Acetoacetate Ameliorates Hepatic Fibrosis by Targeting Peroxisome Proliferator-Activated Receptor Gamma to Restore Lipid Droplets in Activated Hepatic Stellate Cells
Abstract
1. Introduction
2. Results
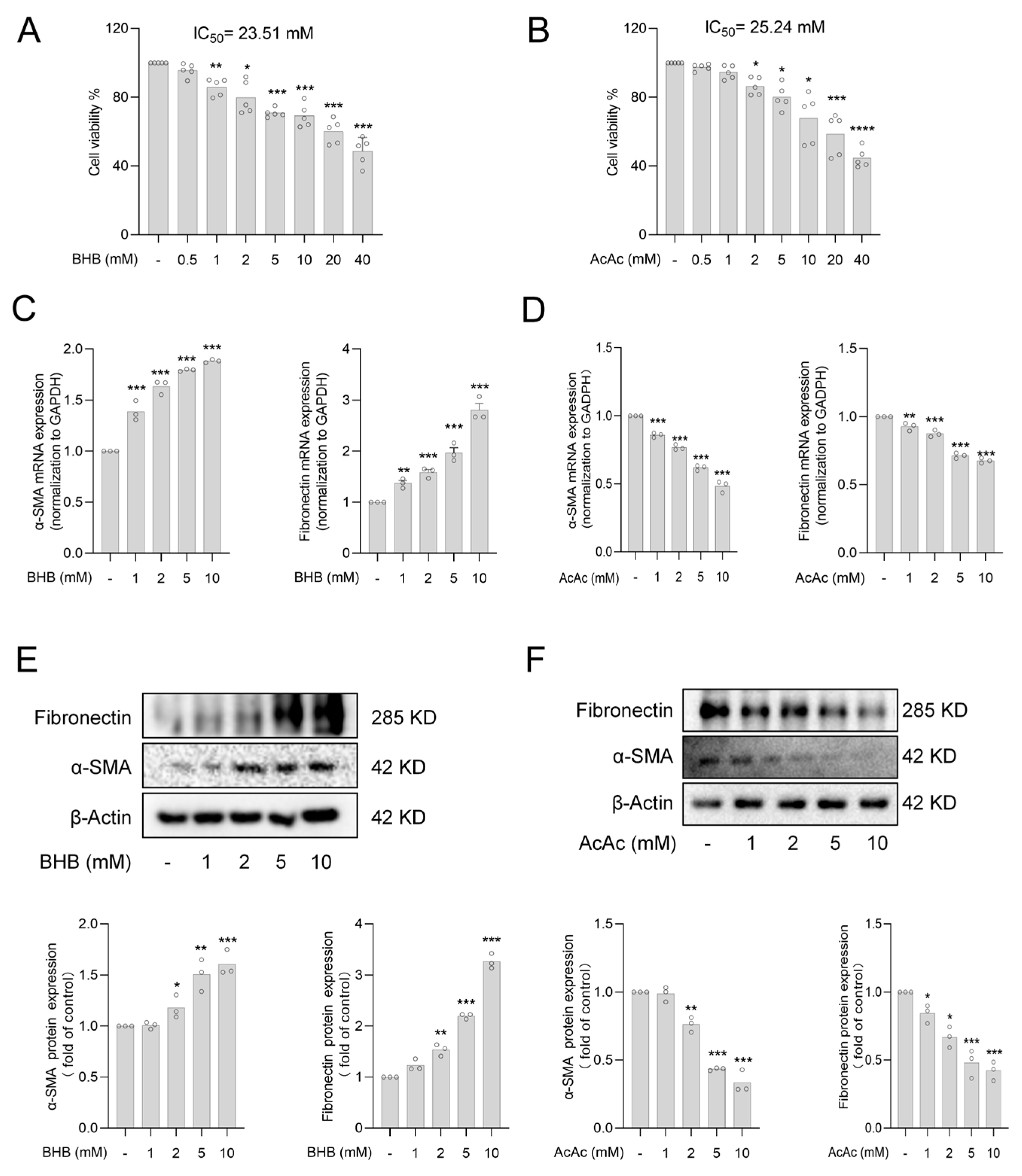
2.1. The Ketone Body Acetoacetate (AcAc) but Not Beta-Hydroxybutyrate (BHB) Inhibits Hepatic Stellate Cells (HSCs) Activation
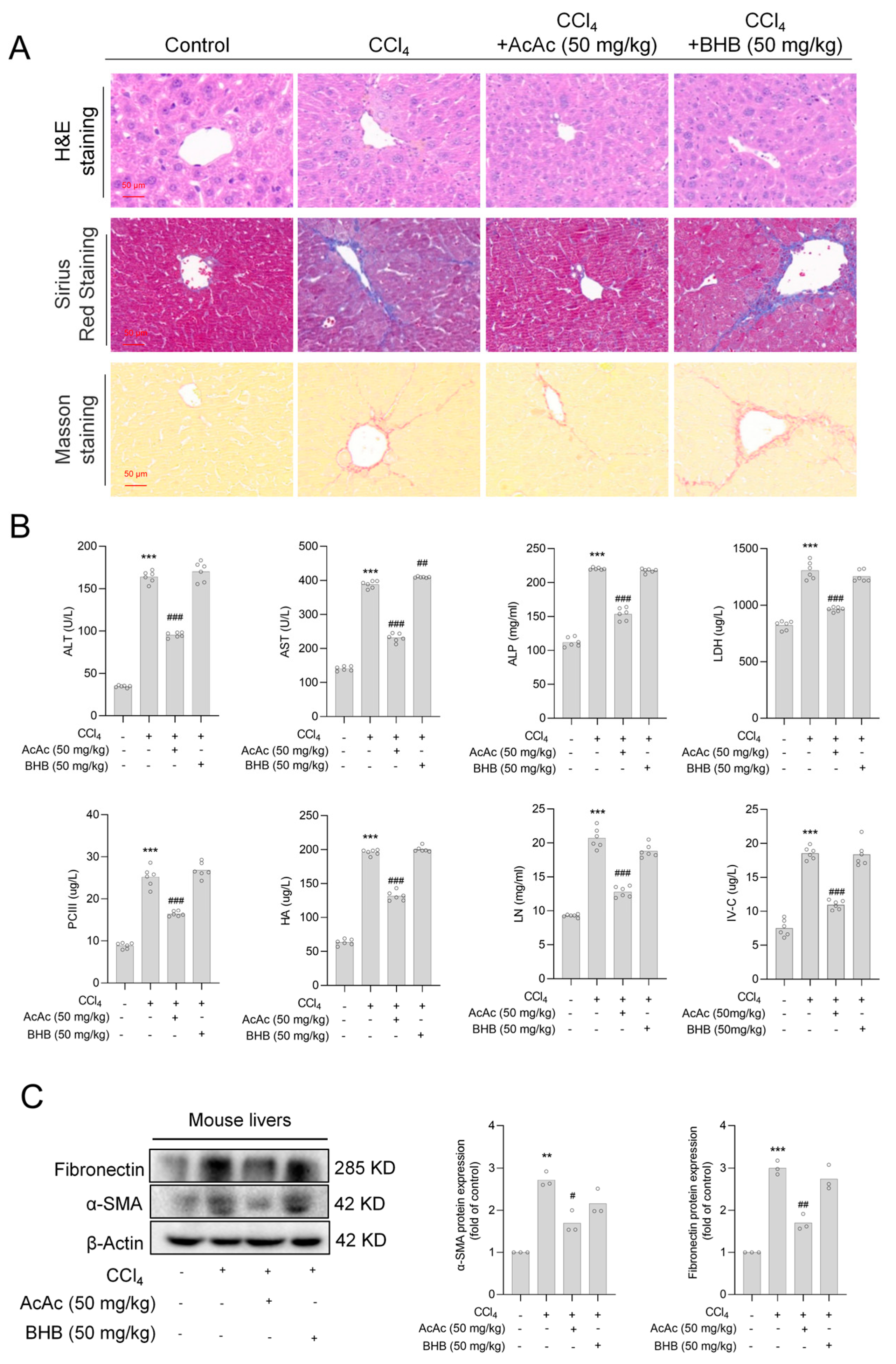
2.2. Acetoacetate (AcAc) Alleviated Hepatic Fibrosis (HF)
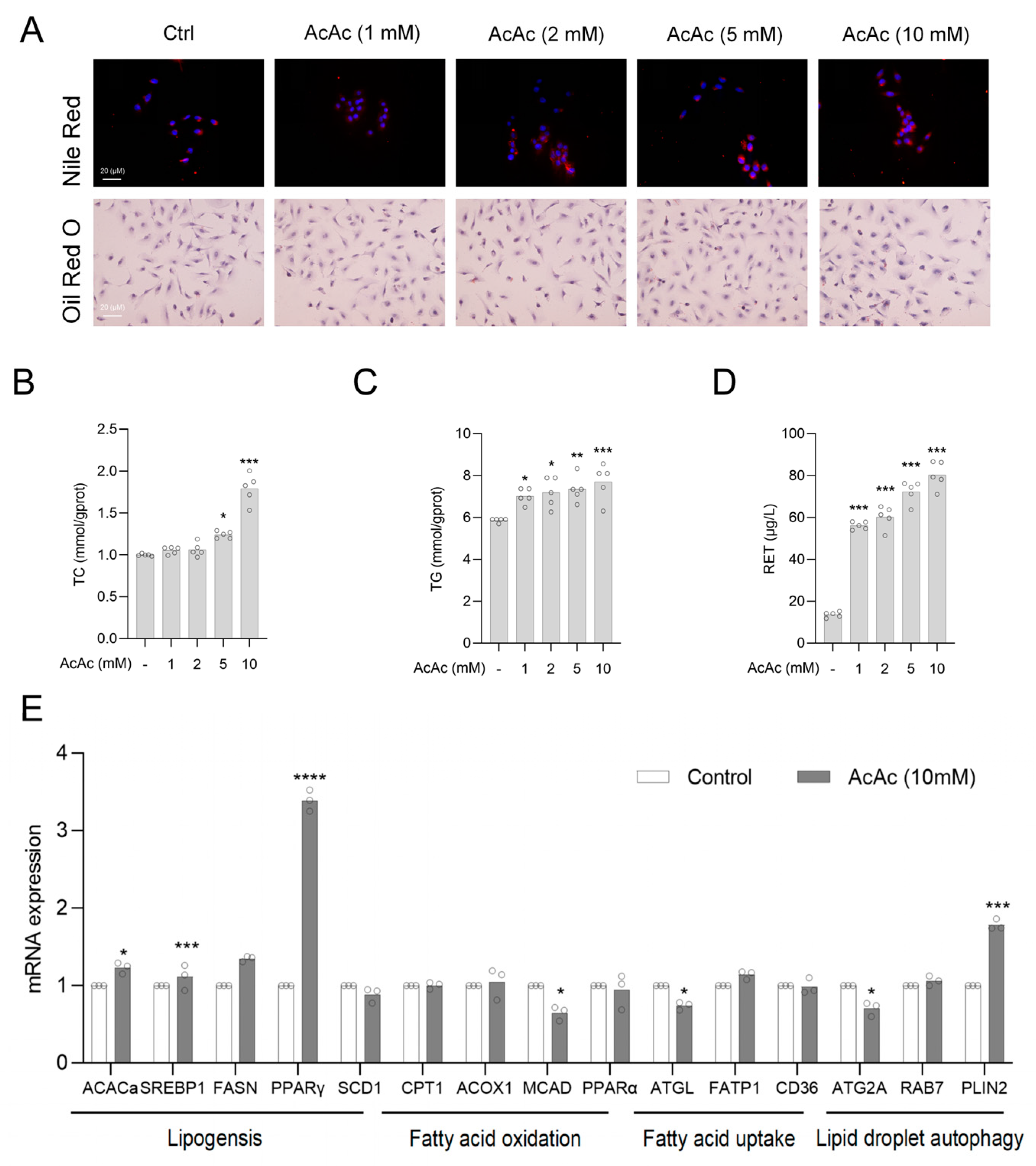
2.3. Acetoacetate (AcAc) Increased the Content of Lipid Droplets in Hepatic Stellate Cells (HSCs)
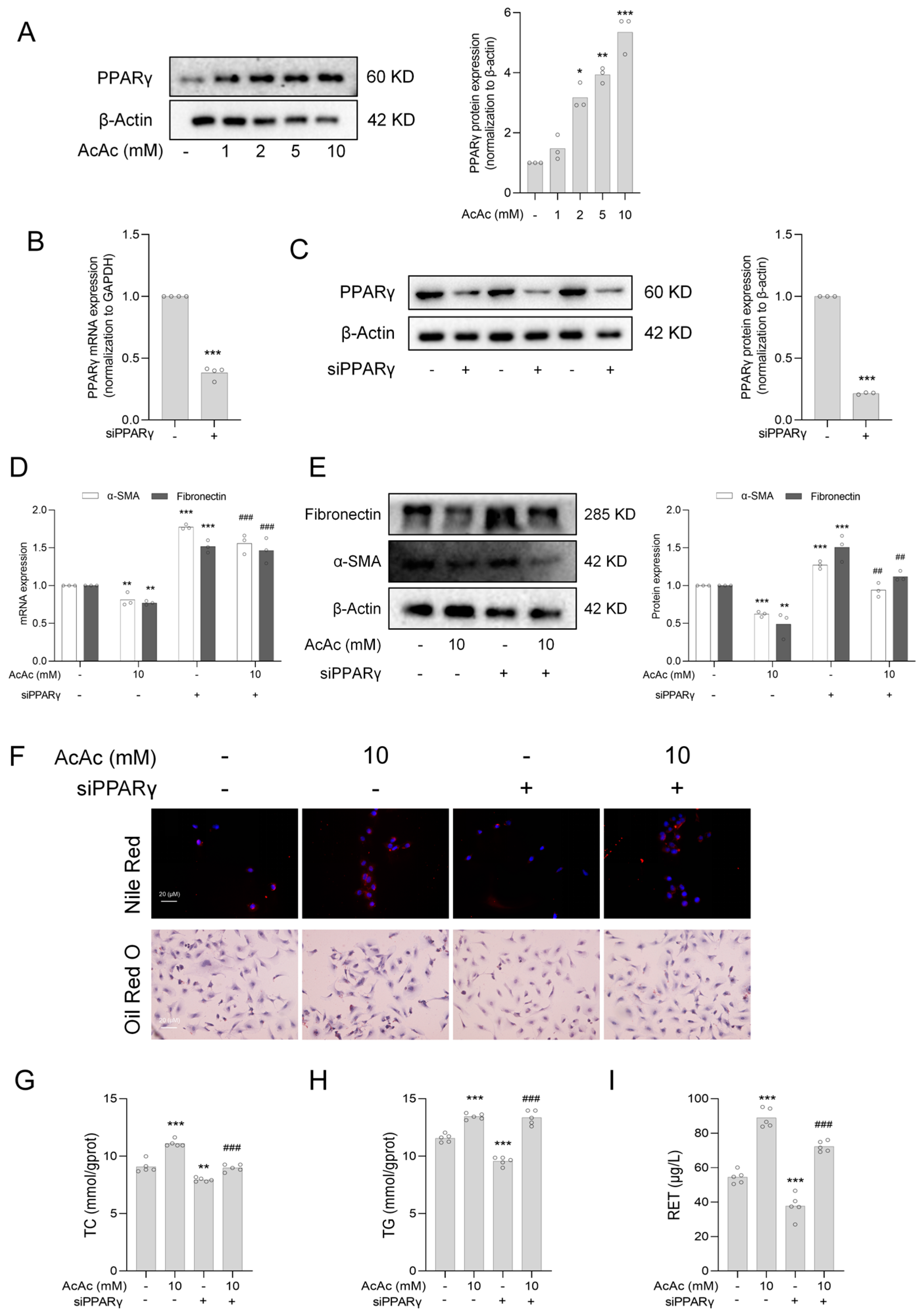
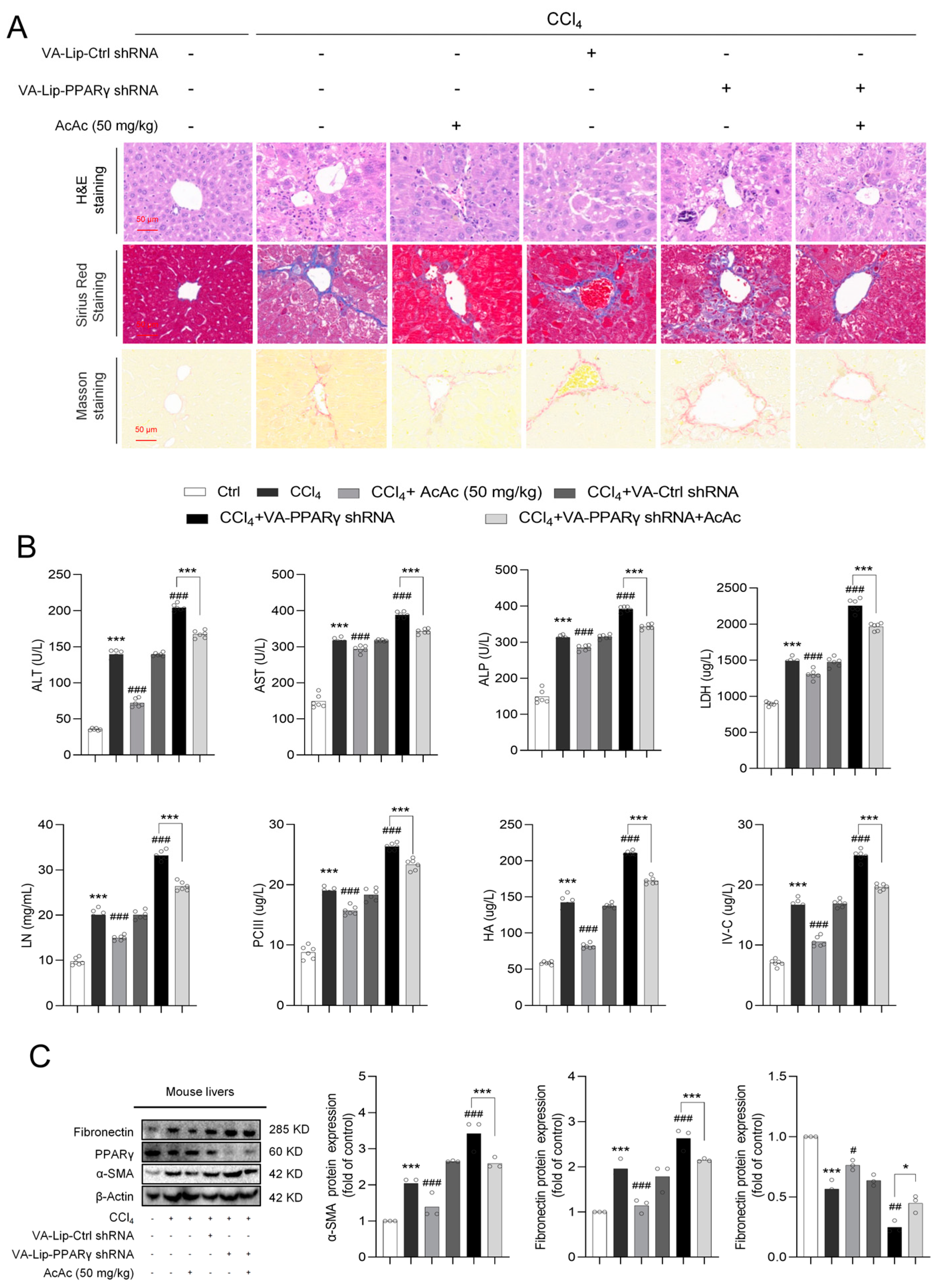
2.4. Interference of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) Reversed the Inhibition of Hepatic Stellate Cells (HSCs) Activation by Acetoacetate (AcAc)
2.5. Interference of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) Reversed the Anti-Fibrotic Effect of Acetoacetate (AcAc)
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Animal Experiments
4.3. Cell Culture and Transfection
4.4. Quantitative Real-Time PCR (RT-qPCR)
4.5. Western Blotting
4.6. Oil Red O Staining and Nile Red Staining
4.7. Cell Viability Assay
4.8. Biochemical Analysis and Enzyme Linked Immunosorbent Assay
4.9. Histological Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HF | Hepatic fibrosis |
| HSCs | Hepatic stellate cells |
| ECM | Extracellular matrix |
