Ilex Guayusa Tea Improves Glycaemia and Autonomic Modulation in Female Streptozotocin-Induced Diabetic Rats
Abstract
1. Introduction
2. Results
2.1. Antioxidant and Phytochemical Properties of Ilex Guayusa
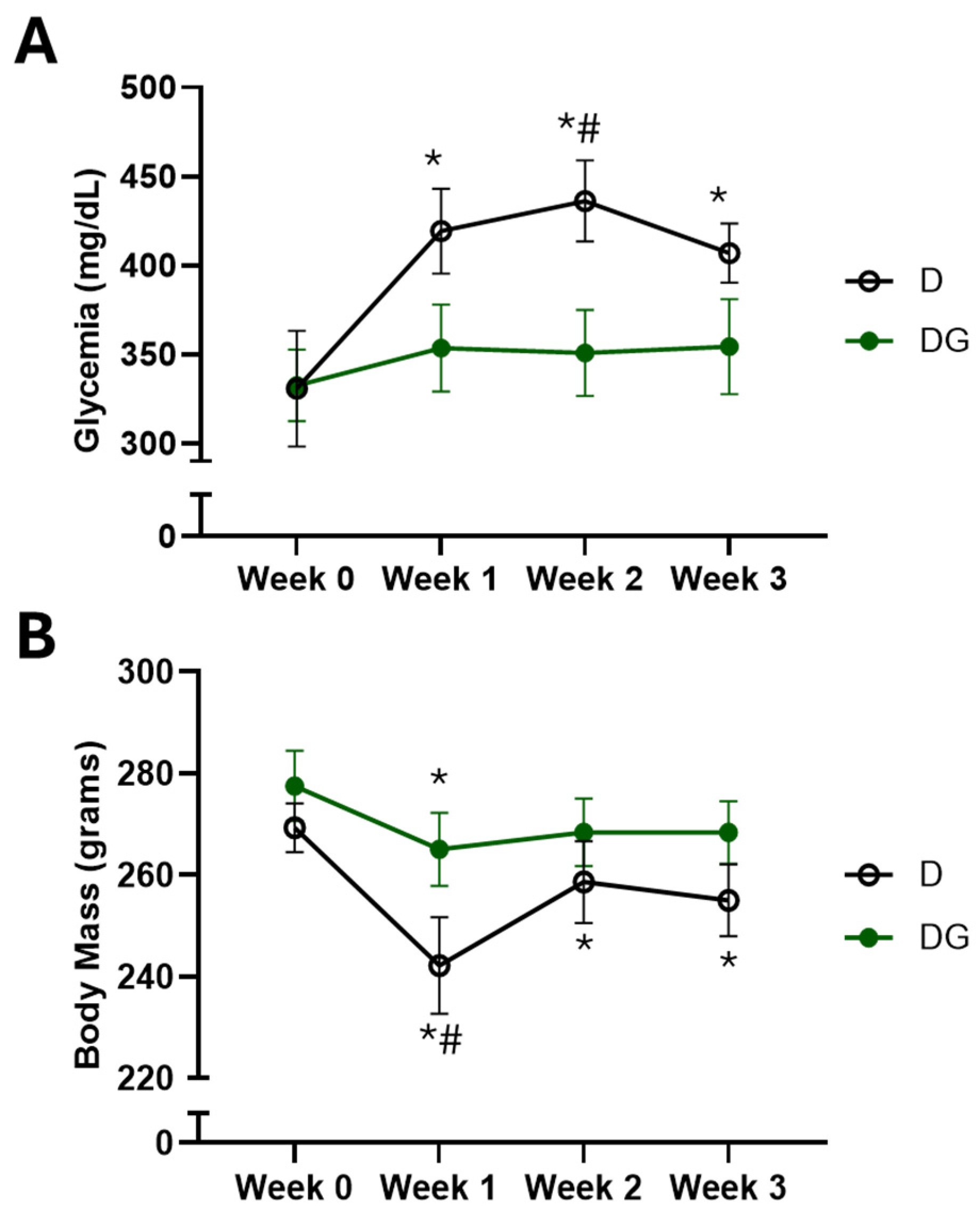
2.2. Metabolic Evaluations
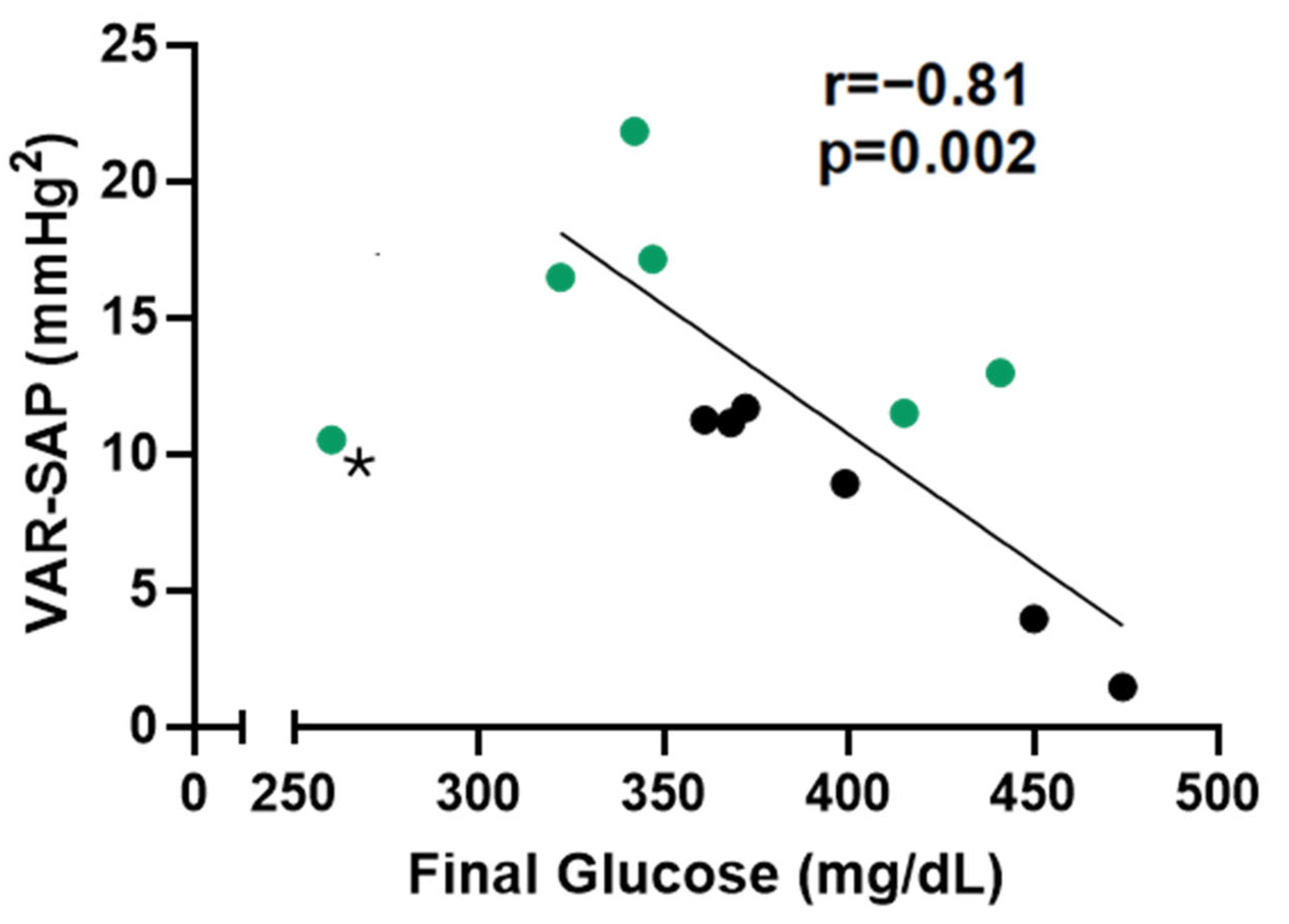
2.3. Hemodynamic and Autonomic Assessments
2.4. Brain and Cardiac Oxidative Stress of STZ-Induced Diabetic Rats Treated with Guayusa Tea
3. Discussion
4. Materials and Methods
4.1. Preparation of Aqueous Extracts of Ilex Guayusa
4.2. Determination of Total Polyphenolic Content
4.3. Determination of Total Flavonoid Content
4.4. Total Reactive Antioxidant Potential (TRAP)
4.5. Scavenging of ABTS Radical Cations
4.6. Scavenging of DPPH Radicals
4.7. Reducing Power (RP) Assay
4.8. Animal Studies
4.9. Diabetes Induction
4.10. Treatment with Ilex Guayusa
4.11. Blood Glucose and Triglyceride Concentrations
4.12. Hemodynamic Measurements
4.13. Heart Rate Variability (HRV) and Systolic Arterial Pressure Variability (SAPV)
4.14. Oxidative Stress Profile on Brain and Cardiac Tissue
4.15. NADPH Oxidase
4.16. Antioxidant Enzyme Activities
4.17. Membrane Lipoperoxidation by Thiobarbituric Acid Reactive Substances
4.18. Determination of Protein Oxidation Using Carbonyls Assay
4.19. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Matheus, A.S.; Tannus, L.R.; Cobas, R.A.; Palma, C.C.; Negrato, C.A.; Gomes, M.B. Impact of diabetes on cardiovascular disease: An update. Int. J. Hypertens. 2013, 2013, 653789. [Google Scholar] [CrossRef] [PubMed]
- Franco, O.H.; Steyerberg, E.W.; Hu, F.B.; Mackenbach, J.; Nusselder, W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch. Intern. Med. 2007, 167, 1145–1151. [Google Scholar] [CrossRef]
- Rivellese, A.A.; Riccardi, G.; Vaccaro, O. Cardiovascular risk in women with diabetes. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Deka, A.; Vita, J.A. Tea and cardiovascular disease. Pharmacol. Res. 2011, 64, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Kapp, R.W., Jr.; Mendes, O.; Roy, S.; McQuate, R.S.; Kraska, R. General and Genetic Toxicology of Guayusa Concentrate (Ilex guayusa). Int. J. Toxicol. 2016, 35, 222–242. [Google Scholar] [CrossRef] [PubMed]
