Evaluation of the Efflux Pump Inhibition Activity of Thiadiazine-Derived Compounds Against the Staphylococcus aureus 1199B Strain
Abstract
1. Introduction
2. Results
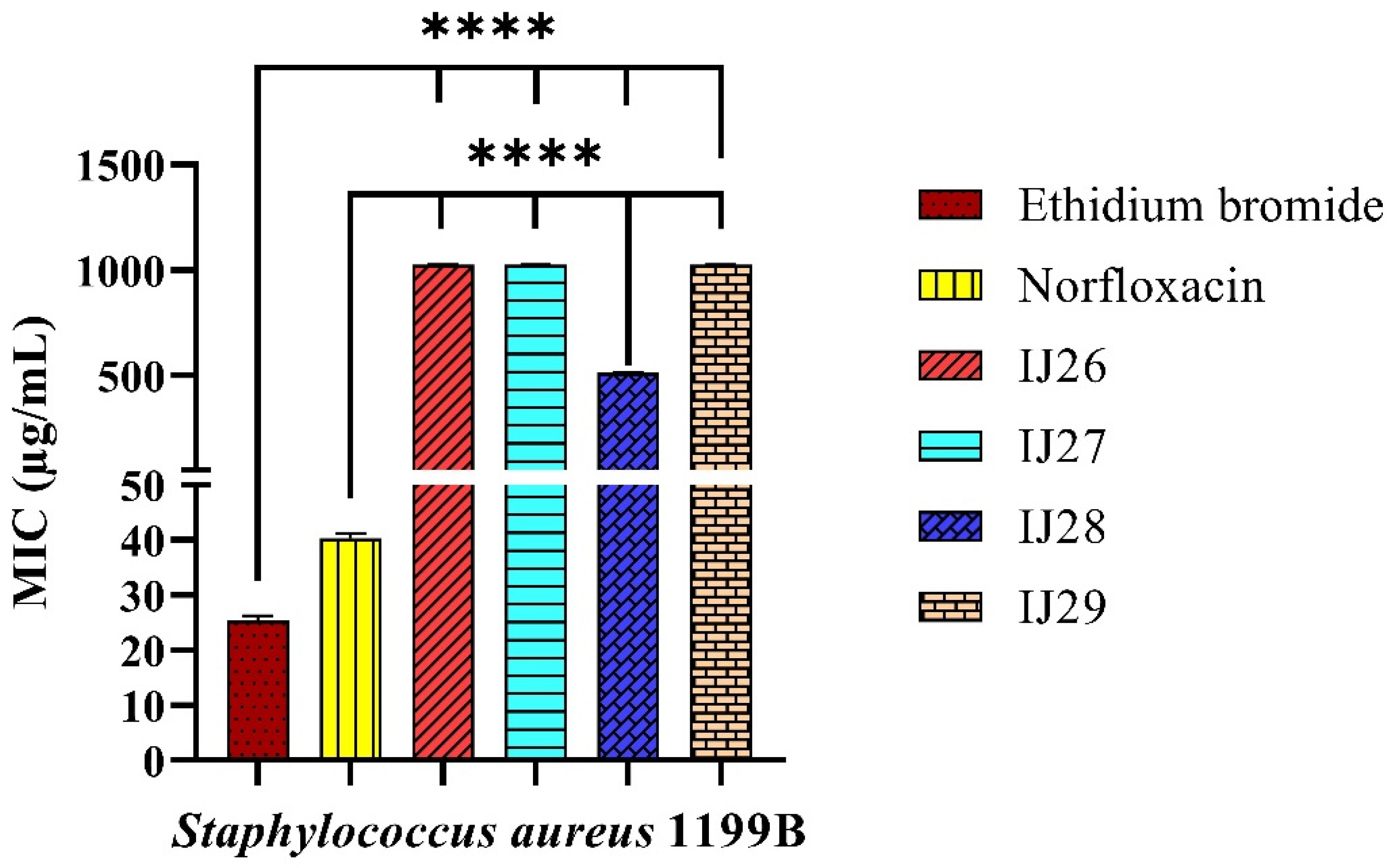
2.1. Antibacterial Activity
2.2. Evaluation of Antibacterial Activity Through Efflux Pump Inhibition
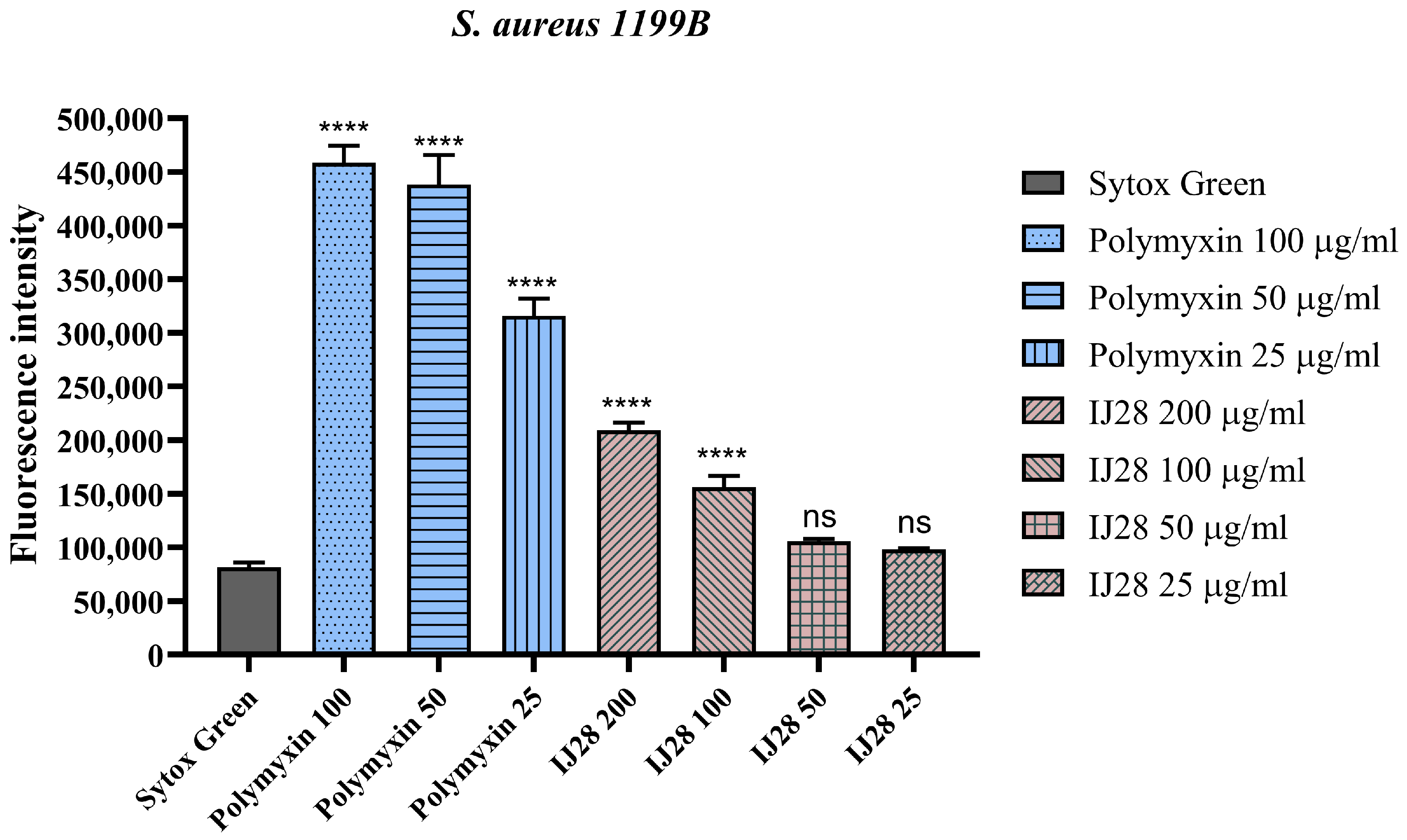
2.3. Evaluation of NorA Efflux Pump Inhibition by Fluorescence Emission
2.4. Molecular Docking Evaluation of Compound IJ28
3. Discussion
4. Materials and Methods
4.1. Material Acquisition
4.2. General Procedure
| 5-(4-Methoxyphenyl)-N-phenyl-6H-1,3,4-thiadiazin-2-amine IJ26 Yellow solid; yield 56%; mp 187–188 °C; HPLC-UV: 3.07 min.; purity of 99%; 1H NMR (600 MHz, DMSO-d6) δ 3.85 (s, 3H, OCH3); 4.30 (s, 2H, CH2); 7.12 (dt, 2H, J = 3.10 and 8.95 Hz, H-Ar); 7.34 (t, 1H, J = 7.38 Hz, H-Ar); 7.45–7.50 (m, 4H, H-Ar); 7.92 (dt, 2H, J = 3.10 and 8.95 Hz, H-Ar); 13C NMR (150 MHz, DMSO-d6) δ 162.2; 154.4; 151.5; 137.7; 129.4; 129.2; 126.7; 123.9; 123.3; 114.5; 114.1; 55.5; 22.7. | 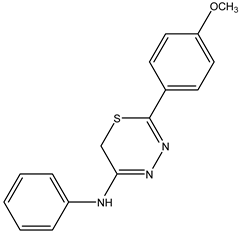 IJ26 |
| 5-(4-Chlorophenyl)-N-phenyl-6H-1,3,4-thiadiazin-2-amine—IJ 27 Yellow solid; yield 60%; mp 179–180 °C; HPLC-UV: 3.27 min.; purity of 99%; 1H NMR (600 MHz, DMSO-d6) δ 3.74 (s, 2H, CH2); 7.06 (dd, 2H, J = 1.18 and 8.62 Hz, H-Ar); 7.18 (tt, 1H, J = 1.18 and 7.46 Hz, H-Ar); 7.37 (dtq, 2H, J = 1.95, 8.62 and 0.76 Hz, H-Ar); 7.43 (dt, 2H, J = 2.58 and 8.70 Hz, H-Ar); 7.71 (dt, 2H, J = 2.58 and 8.70 Hz, H-Ar); 13C NMR (150 MHz, DMSO-d6) δ 153.4; 145.9; 145.5; 136.1; 133.0; 129.1; 129.0; 127.4; 124.7; 122.4; 23.2. | 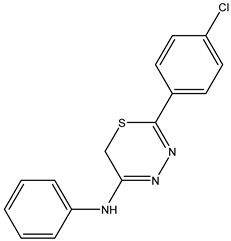 IJ 27 |
