Application of Microneedles for High-Molecular-Weight Dextran Penetration Across the Buccal Mucosa
Abstract
1. Introduction
2. Results and Discussion
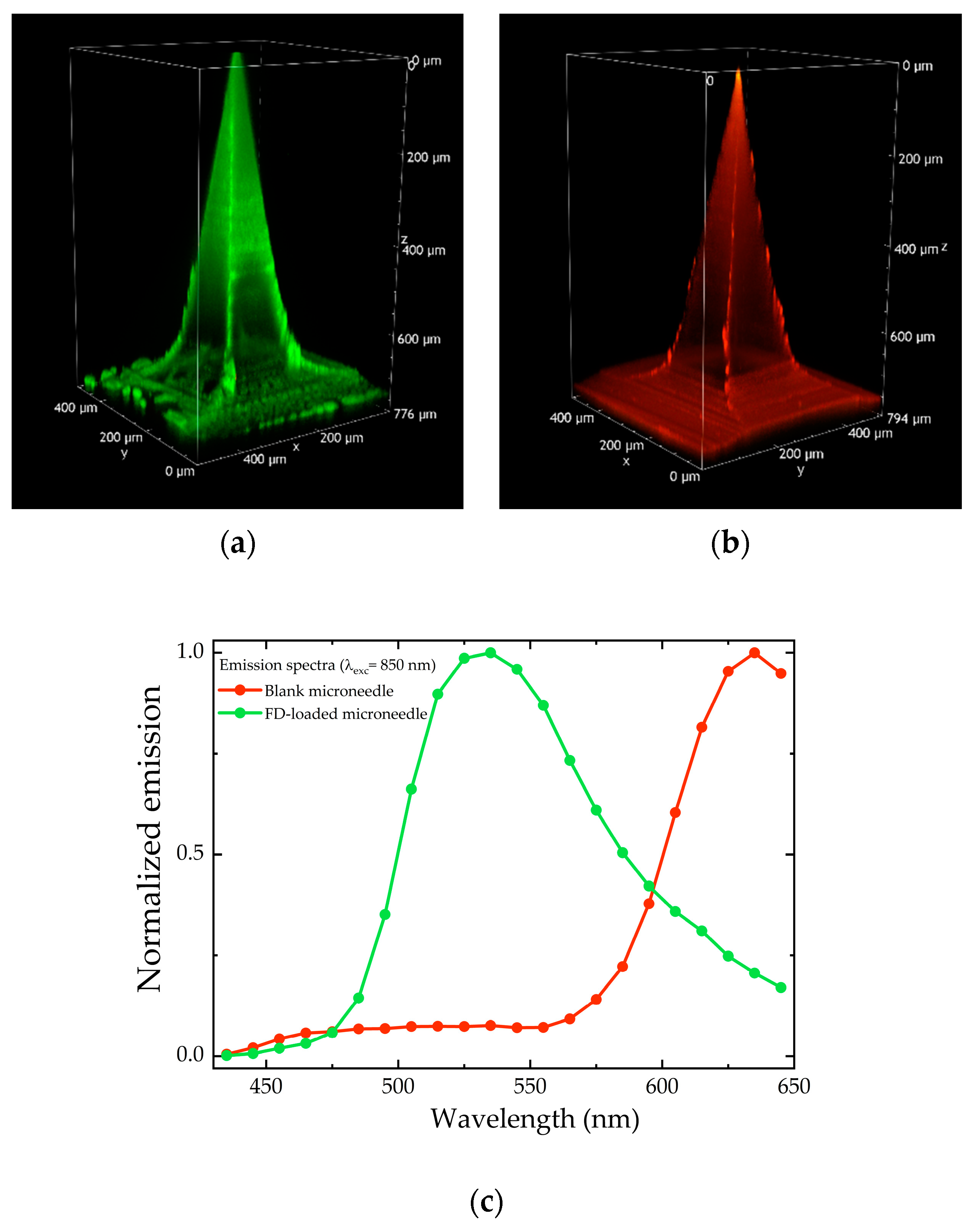
2.1. Soluble MN Characterization
2.2. Permeation Studies
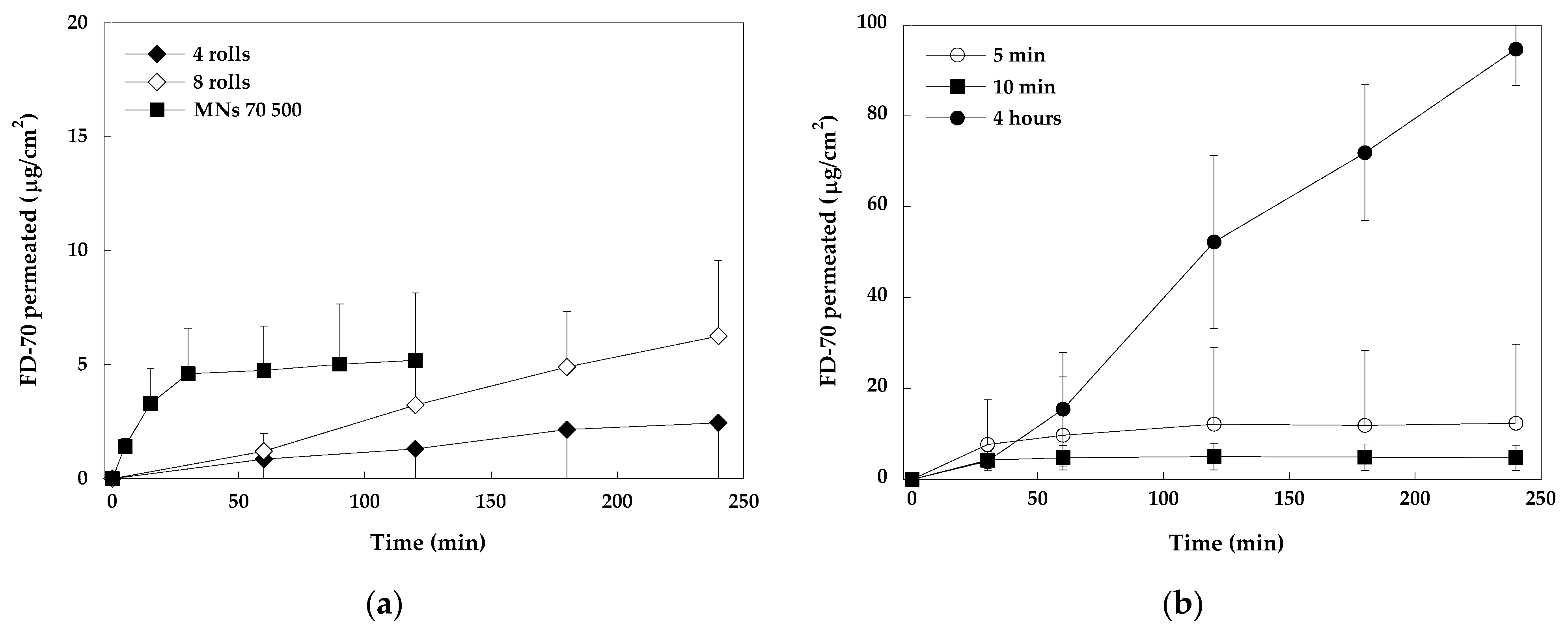
2.2.1. Stainless Steel MNs vs. Soluble MNs
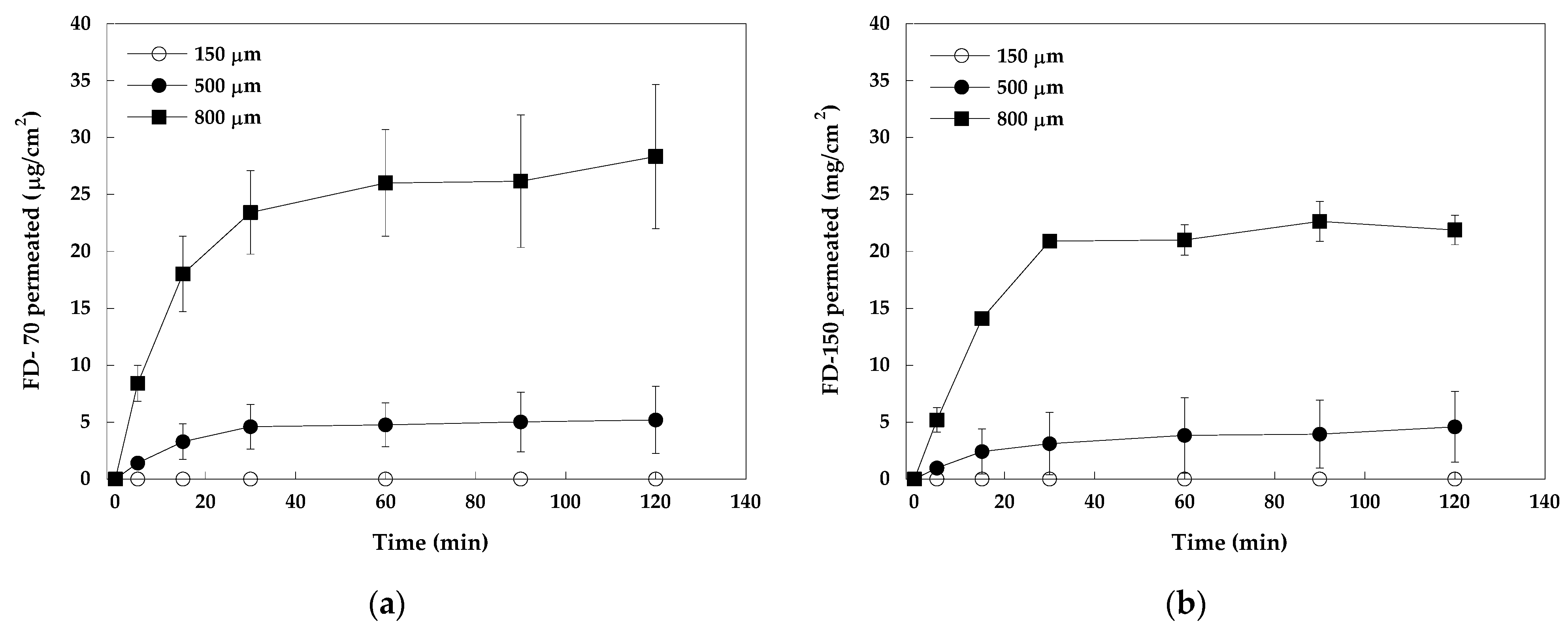
2.2.2. In Vitro Evaluation of Dissolving Microneedle Arrays
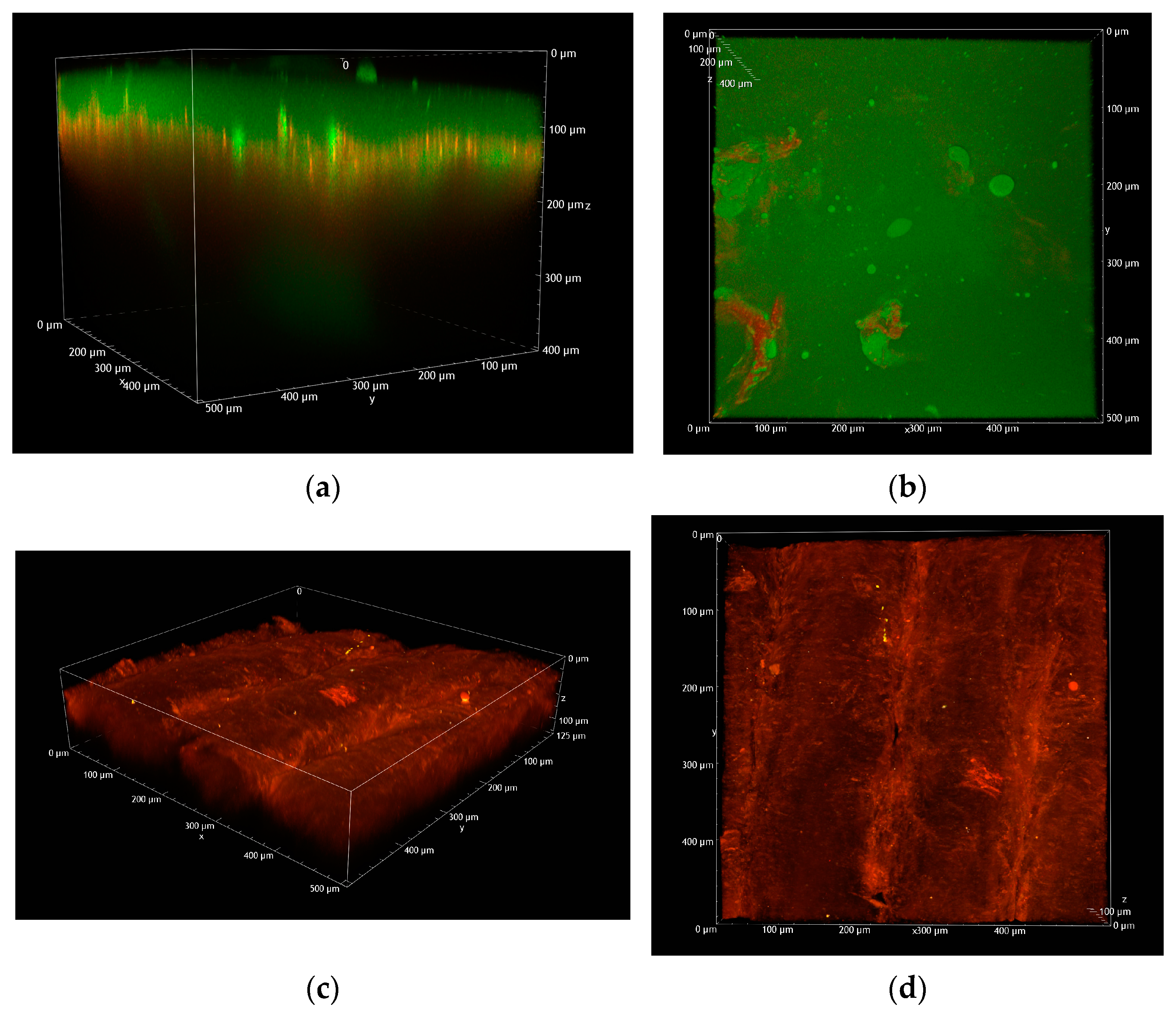
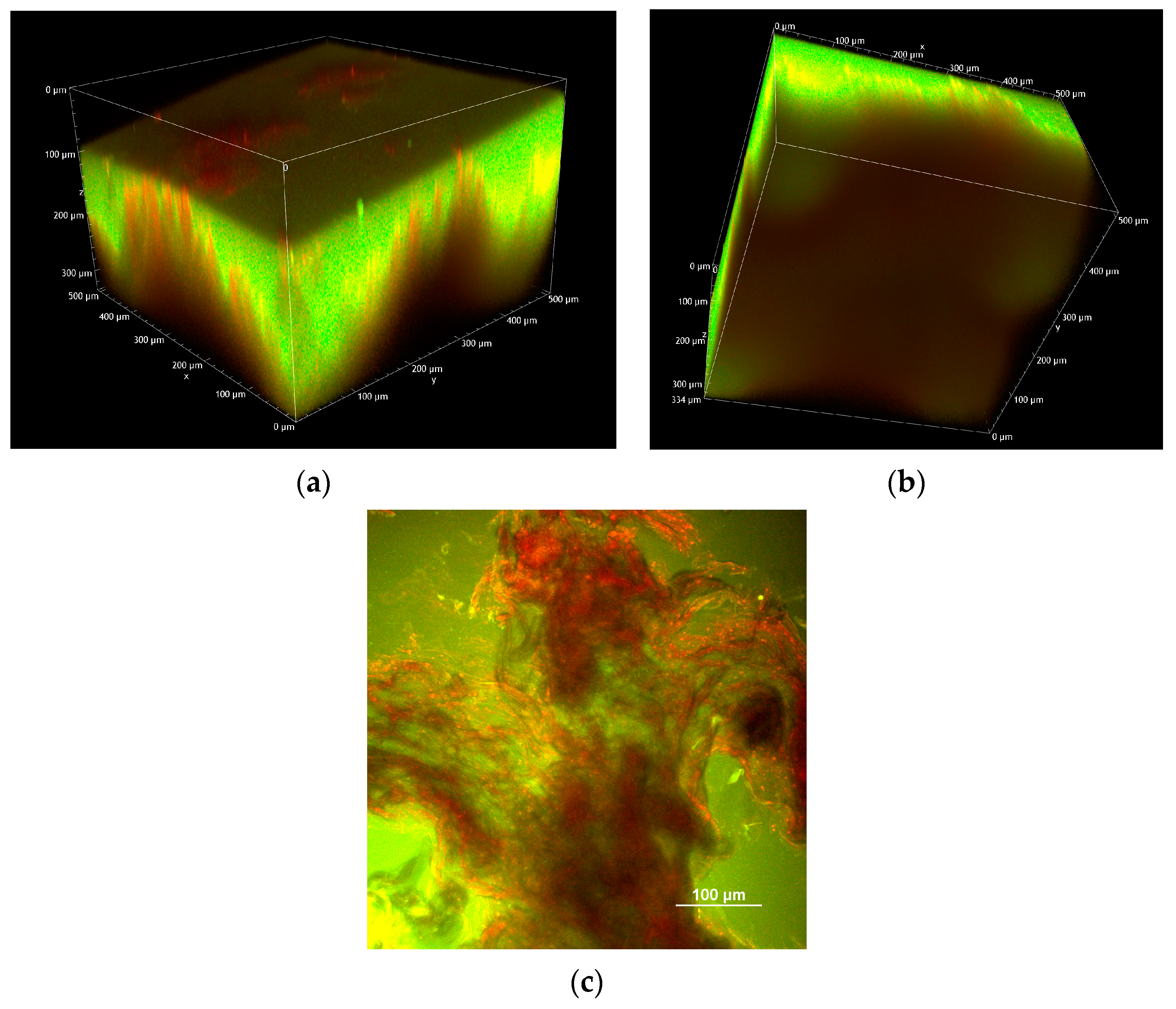
2.2.3. Two-Photon Microscopy Analysis
- 150 µm MNs loaded with FD-70
- 500 µm MNs loaded with FD-70
- 800 µm microneedles loaded with FD-70
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Analytical Method
3.2.2. Preparation of PVP Dissolving Microneedles
3.2.3. Permeation Studies
In Vitro Evaluation of Dissolving Microneedle Patches
In Vitro Evaluation of Stainless Steel Microneedles
3.2.4. Two-Photon Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawas-Qalaji, M.; Thu, H.E.; Hussain, Z. Oromucosal delivery of macromolecules: Challenges and recent developments to improve bioavailability. J. Control. Release 2022, 352, 726–746. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2023: An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2024, 29, 585. [Google Scholar] [CrossRef]