- Pardau, M.D.; Pereira, A.S.P.; Apostolides, Z.; Serem, J.C.; Bester, M.J. Antioxidant and anti-inflammatory properties of Ilex guayusa tea preparations: A comparison to Camellia sinensis teas. Food Funct. 2017, 8, 4601–4610. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Ong, L.; Smith, M.T.; Ross, F.B.; Schmid, K.; Hoey, A.J.; Burstow, D.; Brown, L. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ. 2003, 12, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, P.; Evangelista, F.S.; Santos, F.; Motter Magri, F.M.; Delorenzi, J.C.; Ginoza, M.; Farah, V. The effects of green tea consumption on cardiometabolic alterations induced by experimental diabetes. Exp. Diabetes Res. 2012, 2012, 309231. [Google Scholar] [CrossRef]
- Freitas, S.C.F.; Dutra, M.R.H.; Dourado, P.M.M.; Miranda, V.H.M.; Dos Santos, C.P.; Sanches, I.C.; Irigoyen, M.C.; De Angelis, K. Insulin Treatment Does Not Prevent EARLY Autonomic Cardiovascular and Diastolic Dysfunctions in Streptozotocin-Induced Diabetic Rats. Pharmaceuticals 2024, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Sanches, I.C.; Conti, F.F.; Bernardes, N.; Brito Jde, O.; Galdini, E.G.; Cavaglieri, C.R.; Irigoyen, M.C.; De Angelis, K. Impact of combined exercise training on cardiovascular autonomic control and mortality in diabetic ovariectomized rats. J. Appl. Physiol. 2015, 119, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, A.; Baenas, N.; Benitez-Gonzalez, A.M.; Stinco, C.M.; Melendez-Martinez, A.J.; Moreno, D.A.; Ruales, J. Guayusa (Ilex guayusa L.) new tea: Phenolic and carotenoid composition and antioxidant capacity. J. Sci. Food Agric. 2017, 97, 3929–3936. [Google Scholar] [CrossRef]
- Villacis-Chiriboga, J.; Garcia-Ruiz, A.; Baenas, N.; Moreno, D.A.; Melendez-Martinez, A.J.; Stinco, C.M.; Jerves-Andrade, L.; Leon-Tamariz, F.; Ortiz-Ulloa, J.; Ruales, J. Changes in phytochemical composition, bioactivity and in vitro digestibility of guayusa leaves (Ilex guayusa Loes.) in different ripening stages. J. Sci. Food Agric. 