| α-SMA | α-smooth muscle actin |
| KD | Ketone body |
| AcAc | Acetoacetate |
| BHB | beta-hydroxybutyric acid |
| LDs | lipid droplets |
| TG | Triglyceride |
| TC | Total Cholesterol |
| RET | Retinol |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| OXCT1 | 3-oxoacid CoA-transferase 1 |
| ACACa | Acetyl-CoA Acetyltransferase 1 |
| FASN | Fatty Acid Synthase |
| SREBP1 | Sterol Regulatory Element-Binding Protein 1 |
| CPT1 | Carnitine Palmitoyltransferase 1 |
| ACOX1 | Acyl-CoA Oxidase 1 |
| MCAD | Medium-Chain Acyl-CoA Dehydrogenase |
| FATP1 | Fatty Acid Transport Protein 1 |
| CD36 | Cluster of Differentiation 36 |
| PLIN2 | Perilipin 2 |
| RAB7 | RAB7—Ras-Related Protein Rab-7 |
| ATG2A | Autophagy-Related Protein 2A |
| CMA | Chaperone mediated autophagy |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| LDH | Lactate dehydrogenase |
| LN | Laminin |
| HA | Hyaluronic acid |
| PC-III | Procollagen III |
| IV-C | Collagen type IV |
References
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of hepatic fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Hammerich, L.; Tacke, F. Hepatic inflammatory responses in hepatic fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Pinzani, M. hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology 2022, 75, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cells activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Cai, X.; Wang, J.; Wang, J.; Zhou, Q.; Yang, B.; He, Q.; Weng, Q. Intercellular crosstalk of hepatic stellate cells in hepatic fibrosis: New insights into therapy. Pharmacol. Res. 2020, 155, 104720. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- Marron, G.; Shah, V.H.; Gracia-Sancho, J. Sinusoidal communication in hepatic fibrosis and regeneration. J. Hepatol. 2016, 65, 608–617. [Google Scholar] [CrossRef]
- Scorletti, E.; Carr, R.M. A new perspective on NAFLD: Focusing on lipid droplets. J. Hepatol. 2022, 76, 934–945. [Google Scholar] [CrossRef]
- Hernández-Gea, V.; Friedman, S.L. Autophagy fuels tissue fibrogenesis. Autophagy 2012, 8, 849–850. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, S.; Yao, Z.; Wang, L.; Shao, J.; Chen, A.; Zhang, F.; Zheng, S. Autophagy regulates turnover of lipid droplets via ROS-dependent Rab25 activation in hepatic stellate cells. Redox Biol. 2017, 11, 322–334. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.; Macwan, V.; Dixon, C.L.; Li, X.; Liu, S.; Yu, Y.; Xu, P.; Sun, Q.; Hu, Q.; et al. S-acylation of ATGL is required for lipid droplet homoeostasis in hepatocytes. Nat. Metab. 2024, 6, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hong, B.; Bian, M.; Jin, H.; Chen, J.; Shao, J.; Zhang, F.; Zheng, S. Docosahexaenoic acid inhibits hepatic stellate cells activation to attenuate hepatic fibrosis in a PPARγ-dependent manner. Int. Immunopharmacol. 2019, 75, 105816. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, S.; Fujimori, K. Promotion of lipogenesis by PPARγ-activated FXR expression in adipocytes. Biochem. Biophys. Res. Commun. 2020, 527, 49–55. [Google Scholar] [CrossRef]
