Potential Benefits of In Silico Methods: A Promising Alternative in Natural Compound’s Drug Discovery and Repurposing for HBV Therapy
Abstract
1. Introduction
2. Reasons for Exploring in Silico Drug Design in Repurposing, Especially for HBV Treatment
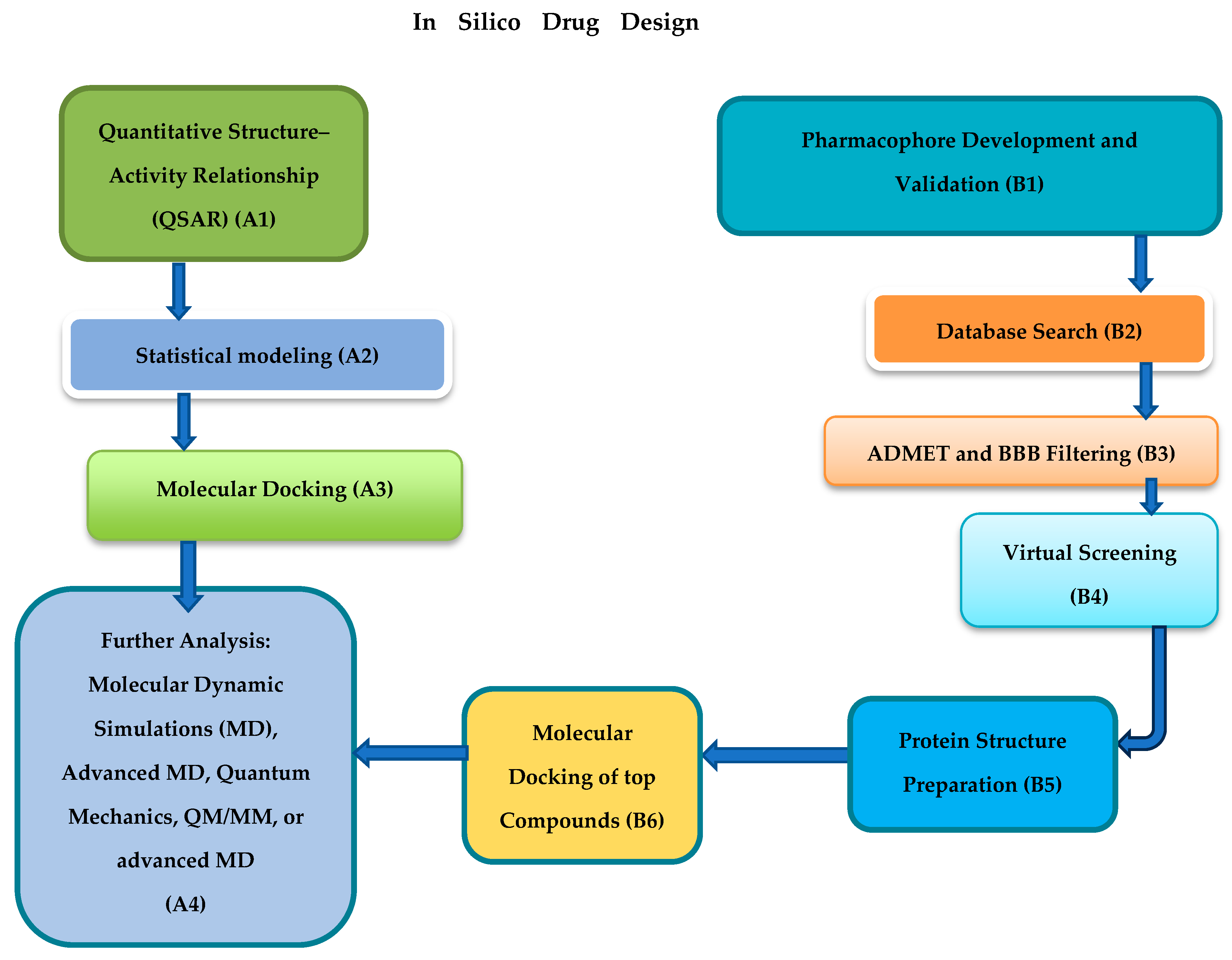
3. Mechanistic Overview of In Silico Methods Employed for Drug Discovery
4. Usefulness of Molecular Docking in Evaluating Binding Affinities and Interactions of Natural Compounds with HBV-Related Targets
5. Pharmacophore Modeling Benefits in Identifying Natural Compound HBV Inhibitors
6. QSAR and ADMET in Natural Compounds Bioactivity Predictions
7. Future Perspectives and Research Directions
8. Importance of Integrative In Silico, In Vitro, and In Vivo Methods in Drug Discovery
9. Potential Role of AI and Machine Learning in Accelerating the Discovery of Effective Natural Products
10. Importance of Collaborative Research Between Computational Scientists, Pharmacologists, and Traditional Medicine Experts
11. Potential Advancements for Predictive Modeling in HBV Therapy
12. Overview of In Silico Successes and Failures in HBV Therapy and Clinical Translation of Computationally Repurposed Natural Compounds
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, I.; Muhammad, S.; Naqvi, S.S.Z.H.; Wei, L.; Yan, W.; Khan, M.F.; Mahmood, A.; Liu, H.; Shah, W. Hepatitis B Virus-Associated Liver Carcinoma: The Role of Iron Metabolism and Its Modulation. J. Viral Hepat. 2024, 32, e14016. [Google Scholar] [CrossRef] [PubMed]
- Roma, K.; Chandler, T.-M.; Dossaji, Z.; Patel, A.; Gupta, K.; Minacapelli, C.D.; Rustgi, V.; Gish, R. A Review of the Systemic Manifestations of Hepatitis B Virus Infection, Hepatitis D Virus, Hepatocellular Carcinoma, and Emerging Therapies. Gastro Hep Adv. 2024, 3, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Al-Busafi, S.A.; Alwassief, A. Global Perspectives on the Hepatitis B Vaccination: Challenges, Achievements, and the Road to Elimination by 2030. Vaccines 2024, 12, 288. [Google Scholar] [CrossRef]
- Asandem, D.A.; Segbefia, S.P.; Kusi, K.A.; Bonney, J.H.K. Hepatitis B Virus Infection: A Mini Review. Viruses 2024, 16, 724. [Google Scholar] [CrossRef]
- WHO. W.H.O. Global Hepatitis Report 2024: Action for Access in Low- and Middle-Income Countries. Available online: https://www.who.int/publications/i/item/9789240091672 (accessed on 9 April 2024).