2018, 98, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Henry-Vitrac, C.; Ibarra, A.; Roller, M.; Merillon, J.M.; Vitrac, X. Contribution of chlorogenic acids to the inhibition of human hepatic glucose-6-phosphatase activity in vitro by Svetol, a standardized decaffeinated green coffee extract. J. Agric. Food Chem. 2010, 58, 4141–4144. [Google Scholar] [CrossRef] [PubMed]
- Swanston-Flatt, S.K.; Day, C.; Flatt, P.R.; Gould, B.J.; Bailey, C.J. Glycaemic effects of traditional European plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetes Res. 1989, 10, 69–73. [Google Scholar] [PubMed]
- Huang, H.; Chen, Y.; Zeng, H.H.; Huang, X.J.; Wang, L.; Wang, Y.; Ma, Z.G.; Zhang, X.Q.; Fan, C.L.; Ye, W.C. Triterpenoids from leaves of Ilex guayusa. Zhongguo Zhong Yao Za Zhi 2021, 46, 3123–3132. [Google Scholar] [CrossRef]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.J.; Frederico, M.J.; Cazarolli, L.H.; Mendes, C.P.; Bretanha, L.C.; Schmidt, E.C.; Bouzon, Z.L.; de Medeiros Pinto, V.A.; da Fonte Ramos, C.; Pizzolatti, M.G.; et al. The mechanism of action of ursolic acid as insulin secretagogue and insulinomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance. Biochim. Biophys. Acta 2015, 1850, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Ewing, D.J.; Campbell, I.W.; Neilson, J.M.; Clarke, B.F. RR interval variations in young male diabetics. Br. Heart J. 1975, 37, 882–885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pop-Busui, R. Cardiac autonomic neuropathy in diabetes: A clinical perspective. Diabetes Care 2010, 33, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995, 5, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Musha, T. 1/f fluctuation of heartbeat period. IEEE Trans. Biomed. Eng. 1982, 29, 456–457. [Google Scholar] [CrossRef]
- Mizobuchi, A.; Osawa, K.; Tanaka, M.; Yumoto, A.; Saito, H.; Fuke, S. Detrended fluctuation analysis can detect the impairment of heart rate regulation in patients with heart failure with preserved ejection fraction. J. Cardiol. 2021, 77, 72–78. [Google Scholar] [CrossRef]