- Ding, H.R.; Wang, J.L.; Ren, H.Z.; Shi, X.L. Lipometabolism and Glycometabolism in Liver Diseases. Biomed. Res. Int. 2018, 2018, 1287127. [Google Scholar] [CrossRef]
- Moya-Garzon, M.D.; Wang, M.; Li, V.L.; Lyu, X.; Wei, W.; Tung, A.S.-H.; Raun, S.H.; Zhao, M.; Coassolo, L.; Islam, H.; et al. A β-hydroxybutyrate shunt pathway generates anti-obesity ketone metabolites. Cell 2025, 188, 175–186.e20. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zingaro, V.A.; Lincoff, J.; Tom, H.; Oikawa, S.; Oses-Prieto, J.A.; Edmondson, Q.; Seiple, I.; Shah, H.; Kajimura, S.; et al. Remodelling of the translatome controls diet and its impact on tumorigenesis. Nature 2024, 633, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.X.; Yan, K.; Chen, L.; Huang, R.R.; Bian, Z.H.; Wei, H.R.; Gu, X.M.; Zhao, Y.Y.; Liu, M.C.; Suo, C.X.; et al. Targeting OXCT1-mediated ketone metabolism reprograms macrophages to promote antitumor immunity via CD8+ T cells in hepatocellular carcinoma. J. Hepatol. 2024, 81, 690–703. [Google Scholar] [CrossRef]
- Puchalska, P.; Martin, S.E.; Huang, X.; Lengfeld, J.E.; Daniel, B.; Graham, M.J.; Han, X.; Nagy, L.; Patti, G.J.; Crawford, P.A. Hepatocyte-Macrophage Acetoacetate Shuttle Protects against Tissue Fibrosis. Cell Metab. 2019, 29, 383–398.e7. [Google Scholar] [CrossRef]