| 4-(2-(Phenylamino)-6H-1,3,4-thiadiazin-5-yl)phenol—IJ28 Yellow solid; yield 60%; mp 111–112 °C; HPLC-UV: 2.85 min.; purity of 99%; 1H NMR (600 MHz, DMSO-d6) δ 3.82 (s, 2H, CH2); 6.82 (dt, 3H, J = 2.86 and 8.86 Hz, H-Ar); 7.01 (t, 1H, J = 7.46 Hz, H-Ar); 7.28 (dt, 3H, J = 1.84 and 8.01 Hz, H-Ar); 7.70 (s, 2H, H-Ar); 9.87 (s, 1H, NH); 10.12 (s, 1H, OH); 13C NMR (150 MHz, DMSO-d6) δ 159.3; 147.2; 129.1; 128.2; 126.4; 123.1; 122.0; 116.5; 115.9; 115.5; 22.6. | 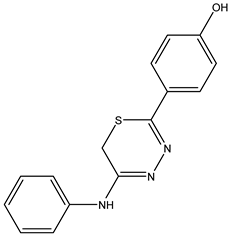 IJ 28 |
| N-phenyl-5-(p-tolyl)-6H-1,3,4-thiadiazin-2-amine—IJ29 Yellow solid; yield 50%; mp 180–181 °C; HPLC-UV: 3.18 min.; purity of 99%; 1H NMR (600 MHz, DMSO-d6) δ 2.39 (s, 3H, CH3); 4.29 (s, 2H, CH2); 7.35–7.38 (m, 3H, H-Ar); 7.42–7.43 (m, 2H, H-Ar); 7.50 (t, 2H, J = 8.09 Hz, H-Ar); 7.84 (d, 2H, J = 8.3 Hz, H-Ar); 13C NMR (150 MHz, DMSO-d6) δ 142.3; 130.6; 130.1; 130.0; 129.2; 127.7; 127.6; 124.8; 123.9; 23.1; 21.5. 13C NMR (150 MHz, DMSO-d6) δ 159.3; 147.2; 129.1; 128.2; 126.4; 123.1; 122.0; 116.5; 115.9; 115.5; 22.6. | 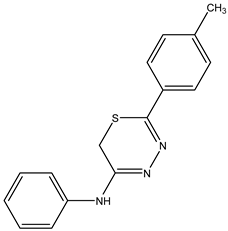 IJ 29 |
4.3. Preparation of Microorganisms and Inoculum for Microdilution
4.4. Preparation of Substances for Microdilution Test
4.5. Determination of Minimum Inhibitory Concentration (MIC)
4.6. Evaluation of Antibacterial Activity Through Efflux Pump Inhibition
4.7. Inhibitory Action of Efflux Pumps NorA Assessed by Increased Fluorescence Emission of Ethidium Bromide
4.8. Molecular Docking Evaluation of Compound IJ28
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alós, J.-I. Antibiotic Resistance: A Global Crisis. Enfermedades Infecc. Microbiol. Clínica 2015, 33, 692–699. [Google Scholar] [CrossRef]