- Sartori, M.; Conti, F.F.; Dias, D.D.S.; Dos Santos, F.; Machi, J.F.; Palomino, Z.; Casarini, D.E.; Rodrigues, B.; De Angelis, K.; Irigoyen, M.C. Association between Diastolic Dysfunction with Inflammation and Oxidative Stress in Females ob/ob Mice. Front. Physiol. 2017, 8, 572. [Google Scholar] [CrossRef] [PubMed]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action 2017.13. J. AOAC Int. 2019, 102, 320–321. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Autolovich, M.; Robards, K. Phenolic Content and Antioxidant Activity of Olive Extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Lissi, E.; Pascual, C.; Del Castillo, M.D. Luminol luminescence induced by 2,2′-azo-bis(2-amidinopropane) thermolysis. Free Radic. Res. Commun. 1992, 17, 299–311. [Google Scholar] [CrossRef]
- Campos, A.M.; Lissi, E.A. Kinetics of the Reaction between 2,2-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Derived Radical Cations and Phenols. Int. J. Chem. Kinet. 1997, 29, 219–224. [Google Scholar] [CrossRef]
- Sanchez Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Lissi, E.A.; Caceres, T.; Videla, L.A. Visible chemiluminescence from rat brain homogenates undergoing autoxidation. I. Effect of additives and products accumulation. J. Free Radic. Biol. Med. 1986, 2, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Rerup, C.C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol. Rev. 1970, 22, 485–518. [Google Scholar] [CrossRef] [PubMed]
- Wichi, R.; Malfitano, C.; Rosa, K.; De Souza, S.B.; Salemi, V.; Mostarda, C.; De Angelis, K.; Irigoyen, M.C. Noninvasive and invasive evaluation of cardiac dysfunction in experimental diabetes in rodents. Cardiovasc. Diabetol. 2007, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, R.D.; de Abreu, L.C.; Adami, F.; Vanderlei, F.M.; de Carvalho, T.D.; Moreno, I.L.; Pereira, V.X.; Valenti, V.E.; Sato, M.A. Heart rate variability in stroke patients submitted to an acute bout of aerobic exercise. Transl. Stroke Res. 2013, 4, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Sandrone, G.; Mortara, A.; Torzillo, D.; La Rovere, M.T.; Signorini, M.G.; Cerutti, S.; Malliani, A. Linear and nonlinear dynamics of heart rate variability after acute myocardial infarction with normal and reduced left ventricular ejection fraction. Am. J. Cardiol. 1996, 77, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef]