- Liao, Y.J.; Wang, Y.H.; Wu, C.Y.; Hsu, F.Y.; Chien, C.Y.; Lee, Y.C. Ketogenic Diet Enhances the Cholesterol Accumulation in Liver and Augments the Severity of CCl4 and TAA-Induced hepatic fibrosis in Mice. Int. J. Mol. Sci. 2021, 22, 2934. [Google Scholar] [CrossRef]
- Walther, T.C.; Chung, J.; Farese, R.V., Jr. Lipid Droplet Biogenesis. Annu. Rev. Cell Dev. Biol. 2017, 33, 491–510. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ning, X.; Wei, L.; Zhou, Y.; Zhao, L.; Ma, F.; Bai, M.; Yang, X.; Wang, D.; Sun, S. Twist1 downregulation of PGC-1α decreases fatty acid oxidation in tubular epithelial cells, leading to kidney fibrosis. Theranostics 2022, 12, 3758–3775. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.D.; Leiria, L.O.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017, 23, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ren, X.; Liu, J.; Lei, Y.; Li, M.; Wang, F.; Cheng, S.; Ying, J.; Ding, J.; Chen, X. Knockdown of the Clock gene in the liver aggravates MASLD in mice via inhibiting lipophagy. Mol. Cell Biochem. 2024. [Google Scholar] [CrossRef]
- Li, Q.; Ni, Y.; Zhang, L.; Jiang, R.; Xu, J.; Yang, H.; Hu, Y.; Qiu, J.; Pu, L.; Tang, J.; et al. HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct. Target. Ther. 2021, 6, 76. [Google Scholar] [CrossRef]
