Current Trends in Clinical Trials of Prodrugs
Abstract
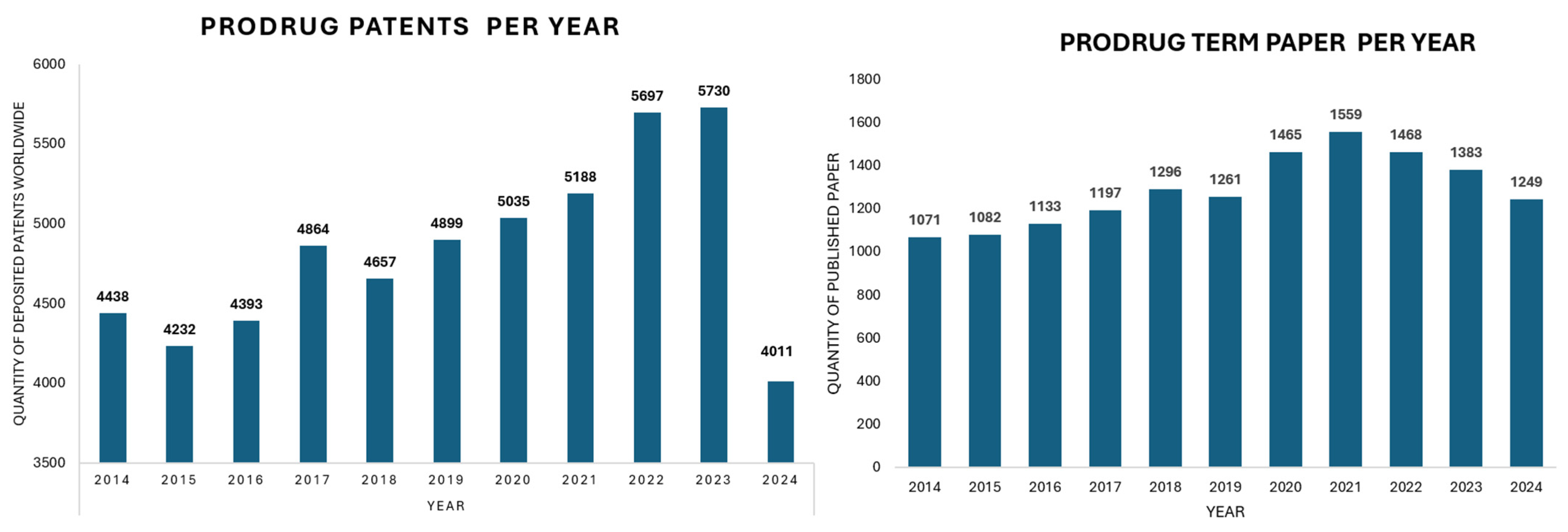
1. Introduction
2. Evolving Landscape of Prodrug Design
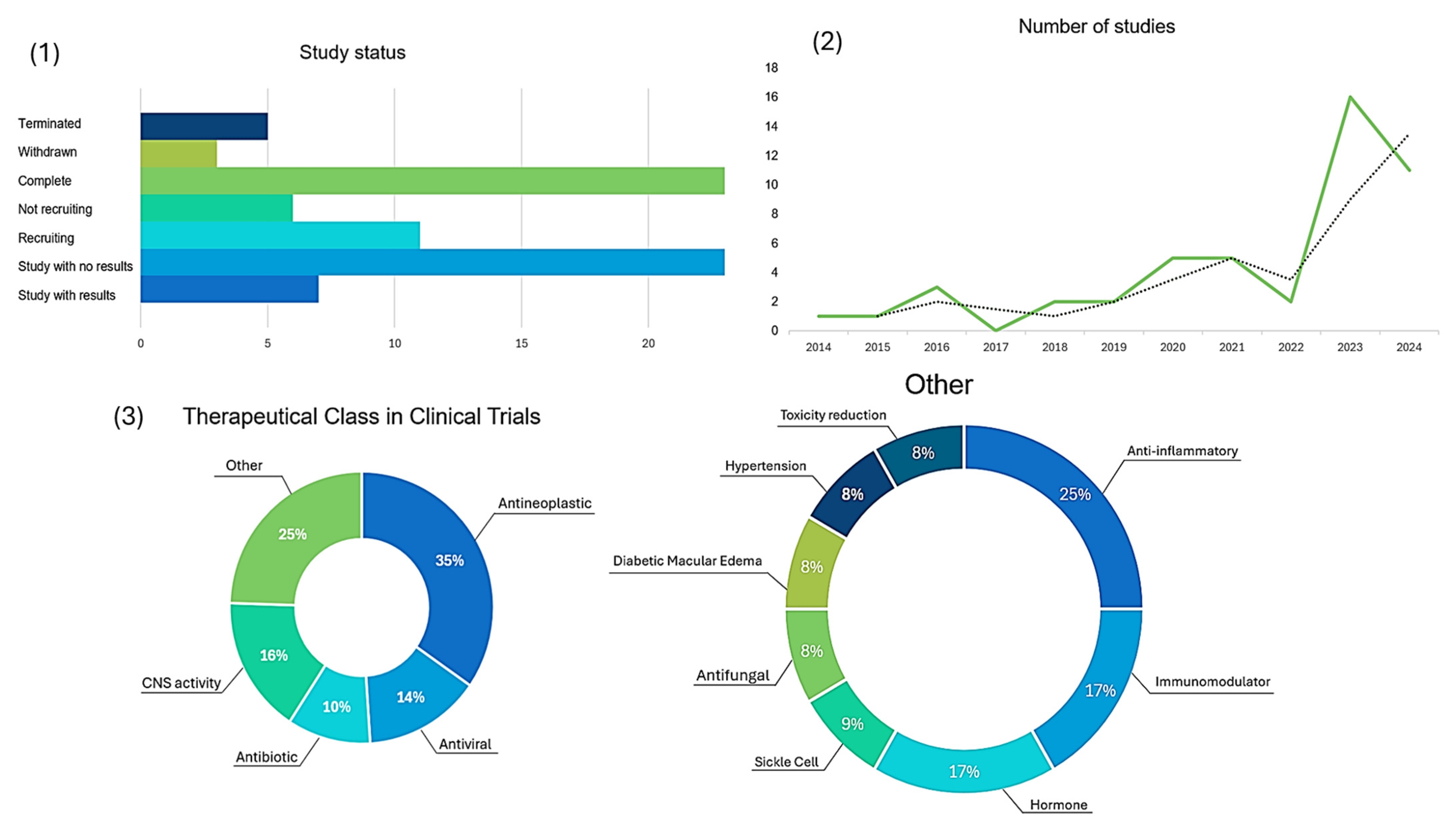
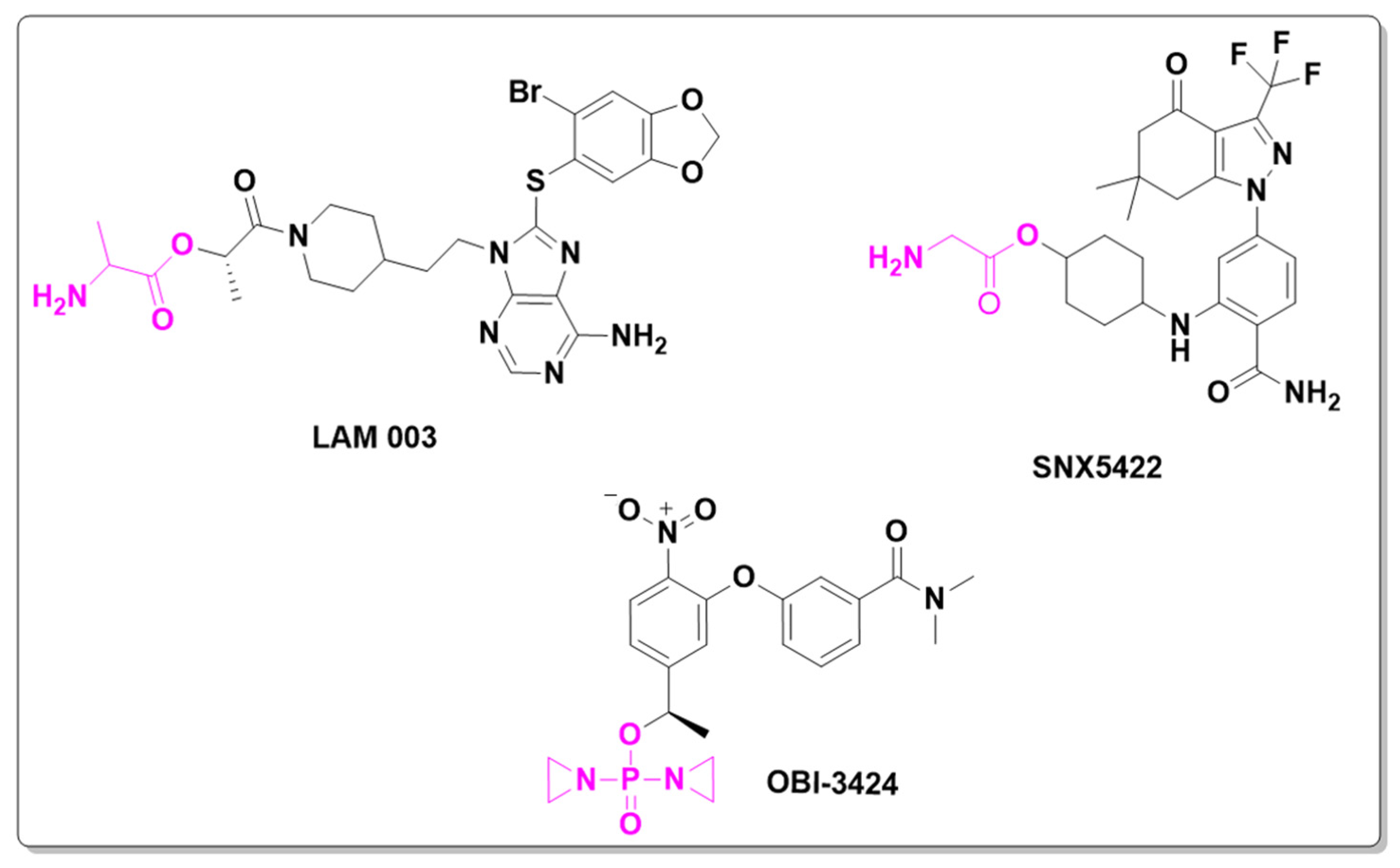
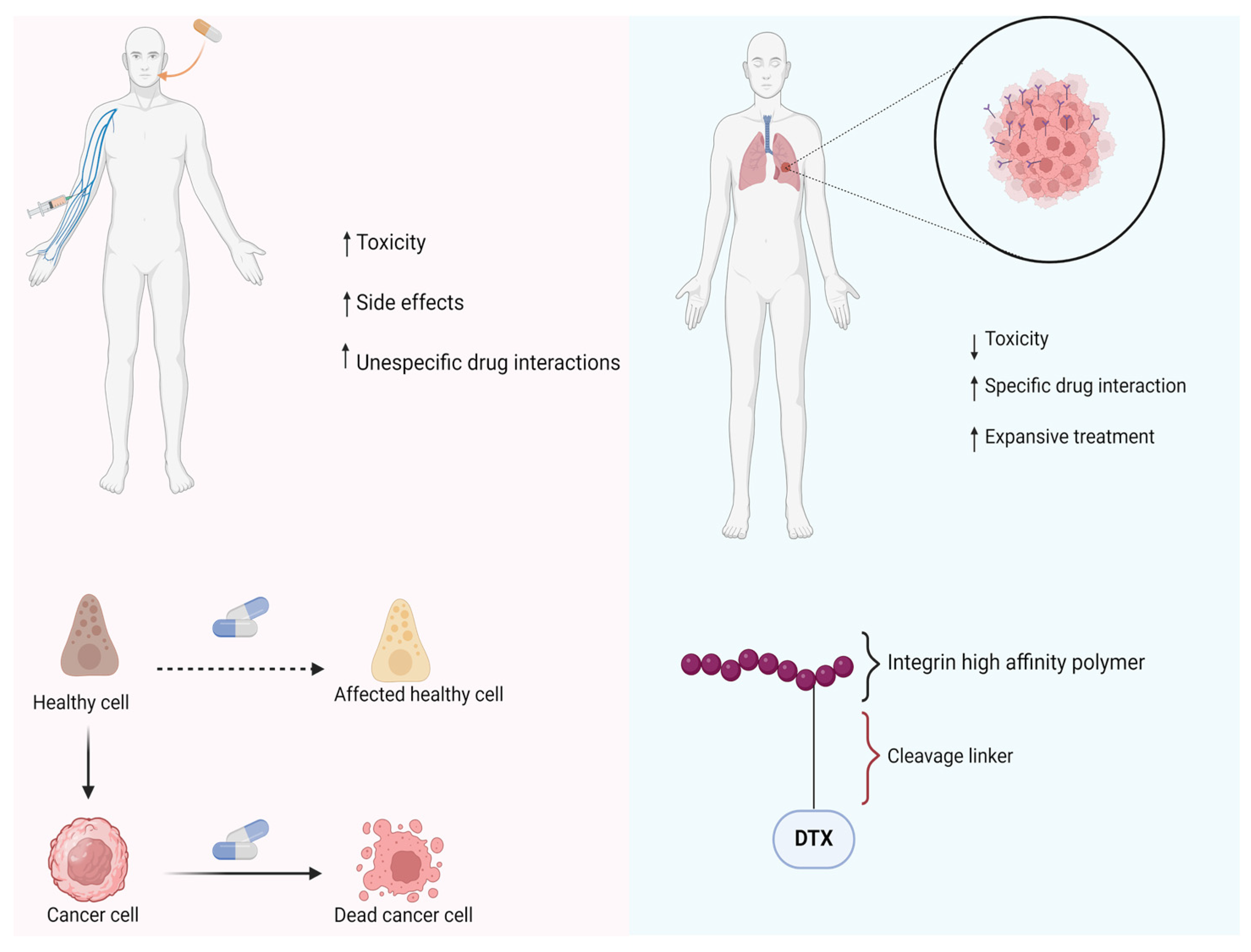
2.1. Antineoplastic Prodrugs
2.2. Antibiotic Prodrugs
2.3. Antiviral Prodrugs
2.4. Central Nervous System Active Prodrugs
2.5. Other Prodrugs
2.5.1. Anti-Inflammatory
2.5.2. Immunomodulator
2.5.3. Hormones
2.5.4. Sickle Cell Disease
2.5.5. Antifungal
2.5.6. Diabetic Macular Edema
2.5.7. Hypertension
2.5.8. Toxicity Reduction
3. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baró, E.L.; Catti, F.; Estarellas, C.; Ghashghaei, O.; Lavilla, R. Drugs from Drugs: New Chemical Insights into a Mature Concept. Drug Discov. Today 2024, 29, 104212. [Google Scholar] [CrossRef] [PubMed]
- Jornada, D.H.; dos Santos Fernandes, G.F.; Chiba, D.E.; de Melo, T.R.F.; dos Santos, J.L.; Chung, M.C. The Prodrug Approach: A Successful Tool for Improving Drug Solubility. Molecules 2015, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.D.R.; Lopes, J.R.; Bonjorno, A.F.; Prates, J.L.B.; Scarim, C.B.; Dos Santos, J.L. The Application of Prodrugs as a Tool to Enhance the Properties of Nucleoside Reverse Transcriptase Inhibitors. Viruses 2023, 15, 2234. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M. Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Monga, J.; Narwal, S.; Singh, G.; Rashid, M.; Afzal, O.; Alatawi, A.; Almadani, N.M. Prodrug Rewards in Medicinal Chemistry: An Advance and Challenges Approach for Drug Designing. Chem. Biodivers. 2023, 20, e202301169. [Google Scholar] [CrossRef]
- He, Z.; Yang, W.; Yang, F.; Zhang, J.; Ma, L. Innovative Medicinal Chemistry Strategies for Enhancing Drug Solubility. Eur. J. Med. Chem. 2024, 279, 116842. [Google Scholar] [CrossRef]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and Clinical Applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Cheng, A.V.; Wuest, W.M. Signed, Sealed, Delivered: Conjugate and Prodrug Strategies as Targeted Delivery Vectors for Antibiotics. ACS Infect. Dis. 2019, 5, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Dean, T.T.; Jelú-Reyes, J.; Allen, A.C.; Moore, T.W. Peptide–Drug Conjugates: An Emerging Direction for the Next Generation of Peptide Therapeutics. J. Med. Chem. 2024, 67, 1641–1661. [Google Scholar] [CrossRef]
- Deng, S.; Wen, X.; Wang, J. Recent Advances in the Linkers of Drug Conjugates. Curr. Med. Chem. 2024, 31. [Google Scholar] [CrossRef]
- Hobson, A.D. The Medicinal Chemistry Evolution of Antibody–Drug Conjugates. RSC Med. Chem. 2024, 15, 809–831. [Google Scholar] [CrossRef]
- Klein, C.; Brinkmann, U.; Reichert, J.M.; Kontermann, R.E. The Present and Future of Bispecific Antibodies for Cancer Therapy. Nat. Rev. Drug Discov. 2024, 23, 301–319. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Singh Cham, P.; Kotwal, P.; Sharma, K.; Dhiman, S.; Singh, L.; Pratap Singh, V.; Kumar, A.; Nandi, U.; Pal Singh, P. Cannabidiol-Based Prodrugs: Synthesis and Bioevaluation. ACS Med. Chem. Lett. 2024, 15, 221–229. [Google Scholar] [CrossRef]
- Banerjee, A.; Hayward, J.J.; Trant, J.F. “Breaking Bud”: The Effect of Direct Chemical Modifications of Phytocannabinoids on Their Bioavailability, Physiological Effects, and Therapeutic Potential. Org. Biomol. Chem. 2023, 21, 3715–3732. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Zhang, W.; Wang, Q.; Wang, C.; Ma, W.; Zhang, C.; Ge, M.; Tian, M.; Yu, J.; Jiao, A.; et al. Cancer SLC6A6-Mediated Taurine Uptake Transactivates Immune Checkpoint Genes and Induces Exhaustion in CD8+ T Cells. Cell 2024, 187, 2288–2304.e27. [Google Scholar] [CrossRef] [PubMed]
- Causes and Prevalence of Visual Impairment Among Adults in the UnitedStates. Arch. Ophthalmol. 2004, 122, 477. [CrossRef]
- Pieken, W.A.; Olsen, D.B.; Benseler, F.; Aurup, H.; Eckstein, F. Kinetic Characterization of Ribonuclease-Resistant 2′-Modified Hammerhead Ribozymes. Science 1991, 253, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Drolet, D.W.; Green, L.S.; Gold, L.; Janjic, N. Fit for the Eye: Aptamers in Ocular Disorders. Nucleic Acid Ther. 2016, 26, 127–146. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T.; Feinsod, M.; Guyer, D.R. Pegaptanib for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef]
- Swanton, C.; Bernard, E.; Abbosh, C.; André, F.; Auwerx, J.; Balmain, A.; Bar-Sagi, D.; Bernards, R.; Bullman, S.; DeGregori, J.; et al. Embracing Cancer Complexity: Hallmarks of Systemic Disease. Cell 2024, 187, 1589–1616. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Wakabayashi, K. Gene Mutations and Altered Gene Expression in Azoxymethane-Induced Colon Carcinogenesis in Rodents. Cancer Sci. 2004, 95, 475–480. [Google Scholar] [CrossRef]
- Armaghany, T.; Wilson, J.D.; Chu, Q.; Mills, G. Genetic Alterations in Colorectal Cancer. Gastrointest. Cancer Res. 2012, 5, 19–27. [Google Scholar] [PubMed]
- Cree, I.A.; Charlton, P. Molecular Chess? Hallmarks of Anti-Cancer Drug Resistance. BMC Cancer 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. The Development and Causes of Cancer. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Alves Junior, A.J.T.; Romero Machuca, M.E.; Ribeiro, R.G.; Garisto, A.M.; de Oliveira, L.H.; Banci, S.O. Malignant Small Bowel Tumor—Case Series and Literature Review. Colorec Cancer 2018, 4. [Google Scholar] [CrossRef][Green Version]
- Lesniewska-Kowiel, M.A.; Muszalska, I. Strategies in the Designing of Prodrugs, Taking into Account the Antiviral and Anticancer Compounds. Eur. J. Med. Chem. 2017, 129, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Beeharry, N.; Landrette, S.; Gayle, S.; Hernandez, M.; Grotzke, J.E.; Young, P.R.; Beckett, P.; Zhang, X.; Carter, B.Z.; Andreeff, M.; et al. LAM-003, a New Drug for Treatment of Tyrosine Kinase Inhibitor-Resistant FLT3-ITD-Positive AML. Blood Adv. 2019, 3, 3661–3673. [Google Scholar] [CrossRef]
- Evans, K.; Duan, J.; Pritchard, T.; Jones, C.D.; McDermott, L.; Gu, Z.; Toscan, C.E.; El-Zein, N.; Mayoh, C.; Erickson, S.W.; et al. OBI-3424, a Novel AKR1C3-Activated Prodrug, Exhibits Potent Efficacy against Preclinical Models of T-ALL. Clin. Cancer Res. 2019, 25, 4493–4503. [Google Scholar] [CrossRef]
- Torabifard, H.; Fattahi, A. Mechanisms and Kinetics of Thiotepa and Tepa Hydrolysis: DFT Study. J. Mol. Model. 2012, 18, 3563–3576. [Google Scholar] [CrossRef] [PubMed]
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of HSP90 in Cancer. Int. J. Mol. Sci. 2021, 22, 10317. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Guo, R.; Giaccone, G.; Liu, S.V.; Hao, Z.; Hilton, C.; Hinson, J.M.; Kris, M.G.; Orlemans, E.O.; Drilon, A. Phase 1 Multicenter Study of the HSP90 Inhibitor SNX-5422 plus Carboplatin and Paclitaxel in Patients with Lung Cancers. Lung Cancer 2021, 162, 23–28. [Google Scholar] [CrossRef]
- Puyo, S.; Montaudon, D.; Pourquier, P. From Old Alkylating Agents to New Minor Groove Binders. Crit. Rev. Oncol. Hematol. 2014, 89, 43–61. [Google Scholar] [CrossRef]
- Bagshawe, K.D. ADEPT and Related Concepts. Cell Biophys. 1994, 24–25, 83–91. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bagshawe, K.D. Antibody Directed Enzyme Prodrug Therapy (ADEPT): Trials and Tribulations. Adv. Drug Deliv. Rev. 2017, 118, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, N.; Wang, J.; Ma, L.; Zhao, Y.; Chen, H.; Wu, H.; Jiang, W. Abstract 4068: In Vitro Masked Effect, in Vivo Anti-Tumor Activity and Toxicology Study of KGX101, an Interleukin-12 Prodrug, in Monotherapy or in Combination with Anti-PD-L1 Antibody. Cancer Res. 2024, 84, 4068. [Google Scholar] [CrossRef]