- Marklund, S.L. Superoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice, nude mice and normal BALB/c mice. Mutat. Res. 1985, 148, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [CrossRef] [PubMed]

| Antioxidant Activity Assay | Infusion | Decoction | p |
|---|---|---|---|
| TRAP (μmol trolox per g dry material) | 30.24 ± 5.10 | 49.64 ± 7.29 | 0.06 |
| ABTS (μmol ascorbic acid per g dry material) | 144.27 ± 20.46 | 324.72 ± 23.97 | 0.0002 |
| DPPH (μmol ascorbic acid per g dry material) | 85.07 ± 0.88 | 183.84 ± 3.66 | >0.0001 |
| RP (μmol trolox per g dry material) | 361.17 ± 68.66 | 778.89 ± 57.86 | 0.0009 |
| Phytochemical Analysis | Infusion | Decoction | p |
|---|---|---|---|
| Total polyphenolic content (mg gallic acid per g of dry material) | 170.33 ± 12.20 | 322.96 ± 9.91 | >0.0001 |
| Total flavonoid content (mg quercetin per g of dry material) | 5.49 ± 0.39 | 11.43 ± 0.47 | >0.0001 |
| D | DG | p | |
|---|---|---|---|
| Hemodynamic | |||
| Mean Arterial Pressure (mmHg) | 103 ± 0.3 | 110 ± 3.4 | 0.33 |
| Heart Rate (bpm) | 295 ± 14 | 311 ± 9 | 0.38 |
| Linear HRV | |||
| SD (ms) | 5.48 ± 1.16 | 7.67 ± 1.11 | 0.20 |
| Variance (ms) | 36.94 ± 14.56 | 64.98 ± 20.39 | 0.29 |
| RMSSD (ms2) | 7.84 ± 1.77 | 9.30 ± 0.55 | 0.45 |
| VLF abs (ms2) | 4.92 ± 1.88 | 13.41 ± 6.97 | 0.22 |
| LF abs (ms2) | 1.98 ± 0.91 | 3.24 ± 0.70 | 0.47 |
| HF abs (ms2) | 20.34 ± 8.96 | 24.66 ± 2.81 | 0.68 |
| VLF % | 20.33 ± 6.70 | 26.83 ± 6.74 | 0.39 |
| LF % | 7.16 ± 0.74 | 7.50 ± 0.71 | 0.87 |
| HF % | 72.50 ± 6.37 | 66.00 ± 6.46 | 0.40 |
| LF (nu) | 9.33 ± 1.05 | 10.33 ± 1.05 | 0.61 |
| HF (nu) | 90.66 ± 1.05 | 89.66 ± 1.05 | 0.61 |
| LF/HF | 0.10 ± 0.01 | 0.12 ± 0.01 | 0.66 |
| Non-Linear HRV | |||
| SD1 (ms) | 5.5 ± 1.2 | 6.6 ± 0.4 | 0.45 |
| SD2 (ms) | 5.3 ± 1.1 | 8.4 ± 1.7 | 0.17 |
| alpha 1 | 0.30 ± 0.02 | 0.40 ± 0.03 | 0.01 |
| alpha 2 | 0.88 ± 0.01 | 0.98 ± 0.08 | 0.47 |
