Therapeutic Potential of Qilianxiaopi Formula: Targeting ADAM17-Mediated Chronic Inflammation in Atrophic Gastritis
Abstract
:1. Introduction
2. Results
2.1. Prediction of the QLXP Potential Targets Using Network Pharmacological Analysis
2.2. Identification of QLXP Targets Using Thermal Proteome Profiling
2.3. ADAM17 Is a Key Candidate Target Associated with CAG and GC
2.4. Validation of ADAM17 as a Target of QLXP
2.5. BLI Analysis Identifies Components Binding to ADAM17 in QLXP
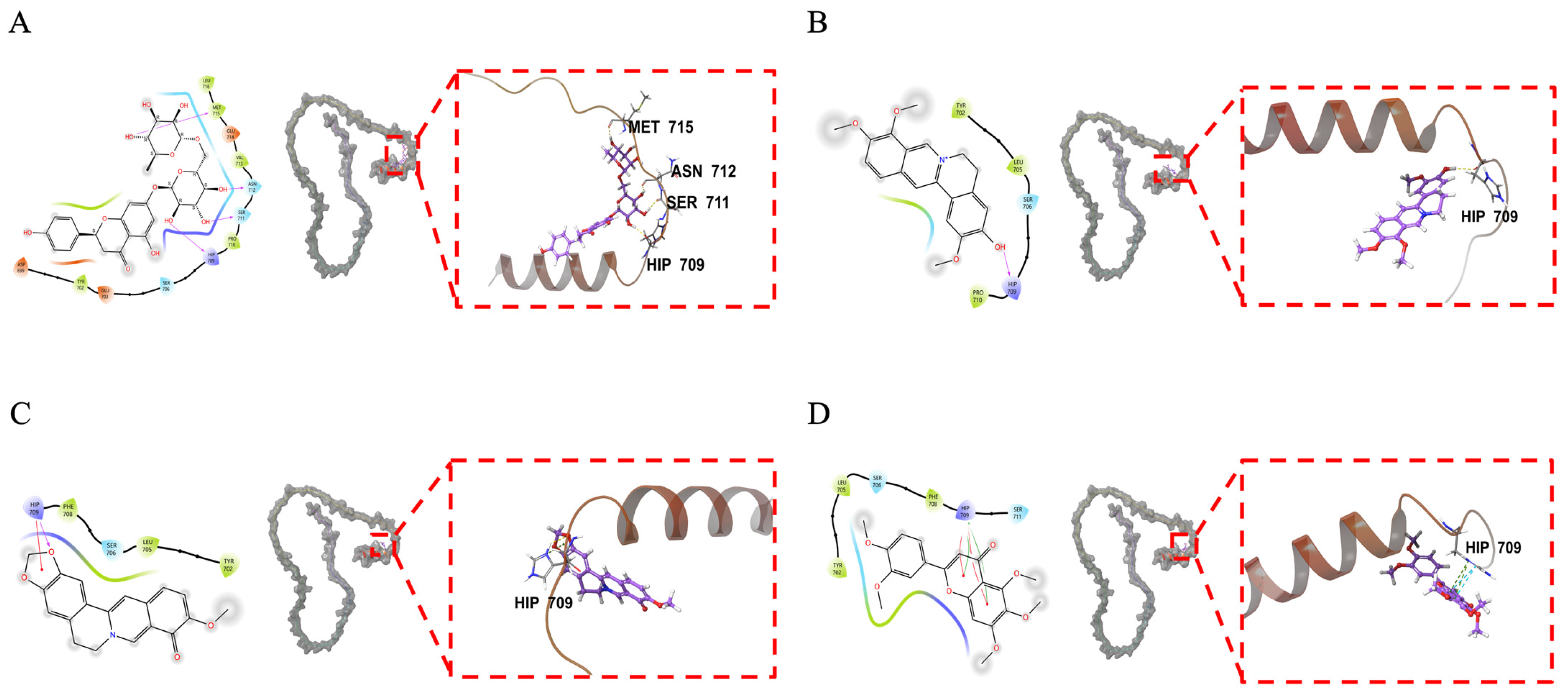
2.6. Molecular Docking Analysis of ADAM17 and Its Binding Components
3. Discussion
4. Materials and Methods
4.1. Network Pharmacology
4.1.1. Aqueous Extraction and Preparation of QLXP
4.1.2. LC-MS/MS Analysis
4.1.3. Prediction of the Potential Targets of the Major Compounds in QLXP
4.1.4. Construction and Analysis of the “Component-Target” Network
4.2. Thermal Proteome Profiling
4.2.1. Cell Culture
4.2.2. Sample Preparation for Thermal Proteome Profiling
4.2.3. LC-MS/MS Analysis and Protein Identification and Quantification
4.2.4. Bioinformatic Analysis
4.3. Western Blotting
4.4. ADAM17 Activity Assay
4.5. Enzyme-Linked Immunosorbent Assay of TNF-α
4.6. BLI Analysis
4.7. Molecular Docking Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gastric cancer |
| CAG | Chronic atrophic gastritis |
| IM | Intestinal metaplasia |
| TCM | Traditional Chinese medicine |
| RCT | Randomized controlled trial |
| QLXP | Qilianxiaopi formula |
| EMT | Epithelial–mesenchymal transition |
| TPP | Thermal proteome profiling |
| CETSA | Cellular thermal shift assay |
| BLI | Bio-layer interferometry |
| HPLC | High-performance liquid chromatography |
| LC-MS | Liquid chromatography-tandem mass spectrometry |
| NPARC | Nonparametric analysis of response curves |
| GO | Gene Ontology |
| PPI | Protein–protein interaction |
| HRP | Horseradish peroxidase |
| OD | Optical density |
| TMT | Tandem mass tag |
| Tm | Melting temperature |
| GSEA | Gene Set Enrichment Analysis |
| TACE | TNF-α-converting enzyme |
| ADAM | A Disintegrin And Metalloproteinase |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- GBD 2017 Stomach Cancer Collaborators. The Global, Regional, and National Burden of Stomach Cancer in 195 Countries, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of Epithelial Precancerous Conditions and Lesions in the Stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) Guideline Update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef]
