Lebanese Medicinal Plants with Ophthalmic Properties
Abstract
1. Introduction
2. Major Ocular Diseases
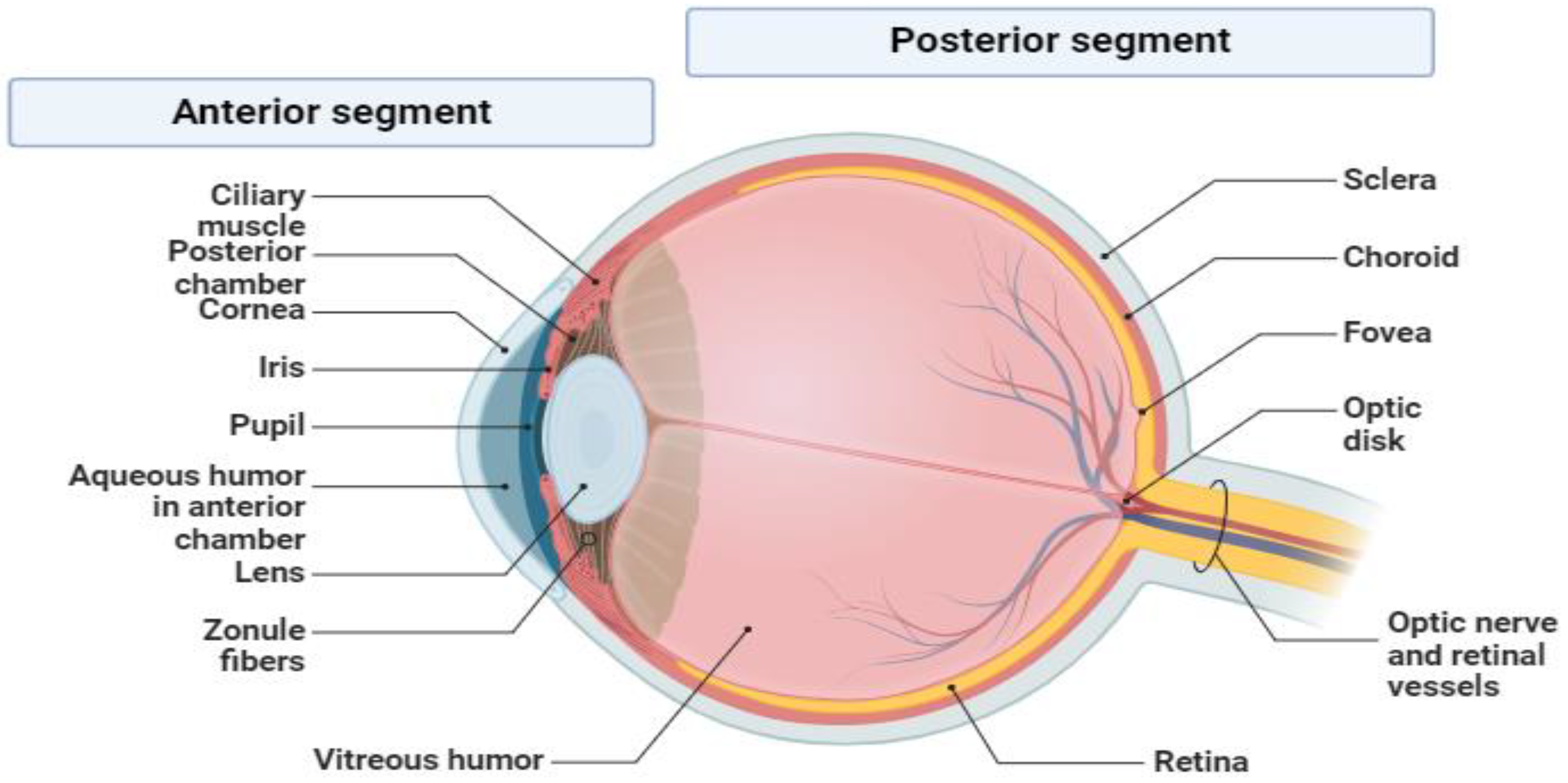
2.1. Eye Infections
2.2. Cataracts, Dry-Eye, and Allergies
2.3. Glaucoma, Eye Cancer, and Diabetic Retinopathy
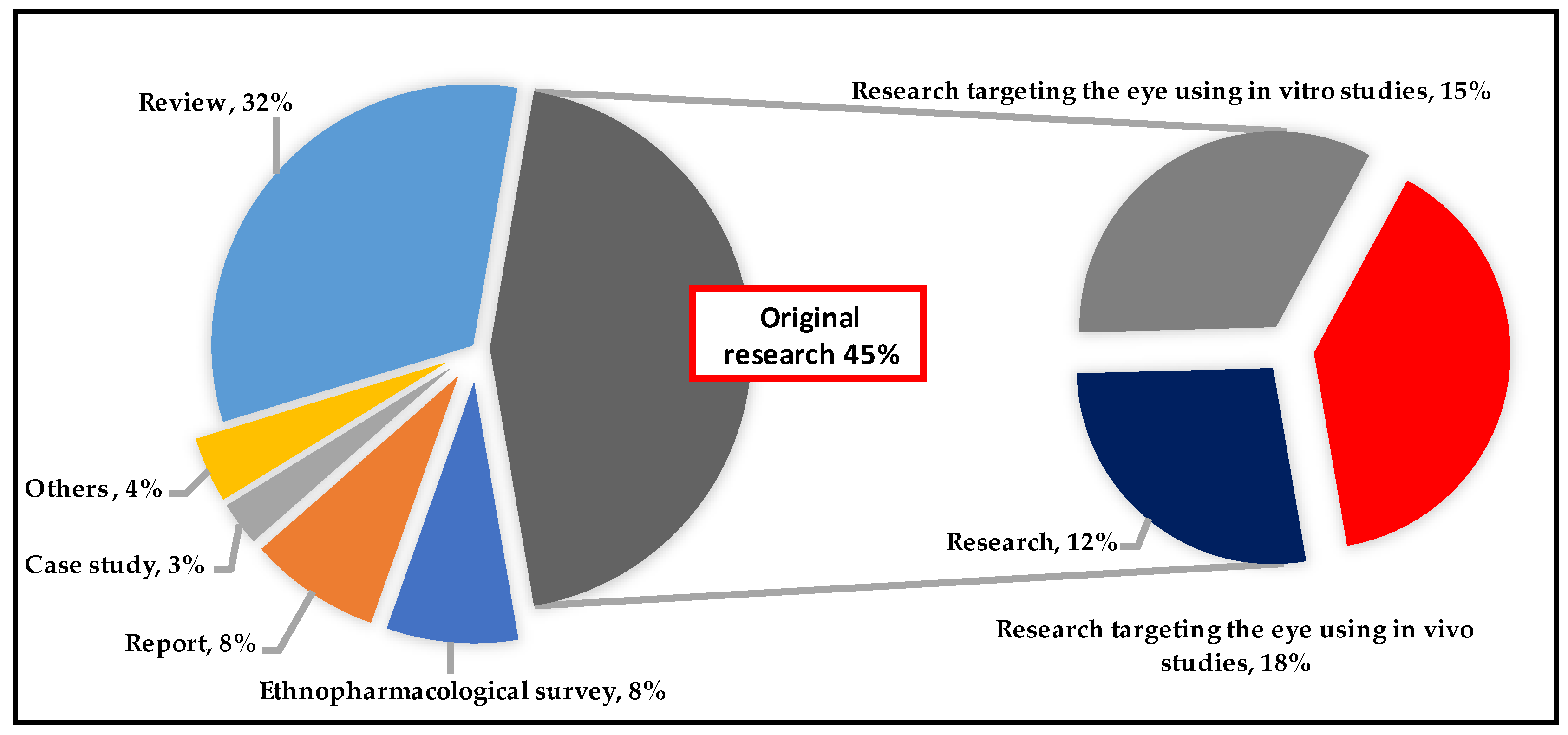
3. Methodology
4. Findings and Discussion
4.1. Family Classification
4.2. Plant Parts
4.3. Ocular Preparation and Administration
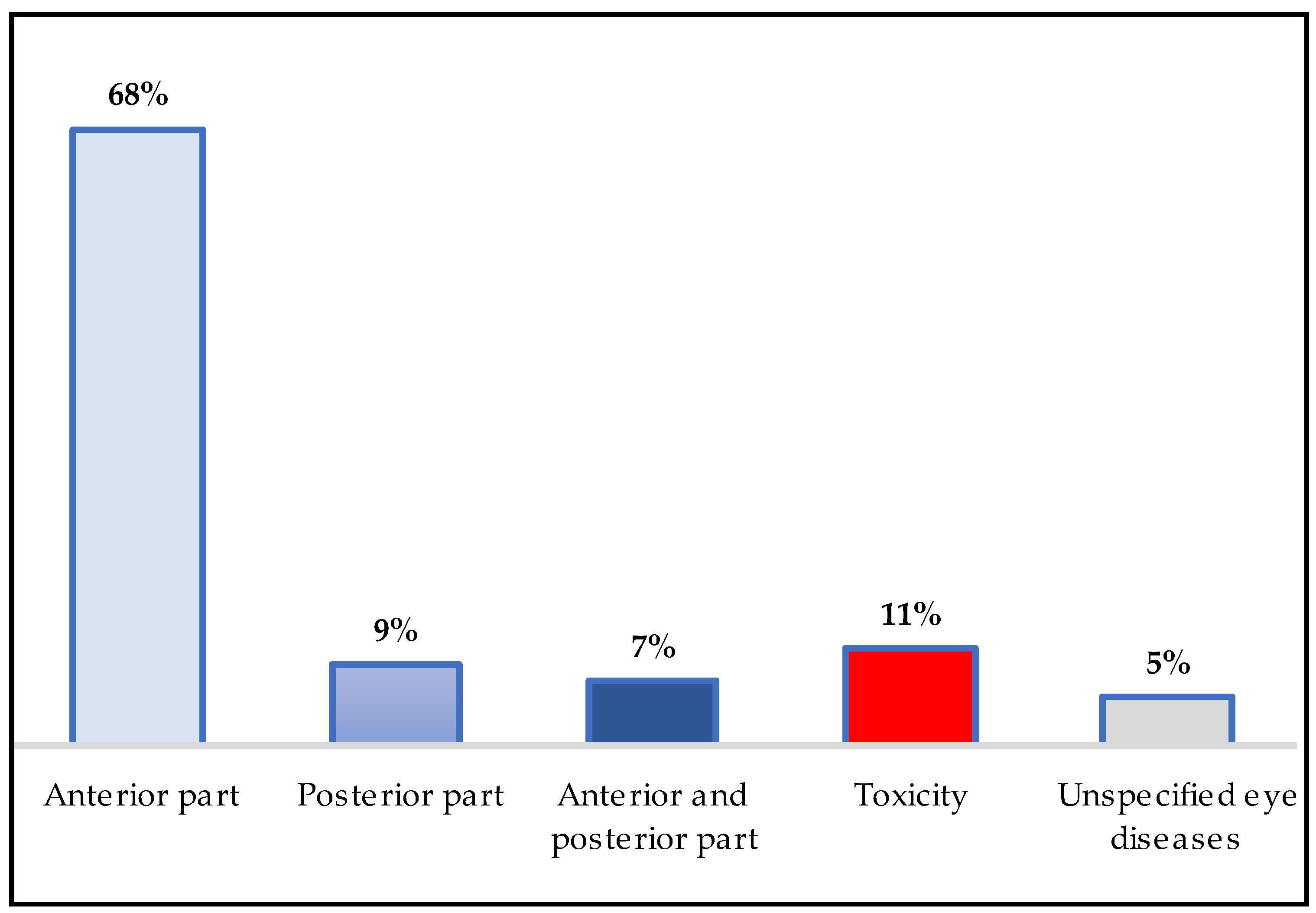
4.4. Disease Treatment Classification
4.5. Toxicology of Some Cited Medicinal Plants
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | American Chemical Society |
| AMD | Age-related macular degeneration |
| DM | Diabetes mellitus |
| DR | Diabetic retinopathy |
| EOs | Essential oil |
| IOP | Internal ocular pressure |
| MAPs | Medicinal and aromatic plants |
| PSED | Posterior-segment eye disease |