- Yang, T.; Qu, X.; Wang, X.; Xu, D.; Sheng, M.; Lin, Y.; Ke, M.; Song, C.; Xia, Q.; Jiang, L.; et al. The macrophage STING-YAP axis controls hepatic steatosis by promoting the autophagic degradation of lipid droplets. Hepatology 2024, 80, 1169–1183. [Google Scholar] [CrossRef]
- Sato, Y.; Murase, K.; Kato, J.; Kobune, M.; Sato, T.; Kawano, Y.; Takimoto, R.; Takada, K.; Miyanishi, K.; Matsunaga, T.; et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 2008, 26, 431–442. [Google Scholar] [CrossRef]
- Tao, J.; Wu, Z.; Liang, Y.; Wang, J.; Tang, M.; Huang, S.; Jiang, F.; Zhou, G.; Guo, L.; Yuan, S.; et al. Lhx2 specifically expressed in hepatic stellate cells promotes liver regeneration and inhibits hepatic fibrosis. Hepatology 2024. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.D. Recent Insights into the Role of PPARs in Disease. Cells 2023, 12, 1572. [Google Scholar] [CrossRef]
- Michalik, L.; Wahli, W. PPARs Mediate Lipid Signaling in Inflammation and Cancer. PPAR Res. 2008, 2008, 134059. [Google Scholar] [CrossRef]
- Ballav, S.; Biswas, B.; Sahu, V.K.; Ranjan, A.; Basu, S. PPAR-γ Partial Agonists in Disease-Fate Decision with Special Reference to Cancer. Cells 2022, 11, 3215. [Google Scholar] [CrossRef]
| Gene | 5′ Forward Primer (5′–3′) | 3′ Reverse Primer (5′–3′) |
|---|---|---|
| Human | ||
| α-SMA | CCGACCGAATGCAGAAGGA | ACAGAGTATTTGCGCTCCGGA |
| Fibronectin | AGGAAGCCGAGGTTTTAACTG | AGGACGCTCATAAGTCACC |
| GADPH | ATTCCACCCATGGCAAATTCC | GACTCCACGACGTACTCAGC |