- Song, H.; Ekheden, I.G.; Zheng, Z.; Ericsson, J.; Nyrén, O.; Ye, W. Incidence of Gastric Cancer among Patients with Gastric Precancerous Lesions: Observational Cohort Study in a Low Risk Western Population. BMJ 2015, 351, h3867. [Google Scholar] [CrossRef]
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Ushiku, T.; Fukayama, M.; Koike, K. Histologic Intestinal Metaplasia and Endoscopic Atrophy Are Predictors of Gastric Cancer Development after Helicobacter pylori Eradication. Gastrointest. Endosc. 2016, 84, 618–624. [Google Scholar] [CrossRef]
- Ortigão, R.; Figueirôa, G.; Frazzoni, L.; Pimentel-Nunes, P.; Hassan, C.; Dinis-Ribeiro, M.; Fuccio, L.; Libânio, D. Risk Factors for Gastric Metachronous Lesions after Endoscopic or Surgical Resection: A Systematic Review and Meta-Analysis. Endoscopy 2022, 54, 892–901. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Garbers, C.; Rose-John, S. ADAM17: A Molecular Switch to Control Inflammation and Tissue Regeneration. Trends Immunol. 2011, 32, 380–387. [Google Scholar] [CrossRef]
- Li, X.; Feng, M.; Yuan, G. Clinical Efficacy of Weisu Granule Combined with Weifuchun Tablet in the Treatment of Chronic Atrophic Gastritis and Its Effect on Serum G-17, PG I and PG II Levels. Am. J. Transl. Res. 2022, 14, 275–284. [Google Scholar]
- Dai, Y.-K.; Zhang, Y.-Z.; Li, D.-Y.; Ye, J.-T.; Zeng, L.-F.; Wang, Q.; Hu, L. The Efficacy of Jianpi Yiqi Therapy for Chronic Atrophic Gastritis: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0181906. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, Y.; Niu, J.; Zhu, C.; Yang, D.; Rong, F.; Liu, G. Efficacy of Banxia Xiexin Decoction for Chronic Atrophic Gastritis: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0241202. [Google Scholar] [CrossRef]
- Zeng, J.; Yan, R.; Pan, H.; You, F.; Cai, T.; Liu, W.; Zheng, C.; Zhao, Z.; Gong, D.; Chen, L.; et al. Weipixiao Attenuate Early Angiogenesis in Rats with Gastric Precancerous Lesions. BMC Complement. Altern. Med. 2018, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Qiao, J.; Kang, D.; Liu, B. Analysis on the Distinguishing Features of Traditional Chinese Therapeutics and Related Statistical Issues. Front. Med. 2011, 5, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network Pharmacology, a Promising Approach to Reveal the Pharmacology Mechanism of Chinese Medicine Formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, D.; Zhou, W.; Wang, L.; Wang, B.; Zhang, T.; Li, S. Network Pharmacology: Towards the Artificial Intelligence-Based Precision Traditional Chinese Medicine. Brief. Bioinform. 