| TFOS DEWS II | Tear Film and Ocular Surface Society Dry Eye Workshop II |
| VEGF | Vascular endothelial growth factor |
| TM | Trabecular meshwork |
| UV | Ultraviolet radiation |
References
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Pinheiro, G.K.L.d.O.; Araújo Filho, I.d.; Araújo Neto, I.d.; Rêgo, A.C.M.; de Azevedo, E.P. Nature as a Source of Drugs for Ophthalmology. Arq. Bras. Oftalmol. 2018, 81, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.; Collison, D.J. Role of the Non-Neuronal Cholinergic System in the Eye. Life Sci. 2003, 72, 2013–2019. [Google Scholar] [CrossRef]
- Cowling, R.M.; Rundel, P.W.; Lamont, B.B.; Kalin Arroyo, M.; Arianoutsou, M. Plant Diversity in Mediterranean-Climate Regions. Trends Ecol. Evol. 1996, 11, 362–366. [Google Scholar] [CrossRef]
- Monari, S.; Ferri, M.; Salinitro, M.; Tassoni, A. Ethnobotanical Review and Dataset Compiling on Wild and Cultivated Plants Traditionally Used as Medicinal Remedies in Italy. Plants 2022, 11, 2041. [Google Scholar] [CrossRef] [PubMed]
