Predictive Value of STC2 Gene Expression in Chemotherapy Response in Breast Cancer
Abstract
1. Introduction
2. Results
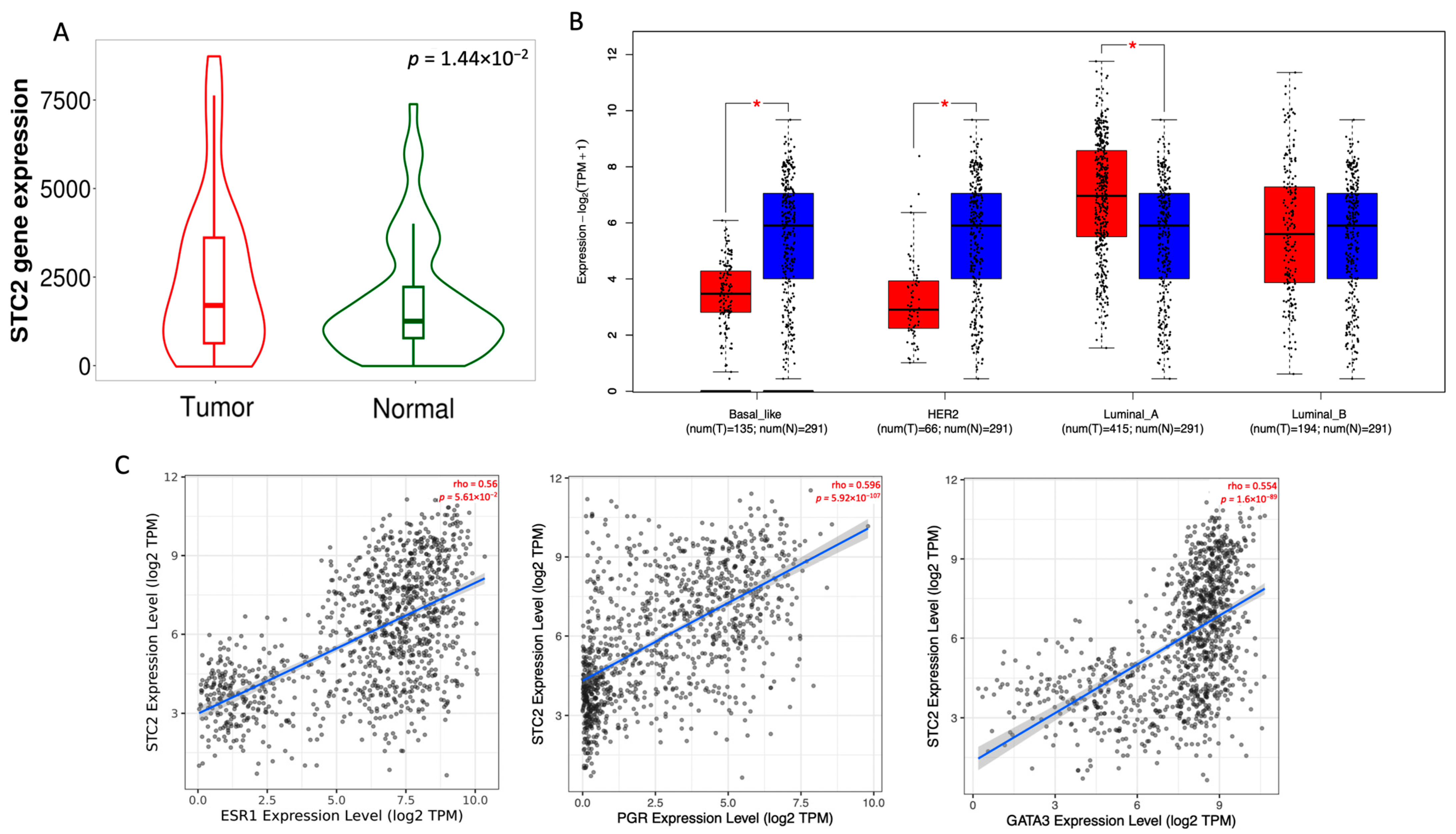
2.1. STC2 Is Overexpressed in Luminal A Breast Cancer
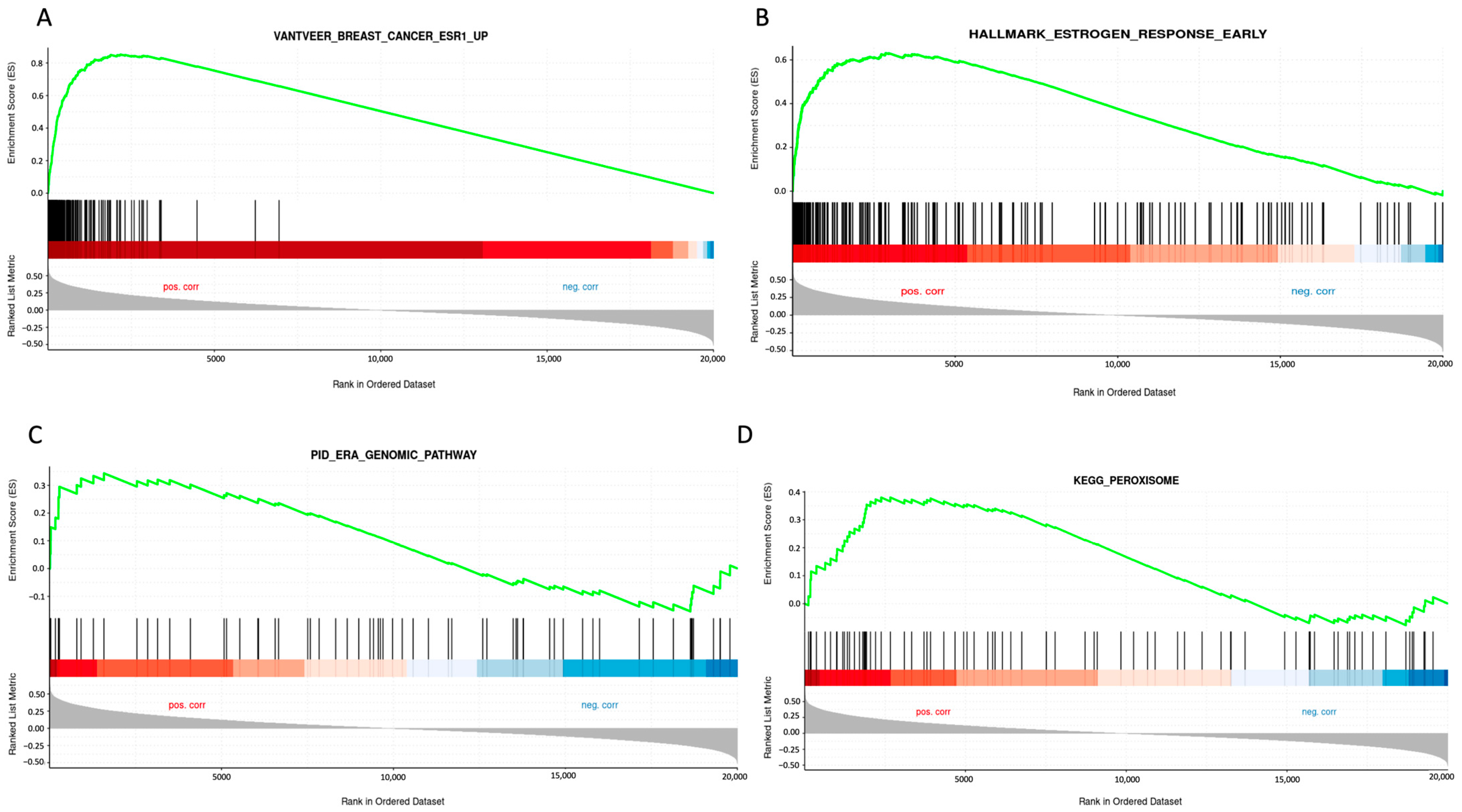
2.2. STC2 Is Associated with ER Regulation
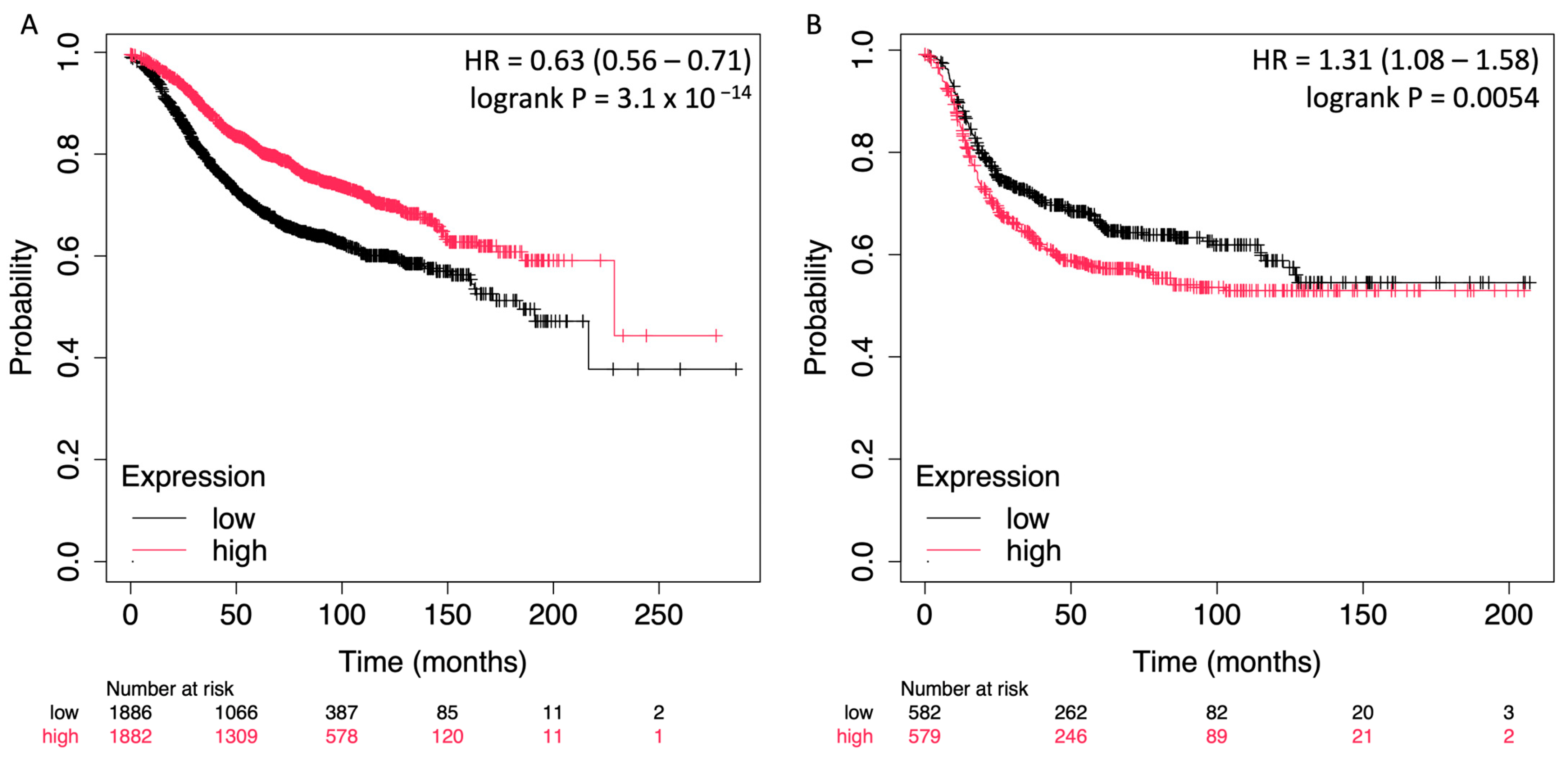
2.3. Prognostic Impact of STC2 in Breast Cancer Is Dependent on ER Status
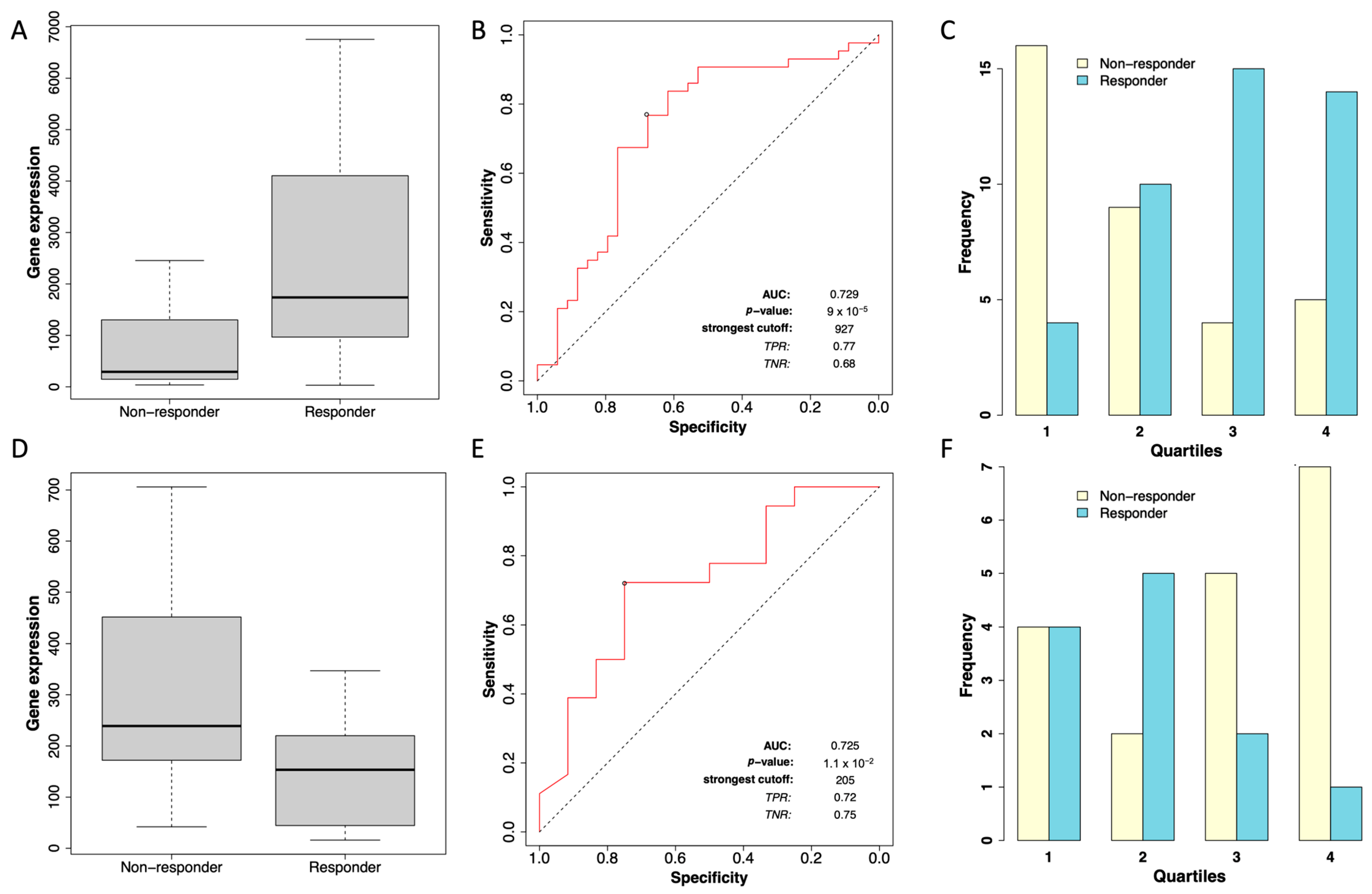
2.4. Differential STC2 Expression as a Predictor of Chemotherapy Response in ER-Positive and ER-Negative Breast Cancer Patients
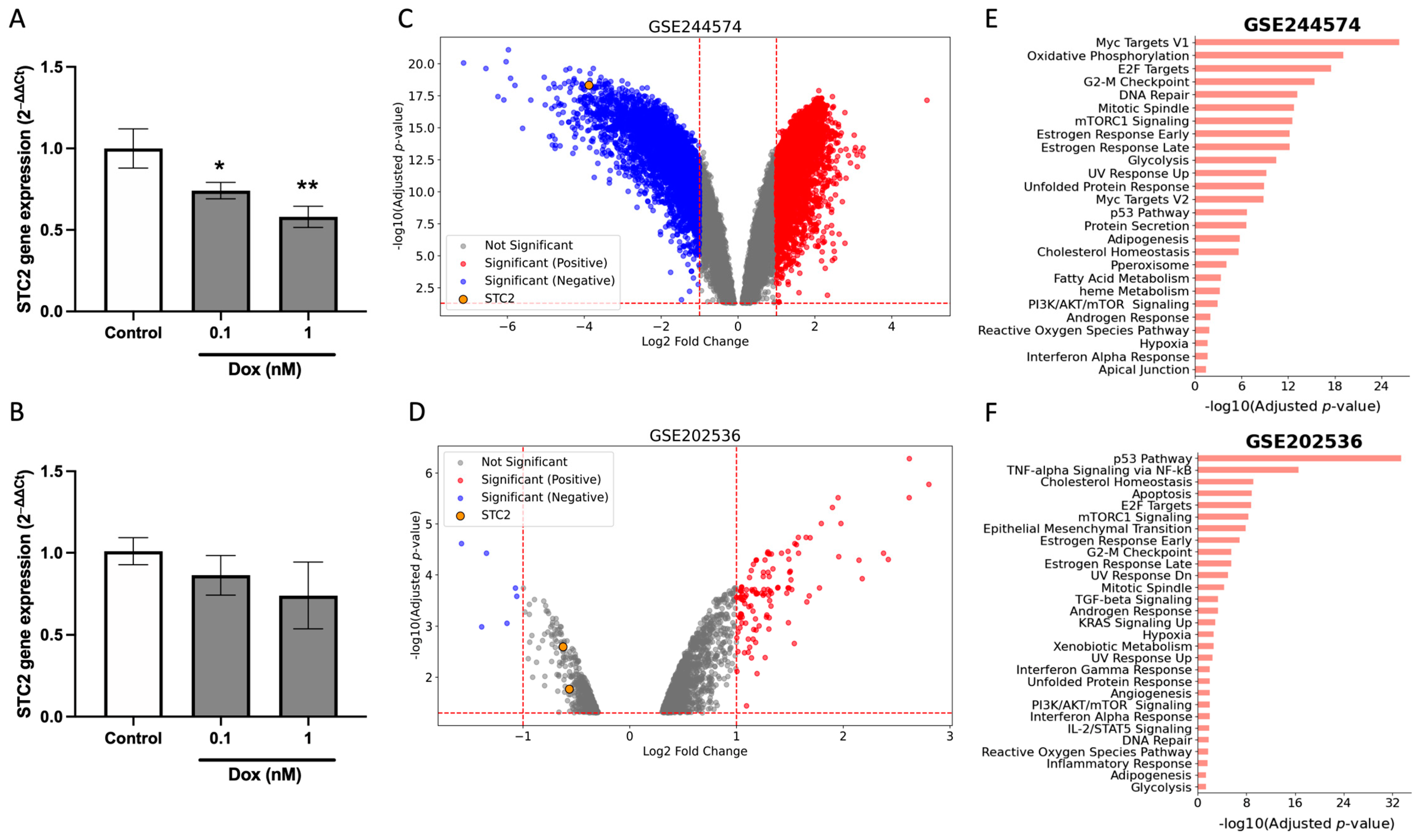
2.5. Dox Suppress STC2 Expression in Breast Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Real-Time Quantitative Reverse Transcription PCR (RT-qPCR)
4.3. Database Analysis
4.4. Datasets
4.5. Identification of DEGs
4.6. Functional Enrichment Analysis of DEGs
4.7. Survival Data Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Qie, S.; Sang, N. Stanniocalcin 2 (STC2): A universal tumour biomarker and a potential therapeutical target. J. Exp. Clin. Cancer Res. 2022, 41, 161. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.D. New Insights Into Physiological and Pathophysiological Functions of Stanniocalcin 2. Front. Endocrinol. 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, W.; Ito, D.; Swetlik, C.; Oh-hora, M.; Villereal, M.L.; Thinakaran, G. Stanniocalcin 2 is a negative modulator of store-operated calcium entry. Mol. Cell Biol. 2011, 31, 3710–3722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jiao, Y.; Song, Y.; Liu, J.; Li, X.; Zhang, H.; Yang, J.; Lu, Y. Stanniocalcin 2 Ameliorates Hepatosteatosis Through Activation of STAT3 Signaling. Front. Physiol. 2018, 9, 873. [Google Scholar] [CrossRef]