| SampEn | 2.17 ± 0.19 | 1.69 ± 0.1 | 0.05 |
| ApEn | 1.34 ± 0.07 | 1.48 ± 0.04 | 0.15 |
| Baroreflex Sensitivity | |||
| Alpha Index (ms/mmHg) | 1.12 ± 0.30 | 1.97 ± 0.55 | 0.67 |
| Heart | Brain | |||||
|---|---|---|---|---|---|---|
| D | DG | p | D | DG | p | |
| CAT (nmol/mg protein) | 2.35 ± 0.18 | 2.02 ± 0.21 | 0.52 | 0.24 ± 0.02 | 0.30 ± 0.02 | 0.09 |
| SOD (USOD/mg protein) | 14.86 ± 0.58 | 13.96 ± 0.53 | 0.30 | 10.47 ± 0.32 | 10.76 ± 0.46 | 0.61 |
| TBARS (μmoles/mg protein) | 2.33 ± 0.16 | 2.24 ± 0.18 | 0.71 | 3.16 ± 0.21 | 3.05 ± 0.16 | 0.69 |
| CARB (nmol/mg protein) | 3.71 ± 0.18 | 3.33 ± 0.12 | 0.13 | 3.20 ± 0.35 | 3.53 ± 0.29 | 0.50 |
| NADPH Oxidase (nmol/mg protein) | 0.192 ± 0.015 | 0.183 ± 0.009 | 0.63 | 0.040 ± 0.004 | 0.037 ± 0.004 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mello, T.C.; Dias, D.d.S.; Bernardes, N.; Araujo, A.A.d.; dos Santos, C.P.; Llesuy, S.; De Angelis, K.; Stoyell-Conti, F.F. Ilex Guayusa Tea Improves Glycaemia and Autonomic Modulation in Female Streptozotocin-Induced Diabetic Rats. Pharmaceuticals 2025, 18, 316. https://doi.org/10.3390/ph18030316
Mello TC, Dias DdS, Bernardes N, Araujo AAd, dos Santos CP, Llesuy S, De Angelis K, Stoyell-Conti FF. Ilex Guayusa Tea Improves Glycaemia and Autonomic Modulation in Female Streptozotocin-Induced Diabetic Rats. Pharmaceuticals. 2025; 18(3):316. https://doi.org/10.3390/ph18030316
Chicago/Turabian StyleMello, Tafne Coelho, Danielle da Silva Dias, Nathalia Bernardes, Amanda Aparecida do Araujo, Camila Paixão dos Santos, Susana Llesuy, Kátia De Angelis, and Filipe F. Stoyell-Conti. 2025. "Ilex Guayusa Tea Improves Glycaemia and Autonomic Modulation in Female Streptozotocin-Induced Diabetic Rats" Pharmaceuticals 18, no. 3: 316. https://doi.org/10.3390/ph18030316
APA StyleMello, T. C., Dias, D. d. S., Bernardes, N., Araujo, A. A. d., dos Santos, C. P., Llesuy, S., De Angelis, K., & Stoyell-Conti, F. F. (2025). Ilex Guayusa Tea Improves Glycaemia and Autonomic Modulation in Female Streptozotocin-Induced Diabetic Rats. Pharmaceuticals, 18(3), 316. https://doi.org/10.3390/ph18030316