- Jena, D.; Srivastava, N.; Chauhan, I.; Verma, M. Challenges and Therapeutic Approaches for the Protein Delivery System: A Review. Pharm. Nanotechnol. 2024, 12, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, J.A.; Reed, B.L.; Finnin, B.C. Buccal penetration enhancers—How do they really work? J. Control. Release 2005, 105, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sohi, H.; Ahuja, A.; Ahmad, F.J.; Khar, R.K. Critical evaluation of permeation enhancers for oral mucosal drug delivery. Drug Dev. Ind. Pharm. 2010, 36, 254–282. [Google Scholar] [CrossRef]
- Padula, C.; Pescina, S.; Nicoli, S.; Santi, P. New Insights on the Mechanism of Fatty Acids as Buccal Permeation Enhancers. Pharmaceutics 2018, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Fantini, A.; Giulio, L.; Delledonne, A.; Pescina, S.; Sissa, C.; Nicoli, S.; Santi, P.; Padula, C. Buccal Permeation of Polysaccharide High Molecular Weight Compounds: Effect of Chemical Permeation Enhancers. Pharmaceutics 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef]
- Gerstel, M.S.; Place, V.A. Drug Delivery Device. U.S. Patent US3964482, 22 June 1976. [Google Scholar]
- Rzhevskiy, A.S.; Singh, T.R.R.; Donnelly, R.F.; Anissimov, Y.G. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J. Control. Release 2018, 270, 184–202. [Google Scholar] [CrossRef]
- Dsouza, L.; Ghate, V.M.; Lewis, S.A. Derma rollers in therapy: The transition from cosmetics to transdermal drug delivery. Biomed. Microdevices 2020, 22, 77. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- De Martino, S.; Battisti, M.; Napolitano, F.; Palladino, A.; Serpico, L.; Amendola, E.; Martone, A.; De Girolamo, P.; Squillace, A.; Dardano, P.; et al. Effect of microneedles shape on skin penetration and transdermal drug administration. Biomater. Adv. 2022, 142, 213169. [Google Scholar] [CrossRef]
- Oliveira, C.; Teixeira, J.A.; Oliveira, N.; Ferreira, S.; Botelho, C.M. Microneedles’ Device: Design, Fabrication, and Applications. Macromol 2024, 4, 320–355. [Google Scholar] [CrossRef]
- Le, Z.; Yu, J.; Quek, Y.J.; Bai, B.; Li, X.; Shou, Y.; Myint, B.; Xu, C.; Tay, A. Design principles of microneedles for drug delivery and sampling applications. Mater. Today 2023, 63, 137–169. [Google Scholar] [CrossRef]
- Al-Qallaf, B.; Das, D.B. Optimizing microneedle arrays for transdermal drug delivery: Extension to non-square distribution of microneedles. J. Drug Target. 