- Zhuang, A.-Q.; Chen, Y.; Chen, S.-M.; Liu, W.-C.; Li, Y.; Zhang, W.-J.; Wu, Y.-H. Current Status and Challenges in Anti-Hepatitis B Virus Agents Based on Inactivation/Inhibition or Elimination of Hepatitis B Virus Covalently Closed Circular DNA. Viruses 2023, 15, 2315. [Google Scholar] [CrossRef]
- Hu, J.; Protzer, U.; Siddiqui, A. Revisiting hepatitis B virus: Challenges of curative therapies. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Angelice, G.P.; Roque, P.H.; Valente, G.; Galvão, K.; Villar, L.M.; Mello, V.M.; Mello, F.C.; Lago, B.V. Evaluation of Interfering RNA Efficacy in Treating Hepatitis B: Is It Promising? Viruses 2024, 16, 1710. [Google Scholar] [CrossRef]
- Quirino, A.; Marascio, N.; Branda, F.; Ciccozzi, A.; Romano, C.; Locci, C.; Azzena, I.; Pascale, N.; Pavia, G.; Matera, G.; et al. Viral Hepatitis: Host Immune Interaction, Pathogenesis and New Therapeutic Strategies. Pathogens 2024, 13, 766. [Google Scholar] [CrossRef]
- Boonstra, A.; Sari, G. HBV cccDNA: The Molecular Reservoir of Hepatitis B Persistence and Challenges to Achieve Viral Eradication. Biomolecules 2025, 15, 62. [Google Scholar] [CrossRef]
- He, W.; Zheng, Z.; Zhao, Q.; Zhang, R.; Zheng, H. Targeting HBV cccDNA Levels: Key to Achieving Complete Cure of Chronic Hepatitis B. Pathogens 2024, 13, 1100. [Google Scholar] [CrossRef]
- Dhamija, N.; Mangla, A. Plant secondary metabolites in antiviral applications. In Plant Secondary Metabolites: Physico-Chemical Properties and Therapeutic Applications; Springer: Singapore, 2022; pp. 459–482. [Google Scholar]
- Conrado, G.G.; da Rosa, R.; Reis, R.D.; Pessa, L.R. Building Natural Product–Based Libraries for Drug Discovery: Challenges and Opportunities from a Brazilian Pharmaceutical Industry Perspective. Rev. Bras. De Farmacogn. 2024, 34, 706–721. [Google Scholar] [CrossRef]
- Dokhale, S.; Garse, S.; Devarajan, S.; Thakur, V.; Kolhapure, S. Rational Design of Antiviral Therapeutics. Comput. Methods Ration. Drug Des. 2025, 423–443. [Google Scholar]
- Idrees, S.; Chen, H.; Panth, N.; Paudel, K.R.; Hansbro, P.M. Exploring Viral–Host Protein Interactions as Antiviral Therapies: A Computational Perspective. Microorganisms 2024, 12, 630. [Google Scholar] [CrossRef]
- Lyu, W.; Qin, H.; Li, Q.; Lu, D.; Shi, C.; Zhao, K.; Zhang, S.; Yu, R.; Zhang, H.; Zhou, X.; et al. Novel mechanistic insights—A brand new Era for anti-HBV drugs. Eur. J. Med. Chem. 2024, 279, 116854. [Google Scholar] [CrossRef]
- Song, X.; Zhu, J.; Sun, F.; Wang, N.; Qiu, X.; Zhu, Q.; Qi, J.; Wang, X. Target-centric analysis of hepatitis B: Identifying key molecules and pathways for treatment. Sci. Rep. 2024, 14, 26858. [Google Scholar] [CrossRef] [PubMed]
- Flores-Holguín, N.; Frau, J.; Glossman-Mitnik, D. Computational Pharmacokinetics Report, ADMET Study and Conceptual DFT-Based Estimation of the Chemical Reactivity Properties of Marine Cyclopeptides. ChemistryOpen 2021, 10, 1142–1149. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Schneider, G.; Böhm, H.-J. Virtual screening and fast automated docking methods. Drug Discov. Today 2002, 7, 64–70. [Google Scholar] [CrossRef]
- Teli, D.M.; Shah, M.B.; Chhabria, M.T. In silico screening of natural compounds as potential inhibitors of SARS-CoV-2 main protease and spike RBD: Targets for COVID-19. Front. Mol. Biosci. 2021, 7, 599079. [Google Scholar] [CrossRef]
- Doman, T.N.; McGovern, S.L.; Witherbee, B.J.; Kasten, T.P.; Kurumbail, R.; Stallings, W.C.; Connolly, D.T.; Shoichet, B.K. Molecular docking and high-throughput screening for novel inhibitors of protein tyrosine phosphatase-1B. J. Med. Chem. 2002, 45, 2213–2221. [Google Scholar] [CrossRef]
- Murugan, N.A.; Podobas, A.; Gadioli, D.; Vitali, E.; Palermo, G.; Markidis, S. A review on parallel virtual screening softwares for high-performance computers. Pharmaceuticals 2022, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Kontoyianni, M. Docking and virtual screening in drug discovery. In Proteomics for Drug Discovery: Methods and Protocols; Springer: New York, NY, USA, 2017; pp. 255–266. [Google Scholar]
- Gozalbes, R.; de Julián-Ortiz, J.V. Applications of chemoinformatics in predictive toxicology for regulatory purposes, especially in the context of the EU REACH legislation. Int. J. Quant. Struct.-Prop. Relatsh. (IJQSPR) 2018, 3, 1–24. [Google Scholar] [CrossRef]
- Carpio, L.E.; Sanz, Y.; Gozalbes, R.; Barigye, S.J. Computational strategies for the discovery of biological functions of health foods, nutraceuticals and cosmeceuticals: A review. Mol. Divers. 2021, 25, 1425–1438. [Google Scholar] [CrossRef]
- Agu, P.; Afiukwa, C.; Orji, O.; Ezeh, E.; Ofoke, I.; Ogbu, C.; Ugwuja, E.; Aja, P. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef] [PubMed]
- Dasmahapatra, U.; Kumar, C.K.; Das, S.; Subramanian, P.T.; Murali, P.; Isaac, A.E.; Ramanathan, K.; Mm, B.; Chanda, K. In-silico molecular modelling, MM/GBSA binding free energy and molecular dynamics simulation study of novel pyrido fused imidazo [4, 5-c] quinolines as potential anti-tumor agents. Front. Chem. 2022, 10, 991369. [Google Scholar] [CrossRef]
- Cousins, H.C.; Nayar, G.; Altman, R.B. Computational Approaches to Drug Repurposing: Methods, Challenges, and Opportunities. Annu. Rev. Biomed. Data Sci. 2024, 7, 15–29. [Google Scholar] [CrossRef]
- Weth, F.R.; Hoggarth, G.B.; Weth, A.F.; Paterson, E.; White, M.P.; Tan, S.T.; Peng, L.; Gray, C. Unlocking hidden potential: Advancements, approaches, and obstacles in repurposing drugs for cancer therapy. Br. J. Cancer 2024, 130, 703–715. [Google Scholar] [CrossRef]
- Tu, T.; Wettengel, J.; Xia, Y.; Testoni, B.; Littlejohn, M.; Le Bert, N.; Ebert, G.; Verrier, E.R.; Tavis, J.E.; Cohen, C. Major open questions in the hepatitis B and D field–Proceedings of the inaugural International emerging hepatitis B and hepatitis D researchers workshop. Virology 2024, 595, 110089. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, H.; Lian, R.; Xie, J.; Feng, R. New perspective of small-molecule antiviral drugs development for RNA viruses. Virology 2024, 594, 110042. [Google Scholar] [CrossRef]
- Wu, X.; Dai, Y.; Nie, K. Research Progress of Liujunzi Decoction in the Treatment of Tumor-Associated Anorexia. Drug Des. Devel. Ther. 2022, 16, 1731–1741. [Google Scholar] [CrossRef]
- Ma, Q.; Li, W.; Wu, W.; Sun, M. Exploring the active ingredients and mechanisms of Liujunzi decoction in treating hepatitis B: A study based on network pharmacology, molecular docking, and molecular dynamics simulations. Comput. Methods Biomech. Biomed. Eng. 2024, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Xiao, S.; Yang, L.; Ma, K.; Shang, H.; Gao, Y.; Wang, Y.; Zhai, F.; Xiang, R. Systematic analysis of the mechanism of Xiaochaihu decoction in hepatitis B treatment via network pharmacology and molecular docking. Comput. Biol. Med. 2021, 138, 104894. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, S.; Tang, K.; Zhan, P.; Liu, X. Recent Advances in Screening Methods Enabling the Discovery of Novel Anti-Hepatitis B Virus Drug Candidates. Eur. J. Med. Chem. 2024, 282, 117093. [Google Scholar] [CrossRef] [PubMed]
- Ogunnaike, M.; Das, S.; Raut, S.S.; Sultana, A.; Nayan, M.U.; Ganesan, M.; Edagwa, B.J.; Osna, N.A.; Poluektova, L.Y. Chronic hepatitis B infection: New approaches towards cure. Biomolecules 2023, 13, 1208. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Huang, D.Q.; Nguyen, M.H. Global burden of hepatitis B virus: Current status, missed opportunities and a call for action. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 524–537. [Google Scholar] [CrossRef]
- Gerlich, W.H. Prophylactic vaccination against hepatitis B: Achievements, challenges and perspectives. Med. Microbiol. Immunol. 2015, 204, 39–55. [Google Scholar] [CrossRef]