- Joshi, A.D.; Carter, D.E.; Harper, T.A., Jr.; Elferink, C.J. Aryl hydrocarbon receptor-dependent stanniocalcin 2 induction by cinnabarinic acid provides cytoprotection against endoplasmic reticulum and oxidative stress. J. Pharmacol. Exp. Ther. 2015, 353, 201–212. [Google Scholar] [CrossRef]
- McCudden, C.R.; James, K.A.; Hasilo, C.; Wagner, G.F. Characterization of mammalian stanniocalcin receptors. Mitochondrial targeting of ligand and receptor for regulation of cellular metabolism. J. Biol. Chem. 2002, 277, 45249–45258. [Google Scholar] [CrossRef]
- Ito, D.; Walker, J.R.; Thompson, C.S.; Moroz, I.; Lin, W.; Veselits, M.L.; Hakim, A.M.; Fienberg, A.A.; Thinakaran, G. Characterization of stanniocalcin 2, a novel target of the mammalian unfolded protein response with cytoprotective properties. Mol. Cell. Biol. 2004, 24, 9456–9469. [Google Scholar] [CrossRef]
- Law, A.Y.; Wong, C.K. Stanniocalcin-1 and -2 promote angiogenic sprouting in HUVECs via VEGF/VEGFR2 and angiopoietin signaling pathways. Mol. Cell. Endocrinol. 2013, 374, 73–81. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Z.; Xu, H.; Yang, L.; Yu, X.; Yang, Z.; Deng, Y.; Meng, J.; Feng, Y.; Guo, X.; et al. Stanniocalicin 2 suppresses breast cancer cell migration and invasion via the PKC/claudin-1-mediated signaling. PLoS ONE 2015, 10, e0122179. [Google Scholar] [CrossRef]