2023, 25, bbad518. [Google Scholar] [CrossRef]
- Martinez Molina, D.; Jafari, R.; Ignatushchenko, M.; Seki, T.; Larsson, E.A.; Dan, C.; Sreekumar, L.; Cao, Y.; Nordlund, P. Monitoring Drug Target Engagement in Cells and Tissues Using the Cellular Thermal Shift Assay. Science 2013, 341, 84–87. [Google Scholar] [CrossRef]
- Leijten, N.M.; Bakker, P.; Spaink, H.P.; den Hertog, J.; Lemeer, S. Thermal Proteome Profiling in Zebrafish Reveals Effects of Napabucasin on Retinoic Acid Metabolism. Mol. Cell. Proteom. 2021, 20, 100033. [Google Scholar] [CrossRef]
- Al-Amin, R.A.; Gallant, C.J.; Muthelo, P.M.; Landegren, U. Sensitive Measurement of Drug-Target Engagement by a Cellular Thermal Shift Assay with Multiplex Proximity Extension Readout. Anal. Chem. 2021, 93, 10999–11009. [Google Scholar] [CrossRef]
- Tu, Y.; Tan, L.; Tao, H.; Li, Y.; Liu, H. CETSA and Thermal Proteome Profiling Strategies for Target Identification and Drug Discovery of Natural Products. Phytomedicine 2023, 116, 154862. [Google Scholar] [CrossRef]
- Petersen, R.L. Strategies Using Bio-Layer Interferometry Biosensor Technology for Vaccine Research and Development. Biosensors 2017, 7, 49. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Huang, K.K.; Ma, H.; Chong, R.H.H.; Uchihara, T.; Lian, B.S.X.; Zhu, F.; Sheng, T.; Srivastava, S.; Tay, S.T.; Sundar, R.; et al. Spatiotemporal Genomic Profiling of Intestinal Metaplasia Reveals Clonal Dynamics of Gastric Cancer Progression. Cancer Cell 2023, 41, 2019–2037.e8. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Wawro, K.; Wawro, M.; Strzelecka, M.; Czarnek, M.; Bereta, J. The Role of NF-κB and Elk-1 in the Regulation of Mouse ADAM17 Expression. Biol. Open 2019, 8, bio039420. [Google Scholar] [CrossRef] [PubMed]
- Vivian, J.; Rao, A.A.; Nothaft, F.A.; Ketchum, C.; Armstrong, J.; Novak, A.; Pfeil, J.; Narkizian, J.; Deran, A.D.; Musselman-Brown, A.; et al. Toil Enables Reproducible, Open Source, Big Biomedical Data Analyses. Nat. Biotechnol. 2017, 35, 314–316. [Google Scholar] [CrossRef]