- Pastuch-Gawołek, G.; Gillner, D.; Król, E.; Walczak, K.; Wandzik, I. Selected nucleos (t) ide-based prescribed drugs and their multi-target activity. Eur. J. Pharmacol. 2019, 865, 172747. [Google Scholar] [CrossRef]
- Kim, H.; Ko, C.; Lee, J.-Y.; Kim, M. Current progress in the development of hepatitis B virus capsid assembly modulators: Chemical structure, mode-of-action and efficacy. Molecules 2021, 26, 7420. [Google Scholar] [CrossRef]
- Yu, X.; Gao, Y.; Zhang, X.; Ji, L.; Fang, M.; Li, M.; Gao, Y. Hepatitis B: Model Systems and Therapeutic Approaches. J. Immunol. Res. 2024, 2024, 4722047. [Google Scholar] [CrossRef]
- Haghir Ebrahim Abadi, M.H.; Ghasemlou, A.; Bayani, F.; Sefidbakht, Y.; Vosough, M.; Mozaffari-Jovin, S.; Uversky, V.N. AI-driven covalent drug design strategies targeting main protease (mpro) against SARS-CoV-2: Structural insights and molecular mechanisms. J. Biomol. Struct. Dyn. 2024, 1–29. [Google Scholar] [CrossRef]
- Shanmuga Sundari, M.; Thotakura, S.A.; Dharmana, M.; Gadela, P.; Ammangatambu, M.M. Process and Applications of Structure-Based Drug Design. Artif. Intell. Mach. Learn. Drug Des. Dev. 2024, 321–368. [Google Scholar] [CrossRef]
- de Sousa Luis, J.A.; Barros, R.P.; de Sousa, N.F.; Muratov, E.; Scotti, L.; Scotti, M.T. Virtual screening of natural products database. Mini Rev. Med. Chem. 2021, 21, 2657–2730. [Google Scholar] [CrossRef]
- Chen, Y.; de Bruyn Kops, C.; Kirchmair, J. Data resources for the computer-guided discovery of bioactive natural products. J. Chem. Inf. Model. 2017, 57, 2099–2111. [Google Scholar] [CrossRef]
- Borges, P.H.O.; Ferreira, S.B.; Silva, F.P., Jr. Recent Advances on Targeting Proteases for Antiviral Development. Viruses 2024, 16, 366. [Google Scholar] [CrossRef] [PubMed]
- Agar, S. De novo Drug Design and Repurposing to suppress Liver Cancer via VEGF-R1 Mechanism: Comprehensive Molecular Docking, Molecular Dynamics Simulations and ADME Estimation. Med. Chem. 2024. [Google Scholar] [CrossRef]
- Costa, B.; Gouveia, M.J.; Vale, N. Safety and Efficacy of Antiviral Drugs and Vaccines in Pregnant Women: Insights from Physiologically Based Pharmacokinetic Modeling and Integration of Viral Infection Dynamics. Vaccines 2024, 12, 782. [Google Scholar] [CrossRef]
- Morgnanesi, D.; Heinrichs, E.J.; Mele, A.R.; Wilkinson, S.; Zhou, S.; Kulp, J.L., III. A computational chemistry perspective on the current status and future direction of hepatitis B antiviral drug discovery. Antivir. Res. 2015, 123, 204–215. [Google Scholar] [CrossRef]
- Mohebbi, A.; Naderi, M.; Sharifian, K.; Behnezhad, F.; Mohebbi, M.; Gholami, A.; Askari, F.S.; Mirarab, A.; Monavari, S.H. Computational-Aided Reproposing Drug (s) and Discovery for Potential Antivirals Targeting Hepatitis B Virus Capsid Protein. Preprint 2023. [Google Scholar] [CrossRef]
- Lourenço, T.; Vale, N. Entecavir: A Review and Considerations for Its Application in Oncology. Pharmaceuticals 2023, 16, 1603. [Google Scholar] [CrossRef]
- Başer, T. Identifying Potential Therapeutic Molecules for Hepatocellular Carcinoma Through Machine Learning-Based Drug Repurposing. Ph.D. Thesis, Middle East Technical University, Ankara, Turkey, 2024. [Google Scholar]
- Kumar, N.; Sharma, S.; Kumar, R.; Tripathi, B.N.; Barua, S.; Ly, H.; Rouse, B.T. Host-directed antiviral therapy. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef]
- Barghash, R.F.; Gemmati, D.; Awad, A.M.; Elbakry, M.M.; Tisato, V.; Awad, K.; Singh, A.V. Navigating the COVID-19 Therapeutic Landscape: Unveiling Novel Perspectives on FDA-Approved Medications, Vaccination Targets, and Emerging Novel Strategies. Molecules 2024, 29, 5564. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Flavonoids as Potential Drugs for Combating SARS-CoV-2: Molecular Docking Studies. In Anti-SARS-CoV-2 Activity of Flavonoids; CRC Press: Boca Raton, FL, USA, 2024; pp. 22–40. [Google Scholar]
- Abdallah, E.A.; Abdulaziz, L.; Elhadi, E.; Alnoor, F.A.; Yousef, B.A. Antiviral Activity of Approved Anti-Inflammatory Drugs and Prospects for Drug Repurposing: A review. J. Adv. Med. Pharm. Sci. 2023, 25, 1–13. [Google Scholar] [CrossRef]
- Namasivayam, V.; Palaniappan, S.; Vanangamudi, M. Repurposing drugs targeting epidemic viruses. Drug Discov. Today 2022, 27, 1874–1894. [Google Scholar] [CrossRef]
- Abiyana, S.R.; Santoso, S.B.; Pribadi, P.; Hapsari, W.S.; Syarifuddin, A. From the Drugbank Application to the Novel Drugs: A Pharmacogenomic Summary. E3S Web Conf. 2024, 500, 04002. [Google Scholar] [CrossRef]
- Attia, Y.M.; Ewida, H.; Ahmed, M.S. Successful stories of drug repurposing for cancer therapy in hepatocellular carcinoma. In Drug Repurposing in Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 213–229. [Google Scholar]
- Rasha, F.; Paul, S.; Simon, T.G.; Hoshida, Y. Hepatocellular carcinoma chemoprevention with generic agents. Semin. Liver Dis. 2022, 42, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Anandamurthy, A.; Varughese, R.E.; Sivaraj, S.; Dasararaju, G. Anti Hepatitis C virus drugs show potential drug repositioning for SARS CoV-2 main protease: An in silico study. Preprint 2020. [Google Scholar] [CrossRef]
- Mercorelli, B.; Palù, G.; Loregian, A. Drug repurposing for viral infectious diseases: How far are we? Trends Microbiol. 2018, 26, 865–876. [Google Scholar] [CrossRef]
- Wahid, B. Successful treatment of HBV, HCV, & HEV, with 12-week long use of tenofovir, sofosbuvir, daclatasvir, and ribavirin: A case report. J. Infect. Public Health 2020, 13, 149–150. [Google Scholar]
- Parvez, M.K.; Rehman, M.T.; Alam, P.; Al-Dosari, M.S.; Alqasoumi, S.I.; Alajmi, M.F. Plant-derived antiviral drugs as novel hepatitis B virus inhibitors: Cell culture and molecular docking study. Saudi Pharm. J. 2019, 27, 389–400. [Google Scholar] [CrossRef]
- Bachar, S.C.; Mazumder, K.; Bachar, R.; Aktar, A.; Al Mahtab, M. A review of medicinal plants with antiviral activity available in Bangladesh and mechanistic insight into their bioactive metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 2021, 12, 732891. [Google Scholar] [CrossRef]
- Bhat, S.A.; Kazim, S.N. A road to contemporary era of hepatitis B virus regimen replacing existing therapeutics exploiting plant secondary metabolites as emerging heroes in exploring drugs: An expedition for a functional cure. Gene Rep. 2023, 30, 101743. [Google Scholar] [CrossRef]
- Kumari, S.; Jain, S. Medicinal Plants and Herbs in Viral Hepatitis. In Promising Antiviral Herbal and Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2024; pp. 121–144. [Google Scholar]
- Ardebili, A.; Pouriayevali, M.H.; Aleshikh, S.; Zahani, M.; Ajorloo, M.; Izanloo, A.; Siyadatpanah, A.; Razavi Nikoo, H.; Wilairatana, P.; Coutinho, H.D.M. Antiviral therapeutic potential of curcumin: An update. Molecules 2021, 26, 6994. [Google Scholar] [CrossRef]
- Winkler, D.A. Computational repurposing of drugs for viral diseases and current and future pandemics. J. Math. Chem. 2024, 62, 2844–2879. [Google Scholar] [CrossRef]
- Jannat, K.; Paul, A.K.; Bondhon, T.A.; Hasan, A.; Nawaz, M.; Jahan, R.; Mahboob, T.; Nissapatorn, V.; Wilairatana, P.; Pereira, M.d.L. Nanotechnology applications of flavonoids for viral diseases. Pharmaceutics 2021, 13, 1895. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Bhutkar, M.; Tomar, S. Therapeutic Antiviral Potential of Flavonoids. In Flavonoids as Nutraceuticals; Apple Academic Press: Waretown, NJ, USA, 2024; pp. 57–101. [Google Scholar]
- Paraiso, I.L.; Revel, J.S.; Stevens, J.F. Potential use of polyphenols in the battle against COVID-19. Curr. Opin. Food Sci. 2020, 32, 149–155. [Google Scholar] [CrossRef]
- Gabbianelli, R.; Shahar, E.; De Simone, G.; Rucci, C.; Bordoni, L.; Feliziani, G.; Zhao, F.; Ferrati, M.; Maggi, F.; Spinozzi, E. Plant-Derived Epi-Nutraceuticals as Potential Broad-Spectrum Anti-Viral Agents. Nutrients 2023, 15, 4719. [Google Scholar] [CrossRef]