2009, 17, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.E.N.; Franz-Montan, M.; Benso, B.; Gill, H.S. Microneedles for oral mucosal delivery—Current trends and perspective on future directions. Expert. Opin. Drug Deliv. 2023, 20, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Creighton, R.L.; Faber, K.A.; Tobos, C.I.; Doan, M.A.; Guo, T.; Woodrow, K.A. Oral mucosal vaccination using integrated fiber microneedles. J. Control. Release 2024, 367, 649–660. [Google Scholar] [CrossRef]
- Caffarel-Salvador, E.; Kim, S.; Soares, V.; Tian, R.Y.; Stern, S.R.; Minahan, D.; Yona, R.; Lu, X.; Zakaria, F.R.; Collins, J.; et al. A microneedle platform for buccal macromolecule delivery. Sci. Adv. 2021, 7, eabe2620. [Google Scholar] [CrossRef]
- Pireddu, R.; Schlich, M.; Marceddu, S.; Valenti, D.; Pini, E.; Fadda, A.M.; Lai, F.; Sinico, C. Nanosuspensions and Microneedles Roller as a Combined Approach to Enhance Diclofenac Topical Bioavailability. Pharmaceutics 2020, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.E.; Zwirner, J.; Ramani, R.S.; Ma, S.; Hussaini, H.M.; Waddell, J.N.; Hammer, N. Mechanical properties of human oral mucosa tissues are site dependent: A combined biomechanical, histological and ultrastructural approach. Clin. Exp. Dent. Res. 2020, 6, 602–611. [Google Scholar] [CrossRef]