- Kita, Y.; Mimori, K.; Iwatsuki, M.; Yokobori, T.; Ieta, K.; Tanaka, F.; Ishii, H.; Okumura, H.; Natsugoe, S.; Mori, M. STC2: A predictive marker for lymph node metastasis in esophageal squamous-cell carcinoma. Ann. Surg. Oncol. 2011, 18, 261–272. [Google Scholar] [CrossRef]
- Meyer, H.A.; Tölle, A.; Jung, M.; Fritzsche, F.R.; Haendler, B.; Kristiansen, I.; Gaspert, A.; Johannsen, M.; Jung, K.; Kristiansen, G. Identification of stanniocalcin 2 as prognostic marker in renal cell carcinoma. Eur. Urol. 2009, 55, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Furihata, M.; Chung, S.Y.; Uemura, M.; Yoshioka, H.; Iiyama, T.; Ashida, S.; Nasu, Y.; Fujioka, T.; Shuin, T.; et al. Stanniocalcin 2 overexpression in castration-resistant prostate cancer and aggressive prostate cancer. Cancer Sci. 2009, 100, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Volland, S.; Kugler, W.; Schweigerer, L.; Wilting, J.; Becker, J. Stanniocalcin 2 promotes invasion and is associated with metastatic stages in neuroblastoma. Int. J. Cancer 2009, 125, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, S.; Ma, X.; Yang, Q.; Su, F.; Shu, X.; Xie, W.; Feng, M.; Xiong, B. Upregulation of STC2 in colorectal cancer and its clinicopathological significance. OncoTargets Ther. 2019, 12, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Bouras, T.; Southey, M.C.; Chang, A.C.; Reddel, R.R.; Willhite, D.; Glynne, R.; Henderson, M.A.; Armes, J.E.; Venter, D.J. Stanniocalcin 2 is an estrogen-responsive gene coexpressed with the estrogen receptor in human breast cancer. Cancer Res. 2002, 62, 1289–1295. [Google Scholar]