- Ghildiyal, R.; Prakash, V.; Chaudhary, V.; Gupta, V.; Gabrani, R. Phytochemicals as antiviral agents: Recent updates. Plant-Deriv. Bioact. Prod. Prop. Ther. Appl. 2020, 279–295. [Google Scholar] [CrossRef]
- Jadhav, A.K.; Karuppayil, S.M. Andrographis paniculata (Burm. F) Wall ex Nees: Antiviral properties. Phytother. Res. 2021, 35, 5365–5373. [Google Scholar] [CrossRef]
- Orosco, F.L.; Wong, J.E. Andrographolides as Antiviral Agents: Insights into Mechanisms, Modifications, and Delivery Innovations. Trop. J. Nat. Prod. Res. 2023, 7. [Google Scholar] [CrossRef]
- Muderawan, I.W.; Widiastuti Giri, M.; Budiawan, I. The Potential of Ayurvedic Medicinal Plants for Prevention and Therapeutic Treatment of Covid-19: A Review Article. Int. J. Ayurvedic Herb. Med. 2021, 11, 3954–3996. [Google Scholar]
- de Oliveira, P.G.; Termini, L.; Durigon, E.L.; Lepique, A.P.; Sposito, A.C.; Boccardo, E. Diacerein: A potential multi-target therapeutic drug for COVID-19. Med. Hypotheses 2020, 144, 109920. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Prabhakar, P.K.; Doble, M. Synergistic Herb-Drug Interactions Against Viral Diseases. In Herb-Drug Combinations: A New Complementary Therapeutic Strategy; Springer: Singapore, 2022; pp. 103–130. [Google Scholar]
- Chen, X.; Han, W.; Wang, G.; Zhao, X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343. [Google Scholar] [CrossRef]
- Ganjhu, R.K.; Mudgal, P.P.; Maity, H.; Dowarha, D.; Devadiga, S.; Nag, S.; Arunkumar, G. Herbal plants and plant preparations as remedial approach for viral diseases. Virusdisease 2015, 26, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Ho, K.L. Phage display creates innovative applications to combat hepatitis B virus. World J. Gastroenterol. WJG 2014, 20, 11650. [Google Scholar] [CrossRef] [PubMed]
- Graciotti, M.; Kandalaft, L.E. Vaccines for cancer prevention: Exploring opportunities and navigating challenges. Nat. Rev. Drug Discov. 2024, 24, 134–150. [Google Scholar] [CrossRef]
- Niazi, S.K.; Mariam, Z. Computer-aided drug design and drug discovery: A prospective analysis. Pharmaceuticals 2023, 17, 22. [Google Scholar] [CrossRef]
- Chen, L.; Liu, W.G.; Xiong, F.; Ma, C.; Sun, C.; Zhu, Y.R.; Zhang, X.G.; Wang, Z.H. 3D-QSAR, molecular docking and molecular dynamics simulations analyses of a series of heteroaryldihydropyrimidine derivatives as hepatitis B virus capsid assembly inhibitors. New J. Chem. 2021, 45, 22062–22076. [Google Scholar] [CrossRef]
- Salo-Ahen, O.M.; Alanko, I.; Bhadane, R.; Bonvin, A.M.; Honorato, R.V.; Hossain, S.; Juffer, A.H.; Kabedev, A.; Lahtela-Kakkonen, M.; Larsen, A.S. Molecular dynamics simulations in drug discovery and pharmaceutical development. Processes 2020, 9, 71. [Google Scholar] [CrossRef]
- Shahab, M.; Khan, A.; Khan, S.A.; Zheng, G. Unraveling the mechanisms of Sofosbuvir resistance in HCV NS3/4A protease: Structural and molecular simulation-based insights. Int. J. Biol. Macromol. 2024, 267, 131629. [Google Scholar] [CrossRef]
- Naithani, U.; Guleria, V. Integrative computational approaches for discovery and evaluation of lead compound for drug design. Front. Drug Discov. 2024, 4, 1362456. [Google Scholar] [CrossRef]
- Akhtar, N.; Husen, A.; Dwibedi, V.; Rath, S.K. Promising Antiviral Herbal and Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Kowalczyk, M.; Golonko, A.; Świsłocka, R.; Kalinowska, M.; Parcheta, M.; Swiergiel, A.; Lewandowski, W. Drug design strategies for the treatment of viral disease. Plant phenolic compounds and their derivatives. Front. Pharmacol. 2021, 12, 709104. [Google Scholar] [CrossRef] [PubMed]
- Weaver, R.J.; Blomme, E.A.; Chadwick, A.E.; Copple, I.M.; Gerets, H.H.; Goldring, C.E.; Guillouzo, A.; Hewitt, P.G.; Ingelman-Sundberg, M.; Jensen, K.G. Managing the challenge of drug-induced liver injury: A roadmap for the development and deployment of preclinical predictive models. Nat. Rev. Drug Discov. 2020, 19, 131–148. [Google Scholar] [CrossRef]
- Asín-Prieto, E.; Parra-Guillen, Z.P.; Mantilla, J.D.G.; Vandenbossche, J.; Stuyckens, K.; de Trixhe, X.W.; Perez-Ruixo, J.J.; Troconiz, I.F. A quantitative systems pharmacology model for acute viral hepatitis B. Comput. Struct. Biotechnol. J. 2021, 19, 4997–5007. [Google Scholar] [CrossRef]
- Maepa, M.B.; Bloom, K.; Ely, A.; Arbuthnot, P. Hepatitis B virus: Promising drug targets and therapeutic implications. Expert Opin. Ther. Targets 2021, 25, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Senerovic, L.; Opsenica, D.; Moric, I.; Aleksic, I.; Spasić, M.; Vasiljevic, B. Quinolines and quinolones as antibacterial, antifungal, anti-virulence, antiviral and anti-parasitic agents. Adv. Microbiol. Infect. Dis. Public Health 2020, 14, 37–69. [Google Scholar]
- Liu, L.; Wang, B.; Ma, Y.; Sun, K.; Wang, P.; Li, M.; Dong, J.; Qin, M.; Li, M.; Wei, C.; et al. A review of Phyllanthus urinaria L. in the treatment of liver disease: Viral hepatitis, liver fibrosis/cirrhosis and hepatocellular carcinoma. Front. Pharmacol. 2024, 15, 1443667. [Google Scholar] [CrossRef]
- Srivastava, R.; Dubey, N.K.; Sharma, M.; Kharkwal, H.; Bajpai, R.; Srivastava, R. Boosting the human antiviral response in conjunction with natural plant products. Front. Nat. Prod. 2025, 3, 1470639. [Google Scholar] [CrossRef]
- Hao, B.; Yang, Z.; Liu, H.; Liu, Y.; Wang, S. Advances in flavonoid research: Sources, biological activities, and developmental prospectives. Curr. Issues Mol. Biol. 2024, 46, 2884–2925. [Google Scholar] [CrossRef]
- Akram, M.; Mandal, A.; Iqbal, M.; Mukherjee, A.; Bandyopadhyay, R.; Rangasamy, S.; Zainab, R.; Khalil, M.T.; Parmar, P.; Laila, U. Inhibitory potential of medicinal plants against viral pathogens and their putative modes of action. Hosts Viruses 2023, 10, 58–71. [Google Scholar] [CrossRef]
- Yadav, J.K.; Ghanchi, M.; Dixit, N.; Sindhav, G.; Patel, S.; Rawal, R. Phytonutrients as a Defensive Barrier Against G Ectodomain Fusion in Chandipura Virus Infection. Mol. Biotechnol. 2025, 1–16. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wu, V.C.-C.; Wang, C.-L.; Wu, C.-L.; Huang, Y.-T.; Chang, S.-H. Silymarin Synergizes with Antiviral Therapy in Hepatitis B Virus-Related Liver Cirrhosis: A Propensity Score Matching Multi-Institutional Study. Int. J. Mol. Sci. 2024, 25, 3088. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xia, H.; He, Y.; Zeng, S.; Shen, Z.; Huang, W. The progress of molecules and strategies for the treatment of HBV infection. Front. Cell. Infect. Microbiol. 2023, 13, 1128807. [Google Scholar] [CrossRef]
- Warowicka, A.; Nawrot, R.; Goździcka-Józefiak, A. Antiviral activity of berberine. Arch. Virol. 2020, 165, 1935–1945. [Google Scholar] [CrossRef]
- Parvez, A.; Das, A.; Mahmud, A.; Biswas, P.; Hasan, M.H.; Tauhida, S.J.; Mostaq, M.S.; Nahid, M.M.H.; Ansari, F.; Fakir, S.; et al. Mechanistic insights of clinically proven natural products in the treatment of hepatitis B focusing on clinical evidence and pathways. Clin. Tradit. Med. Pharmacol. 2024, 5, 200183. [Google Scholar] [CrossRef]
- Deniz, F.S.Ş.; Tuğcu Demiröz, F.N.; Ulutaş, O.K.; Orhan, I.E. Phytosomes-Unraveling the Unique Properties of Plant-Derived Nanotechnological Drug Delivery Systems: A Review. Curr. Med. Chem. 2024. [Google Scholar] [CrossRef] [PubMed]
- Debnath, P.; Deb, V.K.; Nath, S.; Guha, A.; Adhikari, S. Structure-Activity Relationship of Plant-Derived Bioactive Compounds in Anti-Cancer Therapy. In Plant Derived Bioactive Compounds in Human Health and Disease; CRC Press: Boca Raton, FL, USA, 2025; pp. 66–94. [Google Scholar]
- Gao, F.; Feng, X.; Li, X. Recent advances in polymeric nanoparticles for the treatment of hepatic diseases. Front. Pharmacol. 2025, 16, 1528752. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Raza, S. Exploration of computational aids for effective drug designing and management of viral diseases: A comprehensive review. Curr. Top. Med. Chem. 2023, 23, 1640–1663. [Google Scholar] [CrossRef]