- Cussolim, P.A.; Salvi Junior, A.; Melo, A.L. Mechanisms of Resistance of Staphylococcus aureus to Antibiotics. Rev. Fac. Saber 2021, 6, 831–843. [Google Scholar]
- Reygaert, W.C. An Overview of Bacterial Antimicrobial Resistance Mechanisms. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Martinez, J.L. General Principles of Antibiotic Resistance in Bacteria. Drug Discov. Today Technol. 2014, 11, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, W. The mechanism of bacterial resistance and potential bacteriostatic strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef]
- Piddock, L.J.V. Clinically Relevant Chromosomally Encoded Multidrug Resistance Efflux Pumps in Bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Neyfakh, A.A.; Borsch, C.M.; Kaatz, G.W. The NorA Fluoroquinolone Resistance Protein of Staphylococcus aureus is a Multidrug Efflux Transporter. Antimicrob. Agents Chemother. 1993, 37, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, M.; Ammini, P.; Adjei, J.; Zhang, F.; Cheng, W. Modulation of Antimicrobial Efflux Pumps of the Major Facilitator Superfamily in Staphylococcus aureus. AIMS Microbiol. 2018, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.H.S.; Rocha, J.E.; de Freitas, T.S.; Pereira, R.L.S.; Junior, F.N.P.; de Oliveira, M.R.C.; Batista, F.L.A.; Coutinho, H.D.M.; de Menezes, I.R.A. Evaluation of the Antibacterial Activity and Reversal of the NorA and MepA Efflux Pump by Estragole Against Staphylococcus aureus Bacteria. Arch. Microbiol. 2021, 203, 3551–3555. [Google Scholar] [CrossRef]