- Farhat, W.; Chatelain, F.; Marret, A.; Faivre, L.; Arakelian, L.; Cattan, P.; Fuchs, A. Trends in 3D bioprinting for esophageal tissue repair and reconstruction. Biomaterials 2021, 267, 120465. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, R.; Patel, K.D.; Lobo, V.M.; Kumbhar, S.S.; Venuganti, V.V.K. Buccal mucosal application of dissolvable microneedle patch containing photosensitizer provides effective localized delivery and phototherapy against oral carcinoma. Int. J. Pharm. 2023, 640, 122991. [Google Scholar] [CrossRef] [PubMed]
- Diaz Del Consuelo, I.; Pizzolato, G.P.; Falson, F.; Guy, R.H.; Jacques, Y. Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J. Pharm. Sci. 2005, 94, 2777–2788. [Google Scholar] [CrossRef]
- Verbaan, F.J.; Bal, S.M.; van den Berg, D.J.; Groenink, W.H.; Verpoorten, H.; Lüttge, R.; Bouwstra, J.A. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J. Control. Release 2007, 117, 238–245. [Google Scholar] [CrossRef]
- Bisgaard, S.I.; Nguyen, L.Q.; Bøgh, K.L.; Keller, S.S. Dermal tissue penetration of in-plane silicon microneedles evaluated in skin-simulating hydrogel, rat skin and porcine skin. Biomater. Adv. 2023, 155, 213659. [Google Scholar] [CrossRef]