- Definition of Anti-CD137 Agonistic Monoclonal Antibody ADG206—NCI Drug Dictionary—NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/anti-cd137-agonistic-monoclonal-antibody-adg206 (accessed on 21 November 2024).
- Otano, I.; Azpilikueta, A.; Glez-Vaz, J.; Alvarez, M.; Medina-Echeverz, J.; Cortés-Domínguez, I.; Ortiz-de-Solorzano, C.; Ellmark, P.; Fritzell, S.; Hernandez-Hoyos, G.; et al. CD137 (4-1BB) Costimulation of CD8+ T Cells Is More Potent When Provided in Cis than in Trans with Respect to CD3-TCR Stimulation. Nat. Commun. 2021, 12, 7296. [Google Scholar] [CrossRef]
- Huang, S.; Fang, R.; Xu, J.; Qiu, S.; Zhang, H.; Du, J.; Cai, S. Evaluation of the Tumor Targeting of a FAPα-Based Doxorubicin Prodrug. J. Drug Target. 2011, 19, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Shmeeda, H.; Tahover, E.; Kornev, G.; Patil, Y.; Amitay, Y.; Ohana, P.; Sapir, E.; Zalipsky, S. Development of Promitil®, a Lipidic Prodrug of Mitomycin c in PEGylated Liposomes: From Bench to Bedside. Adv. Drug Deliv. Rev. 2020, 154–155, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Lewis, K. The Science of Antibiotic Discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Erah, P.O.; Goddard, A.F.; Barrett, D.A.; Shaw, P.N.; Spiller, R.C. The Stability of Amoxycillin, Clarithromycin and Metronidazole in Gastric Juice: Relevance to the Treatment of Helicobacter Pylori Infection. J. Antimicrob. Chemother. 1997, 39, 5–12. [Google Scholar] [CrossRef]
- Reddy, K.R.; Parkinson, J.; Sabet, M.; Tarazi, Z.; Boyer, S.H.; Lomovskaya, O.; Griffith, D.C.; Hecker, S.J.; Dudley, M.N. Selection of QPX7831, an Orally Bioavailable Prodrug of Boronic Acid β-Lactamase Inhibitor QPX7728. J. Med. Chem. 2021, 64, 17523–17529. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Jain, A.; Walpole, S.; Moore, G.; Utley, L.; Manyak, E.; Dane, A.; Melnick, D. Safety, Pharmacokinetics, and Food Effect of Tebipenem Pivoxil Hydrobromide after Single and Multiple Ascending Oral Doses in Healthy Adult Subjects. Antimicrob. Agents Chemother. 2019, 63, e00618-19. [Google Scholar] [CrossRef] [PubMed]
- Lavis, L.D. Ester Bonds in Prodrugs. ACS Chem. Biol. 2008, 3, 203–206. [Google Scholar] [CrossRef]
- Cotroneo, N.; Stokes, S.S.; Pucci, M.J.; Rubio, A.; Hamed, K.A.; Critchley, I.A. Efficacy of SPR720 in Murine Models of Non-Tuberculous Mycobacterial Pulmonary Infection. J. Antimicrob. Chemother. 2024, 79, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Flamm, R.K.; Rhomberg, P.R.; Kaplan, N.; Jones, R.N.; Farrell, D.J. Activity of Debio1452, a FabI Inhibitor with Potent Activity against Staphylococcus Aureus and Coagulase-Negative Staphylococcus Spp., Including Multidrug-Resistant Strains. Antimicrob. Agents Chemother. 2015, 59, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Hafkin, B.; Berg, J.K.; Kaplan, N.; Métral, V.N.; Ménétrey, A.; Alcorn, H.; Wittke, F. Single-Dose Escalation Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of a FabI Inhibitor, the Prodrug Debio 1450 and Its Active Moiety Debio 1452, Administered Intravenously in Healthy Subjects. Open Forum Infect. Dis. 2015, 2, 797. [Google Scholar] [CrossRef]
- Trout, R.E.; Zulli, A.; Mesaros, E.; Jackson, R.W.; Boyd, S.; Liu, B.; Hamrick, J.; Daigle, D.; Chatwin, C.L.; John, K.; et al. Discovery of VNRX-7145 (VNRX-5236 Etzadroxil): An Orally Bioavailable β-Lactamase Inhibitor for Enterobacterales Expressing Ambler Class A, C, and D Enzymes. J. Med. Chem. 2021, 64, 10155–10166. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Milioudi, A.; Wicha, S.G. Pharmacokinetics and Pharmacodynamics of Tedizolid. Clin. Pharmacokinet. 2022, 61, 489–503. [Google Scholar] [CrossRef]
- Kilmarx, P.H. Global Epidemiology of HIV. Curr. Opin. HIV AIDS 2009, 4, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Greene, W.C. A History of AIDS: Looking Back to See Ahead. Eur. J. Immunol. 2007, 37, S94–S102. [Google Scholar] [CrossRef] [PubMed]
- Agarwal-Jans, S. Timeline: HIV. Cell 2020, 183, 550. [Google Scholar] [CrossRef]
- Nowicka-Sans, B.; Gong, Y.-F.; McAuliffe, B.; Dicker, I.; Ho, H.-T.; Zhou, N.; Eggers, B.; Lin, P.-F.; Ray, N.; Wind-Rotolo, M.; et al. In Vitro Antiviral Characteristics of HIV-1 Attachment Inhibitor BMS-626529, the Active Component of the Prodrug BMS-663068. Antimicrob. Agents Chemother. 2012, 56, 3498–3507. [Google Scholar] [CrossRef]
- Zhou, N.; Nowicka-Sans, B.; McAuliffe, B.; Ray, N.; Eggers, B.; Fang, H.; Fan, L.; Healy, M.; Langley, D.R.; Hwang, C.; et al. Genotypic Correlates of Susceptibility to HIV-1 Attachment Inhibitor BMS-626529, the Active Agent of the Prodrug BMS-663068. J. Antimicrob. Chemother. 2014, 69, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.L.; Kania, R.S.; Brothers, M.A.; Davies, J.F.; Ferre, R.A.; Gajiwala, K.S.; He, M.; Hogan, R.J.; Kozminski, K.; Li, L.Y.; et al. Discovery of Ketone-Based Covalent Inhibitors of Coronavirus 3CL Proteases for the Potential Therapeutic Treatment of COVID-19. J. Med. Chem. 2020, 63, 12725–12747. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Balabanska, R.; Cottreel, E.; Chen, E.; Duan, D.; Jiang, Q.; Patil, A.; Triyatni, M.; Upmanyu, R.; Zhu, Y.; et al. TLR7 Agonist RO7020531 versus Placebo in Healthy Volunteers and Patients with Chronic Hepatitis B Virus Infection: A Randomised, Observer-Blind, Placebo-Controlled, Phase 1 Trial. Lancet Infect. Dis. 2023, 23, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.M.; Stephenson, F.A. CNS Drug Discovery: Challenges and Solutions. Drug News Perspect. 2005, 18, 51–57. [Google Scholar]
- Central Nervous System Drugs Global Market Report 2022. Available online: https://finance.yahoo.com/news/central-nervous-system-drugs-global-075400795.html (accessed on 26 September 2024).
- Ruiz-López, E.; Schuhmacher, A.J. Transportation of Single-Domain Antibodies through the Blood–Brain Barrier. Biomolecules 2021, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Rankovic, Z. CNS Drug Design: Balancing Physicochemical Properties for Optimal Brain Exposure. J. Med. Chem. 2015, 58, 2584–2608. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Gray, J.D.; Larson, C.S.; Kazim, S.F.; Soya, H.; McEwen, B.S.; Pereira, A.C. Riluzole Reduces Amyloid Beta Pathology, Improves Memory, and Restores Gene Expression Changes in a Transgenic Mouse Model of Early-Onset Alzheimer’s Disease. Transl. Psychiatry 2018, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Silk, A.W.; Saraiya, B.; Groisberg, R.; Chan, N.; Spencer, K.; Girda, E.; Shih, W.; Palmeri, M.; Saunders, T.; Berman, R.M.; et al. A Phase Ib Dose-Escalation Study of Troriluzole (BHV-4157), an Oral Glutamatergic Signaling Modulator, in Combination with Nivolumab in Patients with Advanced Solid Tumors. Eur. J. Med. Res. 2022, 27, 107. [Google Scholar] [CrossRef]
- Grassi, G.; Cecchelli, C.; Vignozzi, L.; Pacini, S. Investigational and Experimental Drugs to Treat Obsessive-Compulsive Disorder. J. Exp. Pharmacol. 2020, 12, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Meglio. Marco Biohaven Submits New Drug Application for Troriluzole as Spinocerebellar Ataxia Type 3 Therapy. Available online: https://www.neurologylive.com/view/biohaven-submits-new-drug-application-for-troriluzole-spinocerebellar-ataxia-type-3-therapy (accessed on 21 November 2024).