- Almeida, R.S.; Freitas, R.P.; Araújo, A.C.J.; Oliveira, I.R.; Santos, E.L.; Oliveira, R.A.; Oliveira, F.F.; Filho, J.R.; Ferreira, V.A.; Silva, A.C.A. GC-MS Profile and Enhancement of Antibiotic Activity by the Essential Oil of Ocotea odorífera and Safrole: Efflux Pump Inhibition in Staphylococcus aureus. Antibiotics 2020, 9, 247. [Google Scholar] [CrossRef]
- Rocha, J.E.; de Freitas, T.S.; da Cunha Xavier, J.; Pereira, R.L.S.; Junior, F.N.P.; Nogueira, C.E.S.; Marinho, M.M.; Bandeira, P.N.; de Oliveira, M.R.; Marinho, E.S.; et al. Antibacterial and Antibiotic-Modifying Activity, ADMET Study, and Molecular Docking of Synthetic Chalcone (E)-1-(2-Hydroxyphenyl)-3-(2,4-Dimethoxyphenyl)prop-2-en-1-one in Staphylococcus aureus Strains with NorA and MepA Efflux Pumps. Biomed. Pharmacother. 2021, 140, 111768. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Cruz, C.D.; Morawska, M.; Ciura, K.; Gilbert-Girard, S.; Mazur, L.; Mäkkylä, H.; Ilina, P.; Savijoki, K.; Fallarero, A.; et al. Antibacterial and antibiofilm activity of permanently ionized quaternary ammonium fluoroquinolones. Eur. J. Med. Chem. 2023, 254, 115373. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, J.; Sączewski, J.; Konopacka, A.; Waleron, K.; Lejnowski, D.; Ciura, K.; Tomašič, T.; Skok, Z.; Savijoki, K.; Morawska, M.; et al. Synthesis and biological evaluation of hybrid quinolone-based quaternary ammonium antibacterial agents. Eur. J. Med. Chem. 2019, 179, 576–590. [Google Scholar] [CrossRef]
- Nascimento, I.J.S. Synthesis, Biological Evaluation, and In Silico Studies of New Thiazolic and Thiadiazine Compounds with Potential Against Trypanosoma cruzi and Leishmania amazonensis. Ph.D. Thesis, Federal University of Alagoas, Maceió, Brazil, 2022. Available online: https://www.repositorio.ufal.br/jspui/handle/123456789/8659 (accessed on 3 February 2021).