- Pullaiah, T. (Ed.) Global Biodiversity: Selected Countries in Asia, 1st ed.; Apple Academic Press: New York, NY, USA, 2018; ISBN 978-0-429-48774-3. [Google Scholar]
- Baydoun, S.; Kanj, D.; Raafat, K.; Aboul Ela, M.; Chalak, L.; Arnold-Apostolides, N. Ethnobotanical and Economic Importance of Wild Plant Species of Jabal Moussa Bioreserve, Lebanon. J. Ecosyst. Ecogr. 2017, 7, 1000245. [Google Scholar] [CrossRef]
- Sangeetha, J.; Asokan, S. A Review on Traditional Medicine Used as Treatment for Conjunctivitis. J. Pharm. Drug Anal. 2018, 6, 191–196. [Google Scholar]
- Perez-Garmendia, R.; Rodriguez, A.L.d.E.; Ivan, R.-M.; Zuñiga, N.M.; Roberto, G.-S. Interplay between Oxidative Stress, Inflammation, and Amyloidosis in the Anterior Segment of the Eye; Its Pathological Implications. Oxidative Med. Cell. Longev. 2020, 2020, 6286105. [Google Scholar] [CrossRef] [PubMed]
- Bastawrous, A.; Burgess, P.I.; Mahdi, A.M.; Kyari, F.; Burton, M.J.; Kuper, H. Posterior Segment Eye Disease in Sub-S Aharan A Frica: Review of Recent Population-based Studies. Trop. Med. Int. Health 2014, 19, 600–609. [Google Scholar] [CrossRef]
- Committee on Public Health Approaches to Reduce Vision Impairment and Promote Eye Health; Board on Population Health and Public Health Practice; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. Making Eye Health a Population Health Imperative: Vision for Tomorrow; Teutsch, S.M., McCoy, M.A., Woodbury, R.B., Welp, A., Eds.; National Academies Press: Washington, DC, USA, 2016; p. 23471. ISBN 978-0-309-43998-5. [Google Scholar]
- WHO. World Report on Vision; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- BioRender. Available online: https://www.biorender.com/ (accessed on 10 October 2004).