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, H.M. The Pharmacology of Psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. Psilocybin: From Ancient Magic to Modern Medicine. J. Antibiot. 2020, 73, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.; Collins, H. Mechanisms of SSRI Therapy and Discontinuation. Emerg. Neurobiol. Antidepress. Treat. 2024, 66, 21–47. [Google Scholar] [CrossRef]
- Mi, W.; Yang, F.; Li, H.; Xu, X.; Li, L.; Tan, Q.; Wang, G.; Zhang, K.; Tian, F.; Luo, J.; et al. Efficacy, Safety, and Tolerability of Ansofaxine (LY03005) Extended-Release Tablet for Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled, Dose-Finding, Phase 2 Clinical Trial. Int. J. Neuropsychopharmacol. 2021, 25, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Di, X.; Wang, Y.; Li, H.; Xu, X.; Li, L.; Wang, H.; Wang, G.; Zhang, K.; Tian, F.; et al. A phase 3, multicenter, double-blind, randomized, placebo-controlled clinical trial to verify the efficacy and safety of ansofaxine (LY03005) for major depressive disorder. Transl. Psychiatry 2023, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- DeLucia, R.; Planeta, C.S. Fencamfamine. Gen. Pharmacol. Vasc. Syst. 1990, 21, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Gorenstein, C.; DeLucia, R.; Gentil, V. Psychostimulant Effects of Fencamfamine in Healthy Volunteers. Braz. J. Med. Biol. Res. 1988, 21, 475–477. [Google Scholar]
- Leon Duque, M.A.; Vallavoju, N.; Woo, C.M. Chemical Tools for the Opioids. Mol. Cell. Neurosci. 2023, 125, 103845. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A.; Rose, M.A. Mechanisms of Opioid-Induced Respiratory Depression. Arch. Toxicol. 2022, 96, 2247–2260. [Google Scholar] [CrossRef]
- Montinari, M.R.; Minelli, S.; De Caterina, R. The First 3500 years of Aspirin History from Its Roots—A Concise Summary. Vasc. Pharmacol. 2019, 113, 1–8. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Anti-Inflammatory Drugs and Their Mechanism of Action. Inflamm. Res. 1998, 47 (Suppl. S2), S78–S87. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Seidelin, J.B.; LaCasse, E.C.; Nielsen, O.H. SMAC Mimetics and RIPK Inhibitors as Therapeutics for Chronic Inflammatory Diseases. Sci. Signal. 2020, 13, eaax8295. [Google Scholar] [CrossRef]
- Tian, E.; Zhou, C.; Quan, S.; Su, C.; Zhang, G.; Yu, Q.; Li, J.; Zhang, J. RIPK2 Inhibitors for Disease Therapy: Current Status and Perspectives. Eur. J. Med. Chem. 2023, 259, 115683. [Google Scholar] [CrossRef] [PubMed]
- Alles, S.R.A.; Smith, P.A. Peripheral Voltage-Gated Cation Channels in Neuropathic Pain and Their Potential as Therapeutic Targets. Front. Pain Res. 2021, 2, 750583. [Google Scholar] [CrossRef]
- Hijma, H.J.; Siebenga, P.S.; de Kam, M.L.; Groeneveld, G.J. A Phase 1, Randomized, Double-Blind, Placebo-Controlled, Crossover Study to Evaluate the Pharmacodynamic Effects of VX-150, a Highly Selective NaV1.8 Inhibitor, in Healthy Male Adults. Pain Med. 2021, 22, 1814–1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, S.; Chen, Y.; Chen, M.; Zhang, D.; Liu, W.; Chen, C.; Gan, Y.; Luo, M.; Ke, B. Discovery of a Novel Series of Pyridone Amides as NaV1.8 Inhibitors. Bioorg. Med. Chem. Lett. 2024, 101, 129655. [Google Scholar] [CrossRef]
- Kornberg, M.D.; Bhargava, P.; Kim, P.M.; Putluri, V.; Snowman, A.M.; Putluri, N.; Calabresi, P.A.; Snyder, S.H. Dimethyl Fumarate Targets GAPDH and Aerobic Glycolysis to Modulate Immunity. Science 2018, 360, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Okuda, D.T.; Kantarci, O.; Lebrun-Frénay, C.; Sormani, M.P.; Azevedo, C.J.; Bovis, F.; Hua, L.H.; Amezcua, L.; Mowry, E.M.; Hotermans, C.; et al. Dimethyl Fumarate Delays Multiple Sclerosis in Radiologically Isolated Syndrome. Ann. Neurol. 2023, 93, 604–614. [Google Scholar] [CrossRef]
- Morand, E.F.; Fernandez-Ruiz, R.; Blazer, A.; Niewold, T.B. Advances in the Management of Systemic Lupus Erythematosus. BMJ 2023, 383, e073980. [Google Scholar] [CrossRef] [PubMed]
- Stefanski, A.-L.; Tomiak, C.; Pleyer, U.; Dietrich, T.; Burmester, G.R.; Dörner, T. The Diagnosis and Treatment of Sjögren’s Syndrome. Dtsch. Aerzteblatt Int. 2017, 114, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Mauro, D.; Thomas, R.; Guggino, G.; Lories, R.; Brown, M.A.; Ciccia, F. Ankylosing Spondylitis: An Autoimmune or Autoinflammatory Disease? Nat. Rev. Rheumatol. 2021, 17, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Camellino, D.; Giusti, A.; Girasole, G.; Bianchi, G.; Dejaco, C. Pathogenesis, Diagnosis and Management of Polymyalgia Rheumatica. Drugs Aging 2019, 36, 1015–1026. [Google Scholar] [CrossRef]
- Simakou, T.; Butcher, J.P.; Reid, S.; Henriquez, F.L. Alopecia Areata: A Multifactorial Autoimmune Condition. J. Autoimmun. 2019, 98, 74–85. [Google Scholar] [CrossRef]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s Disease Inflammation and Recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkar, M.K.; Tsoi, L.C.; Gudjonsson, J.E. Psoriasis: A Mixed Autoimmune and Autoinflammatory Disease. Curr. Opin. Immunol. 2017, 49, 1–8. [Google Scholar] [CrossRef]
- Swiecicki, P.L.; Hegerova, L.T.; Gertz, M.A. Cold Agglutinin Disease. Blood 2013, 122, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Lahita, R.G. Sex Hormones as Immunomodulators of Disease. Ann. N. Y. Acad. Sci. 1993, 685, 278–287. [Google Scholar] [CrossRef]
- Güçlü-Ustündağ, O.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Rejnmark, L.; Rubin, M.; Schwarz, P.; Vokes, T.; Clarke, B.; Ahmed, I.; Hofbauer, L.; Marcocci, C.; Pagotto, U.; et al. PaTH Forward: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial of TransCon PTH in Adult Hypoparathyroidism. J. Clin. Endocrinol. Metab. 2022, 107, e372–e385. [Google Scholar] [CrossRef]
- Holten-Andersen, L.; Pihl, S.; Rasmussen, C.E.; Zettler, J.; Maitro, G.; Baron, J.; Heinig, S.; Hoffmann, E.; Wegge, T.; Krusch, M.; et al. Design and Preclinical Development of TransCon PTH, an Investigational Sustained-Release PTH Replacement Therapy for Hypoparathyroidism. J. Bone Miner. Res. 2019, 34, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Pavan, A.R.; Dos Santos, J.L. Advances in Sickle Cell Disease Treatments. Curr. Med. Chem. 2021, 28, 2008–2032. [Google Scholar] [CrossRef] [PubMed]
- Ferreira De Melo, T.R.; Chin, C.M.; Dos Santos, J.L. What Are the Most Promising Emerging Therapies for Sickle Cell Disease? Future Med. Chem. 2014, 6, 979–982. [Google Scholar] [CrossRef]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle Cell Disease. Nat. Rev. Dis. Primers 2018, 4, 18010. [Google Scholar] [CrossRef]
- Riley, C.; Kraft, W.K.; Miller, R. Hydroxyurea in the Sickle Cell Disease Modern Era. Expert Rev. Clin. Pharmacol. 2024, 17, 777–791. [Google Scholar] [CrossRef]