- Freitas, P.R.; Araújo, A.C.J.; Araújo, I.M.; Siqueira, G.M.; Alves, D.S.; Borges, J.A.O.; Miranda, G.M.; Nascimento, I.J.S.; Araújo-Júnior, J.X.; Silva-Júnior, E.F.; et al. ADMET Analysis, Antibacterial Activities, and Antibiotic Modifications of Thiadiazine Derivatives. J. Biol. Regul. Homeost. Agents 2024, 38, 123–135. [Google Scholar] [CrossRef]
- De Araújo, A.C.J.; Freitas, P.R.; Araújo, I.M.; Siqueira, G.M.; de Oliveira Borges, J.A.; Alves, D.S.; Miranda, G.M.; dos Santos Nascimento, I.J.; de Araújo-Júnior, J.X.; da Silva-Júnior, E.F.; et al. Enhancement of Antibiotic Activity and Analysis of Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) Properties of Synthetic Thiadiazines Against Multidrug-Resistant (MDR) Strains. Fundam. Clin. Pharmacol. 2023, 38, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.S. Anticancer, Antibacterial, and Toxicological Effects of Thiazole and Thiazolidinedione Derivatives. Ph.D. Thesis, Biotechnology—Federal University of Pernambuco, Recife, Brazil, 2020. [Google Scholar]
- Jain, V.S.; Vora, D.K.; Ramma, C.S. Thiazolidine-2,4-dione: Progress in Multifaceted Applications. Bioorg. Med. Chem. 2013, 21, 1599–1620. [Google Scholar] [CrossRef]
- Aufort, M.; Herscovici, J.; Bouhours, P.; Moreau, N.; Girard, C. Synthesis and Antibiotic Activity of a Library of Small Molecules Derived from 1,2,3-Triazole. Bioorg. Med. Chem. Lett. 2008, 18, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Couto, I.; Viveiros, M.; Amaral, L. Identification of Multidrug Resistance Mediated by Efflux in Clinical Bacterial Isolates by Two Simple Methods. Methods Mol. Biol. 2010, 642, 143–157. [Google Scholar] [CrossRef]
- Santos, M.; Santos, R.; Soeiro, P.; Silvestre, S.; Ferreira, S. Resveratrol as an Inhibitor of the NorA Efflux Pump and Resistance Modulator in Staphylococcus aureus. Antibiotics 2023, 12, 1168. [Google Scholar] [CrossRef]
- Brawley, D.N.; Sauer, D.B.; Li, J.; Zheng, X.; Koide, A.; Jedhe, G.S.; Suwatthee, T.; Song, J.; Liu, Z.; Arora, P.S.; et al. Structural Basis for the Inhibition of the Staphylococcus aureus NorA Efflux Pump. Nat. Chem. Biol. 2022, 18, 706–712. [Google Scholar] [CrossRef]