- Wang, W.; Di, T.; Wang, W.; Jiang, H. EGCG, GCG, TFDG, or TSA Inhibiting Melanin Synthesis by Downregulating MC1R Expression. Int. J. Mol. Sci. 2023, 24, 11017. [Google Scholar] [CrossRef]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; Wolfson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S.; et al. A Metalloproteinase Disintegrin That Releases Tumour-Necrosis Factor-Alpha from Cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef]
- Moss, M.L.; Jin, S.L.; Milla, M.E.; Bickett, D.M.; Burkhart, W.; Carter, H.L.; Chen, W.J.; Clay, W.C.; Didsbury, J.R.; Hassler, D.; et al. Cloning of a Disintegrin Metalloproteinase That Processes Precursor Tumour-Necrosis Factor-Alpha. Nature 1997, 385, 733–736. [Google Scholar] [CrossRef]
- Zunke, F.; Rose-John, S. The Shedding Protease ADAM17: Physiology and Pathophysiology. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 2059–2070. [Google Scholar] [CrossRef]
- Schumacher, N.; Rose-John, S. ADAM17 Activity and IL-6 Trans-Signaling in Inflammation and Cancer. Cancers 2019, 11, 1736. [Google Scholar] [CrossRef]
- Schumacher, N.; Rose-John, S. ADAM17 Orchestrates Interleukin-6, TNFα and EGF-R Signaling in Inflammation and Cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2022, 1869, 119141. [Google Scholar] [CrossRef]
- Saha, A.; Backert, S.; Hammond, C.E.; Gooz, M.; Smolka, A.J. Helicobacter pylori CagL Activates ADAM17 to Induce Repression of the Gastric H, K-ATPase α Subunit. Gastroenterology 2010, 139, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, H.G.; Qazi, K.R.; Somiah, T.; Pathak, S.K.; Sjölinder, H.; Sverremark Ekström, E.; Jonsson, A.-B. Lactobacillus Gasseri Suppresses the Production of Proinflammatory Cytokines in Helicobacter pylori-Infected Macrophages by Inhibiting the Expression of ADAM17. Front. Immunol. 2019, 10, 2326. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, H.; Zhang, C.; He, J.; Wei, H.; Zhou, M.; Lu, Y.; Sun, Y.; Ding, J.W.; Zeng, J.; et al. ADAM17 Promotes Epithelial-Mesenchymal Transition via TGF-β/Smad Pathway in Gastric Carcinoma Cells. Int. J. Oncol. 2016, 49, 2520–2528. [Google Scholar] [CrossRef]
- Satoh, M.; Nakamura, M.; Satoh, H.; Saitoh, H.; Segawa, I.; Hiramori, K. Expression of Tumor Necrosis Factor-Alpha--Converting Enzyme and Tumor Necrosis Factor-Alpha in Human Myocarditis. J. Am. Coll. Cardiol. 2000, 36, 1288–1294. [Google Scholar] [CrossRef]