- Dunn, R.; Wetten, A.; McPherson, S.; Donnelly, M.C. Viral hepatitis in 2021: The challenges remaining and how we should tackle them. World J. Gastroenterol. 2022, 28, 76. [Google Scholar] [CrossRef]
- Pisano, M.B.; Giadans, C.G.; Flichman, D.M.; Ré, V.E.; Preciado, M.V.; Valva, P. Viral hepatitis update: Progress and perspectives. World J. Gastroenterol. 2021, 27, 4018. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Liu, A.; Xiao, B.; Li, S.; Wen, Z.; Lu, Y.; Du, G. Research progress of the antiviral bioactivities of natural flavonoids. Nat. Prod. Bioprospect. 2020, 10, 271–283. [Google Scholar] [CrossRef]
- Fredsgaard, M.; Kaniki, S.E.K.; Antonopoulou, I.; Chaturvedi, T.; Thomsen, M.H. Phenolic Compounds in Salicornia spp. and Their Potential Therapeutic Effects on H1N1, HBV, HCV, and HIV: A Review. Molecules 2023, 28, 5312. [Google Scholar] [CrossRef]
- Moianos, D.; Makri, M.; Prifti, G.-M.; Chiotellis, A.; Pappas, A.; Woodson, M.E.; Tajwar, R.; Tavis, J.E.; Zoidis, G. N-Hydroxypiridinedione: A Privileged Heterocycle for Targeting the HBV RNase H. Molecules 2024, 29, 2942. [Google Scholar] [CrossRef]
- Li, Q.; Lomonosova, E.; Donlin, M.J.; Cao, F.; O’Dea, A.; Milleson, B.; Berkowitz, A.J.; Baucom, J.-C.; Stasiak, J.P.; Schiavone, D.V. Amide-containing α-hydroxytropolones as inhibitors of hepatitis B virus replication. Antivir. Res. 2020, 177, 104777. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.L.; Guo, H. New insights on molecular mechanism of hepatitis B virus covalently closed circular DNA formation. Cells 2020, 9, 2430. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Kalaimathi, K.; Thiruvengadam, M.; Ayyanar, M.; Shine, K.; Amalraj, S.; Ceasar, S.A.; Priya, S.P.; Prakash, N. Antiviral mechanisms of dietary polyphenols: Recent developments as antiviral agents and future prospects in combating Nipah virus. Phytochem. Rev. 2024, 1–37. [Google Scholar] [CrossRef]
- Samynathan, R.; Thiruvengadam, M.; Nile, S.H.; Shariati, M.A.; Rebezov, M.; Mishra, R.K.; Venkidasamy, B.; Periyasamy, S.; Chung, I.-M.; Pateiro, M. Recent insights on tea metabolites, their biosynthesis and chemo-preventing effects: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 3130–3149. [Google Scholar] [CrossRef]
- Odukoya, J.O.; Odukoya, J.O.; Mmutlane, E.M.; Ndinteh, D.T. Ethnopharmacological study of medicinal plants used for the treatment of cardiovascular diseases and their associated risk factors in sub-Saharan Africa. Plants 2022, 11, 1387. [Google Scholar] [CrossRef]
- Parvez, M.K.; Ahmed, S.; Al-Dosari, M.S.; Abdelwahid, M.A.; Arbab, A.H.; Al-Rehaily, A.J.; Al-Oqail, M.M. Novel anti-hepatitis B virus activity of Euphorbia schimperi and its quercetin and kaempferol derivatives. ACS Omega 2021, 6, 29100–29110. [Google Scholar] [CrossRef]
- Parvez, M.K.; Al-Dosari, M.S.; Basudan, O.A.; Herqash, R.N. The anti-hepatitis B virus activity of sea buckthorn is attributed to quercetin, kaempferol and isorhamnetin. Biomed. Rep. 2022, 17, 89. [Google Scholar] [CrossRef]
- Akbar, S. Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Ramesh, D.; Vijayakumar, B.G.; Kannan, T. Advances in nucleoside and nucleotide analogues in tackling human immunodeficiency virus and hepatitis virus infections. ChemMedChem 2021, 16, 1403–1419. [Google Scholar] [CrossRef]
- Ranga, A.; Gupta, A.; Yadav, L.; Kumar, S.; Jain, P. Advancing beyond reverse transcriptase inhibitors: The new era of hepatitis B polymerase inhibitors. Eur. J. Med. Chem. 2023, 257, 115455. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.L.; Smith, S.J.; Zhao, X.Z.; Das, K.; Gruber, K.; Arnold, E.; Burke, T.R., Jr.; Hughes, S.H. Developing and Evaluating Inhibitors against the RNase H Active Site of HIV-1 Reverse Transcriptase. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Buss, A.D.; Butler, M.S. Natural Product Chemistry for Drug Discovery; Royal Society of Chemistry: London, UK, 2010; Volume 18. [Google Scholar]
- Seto, W.-K.; Lo, Y.-R.; Pawlotsky, J.-M.; Yuen, M.-F. Chronic hepatitis B virus infection. Lancet 2018, 392, 2313–2324. [Google Scholar] [CrossRef]
- Niklasch, M.; Zimmermann, P.; Nassal, M. The hepatitis B virus nucleocapsid—Dynamic compartment for infectious virus production and new antiviral target. Biomedicines 2021, 9, 1577. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.K.; Al-Dosari, M.S.; Arbab, A.H.; Al-Rehaily, A.J.; Abdelwahid, M.A. Bioassay-guided isolation of anti-hepatitis B virus flavonoid myricetin-3-O-rhamnoside along with quercetin from Guiera senegalensis leaves. Saudi Pharm. J. 2020, 28, 550–559. [Google Scholar] [CrossRef]
- Dey, D.; Biswas, P.; Paul, P.; Mahmud, S.; Ema, T.I.; Khan, A.A.; Ahmed, S.Z.; Hasan, M.M.; Saikat, A.S.M.; Fatema, B. Natural flavonoids effectively block the CD81 receptor of hepatocytes and inhibit HCV infection: A computational drug development approach. Mol. Divers. 2023, 27, 1309–1322. [Google Scholar] [CrossRef]
- Sharif, M.A.; Hossen, M.; Shaikat, M.M.; Haidary, F.; Ema, T.I.; Dey, D.; Paul, P.K.; Al Azad, S.; Al Mazid, M.F.; Badal, M. Molecular Optimization, Docking and Dynamic Simulation Study of Selective Natural Aromatic Components to Block E2-CD81 Complex Formation in Predating Protease Inhibitor Resistant HCV Influx. Int. J. Pharm. Res. 2021, 13, 3511–3525. [Google Scholar]
- Albahri, G.; Badran, A.; Abdel Baki, Z.; Alame, M.; Hijazi, A.; Daou, A.; Baydoun, E. Potential Anti-Tumorigenic Properties of Diverse Medicinal Plants against the Majority of Common Types of Cancer. Pharmaceuticals 2024, 17, 574. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Eichler, B.; Klausner, E.A.; Duffy-Matzner, J.; Zheng, W. Lead/drug discovery from natural resources. Molecules 2022, 27, 8280. [Google Scholar] [CrossRef]
- Jia, H.; Rai, D.; Zhan, P.; Chen, X.; Jiang, X.; Liu, X. Recent advance of the hepatitis B virus inhibitors: A medicinal chemistry overview. Future Med. Chem. 2015, 7, 587–607. [Google Scholar] [CrossRef]
- Batra, A.; Nandi, S.; Bagchi, M.C. QSAR and pharmacophore modeling of indole-based C-3 pyridone compounds as HCV NS5B polymerase inhibitors utilizing computed molecular descriptors. Med. Chem. Res. 2015, 24, 2432–2440. [Google Scholar] [CrossRef]
- Ejeh, S.; Uzairu, A.; Shallangwa, G.A.; Abechi, S.E. Computational insight to design new potential hepatitis C virus NS5B polymerase inhibitors with drug-likeness and pharmacokinetic ADMET parameters predictions. Future J. Pharm. Sci. 2021, 7, 1–13. [Google Scholar] [CrossRef]
- Dultz, G.; Shimakami, T.; Schneider, M.; Murai, K.; Yamane, D.; Marion, A.; Zeitler, T.M.; Stross, C.; Grimm, C.; Richter, R.M.; et al. Extended interaction networks with HCV protease NS3-4A substrates explain the lack of adaptive capability against protease inhibitors. J. Biol. Chem. 2020, 295, 13862–13874. [Google Scholar] [CrossRef]
- Chongjun, Y.; Nasr, A.; Latif, M.; Rahman, M.; Marlisah, E.; Tejo, B. Predicting repurposed drugs targeting the NS3 protease of dengue virus using machine learning-based QSAR, molecular docking, and molecular dynamics simulations. SAR QSAR Environ. Res. 2024, 35, 707–728. [Google Scholar] [CrossRef] [PubMed]
- De Borja, J.R.; Cabrera, H.S. In Silico Drug Screening for Hepatitis C Virus Using QSAR-ML and Molecular Docking with Rho-Associated Protein Kinase 1 (ROCK1) Inhibitors. Computation 2024, 12, 175. [Google Scholar] [CrossRef]
- Nalini, S.; Lampa, S.; Saw, S.; Gleeson, M.P.; Ola, S.; Chanin, N. Towards reproducible computational drug discovery. J. Cheminform. 2020, 12, 9. [Google Scholar]
- O’Connell, A.K.; Douam, F. Humanized mice for live-attenuated vaccine research: From unmet potential to new promises. Vaccines 2020, 8, 36. [Google Scholar] [CrossRef]