| PPARγ | CATAAAGTCCTTCCCGCTGA | TCTGTGATCTCCTGCACAGC |
| ACACa | TTCTGCACACGTTCCTTGTC | TGCAGCAGCAACACTGAAAT |
| SREBP1c | TGAGGACAGCAAGGCAAAG | CAGGACAGGCAGAGGAAGAC |
| FASN | GTCCACCAGCAACATCAGC | GTTCTCCAGCAAGCCATCTC |
| PGC-1 | TCCTTTGGGGTCTTTGAGAA | GGCACGCAATCCTATTCATT |
| CPT1a | GCACATCGTCGTGTACCATC | AATAGGCCTGACGACACCTG |
| ACOX1 | CTGTGAGGCACCAGTCTGAA | AGGTGAAAGCCTTCAGTCCA |
| MCAD | ACAGGGGTTCAGACTGCTATT | TCCTCCGTTGGTTATCCACAT |
| FATP1 | ACGCGATATACCAGGAGCTG | ATCTTGAAGGTGCCTGTGGT |
| CD36 | ACGCTGAGGACAACACAGTCT | GCCACAGCCAGATTGAGAAC |
| FAbP1 | GCTGGGTCCAAAGTGATCCA | TGTCACCTTCCAACTGAACCA |
| ATGL | CAACGCCACGCACATCTAC | CAATGAACTTGGCACCAGCC |
| SCD1 | TCTAGCTCCTATACCACCACCA | TCGTCTCCAACTTATCTCCTCC |
| RAB7 | GTGTTGCTGAAGGTTATCATCCT | GCTCCTATTGTGGCTTTGTACTG |
| PLIN2 | TTGCAGTTGCCAATACCTATGC | CCAGTCACAGTAGTCGTCACA |
| siPPARγ | GGAUGCAAGGGUUUCUUCCTT | GGAAGAAACCCUUGCAUCCTT |
| Mouse | ||
| GADPH | GGAGAGTGTTTCCTCGTCCC | ACTGTGCCGTTGAATTTGCC |
| α-SMA | GTACCACCATGTACCCAGGC | GCTGGAAGGTAGACAGCGAA |
| Fibronectin | ATGTGGACCCCTCCTGATAGT | GCCCAGTGATTTCAGCAAAGG |
| PPARγ | GGAAGACCACTCGCATTCCTT | GTAATCAGCAACCATTGGGTCA |
| Antibodies | Host | Dilution | Catalogue | Company |
|---|---|---|---|---|
| anti-β-Actin | Rabbit pAb | 1:2000–1:3000 | 66009-1-AP | Proteintech |
| anti-α-SMA | Rabbit pAb | 1:1000–1:4000 | ab32575 | Abcam |
| anti-Fibronectin | Rabbit pAb | 1:1000–1:4000 | ab2413 | Abcam |
