Chang, Y.; Hsia, Y.P.; Chiang, C.H.; Ghamari, A.; Reynolds, K.L.; et al. Glucagon-like Peptide-1 Agonists Reduce Cardiovascular Events in Cancer Patients on Immune Checkpoint Inhibitors. Eur. J. Cancer. 2025, 216, 115170. [Google Scholar] [CrossRef] [PubMed]
- Biondi, F.; Madonna, R. The Potential Role of GLP1-RAs Against Anticancer-Drug Cardiotoxicity: A Scoping Review. J. Clin. Med. 2025, 14, 2705. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Peng, T.; Chen, Y.; Sha, L.; Dai, H.; Xiang, Y.; Zou, Z.; He, H.; Wang, S. Effects of GLP-1 Receptor Agonists on Biological Behavior of Colorectal Cancer Cells by Regulating PI3K/Akt/mTOR Signaling Pathway. Front. Pharmacol. 2022, 13, 901559. [Google Scholar] [CrossRef]
| First Author, Year, Ref., Country | Study Population | Tumor Type | Treatment | Mortality in SGLT2i Group vs. Non-SGLT2i | Heart Failure Hospitalizations in SGLT2i Group vs. Non-SGLT2i | Other Outcomes | Follow-Up | SGLT2i Used |
|---|---|---|---|---|---|---|---|---|
| Retrospective Studies | ||||||||
| Gongora et al., 2022 [64], USA | 128 (32 diabetic patients on SGLT2i vs. 96 matched controls) | Various, including lymphoma, breast, genitourinary, and others | AC (DOX) and others | Lower | Lower | Reduced HF incidence and exacerbation, development of cardiomyopathy or arrhythmia, sepsis, and neutropenic fever | 1.5 years | Empa, Cana, and Dapa |
| Abdel-Qadir et al., 2023 [65], Canada | 933 (99 diabetic patients on SGLT2i vs. 843 non-SGLT2i controls) | Various (lymphoma, gastrointestinal, breast, and others) | AC (DOX and epirubicin) | No difference | Lower | Reduced HF incidence | 1.6 years | Dapa, Empa, and Cana |
| Chiang et al., 2023 [66], Taiwan | 8640 (848 diabetic SGLT patients vs. 878 non-SGLT2i controls) | Various, including gastrointestinal, genitourinary, thoracic, and others | AC, alkylating agents, antimetabolites, platinum, and plant alkaloids | Lower | Lower | Reduced HF incidence | 18.8 months | Dapa, Empa, and Cana |
| Hwang et al., 2023 [67], Republic of Korea | 81,527 (779 diabetic patients on SGLT2i vs. 77,337 non-DM controls and 3455 T2DM non-SGLT2i controls) | Various, including lymphoma, breast, genitourinary, and others | AC, HER2 inhibitors, alkylating agents, and VEGF-targeting agents | Lower | Lower | Reduced composite of HF hospitalization, acute myocardial infarction, ischemic stroke, and death | Not specified | Not specified |
| Avula et al., 2024 [68], Global | 1280 (640 diabetic on SGLT2i vs. 640 non-SGLT2i diabetic) | Various, including lymphoma, gastrointestinal, breast, and others | AC (DOX, idarubicin, liposomal DOX, and daunorubicin), alkylating agents, antimetabolites, small-molecule tyrosine kinase inhibitors, proteasome inhibitors, radiotherapy, and others | Lower | Lower rate of heart failure admissions | Reduced hospitalizations, HF incidence and exacerbation, AF burden, AKI, and renal replacement therapy | 2 years | Dapa, Empa, and Cana |
| Bhatti et al.; 2024 [69], USA | 8675 patients on SGLT2 vs. 8675 controls | Various, including gastrointestinal, and others | AC, monoclonal Ab, proteasome inhibitors, antimetabolites, alkylating agents, small-molecule tyrosine kinase inhibitors, and others | Lower | N/A | Lower rate of CTRCD in diabetic patients treated with SGLT2i, lower rate of HF exacerbations, all-cause hospitalization, AF, and new-onset AFl | 12 months | Empa, Dapa, and Cana |
