Effect of Curcumin Pretreatment on the Susceptibility of Cryptococcus neoformans to Photodynamic Therapy Mediated by Aluminum Phthalocyanine in Nanoemulsion
Abstract
1. Introduction
2. Results and Discussion
2.1. Curcumin MIC for C. neoformans Strains
2.2. MIC of AlPc-NE in PDT Against C. neoformans Strains
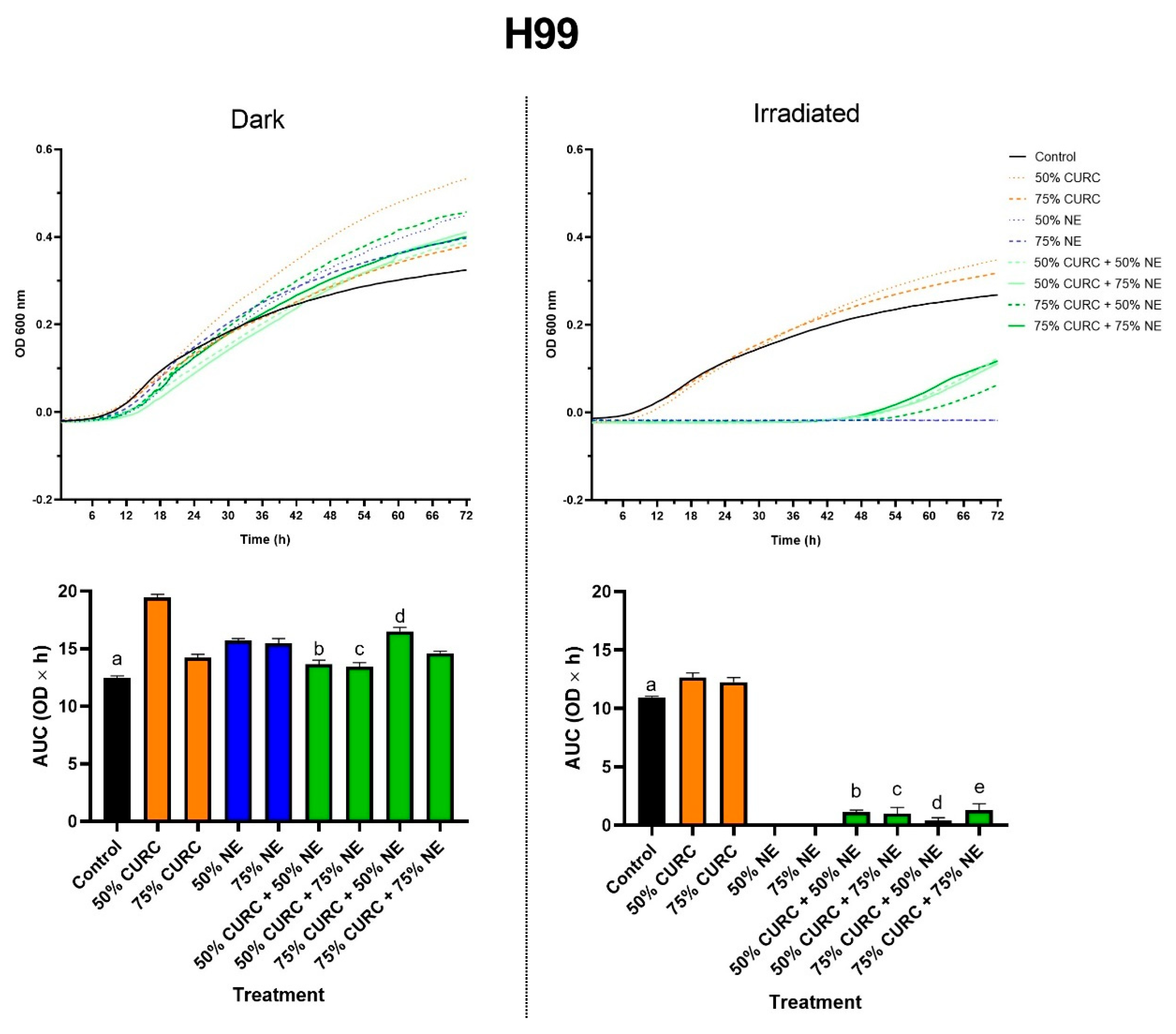
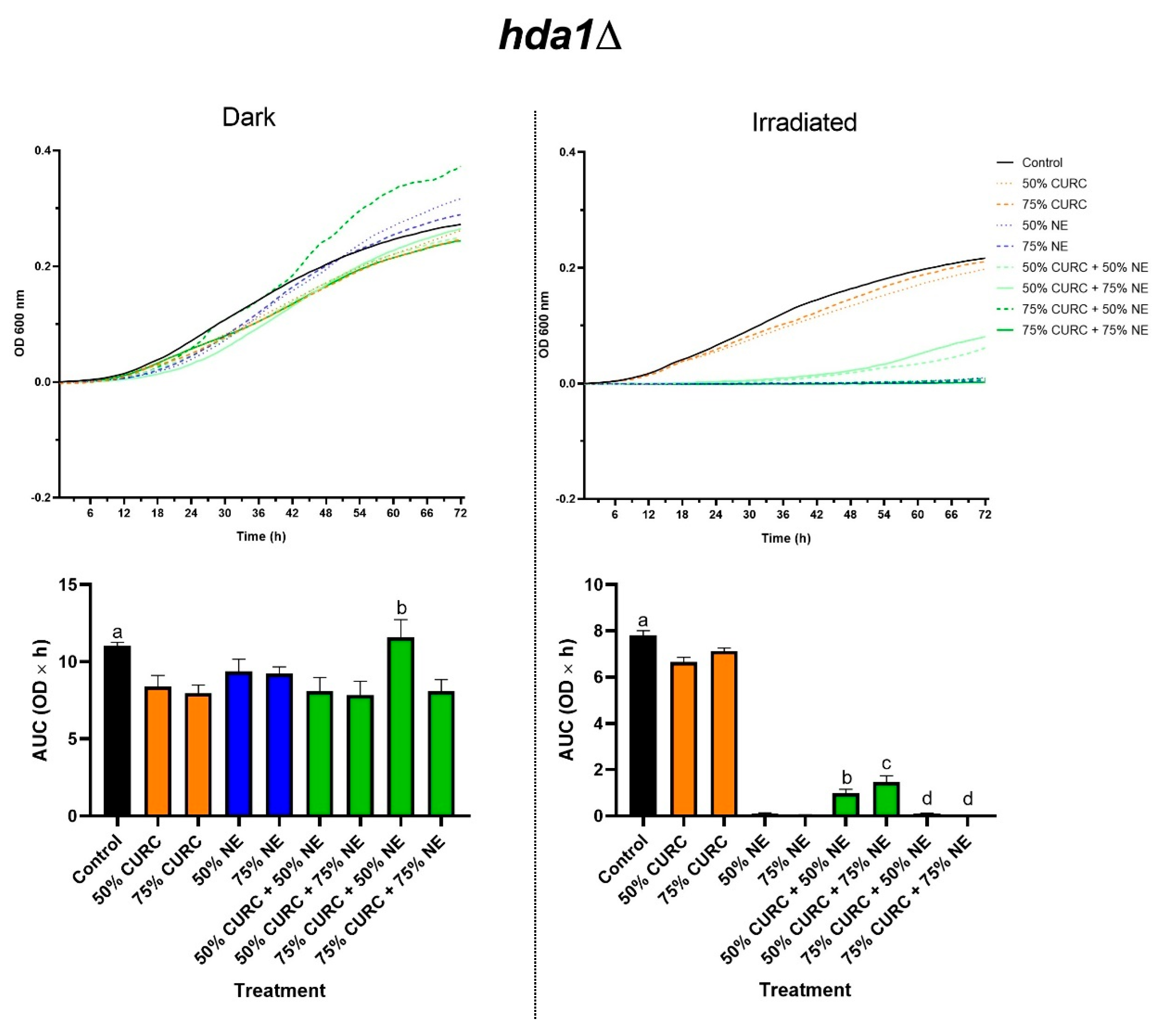
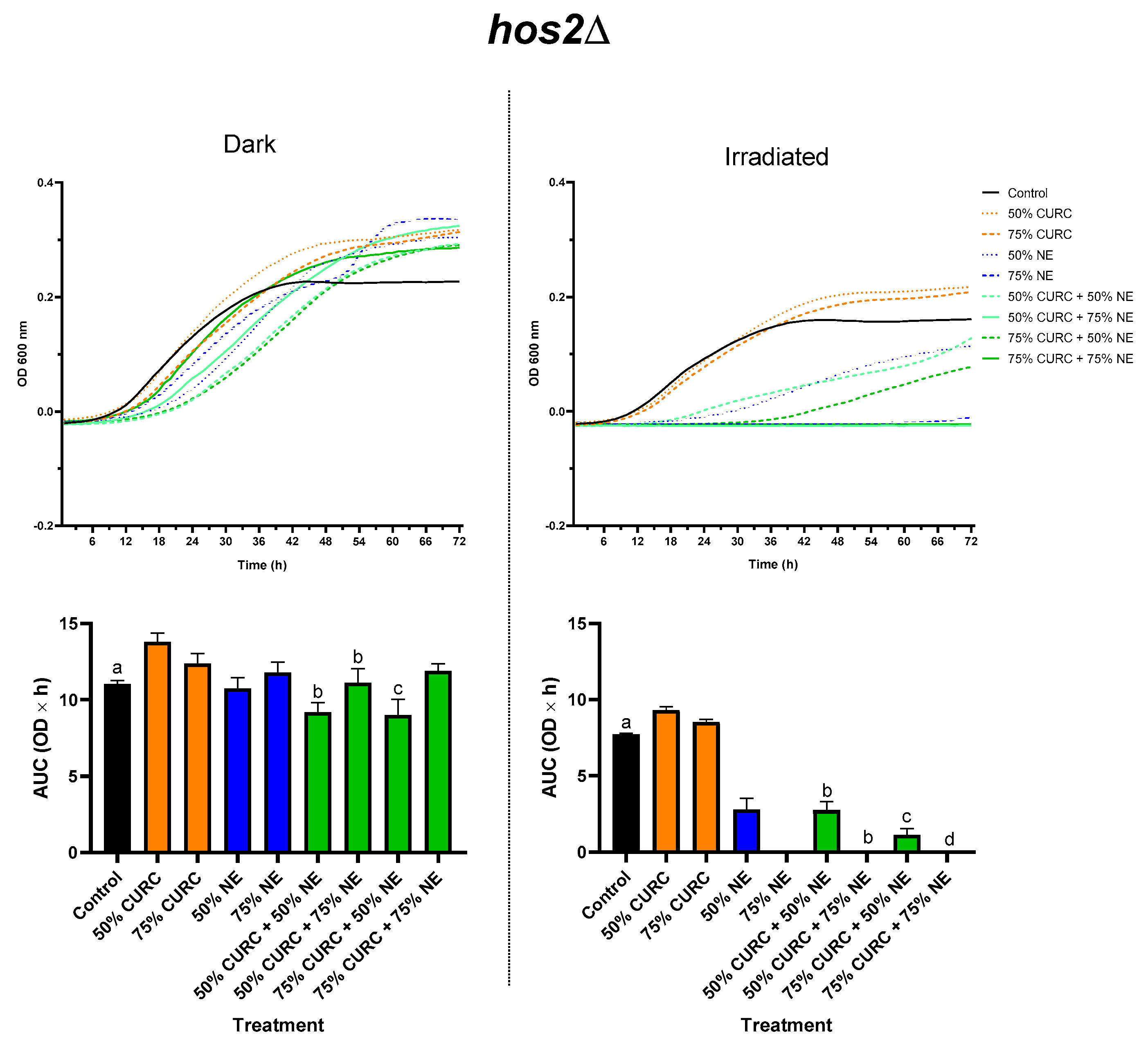
2.3. Combined Effect of Curcumin and PDT
3. Material and Methods
3.1. Reagents and Equipment
3.2. Strains and Growth Conditions
3.3. Assessment of Curcumin MIC
3.4. Assessment of AlPc-NE MIC in PDT
3.5. Combined Effect of PDT and Curcumin
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef]
- Bongomin, F.; Oladele, R.O.; Gago, S.; Moore, C.B.; Richardson, M.D. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses 2018, 61, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.A.; Rodrigues, M.C.; Ferreira, F.F.; Ranjan, K.; Azevedo, R.B.; Poças-Fonseca, M.J.; Muehlmann, L.A. Photodynamic therapy inhibits cell growth and enhances the histone deacetylase-mediated viability impairment in Cryptococcus spp. in vitro. Photodiagnosis Photodyn. Ther. 2020, 29, 101583. [Google Scholar] [CrossRef] [PubMed]
- Mello, V.C.; Araújo, V.H.S.; de Paiva, K.L.R.; Simões, M.M.; Marques, D.C.; Costa, N.R.d.S.; de Souza, I.F.; da Silva, P.B.; Santos, I.; Almeida, R.; et al. Development of New Natural Lipid-Based Nanoparticles Loaded with Aluminum-Phthalocyanine for Photodynamic Therapy against Melanoma. Nanomaterials 2022, 12, 3547. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.L.; da Silva, P.B.; Fonseca-Santos, B.; Báo, S.N.; Chorilli, M.; de Souza, P.E.N.; Muehlmann, L.A.; Azevedo, R.B. Photodynamic Therapy Directed to Melanoma Skin Cancer by Thermosensitive Hydrogel Containing Chlorophyll A. Pharmaceuticals 2023, 16, 1659. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.A.; Barros, P.H.; Brigido, M.D.; Marina, C.L.; Bocca, A.; Mariano, A.D.; Souza, P.E.; Paiva, K.L.; Simões, M.M.; Bao, S.N.; et al. Direct and Abscopal Antitumor Responses Elicited by AlPcNE-Mediated Photodynamic Therapy in a Murine Melanoma Model. Pharmaceutics 2024, 16, 1177. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, K.; Brandão, F.; Morais, J.A.V.; Muehlmann, L.A.; Silva-Pereira, I.; Bocca, A.L.; Matos, L.F.; Poças-Fonseca, M.J. The role of Cryptococcus neoformans histone deacetylase genes in the response to antifungal drugs, epigenetic modulators and to photodynamic therapy mediated by an aluminium phthalocyanine chloride nanoemulsion in vitro. J. Photochem. Photobiol. B Biol. 2021, 216, 112131. [Google Scholar] [CrossRef] [PubMed]
- Pfannenstiel, B.T.; Keller, N.P. On top of biosynthetic gene clusters: How epigenetic machinery influences secondary metabolism in fungi. Biotechnol. Adv. 2019, 37, 107345. [Google Scholar] [CrossRef] [PubMed]