- Sandhu, P.S.; Singh, B.; Gupta, V.; Bansal, P.; Kumar, D. Potential Herbs Used in Ocular Diseases. J. Pharm. Sci. 2011, 3, 1127. [Google Scholar]
- Teweldemedhin, M.; Gebreyesus, H.; Atsbaha, A.H.; Asgedom, S.W.; Saravanan, M. Bacterial Profile of Ocular Infections: A Systematic Review. BMC Ophthalmol. 2017, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Samoilă, O.; Gocan, D.; Mocan, A.; Moldovan, C. Medicinal Plants and Natural Products Used in Cataract Management. Front. Pharmacol. 2019, 10, 466. [Google Scholar] [CrossRef]
- Chu, K.-O.; Pang, C.-P. Herbal Molecules in Eye Diseases. Taiwan J. Ophthalmol. 2014, 4, 103–109. [Google Scholar] [CrossRef]
- Leonardi, A.; Motterle, L.; Bortolotti, M. Allergy and the Eye. Clin. Exp. Immunol. 2008, 153, 17–21. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Donald, K.; Benitez-del-Castillo, J.M.; Sophie, X.D. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C. Oxidative DNA Damage in the Human Trabecular Meshwork: Clinical Correlation in Patients with Primary Open-Angle Glaucoma. Arch. Ophthalmol. 2005, 123, 458. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Dimas, K.; Ulukaya, E.; Sakellaridis, N. Cancer of the Eye (Intraocular Cancer). In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2015; p. B978012801238399473X. ISBN 978-0-12-801238-3. [Google Scholar]
- Mouterde, P.; Charpin, A.; Greuter, W. Nouvelle Flore Du Liban et de La Syrie; Dar El-Machreq: Beyrouth, Lebanon, 1966. [Google Scholar]
- Choopani, R.; Saeed, S.; Kaveh, S.; Kaveh, N.; Dehghan, S. Pharmacological Treatment of Catarrh in Iranian Traditional Medicine. J. Tradit. Complement. Med. 2015, 5, 71–74. [Google Scholar] [CrossRef]
- Kianitalaei, A.; Feyzabadi, Z.; Hamedi, S.; Qaraaty, M. Althaea Officinalis in Traditional Medicine and Modern Phytotherapy. J Adv Pharm Edu Res 2019, 9, 154–161. [Google Scholar]
- Aslam, M.S.; Ahmad, M.S. Alhagi Maurorum and Tamarix Alphylla-Two Medicinal Weeds Mentioned in Holy Quran and Ahadith and Their Ethnomedicinal Uses in District Rajhanpur of Pakistan. Univers. J. Pharm. Res. 2016, 1, 80–84. [Google Scholar] [CrossRef]
- Raju, T.N.; Kanth, V.R.; Lavanya, K. Effect of Methanolic Extract of Allium Sativum (AS) in Delaying Cataract in STZ-Induced Diabetic Rats. J. Ocul. Biol. Dis. Inform. 2008, 1, 46. [Google Scholar] [CrossRef] [PubMed]
- Afarid, M.; Sadeghi, E.; Johari, M.; Namvar, E.; Sanie-Jahromi, F. Evaluation of the Effect of Garlic Tablet as a Complementary Treatment for Patients with Diabetic Retinopathy. J. Diabetes Res. 2022, 2022, 6620661. [Google Scholar] [CrossRef] [PubMed]
- Sheikh Saeed, A. Medicinal Wild Plant from Lahore-Islamabad Motorway (M-2). Pak. J. Bot. 2007, 39, 355–375. [Google Scholar]
- Tudor, C.; Pintea, A. A Brief Overview of Dietary Zeaxanthin Occurrence and Bioaccessibility. Molecules 2020, 25, 4067. [Google Scholar] [CrossRef]
- Xuan, T.D.; Khanh, T.D. Chemistry and Pharmacology of Bidens Pilosa: An Overview. J. Pharm. Investig. 2016, 46, 91–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, K.; Yang, Z.; Wang, Y.; Si, H. The Effect of the Aqueous Extract of Bidens Pilosa L. on Androgen Deficiency Dry Eye in Rats. Cell. Physiol. Biochem. 2016, 39, 266–277. [Google Scholar] [CrossRef]
- Tene, V.; Malagón, O.; Finzi, P.V.; Vidari, G.; Armijos, C.; Zaragoza, T. An Ethnobotanical Survey of Medicinal Plants Used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007, 111, 63–81. [Google Scholar] [CrossRef]