- Hooper, N.M.; Turner, A.J. The Search for Alpha-Secretase and Its Potential as a Therapeutic Approach to Alzheimer s Disease. Curr. Med. Chem. 2002, 9, 1107–1119. [Google Scholar] [CrossRef]
- Moss, M.L.; Sklair-Tavron, L.; Nudelman, R. Drug Insight: Tumor Necrosis Factor-Converting Enzyme as a Pharmaceutical Target for Rheumatoid Arthritis. Nat. Clin. Pract. Rheumatol. 2008, 4, 300–309. [Google Scholar] [CrossRef]
- Calligaris, M.; Cuffaro, D.; Bonelli, S.; Spanò, D.P.; Rossello, A.; Nuti, E.; Scilabra, S.D. Strategies to Target ADAM17 in Disease: From Its Discovery to the iRhom Revolution. Molecules 2021, 26, 944. [Google Scholar] [CrossRef]
- Yu, L.; Wu, A.-G.; Wong, V.K.-W.; Qu, L.-Q.; Zhang, N.; Qin, D.-L.; Zeng, W.; Tang, B.; Wang, H.-M.; Wang, Q.; et al. The New Application of UHPLC-DAD-TOF/MS in Identification of Inhibitors on β-Amyloid Fibrillation From Scutellaria Baicalensis. Front. Pharmacol. 2019, 10, 194. [Google Scholar] [CrossRef]
- Guo, M.; Zhu, F.; Qiu, W.; Qiao, G.; Law, B.Y.-K.; Yu, L.; Wu, J.; Tang, Y.; Yu, C.; Qin, D.; et al. High-Throughput Screening for Amyloid-β Binding Natural Small-Molecules Based on the Combinational Use of Biolayer Interferometry and UHPLC-DAD-Q/TOF-MS/MS. Acta Pharm. Sin B 2022, 12, 1723–1739. [Google Scholar] [CrossRef]
- Mitra, S.; Lami, M.S.; Uddin, T.M.; Das, R.; Islam, F.; Anjum, J.; Hossain, M.J.; Emran, T.B. Prospective Multifunctional Roles and Pharmacological Potential of Dietary Flavonoid Narirutin. Biomed. Pharmacother. 2022, 150, 112932. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Yu, Y.-Q.; Yan, X.-X.; Wang, W.-J.; Tian, X.-T.; Wang, L.; Zhu, W.-L.; Gong, L.-K.; Pan, G.-Y. Different Structures of Berberine and Five Other Protoberberine Alkaloids That Affect P-Glycoprotein-Mediated Efflux Capacity. Acta Pharmacol. Sin. 2019, 40, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Han Jie, L.; Jantan, I.; Yusoff, S.D.; Jalil, J.; Husain, K. Sinensetin: An Insight on Its Pharmacological Activities, Mechanisms of Action and Toxicity. Front. Pharmacol. 2021, 11, 553404. [Google Scholar] [CrossRef] [PubMed]
- Editorial: ChemSpider—A Tool for Natural Products Research. Nat. Prod. Rep. 2015, 32, 1163–1164. [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Yang, C. Flavonoid 4,4′-Dimethoxychalcone Suppresses Cell Proliferation via Dehydrogenase Inhibition and Oxidative Stress Aggravation. Free Radic. Biol. Med. 2021, 175, 206–215. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