| Fath, 2024 [70], USA | 1,412 (706 patients on SGLT2i (91% diabetic) vs. 706 controls) | Various (breast, lymphoma, gastrointestinal, genitourinary, mesothelial tissue, and soft tissue) | AC (DOX, epirubicin, idarubicin, and alrubicin) and mitoxantrone | No reduction | N/A | Reduced new-onset HF, HF exacerbation, and arrhythmia | 2 years | Empa, Cana, Dapa, and Ertu |
| Perelman et al., 2024 [44], Israel | 119 (24 vs. 95) | Various, including breast, melanoma, lung, hepatoma, and others | ICI | Lower | N/A | No significant differences in MACE; we observed 0 cases of myocarditis and AF in the SGLT2i compared to 2 and 6 cases in the non-SGLT2i group, respectively | 28 months | Empa and Dapa |
| Koutroumpakis et al., 2024 [47], USA | 26,848 (2155 vs. 2155) | Prostate cancer | Hormone therapy | Lower | N/A | Lower odds of new-onset HF, HF exacerbation, PAD, AF, cardiac arrest, need for renal replacement therapy, and overall emergency room visits/hospitalizations | 2 years | Cana, Dapa, and Empa |
| Tang, 2024 [48], USA | 4312 (452 vs. 452) | Prostate cancer | Hormone therapy (GnRH agonist) | Lower | N/A | Lower incidence of HF and MI | 2 years | Not specified |
| Henson et al., 2024 [74], USA | (1323 vs. 1323) | Various | AC | Lower | N/A | Improved survival, cachexia, and malnutrition in HF cancer survivors | 5 years | Not specified |
| Huang et al., 2024 [86], Taiwan | 50,133 (16,711 vs. 33,422) | Various (lymphoma, breast, genitourinary, and others) | AC, alkylating agents, antimicrobial agents, HER2 inhibitors, and VEGF-targeting agents | Lower | Reduction | Reduced all-cause mortality, cancer mortality, MI, and ischemic stroke | 4.5 years | Not specified |
| Chiang et al., 2024 [87], Taiwan | 1347 (92 vs. 92) | Colorectal adenocarcinoma | Monoclonal antibody, tegafur/uracil, and others | Lower | N/A | Reduced all-cause mortality | 5 years | Empa, Dapa, Cana, and Ertu |
| Hendryx et al., 2022 [88], USA | 274 (137 vs. 137) | Hepatocellular carcinoma | Surgery, chemotherapy, and radiation | Lower | N/A | Reduced all-cause mortality | 1.7 years | Cana, Dapa, Empa, and Ertugliflozin |
| Luo et al., 2023 [89], USA | 24,915 (531 on SGLT2i vs. 24,384) | Non-small cell lung cancer | Various (chemotherapy, radiation, and immunotherapy) | Lower | N/A | Improved overall survival | 1.5 years | Cana, Dapa, and Empa |
| Prospective Case–Control Study | ||||||||
| Daniele et al., 2024 [73], EMPACARD RCT | 38 vs. 38 placebo | Breast cancer | AC | No difference | No difference | Reduction in CTRCD (reduced decline in LVEF and reduced GLS impairment) | 6 months | Empa 10 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zebrowska, A.M.; Borowiec, A. Potential New Applications of Sodium–Glucose Cotransporter-2 Inhibitors Across the Continuum of Cancer-Related Cardiovascular Toxicity. Pharmaceuticals 2025, 18, 857. https://doi.org/10.3390/ph18060857
Zebrowska AM, Borowiec A. Potential New Applications of Sodium–Glucose Cotransporter-2 Inhibitors Across the Continuum of Cancer-Related Cardiovascular Toxicity. Pharmaceuticals. 2025; 18(6):857. https://doi.org/10.3390/ph18060857
Chicago/Turabian StyleZebrowska, Agnieszka Maria, and Anna Borowiec. 2025. "Potential New Applications of Sodium–Glucose Cotransporter-2 Inhibitors Across the Continuum of Cancer-Related Cardiovascular Toxicity" Pharmaceuticals 18, no. 6: 857. https://doi.org/10.3390/ph18060857
APA StyleZebrowska, A. M., & Borowiec, A. (2025). Potential New Applications of Sodium–Glucose Cotransporter-2 Inhibitors Across the Continuum of Cancer-Related Cardiovascular Toxicity. Pharmaceuticals, 18(6), 857. https://doi.org/10.3390/ph18060857