- Weaver, S.B.; Singh, D.; Wilson, K.M. Gene Therapies for Sickle Cell Disease. J. Pharm. Technol. 2024, 40, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Pavan, A.R.; Lopes, J.R.; Dos Santos, J.L. The State of the Art of Fetal Hemoglobin-Inducing Agents. Expert Opin. Drug Discov. 2022, 17, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Perrine, S.P.; Ginder, G.D.; Faller, D.V.; Dover, G.H.; Ikuta, T.; Witkowska, H.E.; Cai, S.P.; Vichinsky, E.P.; Olivieri, N.F. A Short-Term Trial of Butyrate to Stimulate Fetal-Globin-Gene Expression in the Beta-Globin Disorders. N. Engl. J. Med. 1993, 328, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Atweh, G.F.; Sutton, M.; Nassif, I.; Boosalis, V.; Dover, G.J.; Wallenstein, S.; Wright, E.; McMahon, L.; Stamatoyannopoulos, G.; Faller, D.V.; et al. Sustained Induction of Fetal Hemoglobin by Pulse Butyrate Therapy in Sickle Cell Disease. Blood 1999, 93, 1790–1797. [Google Scholar]
- Kutlar, A.; Reid, M.; Taher, A.T.; Inati, A.; Abboud, M.R.; El-Beshlawy, A.; Buchanan, G.R.; Smith, H.; Ataga, K.I.; Koshy, N.; et al. A Randomized, Open-Label, Multicenter, Dose Escalation Study of HQK-1001 (2,2-Dimethylbutyrate, Sodium Salt) in Sickle Cell Disease. Blood 2012, 120, 998. [Google Scholar] [CrossRef]
- Kutlar, A.; Ataga, K.; Reid, M.; Vichinsky, E.P.; Neumayr, L.; Blair-Britt, L.; Labotka, R.; Glass, J.; Keefer, J.R.; Wargin, W.A.; et al. A Phase 1/2 Trial of HQK-1001, an Oral Fetal Globin Inducer, in Sickle Cell Disease. Am. J. Hematol. 2012, 87, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Kutlar, A.; Reid, M.E.; Inati, A.; Taher, A.T.; Abboud, M.R.; El-Beshlawy, A.; Buchanan, G.R.; Smith, H.; Ataga, K.I.; Perrine, S.P.; et al. A Dose-Escalation Phase IIa Study of 2,2-Dimethylbutyrate (HQK-1001), an Oral Fetal Globin Inducer, in Sickle Cell Disease. Am. J. Hematol. 2013, 88, E255–E260. [Google Scholar] [CrossRef] [PubMed]
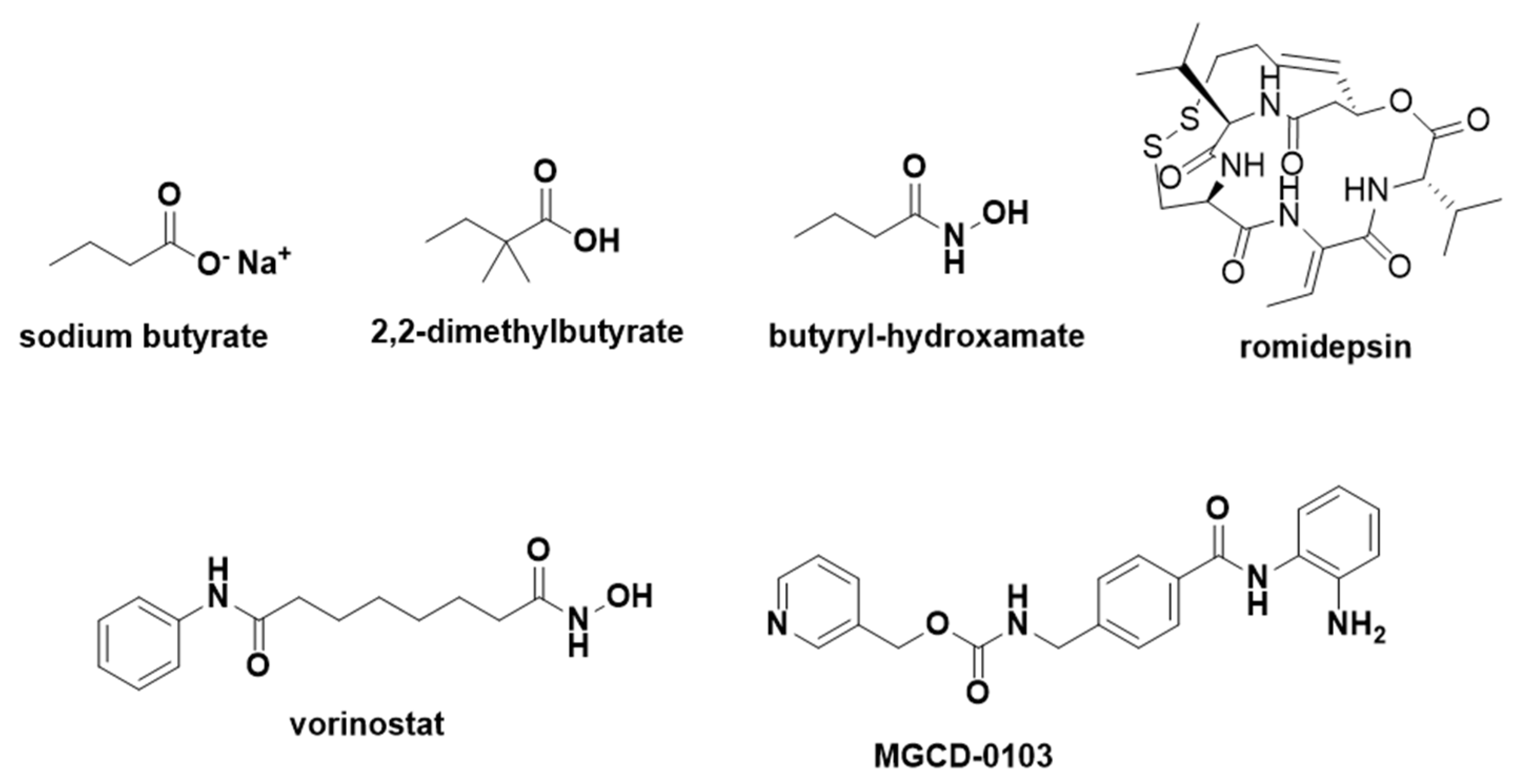
- Skarpidi, E.; Cao, H.; Heltweg, B.; White, B.F.; Marhenke, R.L.; Jung, M.; Stamatoyannopoulos, G. Hydroxamide Derivatives of Short-Chain Fatty Acids Are Potent Inducers of Human Fetal Globin Gene Expression. Exp. Hematol. 2003, 31, 197–203. [Google Scholar] [CrossRef] [PubMed]
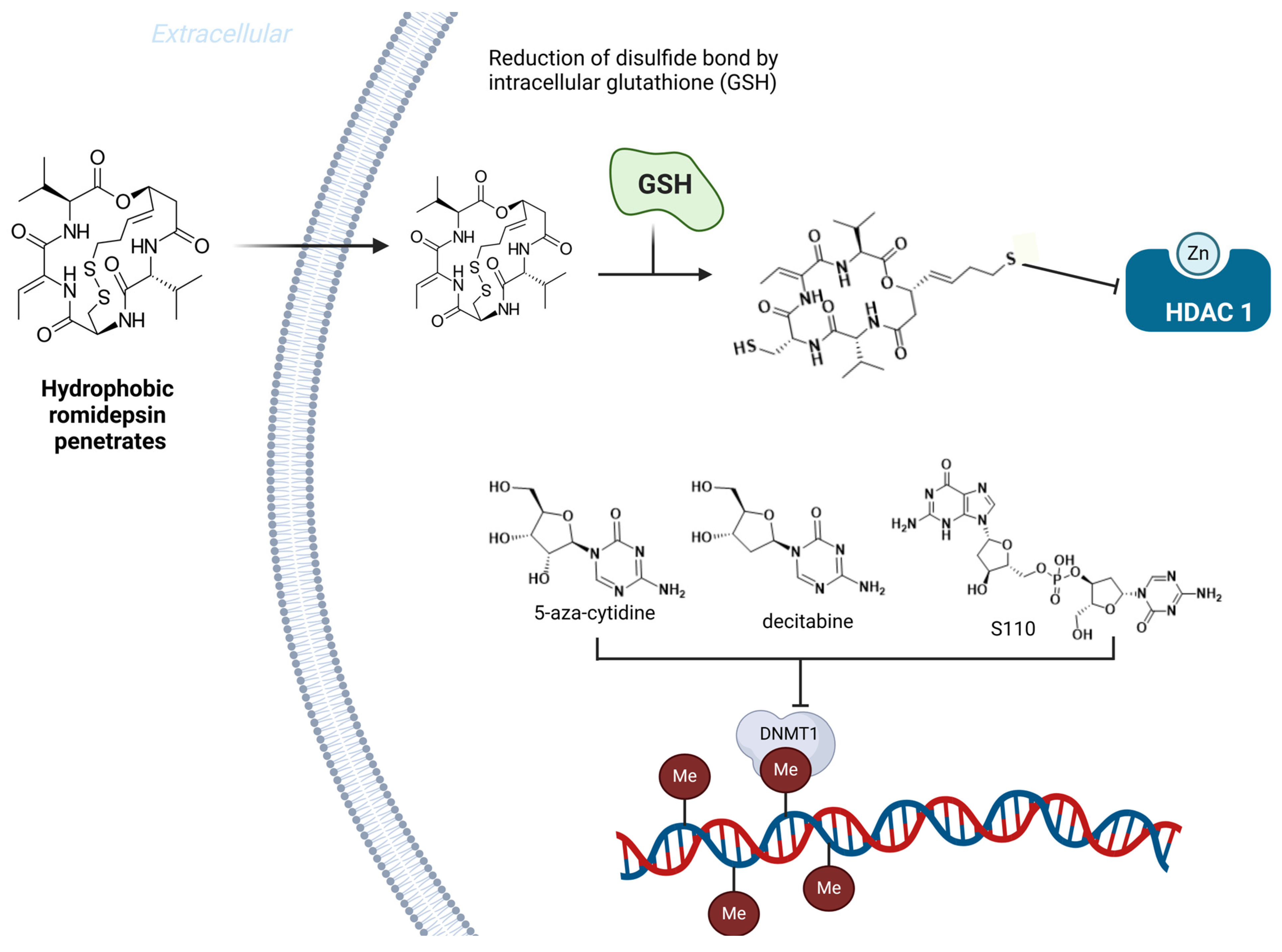
- Cao, H.; Stamatoyannopoulos, G. Histone Deacetylase Inhibitor FK228 Is a Potent Inducer of Human Fetal Hemoglobin. Am. J. Hematol. 2006, 81, 981–983. [Google Scholar] [CrossRef]
- Okam, M.M.; Esrick, E.B.; Mandell, E.; Campigotto, F.; Neuberg, D.S.; Ebert, B.L. Phase 1/2 Trial of Vorinostat in Patients with Sickle Cell Disease Who Have Not Benefitted from Hydroxyurea. Blood 2015, 125, 3668–3669. [Google Scholar] [CrossRef] [PubMed]
- Bradner, J.E.; Mak, R.; Tanguturi, S.K.; Mazitschek, R.; Haggarty, S.J.; Ross, K.; Chang, C.Y.; Bosco, J.; West, N.; Morse, E.; et al. Chemical Genetic Strategy Identifies Histone Deacetylase 1 (HDAC1) and HDAC2 as Therapeutic Targets in Sickle Cell Disease. Proc. Natl. Acad. Sci. USA 2010, 107, 12617–12622. [Google Scholar] [CrossRef] [PubMed]
- Shearstone, J.R.; Golonzhka, O.; Chonkar, A.; Tamang, D.; van Duzer, J.H.; Jones, S.S.; Jarpe, M.B. Chemical Inhibition of Histone Deacetylases 1 and 2 Induces Fetal Hemoglobin through Activation of GATA2. PLoS ONE 2016, 11, e0153767. [Google Scholar] [CrossRef]
- Chonkar, A.; Jarpe, M.; Bhol, K.; Jones, S.S.; Shearstone, J.R. The Histone Deacetylase 1 and 2 (HDAC1/2) Inhibitor ACY-957: Impact of Dosing Schedule on Pharmacokinetics (PK), Pharmacodynamics (PD), Hematopoietic Toxicity, and Gamma Globin (HBG, γ) Expression in Monkey. Blood 2016, 128, 323. [Google Scholar] [CrossRef]
- Ley, T.; DeSimone, J.; Noguchi, C.; Turner, P.; Schechter, A.; Heller, P.; Nienhuis, A. 5-Azacytidine Increases Gamma-Globin Synthesis and Reduces the Proportion of Dense Cells in Patients with Sickle Cell Anemia. Blood 1983, 62, 370–380. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, J.; Koshy, M.; Dorn, L.; Lavelle, D.; Bressler, L.; Molokie, R.; Talischy, N. Maintenance of Elevated Fetal Hemoglobin Levels by Decitabine during Dose Interval Treatment of Sickle Cell Anemia. Blood 2002, 99, 3905–3908. [Google Scholar] [CrossRef] [PubMed]
- Molokie, R.; Lavelle, D.; Gowhari, M.; Pacini, M.; Krauz, L.; Hassan, J.; Ibanez, V.; Ruiz, M.A.; Ng, K.P.; Woost, P.; et al. Oral Tetrahydrouridine and Decitabine for Non-Cytotoxic Epigenetic Gene Regulation in Sickle Cell Disease: A Randomized Phase 1 Study. PLoS Med. 2017, 14, e1002382. [Google Scholar] [CrossRef]
- Lavelle, D.; Saunthararajah, Y.; Vaitkus, K.; Singh, M.; Banzon, V.; Phiasivongsva, P.; Redkar, S.; Kanekal, S.; Bearss, D.; Shi, C.; et al. S110, a Novel Decitabine Dinucleotide, Increases Fetal Hemoglobin Levels in Baboons (P. Anubis). J. Transl. Med. 2010, 8, 92. [Google Scholar] [CrossRef][Green Version]
- Singh, V.; Sharma, P.; Capalash, N. DNA Methyltransferase-1 Inhibitors as Epigenetic Therapy for Cancer. Curr. Cancer Drug Targets 2013, 13, 379–399. [Google Scholar] [CrossRef]
- Terra, L.; Abreu, P.A.; Teixeira, V.L.; Paixão, I.C.P.; Pereira, R.; Leal, B.; Lourenço, A.L.; Rampelotto, P.H.; Castro, H.C. Mycoses and Antifungals: Reviewing the Basis of a Current Problem That Still Is a Biotechnological Target for Marine Products. Front. Mar. Sci. 2014, 1, 12. [Google Scholar] [CrossRef]
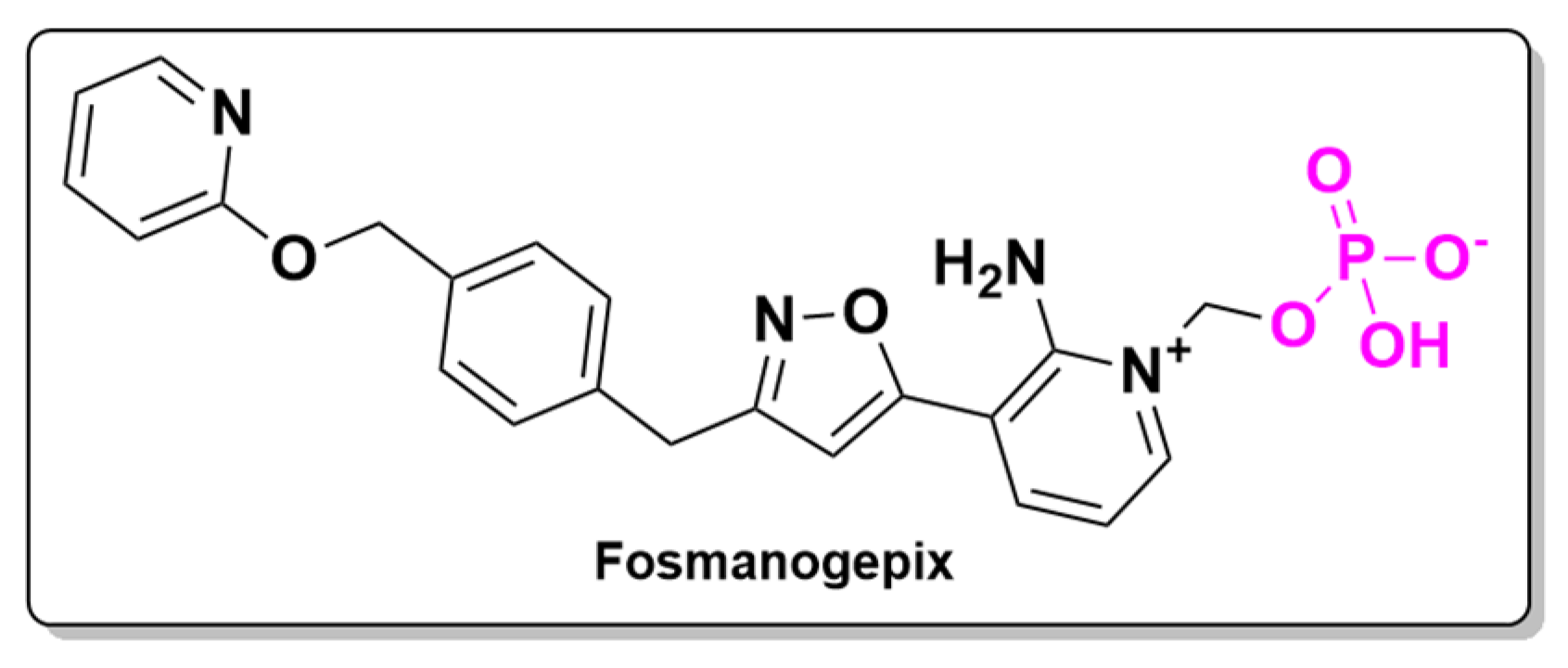
- Shaw, K.J.; Ibrahim, A.S. Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections. J. Fungi 2020, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Badali, H.; Patterson, H.P.; Sanders, C.J.; Mermella, B.; Gibas, C.F.C.; Ibrahim, A.S.; Shaw, K.J.; Wiederhold, N.P. Manogepix, the Active Moiety of the Investigational Agent Fosmanogepix, Demonstrates In Vitro Activity against Members of the Fusarium Oxysporum and Fusarium Solani Species Complexes. Antimicrob. Agents Chemother. 2021, 65, e02343-20. [Google Scholar] [CrossRef]
- Musat, O.; Cernat, C.; Labib, M.; Gheorghe, A.; Toma, O.; Zamfir, M.; Boureanu, A.M. Diabetic Macular Edema. Rom. J. Ophthalmol. 2015, 59, 133–136. [Google Scholar]
- Tatsumi, T. Current Treatments for Diabetic Macular Edema. Int. J. Mol. Sci. 2023, 24, 9591. [Google Scholar] [CrossRef]
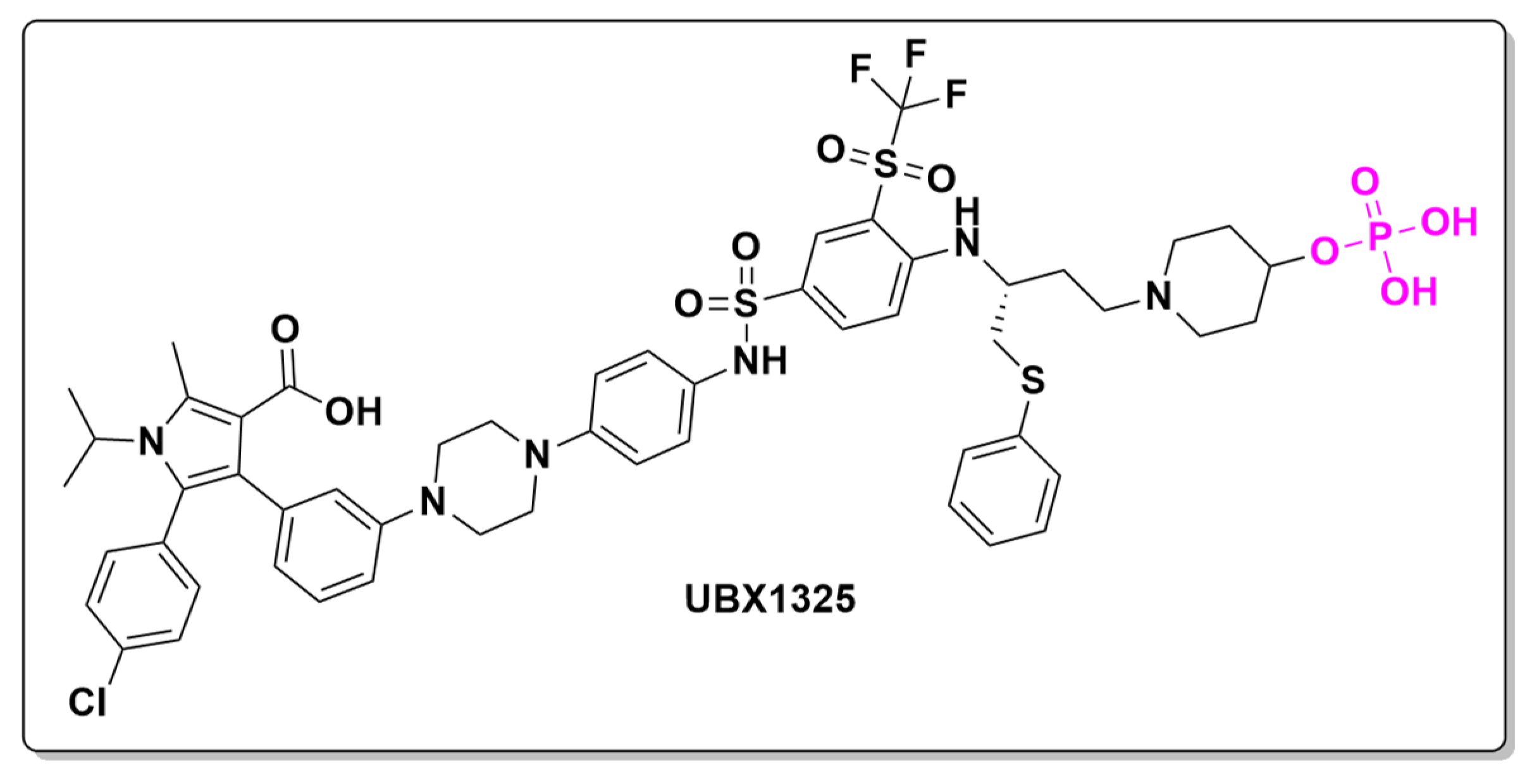
- Bhisitkul, R.; Klier, S.; Tsuruda, P.; Xie, B.; Masaki, L.; Bautista, J.; Khan, A.; Dananberg, J. UBX1325, A Novel Senolytic Treatment for Patients with Advanced DME or Wet AMD: 24-Week Results of a Phase 1 Study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4287. [Google Scholar]
- Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 21 November 2024).