- Ugbaja, S.C.; Omerigwe, S.A.; Ndlovu, S.M.Z.; Ngcobo, M.; Gqaleni, N. Evaluating the Efficacy of Repurposed Antiretrovirals in Hepatitis B Virus Treatment: A Narrative Review of the Pros and Cons. Int. J. Mol. Sci. 2025, 26, 925. [Google Scholar] [CrossRef]
- Furutani, Y.; Hirano, Y.; Toguchi, M.; Higuchi, S.; Qin, X.Y.; Yanaka, K.; Sato-Shiozaki, Y.; Takahashi, N.; Sakai, M.; Kongpracha, P.; et al. A small molecule iCDM-34 identified by in silico screening suppresses HBV DNA through activation of aryl hydrocarbon receptor. Cell Death Discov. 2023, 9, 467. [Google Scholar] [CrossRef]
- Elmessaoudi-Idrissi, M.; Blondel, A.; Kettani, A.; Windisch, M.P.; Benjelloun, S.; Ezzikouri, S. Virtual screening in hepatitis B virus drug discovery: Current stateof-the-art and future perspectives. Curr. Med. Chem. 2018, 25, 2709–2721. [Google Scholar] [CrossRef]
- Patel, R.; Jain, A.; Patel, Z.; Kavani, H.; Patel, M.; Gadhiya, D.K.; Patel, D.; Yang, C. Artificial Intelligence and Machine Learning in Hepatocellular Carcinoma Screening, Diagnosis and Treatment-A Comprehensive Systematic Review. Glob. Acad. J. Med. Sci. 2024, 6, 83–97. [Google Scholar] [CrossRef]
- Gupta, S.; Parveen, S. Potential role of microRNAs in personalized medicine against hepatitis: A futuristic approach. Arch. Virol. 2024, 169, 33. [Google Scholar] [CrossRef]
- Naderi, M.; Salavatiha, Z.; Gogoi, U.; Mohebbi, A. An overview of anti-Hepatitis B virus flavonoids and their mechanisms of action. Front. Cell. Infect. Microbiol. 2024, 14, 1356003. [Google Scholar] [CrossRef] [PubMed]
- Vilar, S.; Costanzi, S. Predicting the biological activities through QSAR analysis and docking-based scoring. Methods Mol. Biol. 2012, 914, 271–284. [Google Scholar] [CrossRef]
- Gangwal, A.; Ansari, A.; Ahmad, I.; Azad, A.K.; Sulaiman, W.M.A.W. Current strategies to address data scarcity in artificial intelligence-based drug discovery: A comprehensive review. Comput. Biol. Med. 2024, 179, 108734. [Google Scholar] [CrossRef]
- Bai, F.J.J.S.; Jasmine, R.A. Optimization of tree-based machine learning algorithms for improving the predictive accuracy of hepatitis C disease. In Decision-Making Models; Elsevier: Amsterdam, The Netherlands, 2024; pp. 523–545. [Google Scholar]
- Ali, G.; Mijwil, M.M.; Adamopoulos, I.; Buruga, B.A.; Gök, M.; Sallam, M. Harnessing the Potential of Artificial Intelligence in Managing Viral Hepatitis. Mesopotamian J. Big Data 2024, 2024, 128–163. [Google Scholar] [CrossRef]
- Liu, J.; Bao, C.; Zhang, J.; Han, Z.; Fang, H.; Lu, H. Artificial intelligence with mass spectrometry-based multimodal molecular profiling methods for advancing therapeutic discovery of infectious diseases. Pharmacol. Ther. 2024, 263, 108712. [Google Scholar] [CrossRef]
- Hamadalnil, Y.; Altayb, H.N. In silico molecular study of hepatitis B virus X protein as a therapeutic target. J. Biomol. Struct. Dyn 2024, 42, 4002–4015. [Google Scholar] [CrossRef]
- Agrwal, A.; Chadha, N.; Sahu, A. Molecular Docking and Virtual Screening to Investigate Lead Compounds for Veterinary Drug Development. In Bioinformatics in Veterinary Science: Vetinformatics; Springer: Singapore, 2025; pp. 277–305. [Google Scholar]
- De Queiroz Simões, R.S.; Ferreira, M.S.; Dumas de Paula, N.; Machado, T.R.; Pascutti, P.G. Computational modeling in virus infections and virtual screening, docking, and molecular dynamics in drug design. In Networks in Systems Biology: Applications for Disease Modeling; Springer: Cham, Switzerland, 2020; pp. 301–337. [Google Scholar]
- Mohanty, M.; Mohanty, P.S. Molecular docking in organic, inorganic, and hybrid systems: A tutorial review. Monatsh. Chem. 2023, 154, 683–707. [Google Scholar] [CrossRef]
- Piyushbhai, M.K.; Sharma, P.; Ramalingam, R.; Binesh, A.; Venkatachalam, K. A Comprehensive Review on Integrated Approach for Discovery and Development of Novel Bioactive Compounds: From Natural Resources to Targeted Therapeutics. In Current Bioactive Compounds; Springer Nature: Singapore, 2024. [Google Scholar]
- Taylor, M.; Ho, J. MM/GBSA prediction of relative binding affinities of carbonic anhydrase inhibitors: Effect of atomic charges and comparison with Autodock4Zn. J. Comput. Aided. Mol. Des. 2023, 37, 167–182. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.; Zhang, Y. Hotspots and trends in global antiviral herbal basic research: A visualization analysis. Eur. J. Integr. Med. 2024, 72, 102419. [Google Scholar] [CrossRef]
- Ahmed, S.; Parvez, M.K.; Zia, K.; Nur-e-Alam, M.; Ul-Haq, Z.; Al-Dosari, M.S.; Al-Rehaily, A.J. Natural anti-hepatitis B virus flavones isolated from schimperi vatke growing in Saudi Arabia: Cell culture and molecular docking study. Pharmacogn. Mag. 2022, 18, 386–392. [Google Scholar]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, S.; Srivastava, T.; Naik, S.; Patravale, V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine 2021, 85, 153286. [Google Scholar] [CrossRef]
- Nikolic, J.; Belot, L.; Raux, H.; Legrand, P.; Gaudin, Y.; A. Albertini, A. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat. Commun. 2018, 9, 1029. [Google Scholar] [CrossRef]
- Rana, H.M.U.; Nisar, H.; Prajapati, J.; Goswami, D.; Rawat, R.; Eyupoglu, V.; Shahid, S.; Javaid, A.; Nisar, W. Integrative bioinformatic analysis to identify potential phytochemical candidates for glioblastoma. Heliyon 2024, 10, e40744. [Google Scholar] [CrossRef]
- Zhong, G.; Chang, X.; Xie, W.; Zhou, X. Targeted protein degradation: Advances in drug discovery and clinical practice. Signal Transduct. Target. Ther. 2024, 9, 308. [Google Scholar] [CrossRef]
- Mishra, A.; Kaur, I.; Sharma, A.; Manu, M.; De, U.K.; Kumar, N.; Malik, Y.S. Antivirals: Approaches and the Way Forward. In Advances in Antiviral Research; Springer Nature: Singapore, 2024; pp. 1–40. [Google Scholar]
- Mahapatra, A.D.; Paul, I.; Dasgupta, S.; Roy, O.; Sarkar, S.; Ghosh, T.; Basu, S.; Chattopadhyay, D. Antiviral potential and in silico insights of polyphenols as sustainable phytopharmaceuticals: A comprehensive review. Chem. Biodivers. 2024, e202401913. [Google Scholar] [CrossRef]
- Darvishi, M.; Nouri, M.; Rahimi, R.; Heidari-Soureshjani, S.; Rafsanjani, S.M.R.H. A systematic review of the impact of resveratrol on viral hepatitis and chronic viral hepatitis-related hepatocellular carcinoma. Curr. Mol. Med. 2025, 24, 1–16. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, Y. Therapeutic potential of flavonoids in gastrointestinal cancer: Focus on signaling pathways and improvement strategies. Mol. Med. Rep. 2025, 31, 1–34. [Google Scholar] [CrossRef]
- Moghe, A.S.; Deshpande, M.M.; Kamyab, S.S.; Chunarkar-Patil, P.; Nandi, S.S.; Bhatt, N.S. Hepatitis C Virus (HCV) and the Role of Phytochemicals in the Antiviral Effects of Different Medicinal Plants Against Infection. In Anti-Viral Metabolites from Medicinal Plants; Springer: Cham, Switzerland, 2023; pp. 1–31. [Google Scholar]
- Navabhatra, A. Molecular Mechanisms of Envelope Protein Inhibitors from Traditional Herbal Medicines. In Traditional and Herbal Medicines for COVID-19; CRC Press: Boca Raton, FL, USA, 2025; pp. 162–178. [Google Scholar]
- Nawaz, A.; Manzoor, A.; Ahmed, S.; Ahmed, N.; Abbas, W.; Mir, M.A.; Bilal, M.; Sheikh, A.; Ahmad, S.; Jeelani, I.; et al. Therapeutic approaches for chronic hepatitis C: A concise review. Front. Pharmacol. 2024, 14, 1334160. [Google Scholar] [CrossRef]
- Mancak, M.; Altintas, D.; Balaban, Y.; Caliskan, U.K. Evidence-based herbal treatments in liver diseases. Hepatol. Forum 2024, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Ugbaja, S.C.; Mokoena, A.T.; Mushebenge, A.G.A.; Kumalo, H.M.; Ngcobo, M.; Gqaleni, N. Evaluation of the Potency of Repurposed Antiretrovirals in HBV Therapy: A Narrative Investigation of the Traditional Medicine Alternatives. Int. J. Mol. Sci. 2025, 26, 1523. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Lv, F.; Quan, T.; Wang, C.; Li, J. Flavonoids in natural products for the therapy of liver diseases: Progress and future opportunities. Front. Pharmacol. 2024, 15, 1485065. [Google Scholar] [CrossRef] [PubMed]