- Makhlouf, S.; Althobiti, M.; Toss, M.; Muftah, A.A.; Mongan, N.P.; Lee, A.H.S.; Green, A.R.; Rakha, E.A. The Clinical and Biological Significance of Estrogen Receptor-Low Positive Breast Cancer. Mod. Pathol. 2023, 36, 100284. [Google Scholar] [CrossRef]
- Yamamura, J.; Miyoshi, Y.; Tamaki, Y.; Taguchi, T.; Iwao, K.; Monden, M.; Kato, K.; Noguchi, S. mRNA expression level of estrogen-inducible gene, alpha 1-antichymotrypsin, is a predictor of early tumor recurrence in patients with invasive breast cancers. Cancer Sci. 2004, 95, 887–892. [Google Scholar] [CrossRef]
- Lei, R.; Yao, C.; Huang, R.; Wu, W.; Ou, L.; Yang, C. STC2 suppresses triple-negative breast cancer migration and invasion by inhibition on EMT and promotion on cell apoptosis. Discov. Oncol. 2024, 15, 339. [Google Scholar] [CrossRef]
- Luque-Bolivar, A.; Pérez-Mora, E.; Villegas, V.E.; Rondón-Lagos, M. Resistance and Overcoming Resistance in Breast Cancer. Breast Cancer Targets Ther. 2020, 12, 211–229. [Google Scholar] [CrossRef]
- Zhu, H.; Sarkar, S.; Scott, L.; Danelisen, I.; Trush, M.A.; Jia, Z.; Li, Y.R. Doxorubicin Redox Biology: Redox Cycling, Topoisomerase Inhibition, and Oxidative Stress. React. Oxyg. Species 2016, 1, 189–198. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Paskeh, M.D.A.; Saebfar, H.; Mahabady, M.K.; Orouei, S.; Hushmandi, K.; Entezari, M.; Hashemi, M.; Aref, A.R.; Hamblin, M.R.; Ang, H.L.; et al. Overcoming doxorubicin resistance in cancer: siRNA-loaded nanoarchitectures for cancer gene therapy. Life Sci. 2022, 298, 120463. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Cui, J.; Wu, C. Breast cancer: Molecular mechanisms of underlying resistance and therapeutic approaches. Am. J. Cancer Res. 2022, 12, 2920–2949. [Google Scholar] [PubMed]
- Yu, Y.; Yu, J.; Pan, Z. Endoplasmic reticulum stress-related features predict the prognosis of osteosarcoma and reveal STC2 as a novel risk indicator for disease progression. Front. Oncol. 2024, 14, 1453173. [Google Scholar] [CrossRef] [PubMed]
- Qie, S.; Xiong, H.; Liu, Y.; Yan, C.; Wang, Y.; Tian, L.; Wang, C.; Sang, N. Stanniocalcin 2 governs cancer cell adaptation to nutrient insufficiency through alleviation of oxidative stress. Cell Death Dis. 2024, 15, 567. [Google Scholar] [CrossRef]
- Tiwari, A.; Trivedi, R.; Lin, S.-Y. Tumor microenvironment: Barrier or opportunity towards effective cancer therapy. J. Biomed. Sci. 2022, 29, 83. [Google Scholar] [CrossRef]
- Győrffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4101–4109. [Google Scholar] [CrossRef]
- Fekete, J.T.; Győrffy, B. ROCplot.org: Validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3,104 breast cancer patients. Int. J. Cancer 2019, 145, 3140–3151. [Google Scholar] [CrossRef]
- Naso, F.D.; Bruqi, K.; Manzini, V.; Chiurchiù, V.; D’Onofrio, M.; Arisi, I.; Strappazzon, F. miR-218-5p and doxorubicin combination enhances anticancer activity in breast cancer cells through Parkin-dependent mitophagy inhibition. Cell Death Discov. 2024, 10, 149. [Google Scholar] [CrossRef]