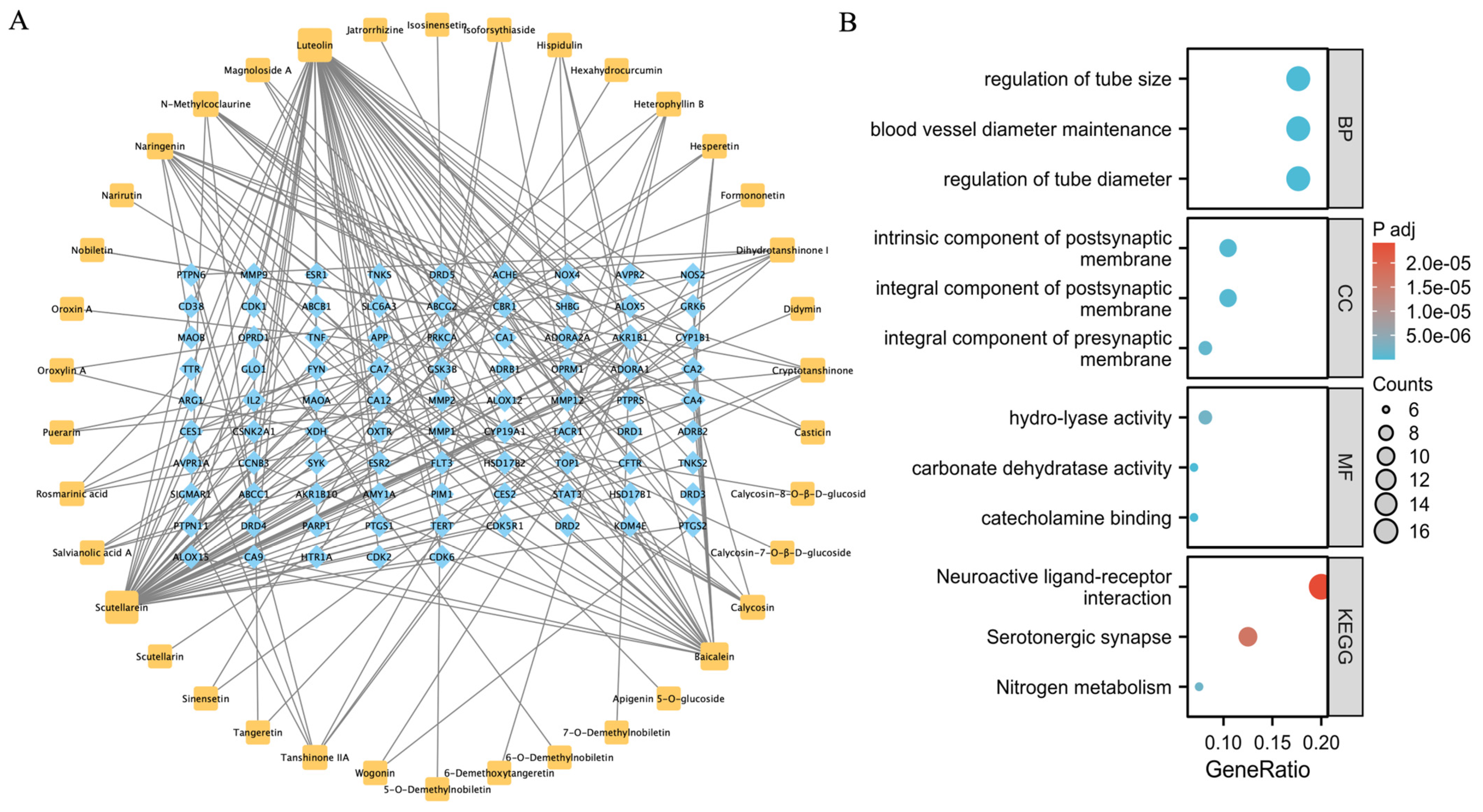

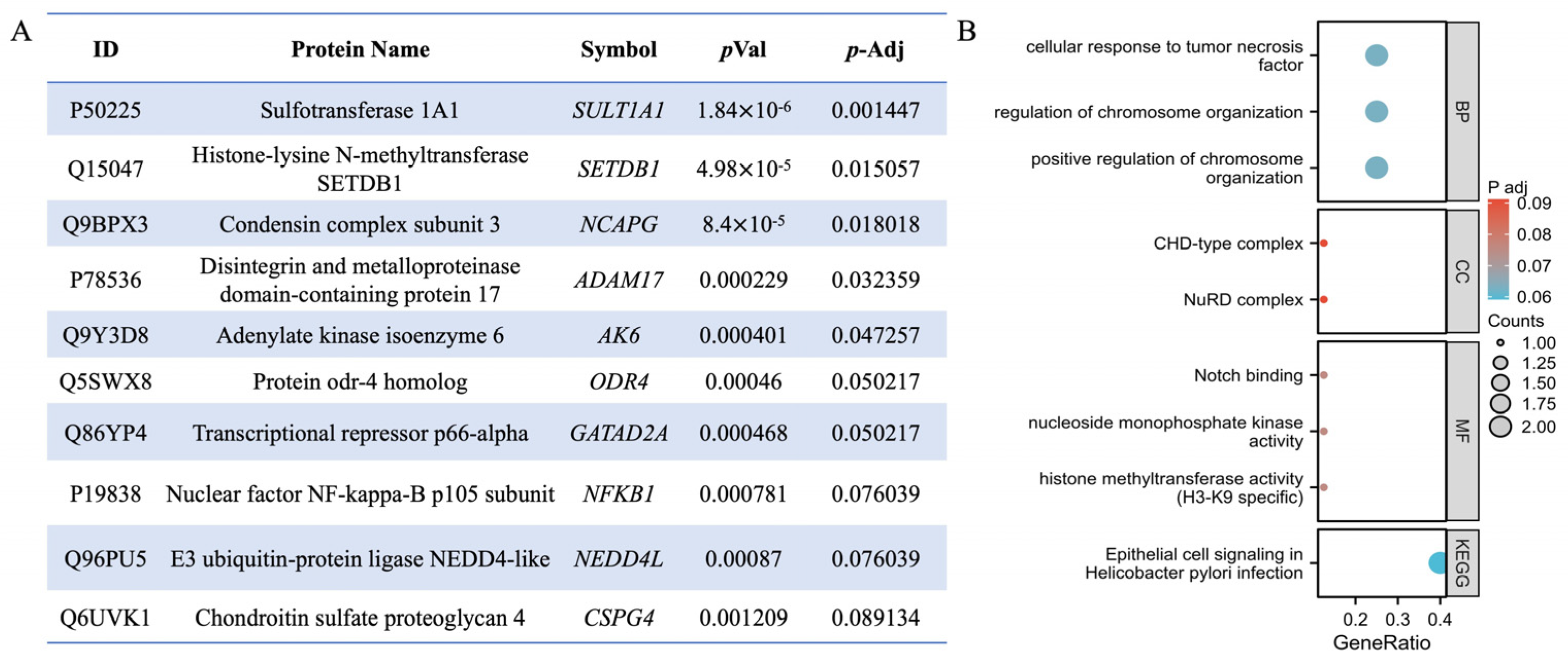
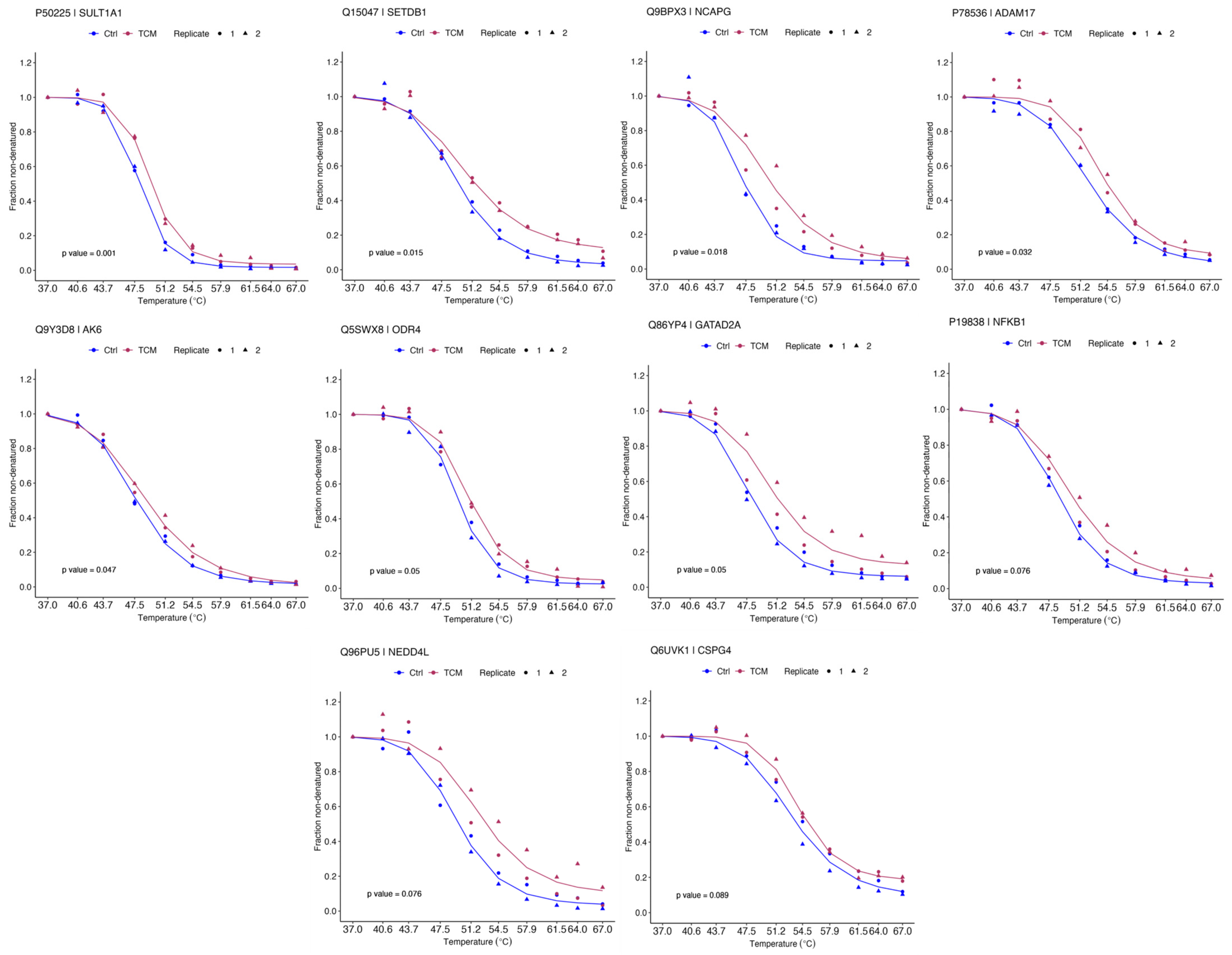
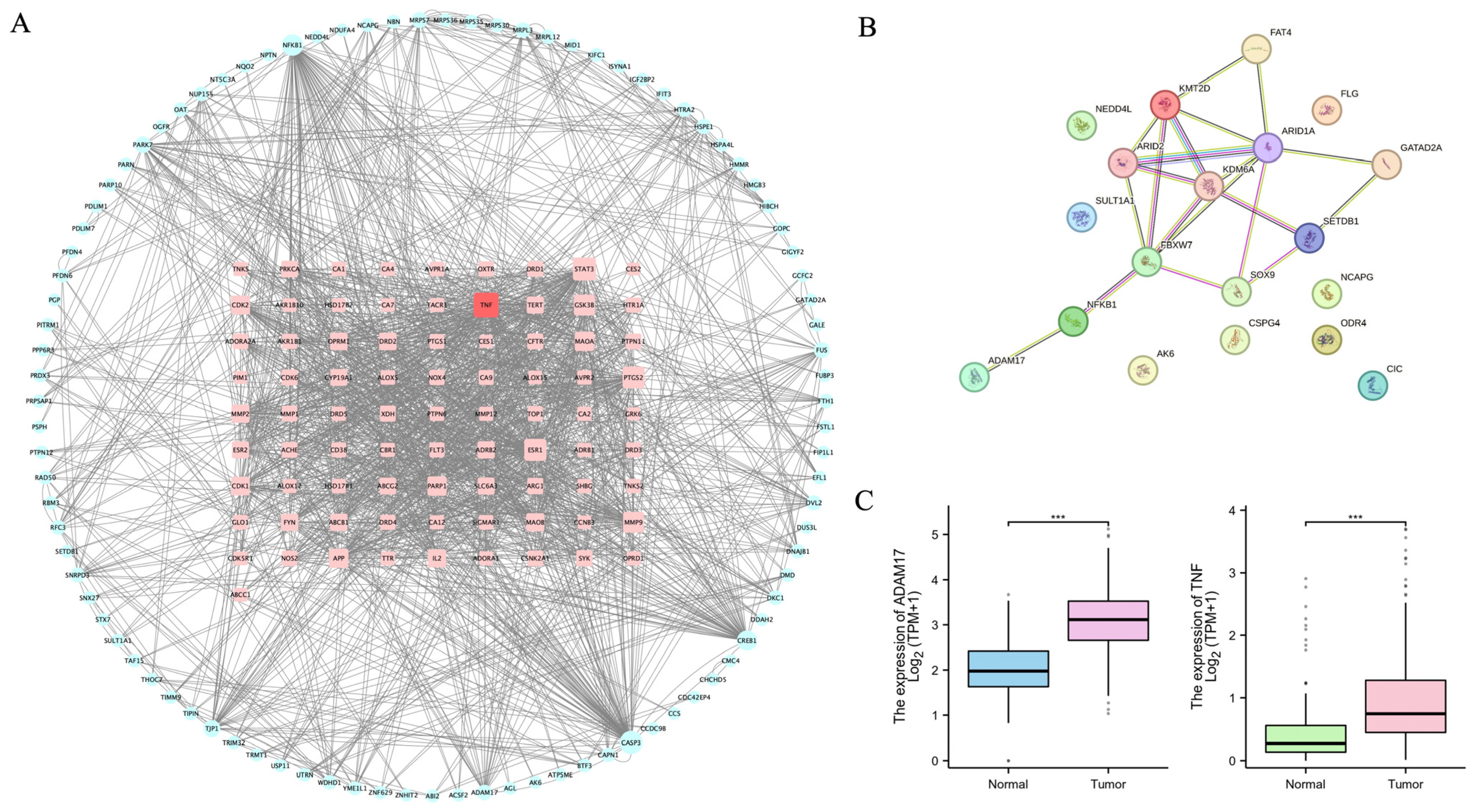

| No. | Name | Formula | m/z | RT [min] | mzVault Best Match | Sample/ Control Ratio |
|---|---|---|---|---|---|---|
| 1 | N-Acetylglutamic acid | C7H11NO5 | 188.05663 | 1.403 | 88.6 | 29.27 |
| 2 | Forsythoside E | C20H30O12 | 461.16685 | 5.795 | 88.3 | 43.39 |
| 3 | Hastatoside | C17H24O11 | 449.13039 | 5.972 | 84.3 | 40.19 |
| 4 | Atractyloside A | C21H36O10 | 493.22952 | 6.266 | 90.5 | 40.27 |
| 5 | Purpureaside C | C35H46O20 | 785.25233 | 7.471 | 90.2 | 50.63 |
| 6 | Vicenin II | C27H30O15 | 593.15181 | 7.583 | 89.5 | 33.36 |
| 7 | Dihydromorin | C15H12O7 | 303.05097 | 7.886 | 92 | 104.08 |
| 8 | Isoforsythiaside | C29H36O15 | 623.19852 | 8.252 | 91.1 | 109.93 |
| 9 | Magnoloside A | C29H36O15 | 623.19843 | 9.288 | 89.2 | 83.28 |
| 10 | Apigenin 5-O-glucoside | C21H20O10 | 431.09853 | 9.289 | 89.2 | 29.35 |
| 11 | Narirutin | C27H32O14 | 579.17223 | 9.721 | 90.9 | 43.45 |
| 12 | Isoacteoside | C29H36O15 | 623.19834 | 9.724 | 88.6 | 109.99 |
| 13 | Rosmarinic acid | C18H16O8 | 359.07738 | 10.035 | 82.8 | 97.41 |
| 14 | Salvianolic acid A | C26H22O10 | 493.11453 | 10.062 | 89.3 | 31.69 |
| 15 | Puerarin | C21H20O9 | 415.10377 | 10.097 | 84.2 | 85.09 |
| 16 | Hesperetin | C16H14O6 | 301.07183 | 10.225 | 92.8 | 40.93 |
| 17 | Scutellarin | C21H18O12 | 461.07282 | 10.605 | 84.9 | 32.96 |
| 18 | Baicalein | C15H10O5 | 269.04536 | 10.82 | 91 | 20.02 |
| 19 | Oroxin A | C21H20O10 | 431.09846 | 11.188 | 88.1 | 35.62 |
| 20 | Dalbergioidin | C15H12O6 | 287.05634 | 11.239 | 84.8 | 150.86 |
| 21 | Baicalin | C21H18O11 | 445.07751 | 11.446 | 87.1 | 33.50 |
| 22 | Luteolin | C15H10O6 | 285.04062 | 11.773 | 87.2 | 23.75 |
| 23 | Oroxylin A | C16H12O5 | 283.06125 | 11.788 | 90.4 | 24.75 |
| 24 | Didymin | C28H34O14 | 593.18798 | 12.009 | 90.1 | 33.26 |
| 25 | Wogonoside | C22H20O11 | 459.093 | 12.188 | 91.5 | 24.93 |
| 26 | Hexahydrocurcumin | C21H26O6 | 373.1659 | 12.613 | 85.4 | 30.01 |
| 27 | Hispidulin | C16H12O6 | 299.0562 | 12.707 | 89.2 | 36.79 |
| 28 | Randaiol | C15H14O3 | 241.08724 | 13.291 | 80.8 | 152.77 |