| anti-PPARγ | Rabbit pAb | 1:500–1:3000 | A6981 | ABclonal |
| anti-rabbit IgG | Rabbit pAb | 1:500–1:2000 | SA00001-2 | ABclonal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, F.; Hu, M.; Xia, S.; Li, Y.; Zheng, S.; Zhang, F. Acetoacetate Ameliorates Hepatic Fibrosis by Targeting Peroxisome Proliferator-Activated Receptor Gamma to Restore Lipid Droplets in Activated Hepatic Stellate Cells. Pharmaceuticals 2025, 18, 219. https://doi.org/10.3390/ph18020219
Zhou Y, Wang F, Hu M, Xia S, Li Y, Zheng S, Zhang F. Acetoacetate Ameliorates Hepatic Fibrosis by Targeting Peroxisome Proliferator-Activated Receptor Gamma to Restore Lipid Droplets in Activated Hepatic Stellate Cells. Pharmaceuticals. 2025; 18(2):219. https://doi.org/10.3390/ph18020219
Chicago/Turabian StyleZhou, Ya, Feixia Wang, Mengru Hu, Siwei Xia, Yang Li, Shizhong Zheng, and Feng Zhang. 2025. "Acetoacetate Ameliorates Hepatic Fibrosis by Targeting Peroxisome Proliferator-Activated Receptor Gamma to Restore Lipid Droplets in Activated Hepatic Stellate Cells" Pharmaceuticals 18, no. 2: 219. https://doi.org/10.3390/ph18020219
APA StyleZhou, Y., Wang, F., Hu, M., Xia, S., Li, Y., Zheng, S., & Zhang, F. (2025). Acetoacetate Ameliorates Hepatic Fibrosis by Targeting Peroxisome Proliferator-Activated Receptor Gamma to Restore Lipid Droplets in Activated Hepatic Stellate Cells. Pharmaceuticals, 18(2), 219. https://doi.org/10.3390/ph18020219