- Kumar, U.; Castellanos-Uribe, M.; May, S.T.; Yagüe, E. Adaptive resistance is not responsible for long-term drug resistance in a cellular model of triple negative breast cancer. Gene 2023, 850, 146930. [Google Scholar] [CrossRef]
- Derouane, F.; Desgres, M.; Moroni, C.; Ambroise, J.; Berlière, M.; Van Bockstal, M.R.; Galant, C.; van Marcke, C.; Vara-Messler, M.; Hutten, S.J.; et al. Metabolic adaptation towards glycolysis supports resistance to neoadjuvant chemotherapy in early triple negative breast cancers. Breast Cancer Res. 2024, 26, 29. [Google Scholar] [CrossRef]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- He, H.; Qie, S.; Guo, Q.; Chen, S.; Zou, C.; Lu, T.; Su, Y.; Zong, J.; Xu, H.; He, D.; et al. Stanniocalcin 2 (STC2) expression promotes post-radiation survival, migration and invasion of nasopharyngeal carcinoma cells. Cancer Manag. Res. 2019, 11, 6411–6424. [Google Scholar] [CrossRef]
- Esseghir, S.; Kennedy, A.; Seedhar, P.; Nerurkar, A.; Poulsom, R.; Reis-Filho, J.S.; Isacke, C.M. Identification of NTN4, TRA1, and STC2 as prognostic markers in breast cancer in a screen for signal sequence encoding proteins. Clin. Cancer Res. 2007, 13, 3164–3173. [Google Scholar] [CrossRef]
- Yun, C.W.; Kim, H.J.; Lim, J.H.; Lee, S.H. Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy. Cells 2020, 9, 60. [Google Scholar] [CrossRef]
- MOREL, Y.; BAROUKI, R. Repression of gene expression by oxidative stress. Biochem. J. 1999, 342, 481–496. [Google Scholar] [CrossRef]