| 29 | Formononetin | C16H12O4 | 267.06628 | 13.61 | 88.7 | 49.00 |
| 30 | Magnaldehyde D | C16H14O3 | 253.0871 | 14.129 | 84.2 | 81.10 |
| 31 | Wogonin | C16H12O5 | 283.0611 | 14.291 | 89.9 | 23.53 |
| 32 | Casticin | C19H18O8 | 373.0929 | 14.435 | 80.1 | 109.01 |
| No. | Name | Formula | m/z | RT [min] | mzVault Best Match | Sample/ Control Ratio |
|---|---|---|---|---|---|---|
| 1 | Hordenine | C10H15NO | 166.1224 | 2.271 | 86.3 | 31.83 |
| 2 | Indoleacrylic acid | C11H9NO2 | 188.0704 | 5.299 | 84.4 | 35.78 |
| 3 | Vicenin II | C27H30O15 | 595.16551 | 7.568 | 86 | 45.71 |
| 4 | N-Methylcoclaurine | C18H21NO3 | 300.15912 | 7.754 | 89.6 | 21.01 |
| 5 | Scutellarein | C15H10O6 | 287.05462 | 8.886 | 81.2 | 26.26 |
| 6 | Calycosin-7-O-β-d-glucoside | C22H22O10 | 447.12807 | 8.968 | 82.9 | 25.81 |
| 7 | Naringenin | C15H12O5 | 273.07539 | 9.718 | 89.1 | 38.50 |
| 8 | Narirutin | C27H32O14 | 581.18599 | 9.718 | 89.8 | 35.54 |
| 9 | Hesperetin | C16H14O6 | 303.08563 | 10.229 | 89.8 | 42.68 |
| 10 | Hesperidin | C28H34O15 | 611.19639 | 10.231 | 89.4 | 34.81 |
| 11 | Baicalein | C15H10O5 | 271.05936 | 10.828 | 83.4 | 23.90 |
| 12 | Baicalin | C21H18O11 | 447.09099 | 10.829 | 80.5 | 24.28 |
| 13 | Atractylenolide I | C15H18O2 | 231.1376 | 11.217 | 83.9 | 31.00 |
| 14 | Formononetin | C16H12O4 | 269.08048 | 11.229 | 84.7 | 21.53 |
| 15 | Jatrorrhizine | C20H19NO4 | 338.13782 | 11.237 | 90.1 | 22.20 |
| 16 | Oroxylin A | C16H12O5 | 285.07512 | 11.791 | 83.1 | 27.36 |
| 17 | Calycosin | C16H12O5 | 285.07516 | 11.931 | 92.5 | 22.71 |
| 18 | Wogonin | C16H12O5 | 285.07495 | 12.195 | 82 | 24.61 |
| 19 | Heterophyllin B | C40H58N8O8 | 779.44451 | 13.048 | 90.1 | 35.10 |
| 20 | Isosinensetin | C20H20O7 | 373.12749 | 13.374 | 93.1 | 46.46 |
| 21 | 5-O-Demethylnobiletin | C20H20O8 | 389.12254 | 13.532 | 87.7 | 55.67 |
| 22 | Sinensetin | C20H20O7 | 373.12687 | 13.853 | 90 | 48.41 |
| 23 | 6-Demethoxytangeretin | C19H18O6 | 343.11711 | 13.93 | 85.5 | 38.92 |
| 24 | Nobiletin | C21H22O8 | 403.13754 | 14.363 | 92.8 | 30.35 |
| 25 | Tangeretin | C20H20O7 | 373.1272 | 14.909 | 90.9 | 49.55 |
| 26 | Curcumenol | C15H22O2 | 235.16861 | 14.924 | 91.1 | 31.01 |
| 27 | Zederone | C15H18O3 | 247.13265 | 15.203 | 84.3 | 31.31 |
| 28 | Dihydrotanshinone I | C18H14O3 | 279.10093 | 15.657 | 87 | 48.06 |