- Jurel, P.; Bahadur, S.; Bajpai, M. Emerging trends in pharmacological and therapeutic potential of glycyrrhizic acids: Traditional and nanotechnological approach. Pharmacol. Res. -Mod. Chin. Med. 2024, 12, 100461. [Google Scholar] [CrossRef]
- Rasool, A.; Dar, T.A. Glycyrrhizin and its derivatives: An emerging secondary metabolite arsenal of Glycyrrhiza glabra. Med. Chem. Res. 2025, 1–19. [Google Scholar] [CrossRef]
| Drug/Compound Name and Structure | Original Indication/Compound Common Use | Target in HBV | Mechanism of Action | In silico Methods Used | Key Findings | Clinical/Preclinical Status | References |
|---|---|---|---|---|---|---|---|
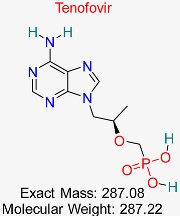 | HIV | HBV Polymerase | Inhibits reverse transcription, reducing viral replication | Molecular docking, molecular dynamics simulation | High binding affinity with HBV polymerase; favorable ADMET profile | Approved for HBV treatment | [50,51] |
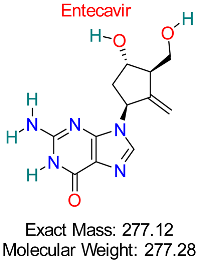 | Herpes Simplex Virus | HBV Polymerase | Competitive inhibition of nucleotide incorporation during HBV replication | Molecular docking, ADMET analysis | Demonstrates strong selectivity and efficacy in HBV pathways | Approved for HBV treatment | [17,52] |
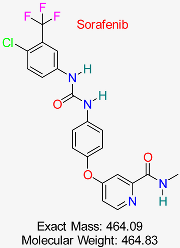 | Hepatocellular Carcinoma | HBV cccDNA transcription | Inhibits tyrosine kinases, reducing cccDNA transcription and replication | Virtual screening, molecular docking | Identified potential off-target effects on HBV cccDNA pathways | Under investigation for HBV treatment | [53,54] |
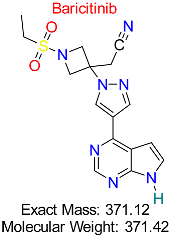 | Rheumatoid Arthritis | HBV Immune Evasion Pathways | Janus kinase (JAK) inhibitor modulating immune responses to HBV infection | Molecular docking, molecular dynamics, AI modeling | High binding affinity to key immune evasion proteins; potential to enhance antiviral immunity | Preclinical stage | [55,56] |
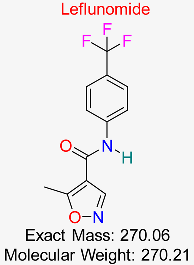 | Rheumatoid Arthritis | HBV DNA Replication | Inhibits pyrimidine synthesis, disrupting HBV DNA synthesis | Docking, MD simulations, pharmacokinetics modeling | Favorable docking scores with HBV polymerase; potential to enhance existing HBV therapies | Preclinical stage | [57,58] |
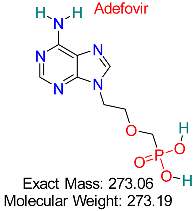 | HIV | HBV Polymerase | Nucleotide analog that inhibits HBV polymerase activity | Structural modeling, molecular dynamics simulations | Effective against wild-type and resistant HBV strains | Approved for HBV treatment | [50,51] |
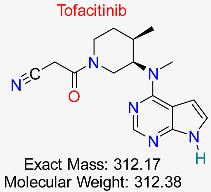 | Rheumatoid Arthritis | HBV-Induced Inflammatory Pathways | Inhibits JAK-STAT pathway involved in HBV-induced liver inflammation | Molecular docking, network pharmacology | Strong inhibitory activity on inflammatory targets; favorable ADMET properties | Preclinical investigations | [57,59] |
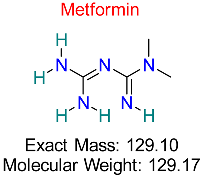 | Type 2 Diabetes Mellitus | HBV cccDNA Regulation | Reduces cccDNA stability by modulating cellular energy metabolism | Molecular docking, ADMET analysis | Demonstrates potential to disrupt cccDNA stability in hepatocytes | Preclinical studies | [60,61] |
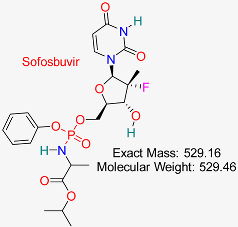 | Hepatitis C Virus | HBV Polymerase | Inhibits HBV RNA-dependent RNA polymerase, disrupting replication | Molecular docking, dynamics, AI modeling | Effective binding to HBV polymerase; potential for combination therapies | Preclinical for HBV | [62,63] |
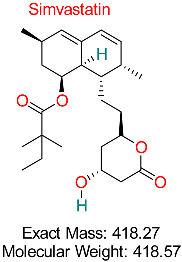 | Hyperlipidemia | HBV Assembly | Reduces viral assembly and secretion by modulating cellular pathways | Docking, network pharmacology | Promising inhibition of HBV secretion pathways; synergistic effects with existing antivirals | Preclinical studies | [63,64] |
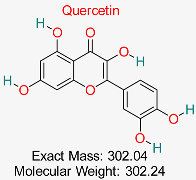 | Natural flavonoid, possessing antioxidant, anti-inflammatory, antiviral, and anticancer effects, promoting cardiovascular wellness, neuroprotection, immune balance, and healing of wounds. | HBV cccDNA and Viral Polymerase | Inhibits key proteins involved in HBV replication and cccDNA maintenance | Molecular docking, dynamics simulations | Strong binding affinity to multiple HBV targets; multitarget action potential | Preclinical validation for HBV | [65,66] |
 | A potent catechin found in green tea, recognized for its antioxidant, anti-inflammatory, antiviral, and anticancer effects, aiding in heart health, brain performance, and immune system support. | HBV Viral Polymerase | Inhibits viral replication through polymerase targeting | Molecular docking, dynamics | High binding affinity to HBV polymerase with low toxicity | Preclinical studies | [67] |
 | A natural triterpenoid exhibiting hepatoprotective, anti-inflammatory, antioxidant, and antiviral effects, commonly utilized for liver wellness and metabolic assistance. | HBV Assembly | Disrupts viral assembly and secretion pathways | Molecular docking, dynamics | Demonstrates effective HBV assembly inhibition; favorable pharmacokinetics | Preclinical investigations | [68] |
 | Natural alkaloid exhibiting antimicrobial, anti-inflammatory, antidiabetic, and cholesterol-reducing effects, frequently utilized to promote metabolic and cardiovascular well-being. | HBV DNA Replication | Interferes with viral replication by targeting HBV DNA synthesis | Virtual screening, molecular dynamics | Potent inhibition of HBV polymerase; synergistic potential with antivirals | Preclinical stage | [12] |
 | Bioactive substance present in turmeric possessing strong anti-inflammatory, antioxidant, and anticancer effects, commonly utilized for enhancing general well-being and controlling chronic illnesses. | HBV cccDNA Transcription | Modulates transcription factors involved in HBV cccDNA regulation | Network pharmacology, docking, dynamics | Identified as a multitarget inhibitor with low cytotoxicity | Preclinical studies | [67,69] |
 | A natural flavonoid possessing antioxidant, anti-inflammatory, and anticancer characteristics, frequently studied for its possible benefits in treating anxiety, enhancing testosterone levels, and promoting cardiovascular wellness. | HBV Immune Modulation | Modulates immune pathways associated with HBV infection | Molecular docking, dynamics | Validated interactions with HBV immune evasion targets | Preclinical studies | [70,71] |
 | Flavonoid exhibiting strong antioxidant, anti-inflammatory, and anticancer effects, frequently researched for its involvement in neuroprotection, immune modulation, and lowering the risk of chronic diseases. | HBV Polymerase | Inhibits viral replication by targeting polymerase | Molecular docking, pharmacokinetics modeling | Demonstrates strong polymerase inhibition with favorable drug-likeness properties | Preclinical stage | [72] |
 | Derived from Artemisia annua (sweet wormwood), is widely known for its potent antimalarial properties and is also being explored for its anticancer, antiviral, and anti-inflammatory activities. | HBV Replication and Transcription | Targets viral replication pathways and transcription regulation | Docking, molecular dynamics | Promising multitarget activity against HBV; synergistic effects with other antivirals | Preclinical validation | [67] |
 | A natural substance present in grapes, berries, and peanuts; shows antioxidant, anti-inflammatory, cardioprotective, neuroprotective, and anticancer effects, with growing possibilities in antiviral treatment. | HBV cccDNA Stability | Reduces HBV cccDNA stability through modulation of host cellular factors | Molecular docking, dynamics, network pharmacology | High binding affinity to HBV cccDNA-related pathways; multitarget potential | Preclinical studies | [73,74] |