- Dhaneshwar, S.S.; Vadnerkar, G. Rational Design and Development of Colon-Specific Prodrugs. Curr. Top. Med. Chem. 2011, 11, 2318–2345. [Google Scholar] [CrossRef] [PubMed]
- Aiello, R.J.; Bourassa, P.-A.; Zhang, Q.; Dubins, J.; Goldberg, D.R.; De Lombaert, S.; Humbert, M.; Guignabert, C.; Cavasin, M.A.; McKinsey, T.A.; et al. Tryptophan Hydroxylase 1 Inhibition Impacts Pulmonary Vascular Remodeling in Two Rat Models of Pulmonary Hypertension. J. Pharmacol. Exp. Ther. 2017, 360, 267–279. [Google Scholar] [CrossRef]
- Paralkar, V.; Pearson, P.; Mason, J.W.; Li, S.-X.; Curry, S.; Aiello, R.; Feig, P.U. KAR5585, a First-in-Class Oral Tryptophan Hydroxylase 1 (TPH1) Inhibitor as a Novel Candidate for the Treatment of Pulmonary Arterial Hypertension. In B71. Pulmonary Hypertension Life: Animal Models And Ex Vivo Studies in Pulmonary Hypertension; American Thoracic Society International Conference Abstracts; American Thoracic Society: New York, NY, USA, 2017; p. A4193. [Google Scholar]
- Kato, Y.; Fuchi, N.; Saburi, H.; Nishimura, Y.; Watanabe, A.; Yagi, M.; Nakadera, Y.; Higashi, E.; Yamada, M.; Aoki, T. Discovery of 2,8-Diazaspiro[4.5]Decane-Based Trisubstituted Urea Derivatives as Highly Potent Soluble Epoxide Hydrolase Inhibitors and Orally Active Drug Candidates for Treating Hypertension. Bioorg. Med. Chem. Lett. 2013, 23, 5975–5979. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial Diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef]
- Gerards, M.; Sallevelt, S.C.E.H.; Smeets, H.J.M. Leigh Syndrome: Resolving the Clinical and Genetic Heterogeneity Paves the Way for Treatment Options. Mol. Genet. Metab. 2016, 117, 300–312. [Google Scholar] [CrossRef]
- Ehinger, J.K.; Piel, S.; Ford, R.; Karlsson, M.; Sjövall, F.; Frostner, E.Å.; Morota, S.; Taylor, R.W.; Turnbull, D.M.; Cornell, C.; et al. Cell-Permeable Succinate Prodrugs Bypass Mitochondrial Complex I Deficiency. Nat. Commun. 2016, 7, 12317. [Google Scholar] [CrossRef] [PubMed]
- Piel, S.; Janowska, J.I.; Ward, J.L.; McManus, M.J.; Jose, J.S.; Starr, J.; Sheldon, M.; Clayman, C.L.; Elmér, E.; Hansson, M.J.; et al. Succinate Prodrugs in Combination with Atropine and Pralidoxime Protect Cerebral Mitochondrial Function in a Rodent Model of Acute Organophosphate Poisoning. Sci. Rep. 2022, 12, 20329. [Google Scholar] [CrossRef]