- Vahedian, Z.; Fang, L.; Fakhraie, G.; Amini, H.; Chiou, A.; Psychias, A.; Bovet, J.; Mozaffarieh, M. The Effect of Borage on Retinal Venous Pressure of Healthy Subjects with the Flammer Syndrome Running Title: Borage and Retinal Venous Pressure. JOJO 2017, 5, 555659. [Google Scholar] [CrossRef]
- Annaz, H.; Sane, Y.; Bitchagno, G.T.M.; Ben Bakrim, W.; Drissi, B. Caper (Capparis spinosa L.): An Updated Review on Its Phytochemistry, Nutritional Value, Traditional Uses, and Therapeutic Potential. Front. Pharmacol. 2022, 13, 878749. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The Pharmacological Importance Of Centaurea Cyanus—A Review. Pharm. Rev. Res. 2015, 5, 379–384. [Google Scholar]
- Ajayi, A.; Tanayen, J.; Magomere, A.; Ezeonwumelu, J. Antinociceptive and Anti-Inflammatory Effects of Aqueous Extract of Chenopodium Opulifolium Schrad Leaves. J. Intercult. Ethnopharmacol. 2017, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Janda, K.; Gutowska, I.; Geszke-Moritz, M.; Jakubczyk, K. The Common Cichory (Cichorium intybus L.) as a Source of Extracts with Health-Promoting Properties—A Review. Molecules 2021, 26, 1814. [Google Scholar] [CrossRef]
- Puhlmann, M.-L.; de Vos, W.M. Back to the Roots: Revisiting the Use of the Fiber-Rich Cichorium intybus L. Taproots. Adv. Nutr. 2020, 11, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.D.; Thorat, K.B. Evaluation of Anticataract Activity of Citrullus colocynthis (L). Fruit Extract on Goat Lens. J. Emerg. Technol. Innov. Res. 2021, 8, b41–b52. [Google Scholar]
- Safaa, B.; Lamis, C.; Helena, D.; Nelly, A. Ethnopharmacological Survey of Medicinal Plants Used in Traditional Medicine by the Communities of Mount Hermon, Lebanon. J. Ethnopharmacol. 2015, 173, 139–156. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Ajayan, S.; Mukkudakkattu, S.; Nechiyil, K.; Nambi, N. Review of Unique Ophthalmic Formulations in Vaidya Manorama: A Traditional Kerala Ayurveda Literature. J. Ayurveda Integr. Med. 2022, 13, 100576. [Google Scholar] [CrossRef]
- Kuete, V. Physical, Hematological, and Histopathological Signs of Toxicity Induced by African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 635–657. ISBN 978-0-12-800018-2. [Google Scholar]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Ariño, A. Phytochemicals in Daucus Carota and Their Health Benefits—Review Article. Foods 2019, 8, 424. [Google Scholar] [CrossRef]
- Taiwo, E.A.; Abdulkareem, T.T.; Fajemisin, E. The Nutraceutical Potential of Carrots Carotenoids in Chronic Eyes Defects (CEDs): A Review. SSRN J. 2021. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, S.K.; Srivastava, S.; Agrawal, S.S.; Saxena, R. Lowering of Intraocular Pressure by Topical Application of Daucus Carota Seed Extract in Rabbits. Indian J. Exp. Biol. 2008, 46, 541–546. [Google Scholar] [PubMed]
- European Medicines Agency. Assessment Report on Euphrasia officinalis L. and Euphrasia Rostkoviana Hayne, Herba, a Report of Committee on Herbal Medicinal Products; European Medicines Agency: London, UK, 2010. [Google Scholar]
- Bigagli, E.; Cinci, L.; D’Ambrosio, M.; Luceri, C. Pharmacological Activities of an Eye Drop Containing Matricaria Chamomilla and Euphrasia Officinalis Extracts in UVB-Induced Oxidative Stress and Inflammation of Human Corneal Cells. J. Photochem. Photobiol. B Biol. 2017, 173, 618–625. [Google Scholar] [CrossRef]