- Santana, L.M.D. The Potential of Antimicrobial Photodynamic Therapy in the Development of Resistance in Candida albicans. Master’s Thesis, School of Dentistry, Oral Rehabilitation—São Paulo State University, Araraquara, Brazil, 2020; 137p. [Google Scholar]
- Carmello, J.C.; Alves, F.G.; Basso, F.; de Souza Costa, C.A.; Bagnato, V.S.; de Oliveira Mima, E.G.; Pavarina, A.C. Treatment of Oral Candidiasis Using Photodynamic Therapy Mediated by Photodithazine® In Vivo. PLoS ONE 2016, 11, e0156947. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Barbosa, C.R.; Macêdo, N.S.; de Sousa Silveira, Z.; Rocha, J.E.; Freitas, T.S.; Muniz, D.F.; Araújo, I.M.; de Morais Oliveira-Tintino, C.D.; Marinho, E.S.; da Rocha, M.N.; et al. Evaluation of the Antibacterial Activity and Inhibition of the MepA Efflux Pump in Staphylococcus aureus by Riparins I, II, III, and IV. Arch. Biochem. Biophys. 2023, 748, 109782. [Google Scholar] [CrossRef]
- Javadpour, M.; Juban, M.; Lo, W.; Bishop, S.; Alberty, J.; Mann, C.; Markhan, J.L. A New Method for Determining the Minimum Inhibitory Concentration of Essential Oils. J. Appl. Microbiol. 1996, 84, 538–544. [Google Scholar]
- Coutinho, H.D.; Costa, J.G.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of Antibiotic Activity Against Multidrug-Resistant Escherichia coli by Mentha arvensis L. and Chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated Comparative Protein Structure Modeling with SWISS-MODEL and Swiss-PdbViewer: A Historical Perspective. Electrophoresis 2009, 30, S162–S173. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Neto, J.B.; Oliveira-Tintino, C.D.M.; Araújo, G.A.; Alves, D.S.; Ribeiro, F.R.; Brancaglion, G.A.; Carvalho, D.T.; Lima, C.M.G.; Mohammed Ali, H.S.H.; Rather, I.A.; et al. Substituted Coumarins Inhibit NorA and MepA Efflux Pumps in Staphylococcus aureus. Antibiotics 2023, 12, 1739. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for Structure Building and Analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]


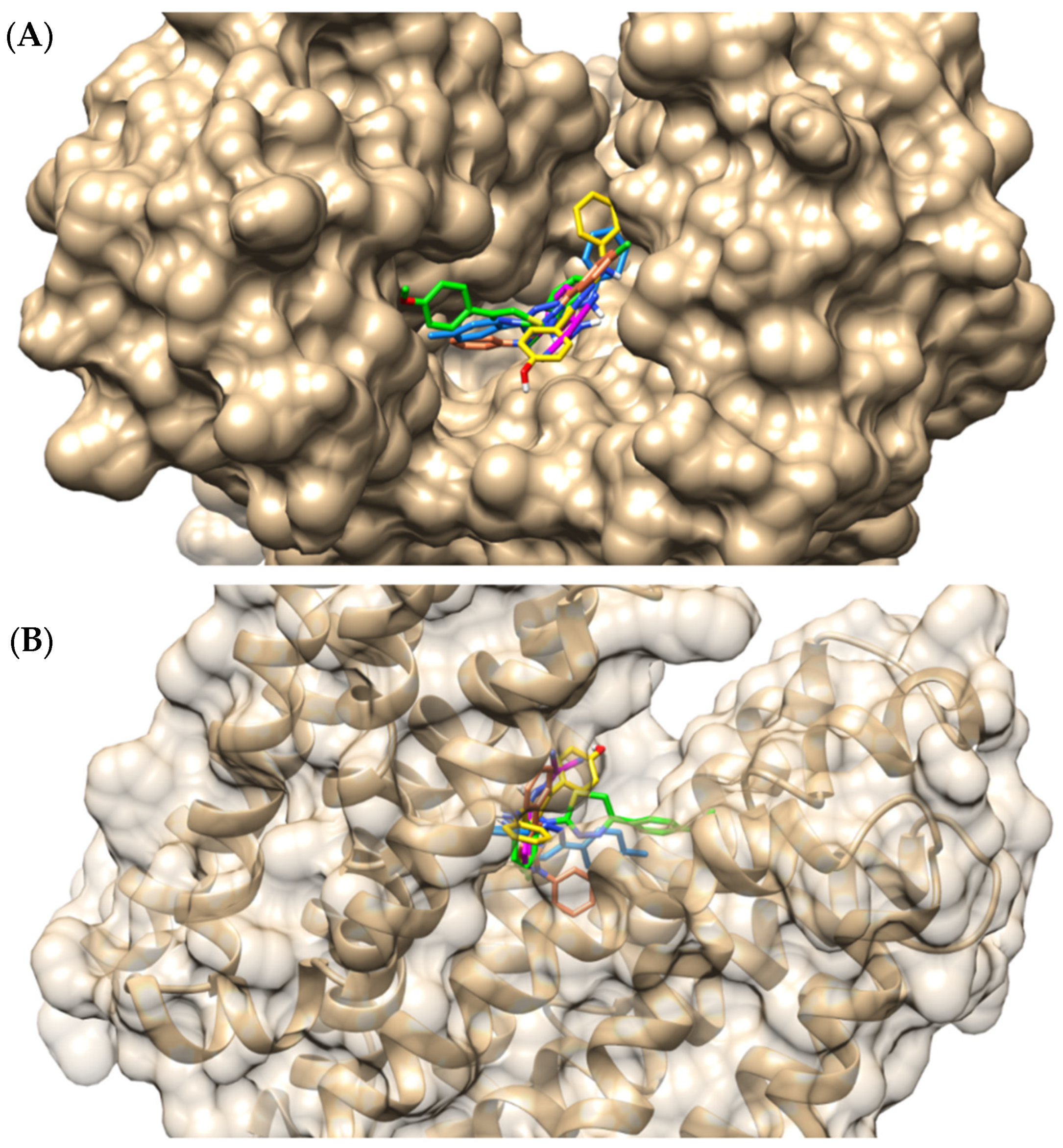

| Compounds | Binding Energy (Kcal/mol) | Inhibition Constant (µM) | Ligand Efficiency |
|---|---|---|---|
| IJ26 | −5.30 | 129.52 | −0.25 |
| IJ27 | −5.46 | 99.54 | −0.27 |
| IJ28 | −5.55 | 84.96 | −0.28 |
| IJ29 | −5.43 | 104.31 | −0.27 |
| CCCP | −4.63 | 403.17 | −0.33 |