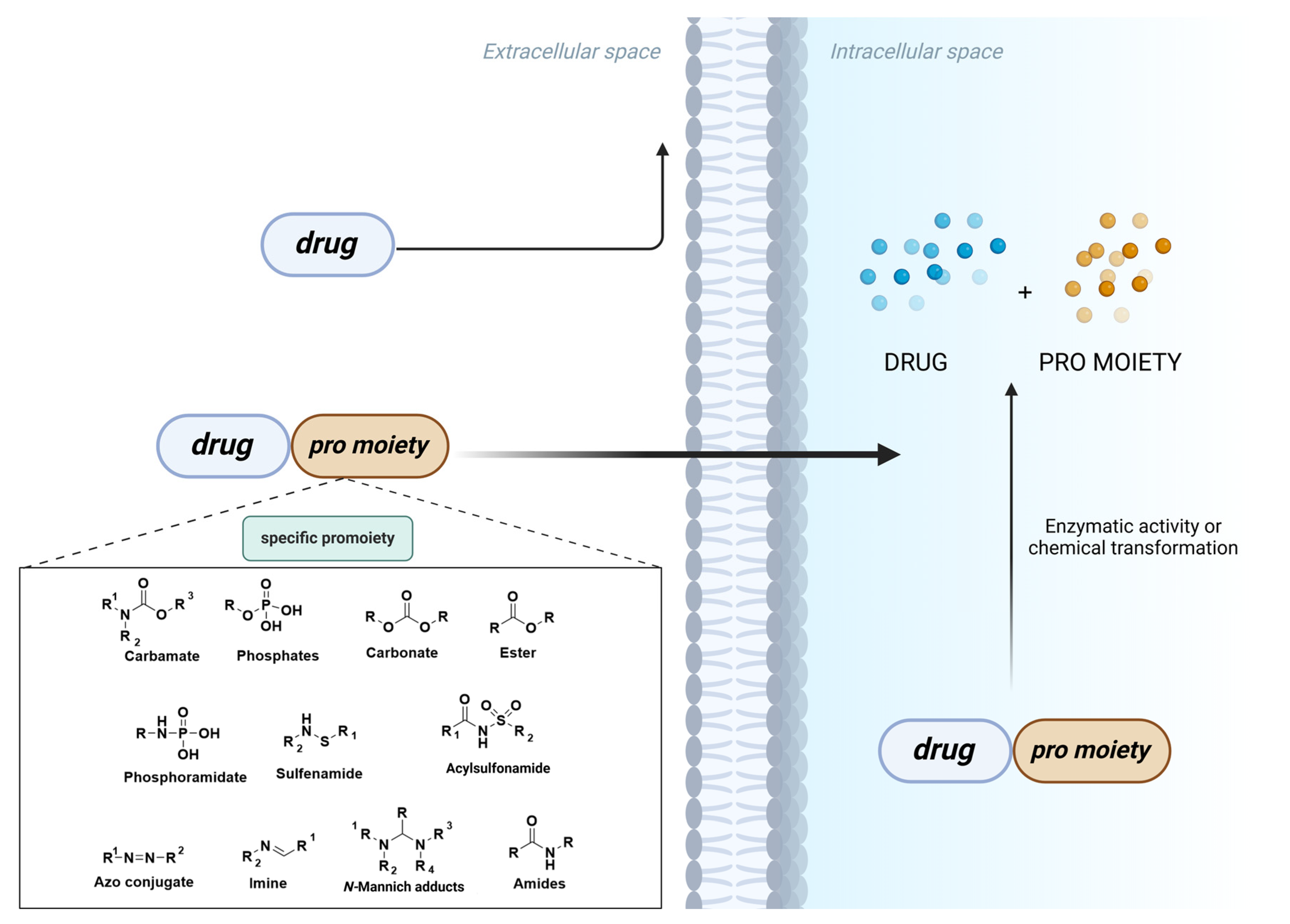
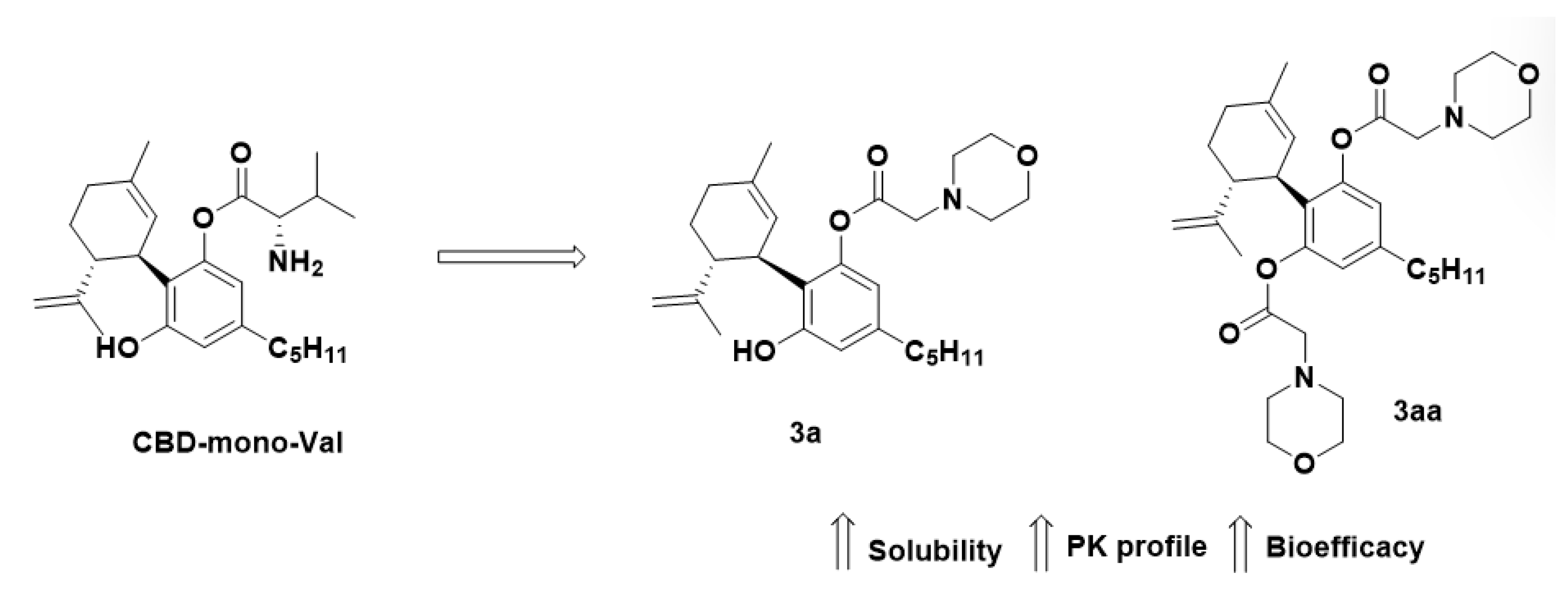
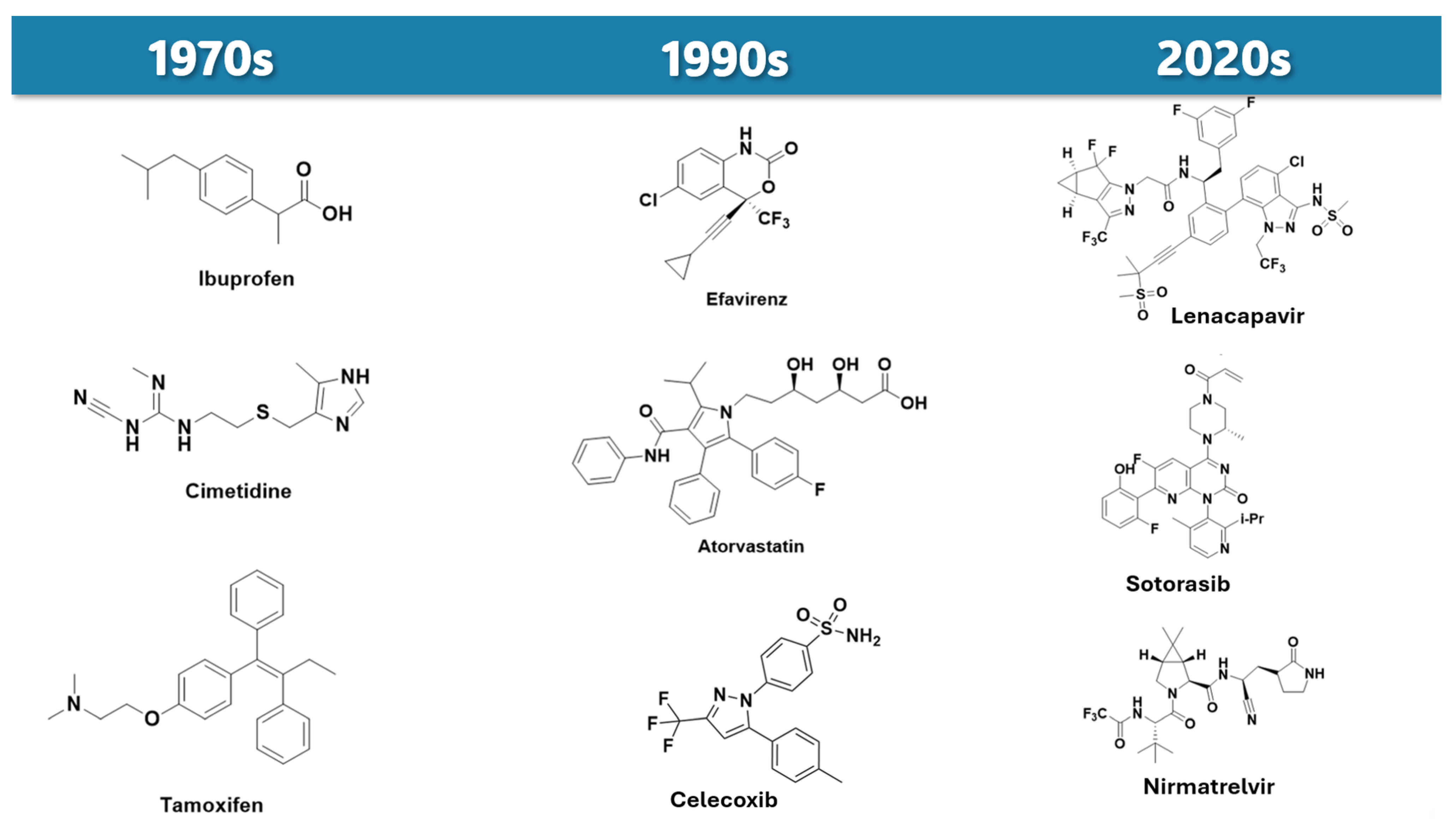
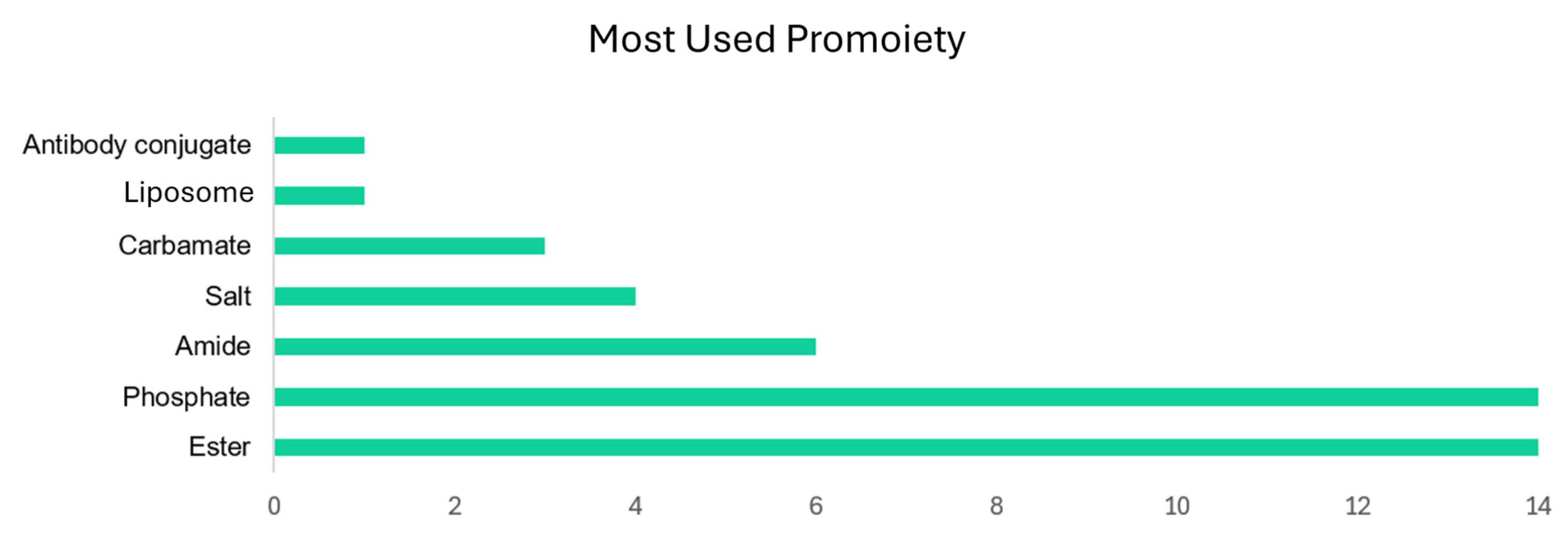

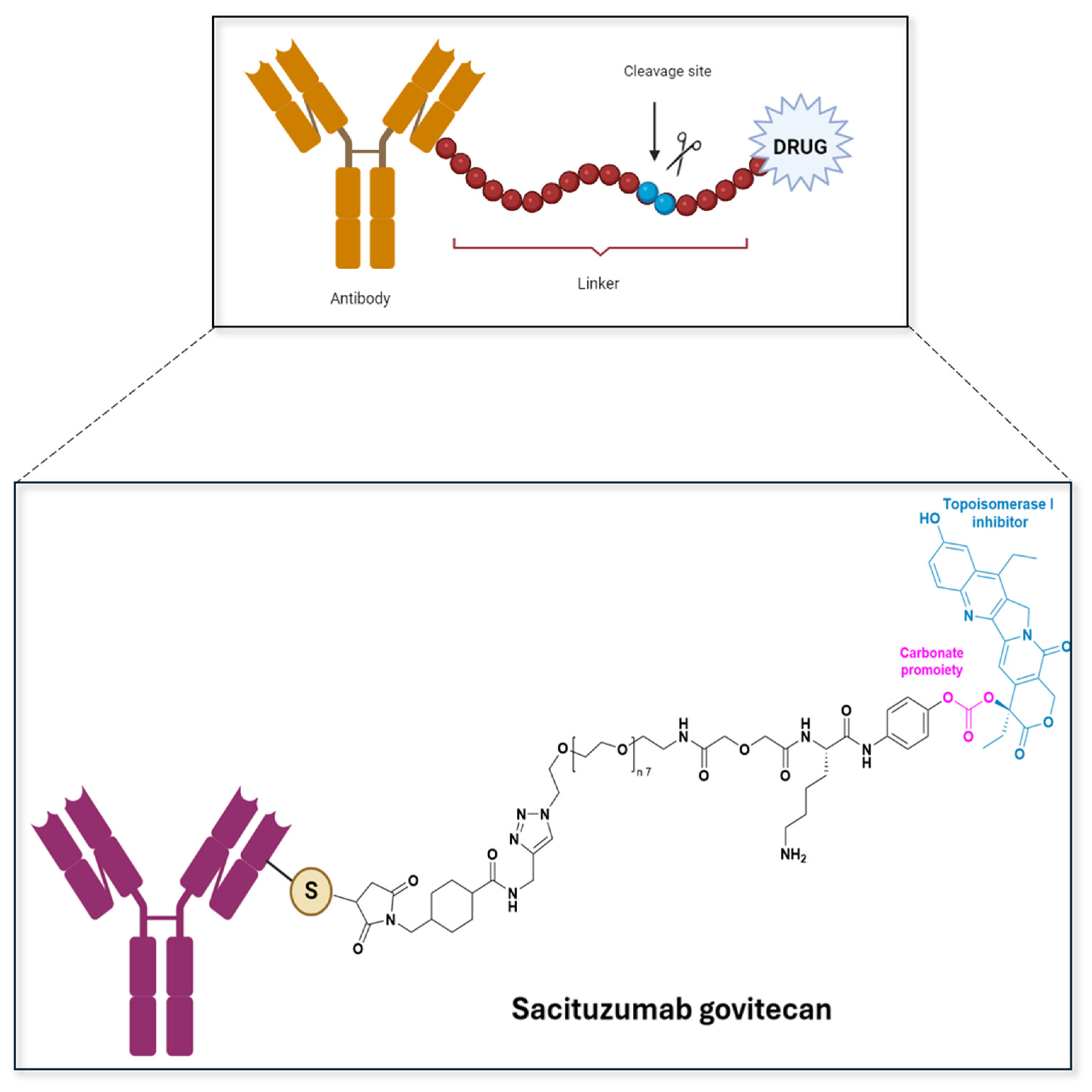
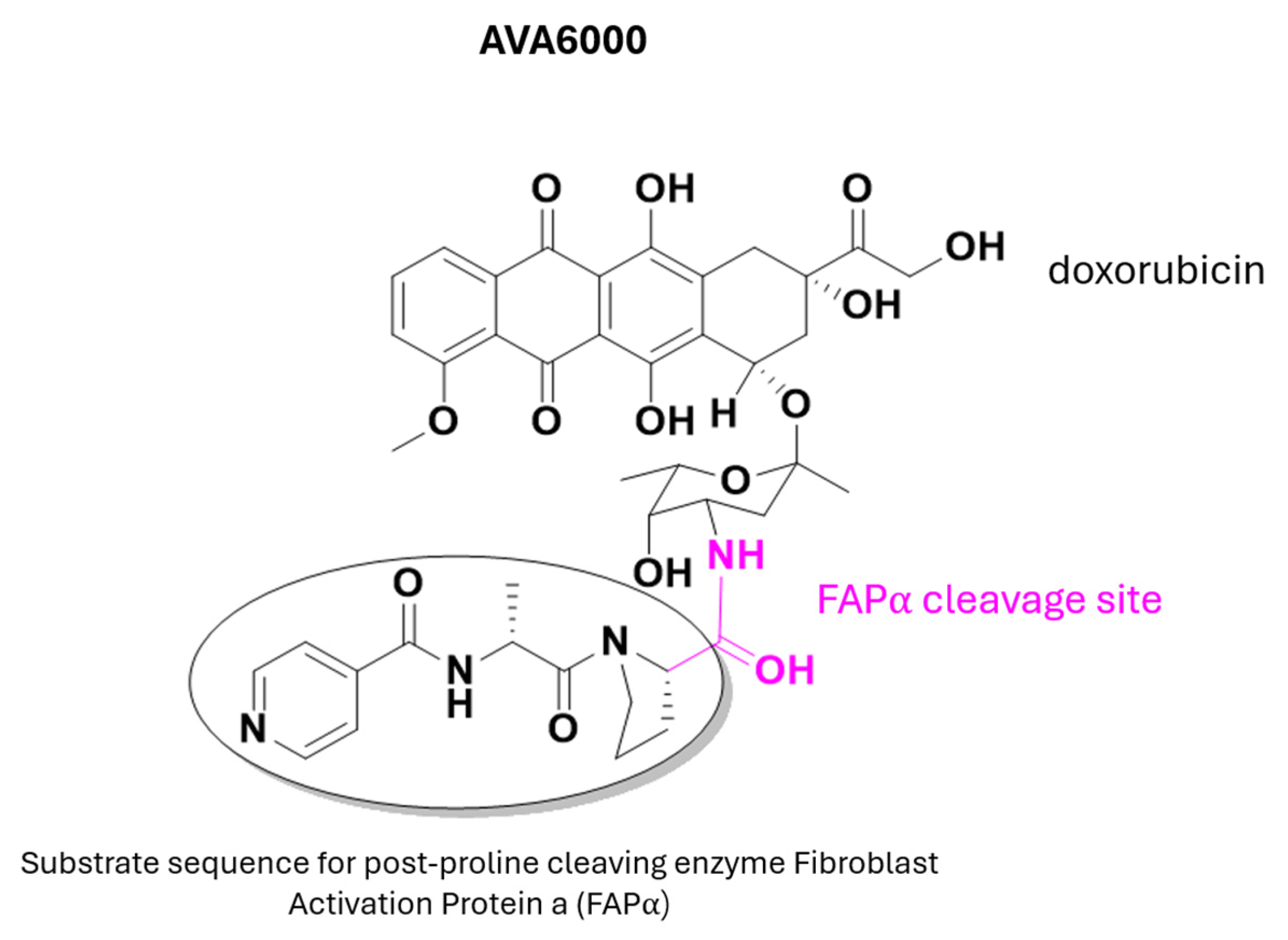
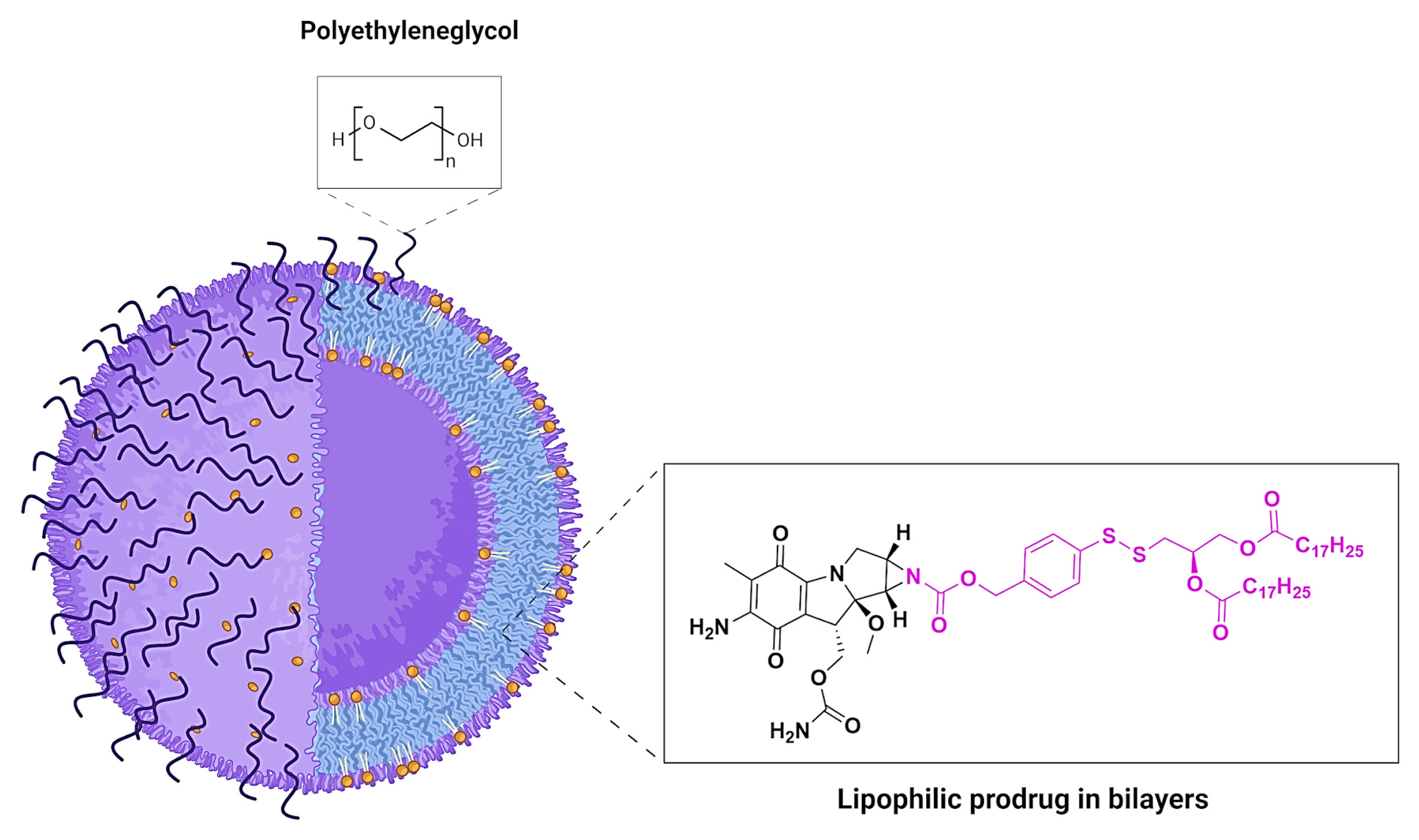
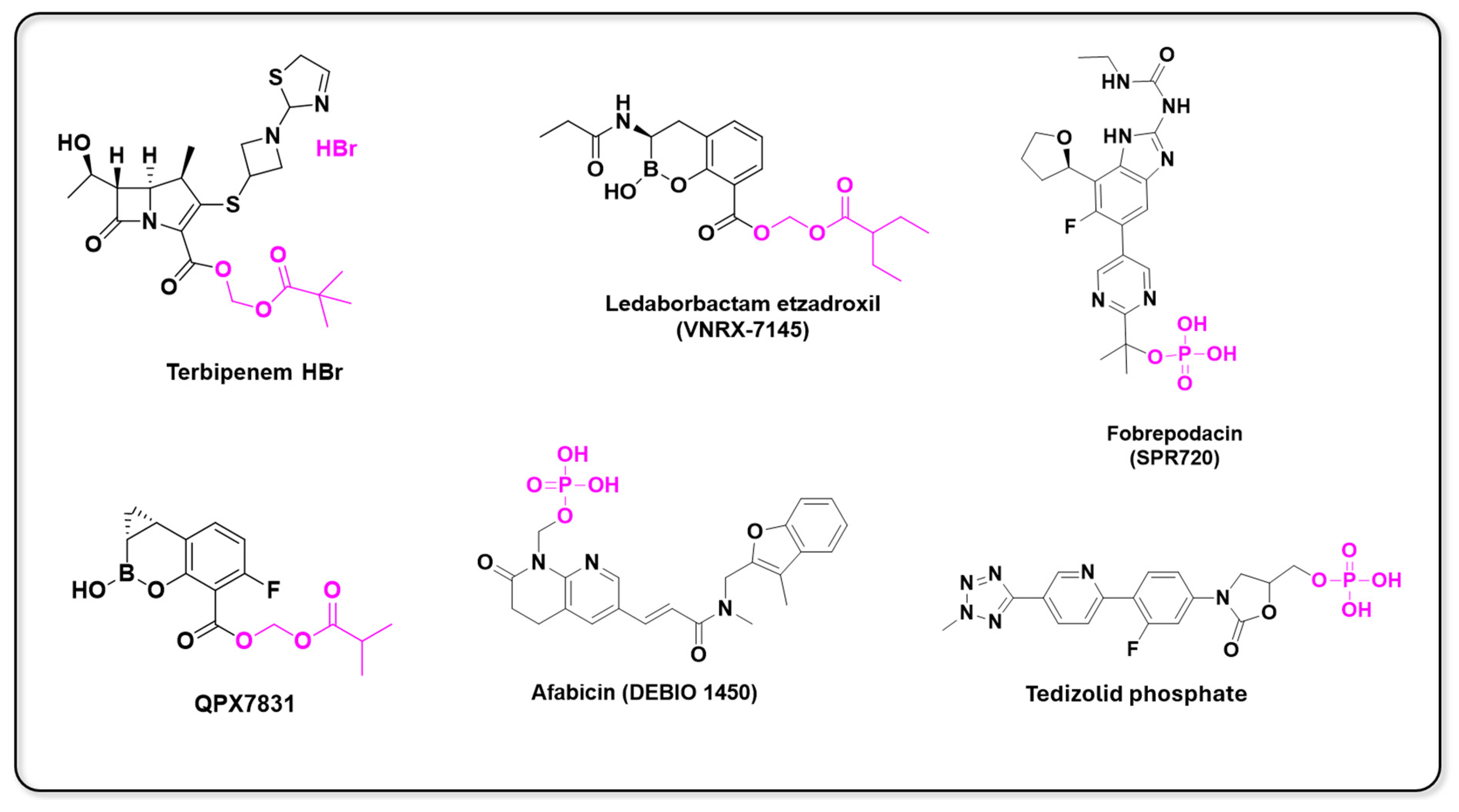
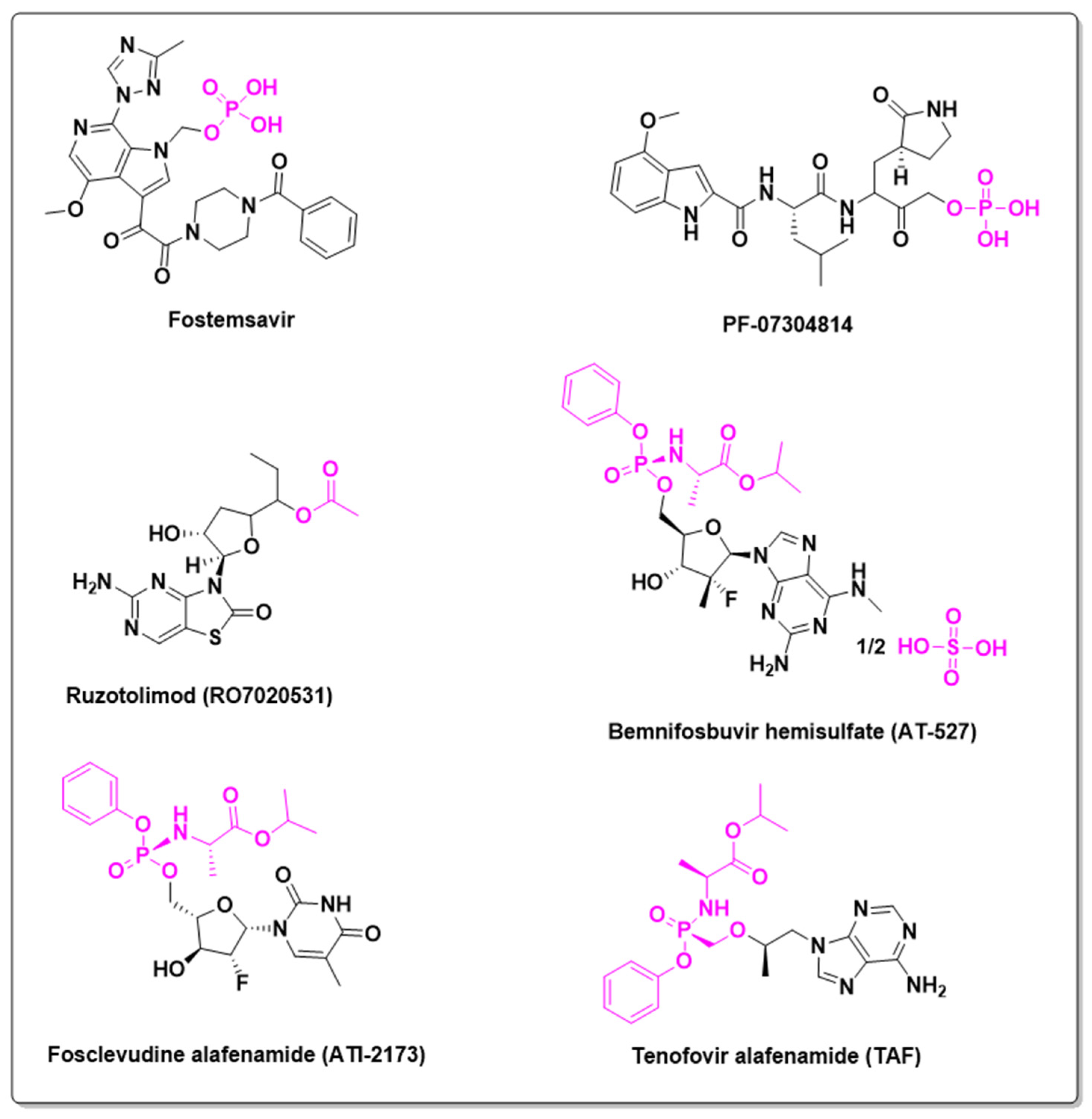
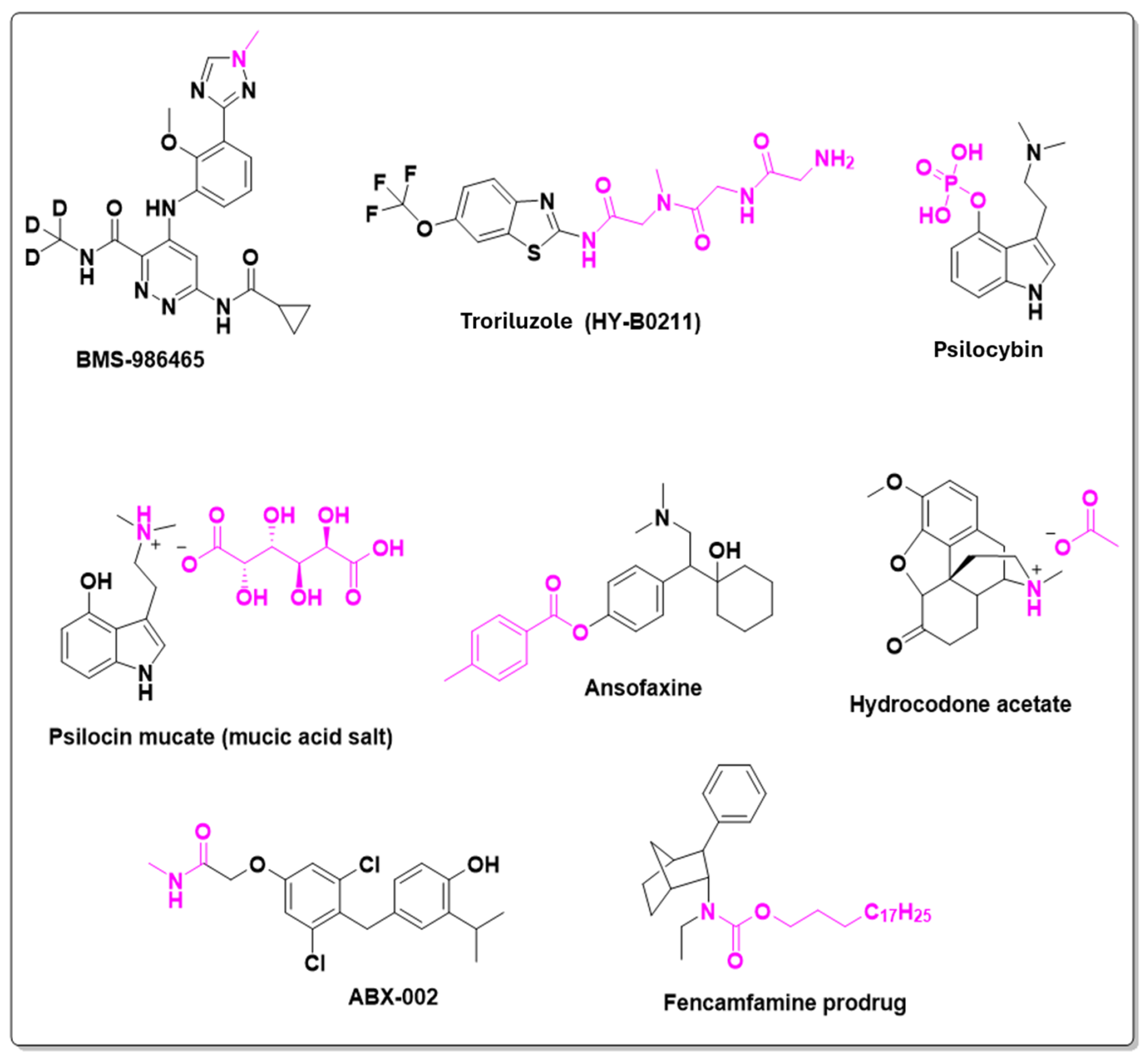
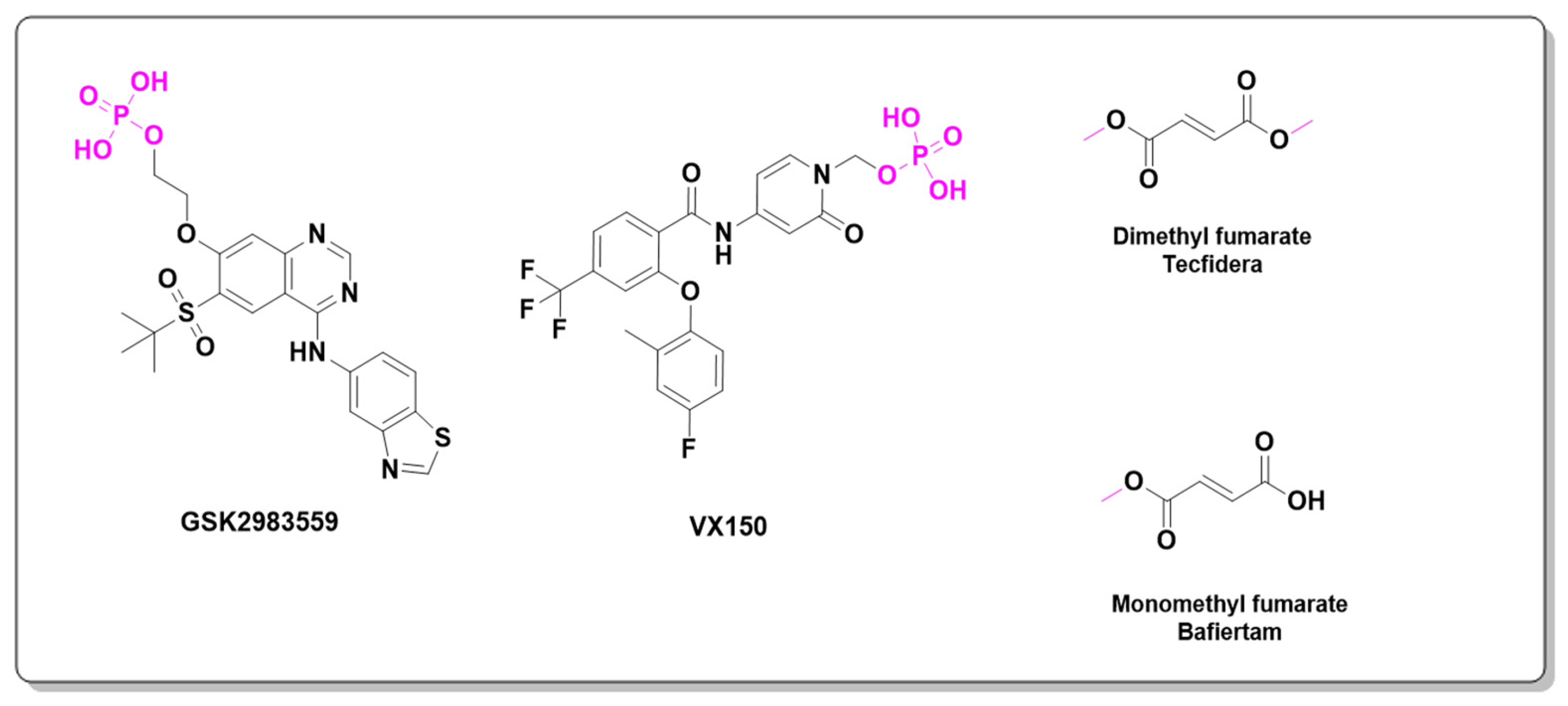
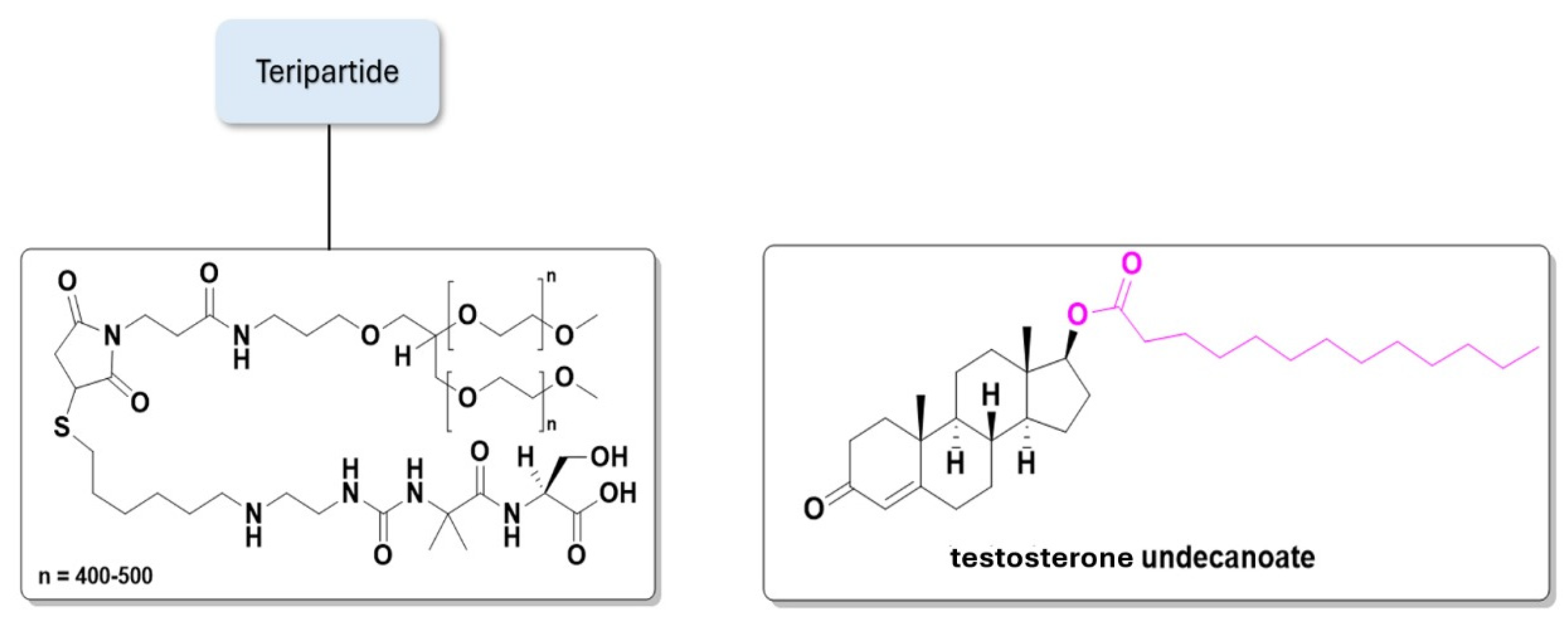
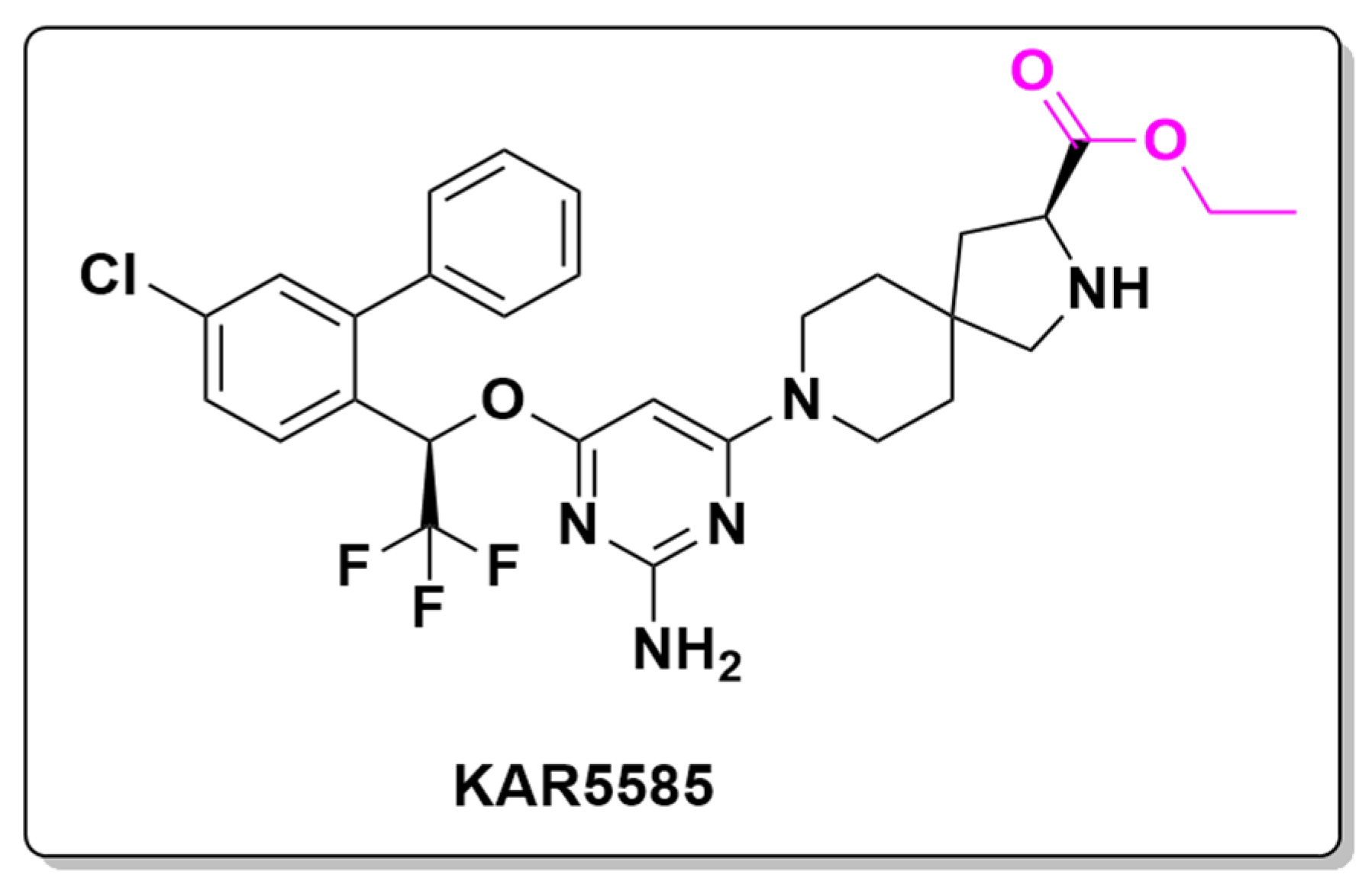
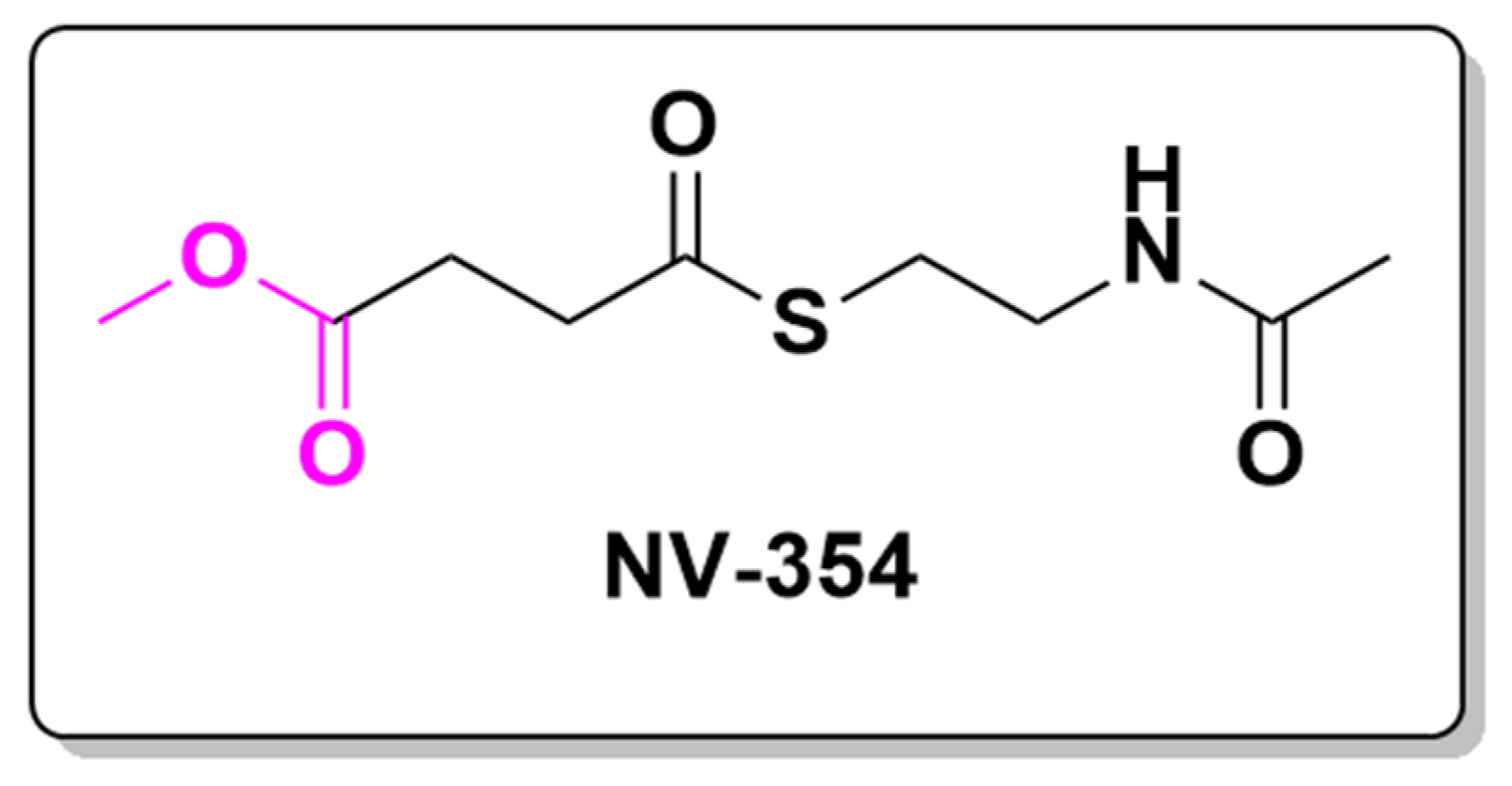
| Prodrug (Moiety Group Is in Magenta) | Name | Condition | Phase | Promoiety |
|---|---|---|---|---|
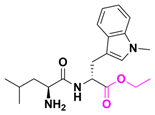 | Indoximod (NLG802) | Solid tumor | I | Ester |
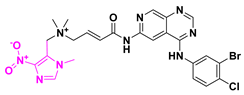 | Tarloxotinib hydrobromide | Solid tumors, head and neck cancers. | II | Bromide salt |
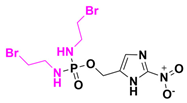 | Evofosfamide | Solid tumors | I | Phosphamide |
 | CP506 | Solid tumor | I/II | Hypoxia-activated prodrug |
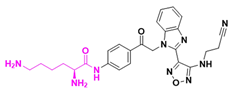 | Lisavandibulin (BAL101553) | Solid tumor/ Glioblastoma | I/II | Amide |
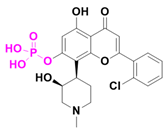 | Alvocidib (TP1287) | CDK9 inhibitor/Advanced solid tumor | I | Phosphate |
| NCT ID | ADC ID | Target | Status | Phase | Sponsor |
|---|---|---|---|---|---|
| NCT06561607 | TQB2102 | Breast cancer with low HER2 expression in the recurrent Metastatic stage | Not yet recruiting | 1 | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. |
| NCT06496490 | TQB2102 | Locally advanced or metastatic non-small cell lung cancer with HER2 gene abnormality | Recruiting | 2 | Chia Tai Tianqing Pharmaceutical Group Nanjing Shunxin Pharmaceutical Co., Ltd. |
| NCT06431490 | TQB2102 | HER2-positive Biliary Tract Cancer | Recruiting | 1/2 | Chia Tai Tianqing Pharmaceutical Group Nanjing Shunxin Pharmaceutical Co., Ltd. |
| NCT06555744 | ZW191 | Folate Receptor Alpha for Advanced Solid Tumors | Recruiting | 1 | Zymeworks BC Inc. |
| NCT06555263 | Luveltamab Tazevibulin— STRO-002 | Advanced or metastatic non-small cell lung cancer expressing FOLR1 (FolRα) | Recruiting | 2 | Sutro Biopharma, Inc. |