- Brandão, F.; Esher, S.K.; Ost, K.S.; Pianalto, K.; Nichols, C.B.; Fernandes, L.; Bocca, A.L.; Poças-Fonseca, M.J.; Alspaugh, J.A. HDAC genes play distinct and redundant roles in Cryptococcus neoformans virulence. Sci. Rep. 2018, 8, 5209. [Google Scholar] [CrossRef] [PubMed]
- Ming, T.; Tao, Q.; Tang, S.; Zhao, H.; Yang, H.; Liu, M.; Ren, S.; Xu, H. Curcumin: An epigenetic regulator and its application in cancer. Biomed. Pharmacother. 2022, 156, 113956. [Google Scholar] [CrossRef]
- Lee, S.J.; Krauthauser, C.; Maduskuie, V.; Fawcett, P.T.; Olson, J.M.; A Rajasekaran, S. Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer 2011, 11, 144. [Google Scholar] [CrossRef]
- Bora-Tatar, G.; Dayangaç-Erden, D.; Demir, A.S.; Dalkara, S.; Yelekçi, K.; Erdem-Yurter, H. Molecular modifications on carboxylic acid derivatives as potent histone deacetylase inhibitors: Activity and docking studies. Bioorg Med. Chem. 2009, 17, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Soflaei, S.S.; Momtazi-Borojeni, A.A.; Majeed, M.; Derosa, G.; Maffioli, P.; Sahebkar, A. Curcumin: A Natural Pan-HDAC Inhibitor in Cancer. Curr. Pharm. Des. 2018, 24, 123–129. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. Curcumin: Modulator of Key Molecular Signaling Pathways in Hormone-Independent Breast Cancer. Cancers 2021, 13, 3427. [Google Scholar] [CrossRef]
- He, T.; Jin, Z.; Hu, W.; Xia, X.; Li, D.; Yao, W.; Li, G.; Zhou, X.; Song, G. Tetrahydrocurcumin (THC) enhanced the clearance of Cryptococcus deneoformans during infection in vivo. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2023, 116, 565–576. [Google Scholar] [CrossRef]