- Bhargavan, B.; Chhunchha, B.; Kubo, E.; Singh, D.P. DNA methylation as an epigenetic mechanism in the regulation of LEDGF expression and biological response in aging and oxidative stress. Cell Death Discov. 2024, 10, 296. [Google Scholar] [CrossRef]
- Posadino, A.M.; Phu, H.T.; Cossu, A.; Giordo, R.; Fois, M.; Thuan, D.T.B.; Piga, A.; Sotgia, S.; Zinellu, A.; Carru, C.; et al. Oxidative stress-induced Akt downregulation mediates green tea toxicity towards prostate cancer cells. Toxicol. In Vitro 2017, 42, 255–262. [Google Scholar] [CrossRef]
- Kim, P.H.; Na, S.S.; Lee, B.; Kim, J.H.; Cho, J.Y. Stanniocalcin 2 enhances mesenchymal stem cell survival by suppressing oxidative stress. BMB Rep. 2015, 48, 702–707. [Google Scholar] [CrossRef]
- Bartlett, J.J.; Trivedi, P.C.; Yeung, P.; Kienesberger, P.C.; Pulinilkunnil, T. Doxorubicin impairs cardiomyocyte viability by suppressing transcription factor EB expression and disrupting autophagy. Biochem. J. 2016, 473, 3769–3789. [Google Scholar] [CrossRef]
- AbuHammad, S.; Zihlif, M. Gene expression alterations in doxorubicin resistant MCF7 breast cancer cell line. Genomics 2013, 101, 213–220. [Google Scholar] [CrossRef]
- Liang, X.; Lu, B.; Scott, G.K.; Chang, C.H.; Baldwin, M.A.; Benz, C.C. Oxidant stress impaired DNA-binding of estrogen receptor from human breast cancer. Mol. Cell. Endocrinol. 1998, 146, 151–161. [Google Scholar] [CrossRef]
- Jiang, S.-t.; Wang, H.-q.; Yang, T.-c.; Wang, D.-w.; Yang, L.-j.; Xi, Y.-q.; Kong, F.-z.; Pan, X.-k.; Xu, L.-h.; Feng, M.-h.; et al. Expression of Stanniocalcin 2 in Breast Cancer and Its Clinical Significance. Curr. Med. Sci. 2019, 39, 978–983. [Google Scholar] [CrossRef]