| Compounds | vdW | Hydrogen Bond | Hidrophobic | Electrostatic |
|---|---|---|---|---|
| IJ28 | Arg310, Asn137, Asp307, Ile141, Phe341, Ser219, Thr223 | Asn340 (1.77 Å) Glu222 (2.23 Å) | Phe140 (5.28 Å) Pro344 (5.29 Å) | - |
| CCCP | Ile136, Leu218, Pro344, Thr223, Thr336 | Glu222 (1.74 Å) Ser219 (3.55 Å) | Phe140 (4.88 Å) | Arg310 (3.96 Å) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roque Paulo, C.L.; Alexandre, P.R.F.; Araujo, A.C.F.; Almeida, R.S.; Albuquerque, E.S.; Oliveira-Tintino, C.D.d.M.; Nascimento, I.J.S.; Araújo-Júnior, J.X.; Silva-Junior, E.F.d.; Aquino, T.M.d.; et al. Evaluation of the Efflux Pump Inhibition Activity of Thiadiazine-Derived Compounds Against the Staphylococcus aureus 1199B Strain. Pharmaceuticals 2025, 18, 323. https://doi.org/10.3390/ph18030323
Roque Paulo CL, Alexandre PRF, Araujo ACF, Almeida RS, Albuquerque ES, Oliveira-Tintino CDdM, Nascimento IJS, Araújo-Júnior JX, Silva-Junior EFd, Aquino TMd, et al. Evaluation of the Efflux Pump Inhibition Activity of Thiadiazine-Derived Compounds Against the Staphylococcus aureus 1199B Strain. Pharmaceuticals. 2025; 18(3):323. https://doi.org/10.3390/ph18030323
Chicago/Turabian StyleRoque Paulo, Cicera Laura, Priscilla Ramos Freitas Alexandre, Ana Carolina Ferreira Araujo, Ray Silva Almeida, Emílio Sousa Albuquerque, Cícera Datiane de Morais Oliveira-Tintino, Igor J. S. Nascimento, João Xavier Araújo-Júnior, Edeildo Ferreira da Silva-Junior, Thiago Mendonça de Aquino, and et al. 2025. "Evaluation of the Efflux Pump Inhibition Activity of Thiadiazine-Derived Compounds Against the Staphylococcus aureus 1199B Strain" Pharmaceuticals 18, no. 3: 323. https://doi.org/10.3390/ph18030323
APA StyleRoque Paulo, C. L., Alexandre, P. R. F., Araujo, A. C. F., Almeida, R. S., Albuquerque, E. S., Oliveira-Tintino, C. D. d. M., Nascimento, I. J. S., Araújo-Júnior, J. X., Silva-Junior, E. F. d., Aquino, T. M. d., Mendonça-Junior, F. J. B., Araújo-Neto, J. B. d., Silva Leandro, M. K. d. N., Menezes, I. R. A. d., Coutinho, H. D. M., & Rocha, J. E. (2025). Evaluation of the Efflux Pump Inhibition Activity of Thiadiazine-Derived Compounds Against the Staphylococcus aureus 1199B Strain. Pharmaceuticals, 18(3), 323. https://doi.org/10.3390/ph18030323