- da Silva, D.L.; Magalhães, T.F.; dos Santos, J.R.; de Paula, T.P.; Modolo, L.V.; de Fatima, A.; Buzanello Martins, C.V.; Santos, D.A.; de Resende-Stoianoff, M.A. Curcumin enhances the activity of fluconazole against Cryptococcus gattii-induced cryptococcosis infection in mice. J. Appl. Microbiol. 2016, 120, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, K.; Morais, J.A.V.; Dixit, M.; Nunes, L.C.; Rodrigues, F.P.; Muehlmann, L.A.; Shukla, P.; Poças-Fonseca, M.J. Antifungal efficacy of photodynamic therapy on Cryptococcus and Candida species is enhanced by Streptomyces spp. extracts in vitro. Lasers Med. Sci. 2024, 39, 255. [Google Scholar] [CrossRef] [PubMed]
- Sreejayan; Rao, M.N.A. Curcuminoids as Potent Inhibitors of Lipid Peroxidation. J. Pharm. Pharmacol. 1994, 46, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer. Pharmaceutics 2021, 13, 1562. [Google Scholar] [CrossRef] [PubMed]
- M27-A3 Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-Third Edition. 2008. Available online: www.clsi.org (accessed on 3 February 2025).
| Strain | MIC (μg/mL) |
|---|---|
| H99 | 21.7 ± 0.3 |
| hda1Δ | 24.7 ± 3.2 |
| hos2Δ | 19.7 ± 0.3 |
| Strain | MIC (nM) |
|---|---|
| H99 | 6.3 ± 0.3 * |
| hda1Δ | 3.0 ± 0.0 |
| hos2Δ | 3.3 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, F.C.; Nunes, L.C.; Ranjan, K.; Silveira, A.P.; Martins da Silva, I.G.; Mariano, A.d.L.e.S.; de Souza, P.E.N.; Báo, S.N.; Poças-Fonseca, M.J.; Muehlmann, L.A. Effect of Curcumin Pretreatment on the Susceptibility of Cryptococcus neoformans to Photodynamic Therapy Mediated by Aluminum Phthalocyanine in Nanoemulsion. Pharmaceuticals 2025, 18, 240. https://doi.org/10.3390/ph18020240