| NCT06549816 | Sigvotatug vedotin SGN-B6A | Advanced solid tumors | Recruiting | 1 | Seagen Inc. |
| NCT06545617 | BAT8006 | Folate receptor α (FRα). Subjects with solid tumors. | Not yet recruiting | 1 | Bio-Thera Solutions |
| NCT06533826 | Datopotamab deruxtecan and Trastuzumab deruxtecan | HER2-low locally advanced unresectable or metastatic breast cancer (MBC) | Not yet recruiting | 2 | Ana C Garrido-Castro, MD |
| NCT06519370 | FDA018-ADC | Locally recurrent inoperable or metastatic Triple-negative Breast cancer (TNBC) who are resistant to or recurring during or after taxane therapy. | Recruiting | 3 | Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co., Ltd. |
| NCT06509997 | MRG003 combined with Dalpicicilip posterior line | Recurrent/metastatic CDKN2A gene variant head and neck squamous cell carcinoma (HNSCC) | Not yet recruiting | 2 | Lei Liu, West China Hospital |
| NCT06465069 | LY4052031 (enfortumab vedotin-ejfv) | Anti-nectin-4 advanced or metastatic solid tumors including urothelial cancer. | Recruiting | 1 | Eli Lilly and Company |
| NCT06457997 | PHN-010 | Advanced solid tumors. | Recruiting | 1b | Pheon Therapeutics |
| NCT06453044 | Polatuzumab vedotin | Relapsed or Refractory grade 1-3a Follicular Lymphoma | Recruiting | 2 | City of Hope Medical Center |
| NCT06440005 | AGX101 | Advanced Solid Tumors | Recruiting | 1 | Angiex, Inc. |
| NCT06384807 | PBI-410 | Advanced Solid Tumors | Recruiting | 1/2 | Biohaven Therapeutics Ltd. |
| NCT06362252 | Ifinatamab deruxtecan (I-DXd), the ADC in combination with immune checkpoint inhibitor (ICI) atezolizumab with or without carboplatin | Extensive stage-small cell lung cancer (ES-SCLC) in the first line (1L) setting. | Recruiting | 1/2 | Daiichi Sankyo |
| NCT06341400 | RC48 Combined with Toripalimab | Platinum-intolerant bladder cancer patients. tumor cells | Recruiting | 1/2 | Zhujiang Hospital |
| NCT06265727 | CRB-701 | Solid tumors that express nectin-4. | Recruiting | 1/2 | Corbus Pharmaceuticals Inc. |