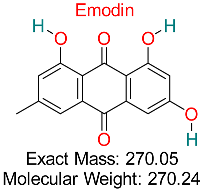 | Naturally occurring anthraquinone compounds discovered in plants such as rhubarb and aloe vera exhibit anti-inflammatory, anticancer, antimicrobial, and antiviral effects, showing potential therapeutic uses for various illnesses, including HBV. | HBV Assembly and cccDNA Regulation | Inhibits HBV assembly and reduces cccDNA stability | Docking, ADMET analysis | Effective against multiple HBV targets; potential for combination therapies | Preclinical validation | [68] |
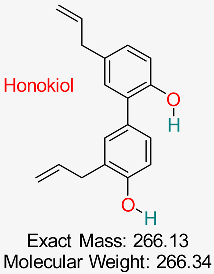 | A biphenolic compound discovered in the bark and seeds of the Magnolia tree demonstrates multiple pharmacological effects, such as anti-inflammatory, antioxidant, anticancer, and antiviral characteristics, positioning it as a potential candidate for drug development, including treatment for HBV. | HBV Replication and Inflammation | Modulates inflammatory and viral replication pathways | Molecular docking, network pharmacology | Potential multitarget inhibitor; low toxicity | Preclinical studies | [67,75] |
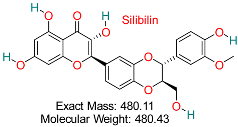 | Flavonoids derived from milk thistle (Silybum marianum) have shown hepatoprotective, anti-inflammatory, antioxidant, and antiviral effects. It is recognized for its ability to block HBV replication and safeguard liver cells from harm, positioning it as a promising natural agent for HBV treatment. | HBV Replication and Oxidative Stress | Inhibits viral replication and modulates oxidative stress in liver cells | Docking, molecular dynamics, ADMET | Synergistic potential with standard antivirals | Preclinical validation | [68,72] |
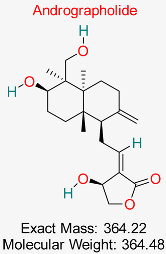 | Diterpenoid lactone obtained from Andrographis paniculata is recognized for its anti-inflammatory, antioxidant, and antiviral capabilities. It has demonstrated promise in suppressing HBV replication, safeguarding liver cells, and regulating immune responses, positioning it as a potential option for therapeutic use in HBV therapy. | HBV Polymerase | Inhibits HBV polymerase activity | Molecular docking, dynamics | Potent HBV polymerase inhibition; favorable drug-likeness | Preclinical studies | [76,77] |
 | A flavonoid derived from Scutellaria baicalensis shows anti-inflammatory, antiviral, and liver-protective properties, possibly hindering HBV replication and liver injury. | HBV Replication | Disrupts viral replication pathways | Docking, dynamics, pharmacokinetics modeling | Promising activity against HBV replication | Preclinical investigations | [68,72] |
 | Lignan extracted from Phyllanthus niruri exhibits antiviral, hepatoprotective, and anti-inflammatory effects, especially against HBV, by suppressing viral replication and enhancing liver health. | HBV cccDNA Stability | Reduces stability of HBV cccDNA | Molecular docking, pharmacokinetics modeling | Effective multitarget activity against HBV pathways | Preclinical stage | [66] |
 | Key active ingredient from Picrorhiza kurroa shows hepatoprotective, anti-inflammatory, and antiviral effects, potentially blocking HBV replication and safeguarding liver cells from harm. | HBV DNA Replication | Disrupts HBV DNA replication pathways | Molecular docking, dynamics simulations | Favorable binding affinity to HBV DNA replication proteins | Preclinical studies | [78] |
 | Extracted from Glycyrrhiza glabra (licorice root), it possesses strong antiviral, anti-inflammatory, and liver-protective effects, demonstrating promise in hindering HBV replication and enhancing liver function. | HBV Replication and Immune Modulation | Modulates immune response and inhibits viral replication | Molecular docking, network pharmacology | Identified as multitarget inhibitor with high safety profile | Preclinical validation | [67,68] |
 | Flavonoids present in tea leaves exhibit antioxidant, anti-inflammatory, and antiviral effects, showing promise in blocking HBV replication and safeguarding liver cells from harm. | HBV cccDNA and Polymerase | Inhibits HBV replication and reduces cccDNA stability | Docking, dynamics simulations, ADMET analysis | Effective multitarget inhibitor against HBV pathways | Preclinical studies | [66,68,72] |
 | Isoflavonoid present in soybeans demonstrates antioxidant, anti-inflammatory, and antiviral abilities and has indicated promise in suppressing HBV replication and encouraging liver cell regeneration. | HBV Replication and Inflammatory Pathways | Modulates HBV replication and reduces inflammation | Docking, molecular dynamics | Strong binding to HBV replication proteins and inflammatory targets | Preclinical studies | [72] |
 | Anthraquinone derivatives discovered in a range of plants, such as Rheum palmatum, have shown anti-inflammatory, antioxidant, and antiviral effects and are being investigated for their ability to block HBV replication and alleviate liver damage. | HBV cccDNA Stability | Reduces HBV cccDNA by modulating cellular factors | Molecular docking, pharmacokinetics modeling | Promising multitarget potential for HBV therapy | Preclinical investigations | [79] |
 | Flavonoids present in numerous plants like parsley, chamomile, and celery demonstrate antioxidant, anti-inflammatory, and antiviral effects, and research has examined their ability to hinder HBV replication and influence immune responses. | HBV Polymerase | Targets viral replication pathways | Molecular docking, network pharmacology | Demonstrates high binding affinity to HBV polymerase | Preclinical studies | [72,74] |
 | Saponin compounds obtained from Astragalus membranaceus have shown antioxidant, anti-inflammatory, and immune-enhancing effects and are being investigated for their ability to improve liver function and inhibit HBV replication. | HBV Immune Modulation | Modulates immune pathways related to HBV infection | Molecular docking, dynamics, pharmacokinetics modeling | Promising multitarget effects on immune evasion pathways | Preclinical studies | [80,81,82] |
 | Polyphenolic compounds present in different fruits and vegetables have demonstrated potential as antiviral, anti-inflammatory, and antioxidant agents, and research is underway to assess their capacity to block HBV replication and safeguard liver cells from damage. | HBV Polymerase and cccDNA | Inhibits HBV replication and destabilizes cccDNA | Docking, dynamics, ADMET analysis | Effective multitarget inhibition of HBV pathways | Preclinical validation | [67,74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugbaja, S.C.; Mushebenge, A.G.-A.; Kumalo, H.; Ngcobo, M.; Gqaleni, N. Potential Benefits of In Silico Methods: A Promising Alternative in Natural Compound’s Drug Discovery and Repurposing for HBV Therapy. Pharmaceuticals 2025, 18, 419. https://doi.org/10.3390/ph18030419
Ugbaja SC, Mushebenge AG-A, Kumalo H, Ngcobo M, Gqaleni N. Potential Benefits of In Silico Methods: A Promising Alternative in Natural Compound’s Drug Discovery and Repurposing for HBV Therapy. Pharmaceuticals. 2025; 18(3):419. https://doi.org/10.3390/ph18030419
Chicago/Turabian StyleUgbaja, Samuel Chima, Aganze Gloire-Aimé Mushebenge, Hezekiel Kumalo, Mlungisi Ngcobo, and Nceba Gqaleni. 2025. "Potential Benefits of In Silico Methods: A Promising Alternative in Natural Compound’s Drug Discovery and Repurposing for HBV Therapy" Pharmaceuticals 18, no. 3: 419. https://doi.org/10.3390/ph18030419
APA StyleUgbaja, S. C., Mushebenge, A. G.-A., Kumalo, H., Ngcobo, M., & Gqaleni, N. (2025). Potential Benefits of In Silico Methods: A Promising Alternative in Natural Compound’s Drug Discovery and Repurposing for HBV Therapy. Pharmaceuticals, 18(3), 419. https://doi.org/10.3390/ph18030419