- Matsukawa, Y.; Lee, V.H.; Crandall, E.D.; Kim, K.J. Size-dependent dextran transport across rat alveolar epithelial cell monolayers. J. Pharm. Sci. 1997, 86, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Canakis, C.S.; Miller, J.W.; Gragoudas, E.S.; Edwards, A.; Weissgold, D.J.; Kim, I.; Delori, F.C.; Adamis, A.P. Diffusion of high molecular weight compounds through sclera. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1181–1185. [Google Scholar]
- Hutton, A.R.J.; McCrudden, M.T.C.; Larrañeta, E.; Donnelly, R.F. Influence of molecular weight on transdermal delivery of model macromolecules using hydrogel-forming microneedles: Potential to enhance the administration of novel low molecular weight biotherapeutics. J. Mater. Chem. B 2020, 8, 4202–4209. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Del Consuelo, I.; Jacques, Y.; Pizzolato, G.P.; Guy, R.H.; Falson, F. Comparison of the lipid composition of porcine buccal and esophageal permeability barriers. Arch. Oral Biol. 2005, 50, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Casula, L.; Pireddu, R.; Cardia, M.C.; Pini, E.; Valenti, D.; Schlich, M.; Sinico, C.; Marceddu, S.; Dragićević, N.; Fadda, A.M.; et al. Nanosuspension-Based Dissolvable Microneedle Arrays to Enhance Diclofenac Skin Delivery. Pharmaceutics 2023, 15, 2308. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantini, A.; Delledonne, A.; Casula, L.; Nicoli, S.; Pescina, S.; Cardia, M.C.; Lai, F.; Sissa, C.; Santi, P.; Padula, C. Application of Microneedles for High-Molecular-Weight Dextran Penetration Across the Buccal Mucosa. Pharmaceuticals 2025, 18, 158. https://doi.org/10.3390/ph18020158
Fantini A, Delledonne A, Casula L, Nicoli S, Pescina S, Cardia MC, Lai F, Sissa C, Santi P, Padula C. Application of Microneedles for High-Molecular-Weight Dextran Penetration Across the Buccal Mucosa. Pharmaceuticals. 2025; 18(2):158. https://doi.org/10.3390/ph18020158
Chicago/Turabian StyleFantini, Adriana, Andrea Delledonne, Luca Casula, Sara Nicoli, Silvia Pescina, Maria Cristina Cardia, Francesco Lai, Cristina Sissa, Patrizia Santi, and Cristina Padula. 2025. "Application of Microneedles for High-Molecular-Weight Dextran Penetration Across the Buccal Mucosa" Pharmaceuticals 18, no. 2: 158. https://doi.org/10.3390/ph18020158
APA StyleFantini, A., Delledonne, A., Casula, L., Nicoli, S., Pescina, S., Cardia, M. C., Lai, F., Sissa, C., Santi, P., & Padula, C. (2025). Application of Microneedles for High-Molecular-Weight Dextran Penetration Across the Buccal Mucosa. Pharmaceuticals, 18(2), 158. https://doi.org/10.3390/ph18020158