| NCT06238479 | LY4101174 | Humanized immunoglobulin G1 (IgG1) Fcg-silent monoclonal antibody directed against the cell surface adhesion molecule and tumor-associated antigen (TAA) nectin-4 (PVRL4) conjugated, via maleimide-beta-glucuronide poly-sarcosine linkers, to the camptothecin analog and topoisomerase 1 inhibitor exatecan, with potential antineoplastic activity. | Recruiting | 1 | Eli Lilly and Company |
| NCT06234423 | CUSP06-1001 | Platinum-Refractory/Resistant Ovarian Cancer and Other Advanced Solid Tumors | Recruiting | 1 | OnCusp Therapeutics, Inc. |
| NCT06210490 | Disitamab Vedotin | Adjuvant Treatment of HER2 Overexpressing UTUC Patients with high risk factors for recurrence after radical surgery | Not yet recruiting | 2 | Peking University First Hospital |
| NCT06210815 | HLX42 | EGFR-targeting ADC, for cetuximab or TKI resistant cancer | Recruiting | Shanghai Henlius Biotech | |
| NCT06144723 | HS-20105 | Targeting Trop-2 advanced solid tumors | Not yet recruiting | 1/2 | Hansoh BioMedical R&D Company |
| NCT06132958 | Sacituzumab Tirumotecan (MK-2870) | Endometrial cancer (EC) that have previously received treatment with platinum-based therapy. | Recruiting | 3 | Merck Sharp & Dohme LLC |
| NCT06112704 | HS-20093 | Advanced esophageal carcinoma and other solid tumor | Recruiting | 2 | Hansoh BioMedical R&D Company |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boreski, D.; Schmid, V.F.; Bosquesi, P.L.; dos Santos, J.L.; Scarim, C.B.; Reshetnikov, V.; Chin, C.M. Current Trends in Clinical Trials of Prodrugs. Pharmaceuticals 2025, 18, 210. https://doi.org/10.3390/ph18020210
Boreski D, Schmid VF, Bosquesi PL, dos Santos JL, Scarim CB, Reshetnikov V, Chin CM. Current Trends in Clinical Trials of Prodrugs. Pharmaceuticals. 2025; 18(2):210. https://doi.org/10.3390/ph18020210
Chicago/Turabian StyleBoreski, Diogo, Valentine Fabienne Schmid, Priscila Longhin Bosquesi, Jean Leandro dos Santos, Cauê Benito Scarim, Viktor Reshetnikov, and Chung Man Chin. 2025. "Current Trends in Clinical Trials of Prodrugs" Pharmaceuticals 18, no. 2: 210. https://doi.org/10.3390/ph18020210
APA StyleBoreski, D., Schmid, V. F., Bosquesi, P. L., dos Santos, J. L., Scarim, C. B., Reshetnikov, V., & Chin, C. M. (2025). Current Trends in Clinical Trials of Prodrugs. Pharmaceuticals, 18(2), 210. https://doi.org/10.3390/ph18020210