- Niu, X.; Zhan, Y.; Zhang, S.; Liu, Z.; Qu, C. Research progress of STC2 in breast cancer. Biophys. Rep. 2021, 7, 185–192. [Google Scholar]
- Huang, F.; Li, H.; Qin, Z.; Wang, A.; Zhang, Y.; Guo, J.; Wei, M.; Guo, H.; Pu, J. SNHG17 Serves as an Oncogenic lncRNA by Regulating the miR-361-3p/STC2 Axis in Rectal Cancer. Front. Genet. 2021, 12, 654686. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef]
- Li, B.; Severson, E.; Pignon, J.C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef]
- Hayashi, A.; Ruppo, S.; Heilbrun, E.E.; Mazzoni, C.; Adar, S.; Yassour, M.; Rmaileh, A.A.; Shaul, Y.D. GENI: A web server to identify gene set enrichments in tumor samples. Comput. Struct. Biotechnol. J. 2023, 21, 5531–5537. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, J.P.; Lampe-Huenul, N. Predictive Value of STC2 Gene Expression in Chemotherapy Response in Breast Cancer. Pharmaceuticals 2025, 18, 235. https://doi.org/10.3390/ph18020235
Muñoz JP, Lampe-Huenul N. Predictive Value of STC2 Gene Expression in Chemotherapy Response in Breast Cancer. Pharmaceuticals. 2025; 18(2):235. https://doi.org/10.3390/ph18020235
Chicago/Turabian StyleMuñoz, Juan P., and Nicolás Lampe-Huenul. 2025. "Predictive Value of STC2 Gene Expression in Chemotherapy Response in Breast Cancer" Pharmaceuticals 18, no. 2: 235. https://doi.org/10.3390/ph18020235
APA StyleMuñoz, J. P., & Lampe-Huenul, N. (2025). Predictive Value of STC2 Gene Expression in Chemotherapy Response in Breast Cancer. Pharmaceuticals, 18(2), 235. https://doi.org/10.3390/ph18020235