Costa FC, Nunes LC, Ranjan K, Silveira AP, Martins da Silva IG, Mariano AdLeS, de Souza PEN, Báo SN, Poças-Fonseca MJ, Muehlmann LA. Effect of Curcumin Pretreatment on the Susceptibility of Cryptococcus neoformans to Photodynamic Therapy Mediated by Aluminum Phthalocyanine in Nanoemulsion. Pharmaceuticals. 2025; 18(2):240. https://doi.org/10.3390/ph18020240
Chicago/Turabian StyleCosta, Fabiana Chagas, Lourival Carvalho Nunes, Kunal Ranjan, Ariane Pandolfo Silveira, Ingrid Gracielle Martins da Silva, André de Lima e Silva Mariano, Paulo Eduardo Narcizo de Souza, Sônia Nair Báo, Marcio Jose Poças-Fonseca, and Luis Alexandre Muehlmann. 2025. "Effect of Curcumin Pretreatment on the Susceptibility of Cryptococcus neoformans to Photodynamic Therapy Mediated by Aluminum Phthalocyanine in Nanoemulsion" Pharmaceuticals 18, no. 2: 240. https://doi.org/10.3390/ph18020240
APA StyleCosta, F. C., Nunes, L. C., Ranjan, K., Silveira, A. P., Martins da Silva, I. G., Mariano, A. d. L. e. S., de Souza, P. E. N., Báo, S. N., Poças-Fonseca, M. J., & Muehlmann, L. A. (2025). Effect of Curcumin Pretreatment on the Susceptibility of Cryptococcus neoformans to Photodynamic Therapy Mediated by Aluminum Phthalocyanine in Nanoemulsion. Pharmaceuticals, 18(2), 240. https://doi.org/10.3390/ph18020240