| 29 | Cryptotanshinone | C19H20O3 | 297.1477 | 16.609 | 87.7 | 104.72 |
| 30 | Tanshinone IIA | C19H18O3 | 295.13229 | 17.508 | 93 | 33.04 |
| Name | Formula | m/z | RT [min] |
|---|---|---|---|
| Epiberberine | C20H17NO4 | 336.12263 | 12.735 |
| Palmatine | C21H21NO4 | 352.15407 | 12.696 |
| Jatrorrhizine | C20H19NO4 | 338.13866 | 11.872 |
| (+)-Magnoflorine | C20H23NO4 | 342.16997 | 8.584 |
| Berberrubine | C19H15NO4 | 322.10752 | 10.559 |
| Demethyleneberberine | C19H17NO4 | 324.12316 | 10.732 |
| Nobiletin | C21H22O8 | 403.13876 | 15.267 |
| Sinensetin | C20H20O7 | 373.12831 | 14.714 |
| Formononetin | C16H12O4 | 269.08093 | 14.401 |
| Dehydroglaucine | C21H23NO4 | 354.16999 | 11.123 |
| 6-Demethoxytangeretin | C19H18O6 | 343.11792 | 15.312 |
| Quinic acid | C7H12O6 | 191.05673 | 1.02 |
| Oroxylin A-7-O-β-d-glucuronide | C22H20O11 | 459.09442 | 13.101 |
| TF-Hesperidin | C28H34O15 | 609.184 | 11.383 |
| Narirutin | C27H32O14 | 579.17336 | 10.778 |
| ChemSpider ID | Compound | XP GScore | MM-GBSA dG Bind (kcal/mol) |
|---|---|---|---|
| 390871 | Narirutin | −6.114 | −34.20 |
| 65269 | Jatrorrhizine | −2.447 | −22.11 |
| 402990 | Berberrubine | −2.200 | −31.28 |
| 128491 | Sinensetin | −1.373 | −30.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Wang, T.; Li, Z.; Li, T.; Miao, Z.; Chen, Y.; Zhu, S.; Wei, W.; Deng, H. Therapeutic Potential of Qilianxiaopi Formula: Targeting ADAM17-Mediated Chronic Inflammation in Atrophic Gastritis. Pharmaceuticals 2025, 18, 435. https://doi.org/10.3390/ph18030435
Du S, Wang T, Li Z, Li T, Miao Z, Chen Y, Zhu S, Wei W, Deng H. Therapeutic Potential of Qilianxiaopi Formula: Targeting ADAM17-Mediated Chronic Inflammation in Atrophic Gastritis. Pharmaceuticals. 2025; 18(3):435. https://doi.org/10.3390/ph18030435
Chicago/Turabian StyleDu, Sijing, Tianxiang Wang, Zhiqiang Li, Ting Li, Zelong Miao, Yuling Chen, Songbiao Zhu, Wei Wei, and Haiteng Deng. 2025. "Therapeutic Potential of Qilianxiaopi Formula: Targeting ADAM17-Mediated Chronic Inflammation in Atrophic Gastritis" Pharmaceuticals 18, no. 3: 435. https://doi.org/10.3390/ph18030435
APA StyleDu, S., Wang, T., Li, Z., Li, T., Miao, Z., Chen, Y., Zhu, S., Wei, W., & Deng, H. (2025). Therapeutic Potential of Qilianxiaopi Formula: Targeting ADAM17-Mediated Chronic Inflammation in Atrophic Gastritis. Pharmaceuticals, 18(3), 435. https://doi.org/10.3390/ph18030435