- Paduch, R.; Woźniak, A.; Niedziela, P.; Rejdak, R. Assessment of Eyebright (Euphrasia Officinalis L.) Extract Activity in Relation to Human Corneal Cells Using In Vitro Tests. Balk. Med. J. 2014, 33, 29–36. [Google Scholar] [CrossRef]
- Mohammad Sajjad, I.; Ali, A.; Seyed Hassan, T.; Alireza, S. The Curious Cases of Burn by Fig Tree Leaves. Indian J. Dermatol. 2019, 64, 71. [Google Scholar] [CrossRef]
- Pinto, A.R.; Machado Cunha, I.; Rebelo Gomes, E. Fig Tree-Induced Phytophotodermatitis: A Case Report on the Perils of a Hobby. Cureus 2023, 15, e41888. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H.; Mahajan, R.T. Traditional Uses, Phytochemistry and Pharmacology of Ficus carica: A Review. Pharm. Biol. 2014, 52, 1487–1503. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, S.K.; Agrawal, S.S.; Srivastava, S.; Saxena, R. Oculohypotensive Effects of Foeniculum Vulgare in Experimental Models of Glaucoma. Indian J. Physiol. Pharmacol. 2008, 52, 77–83. [Google Scholar]
- Omar Adil, H.; Abu-Raghif, A.R.; Rasheed, A.M.; Al-Yawer, M.A. Effect of Foeniculum Vulgare Seed Aqueous Extract Eye Drops on Selenite Induced Cataract in Rabbits. Int. J. Pharm. Sci. Rev. Res 2007, 47, 83–87. [Google Scholar]
- Manzoor, A.R.; Bilal, A.D.; Shahnawaz, N.S.; Bilal, A.B.; Mushtaq, A.Q. Foeniculum Vulgare: A Comprehensive Review of Its Traditional Use, Phytochemistry, Pharmacology, and Safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- David, S.R. Fumaria Officinalis: Beggary, Common Fumitory, Earth Smoke [Fumaria officinalis L.]. In Materia Medica of New and Old Homeopathic Medicines; David, S.R., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 119–120. ISBN 978-3-030-65919-6. [Google Scholar]
- Küpeli Akkol, E.; Ilhan, M.; Kozan, E.; Gürağaç Dereli, F.T.; Sak, M.; Eduardo, S.-S. Insecticidal Activity of Hyoscyamus Niger L. on Lucilia Sericata Causing Myiasis. Plants 2020, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Moshiri, M.; Alizadeh, J.; Balali-Mood, M. Black Henbane and Its Toxicity—A Descriptive Review. Avicenna J. Phytomed 2014, 4, 297–311. [Google Scholar] [PubMed]
- Meher, S.; Ali, I.; Sami, A.; Ismail, M.; Naheed, N.; Khan, S.A. Screening of Some Medicinal Plants for Antibacterial Activity against Conjunctivitis. J. Anim. Plant Sci. 2017, 27, 2069–2074. [Google Scholar]
- Committee on Herbal Medicinal Products. Assessment Report on Linum usitatissimum L., Semen; 2015. Available online: https://www.ema.europa.eu/en/medicines/herbal/lini-semen (accessed on 10 October 2024).
- Pinheiro, M.N., Jr.; Santos, P.M.D.; Santos, R.C.R.D.; Barros, J.D.N.; Passos, L.F.; Cardoso Neto, J. Oral Flaxseed Oil (Linum usitatissimum) in the Treatment for Dry-Eye Sjögren’s Syndrome Patients. Arq. Bras. Oftalmol. 2007, 70, 649–655. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Renu, A.; Sk, G.; Sushma, S.; Rohit, S. IOP Lowering Effects of Ocimum Basilicum Seed Extract in Two Rabbit Models of Ocular Hypertension. J. Clin. Health Sci. 2019, 4, 39. [Google Scholar] [CrossRef]
- Ferreira, D.M.; de Oliveira, N.M.; Chéu, M.H.; Meireles, D.; Lopes, L.; Oliveira, M.B.; Machado, J. Updated Organic Composition and Potential Therapeutic Properties of Different Varieties of Olive Leaves from Olea Europaea. Plants 2023, 12, 688. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid.-Based Complement. Altern. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef]
- Degerli, S.; Tepe, B.; Celiksoz, A.; Berk, S.; Malatyali, E. In Vitro Amoebicidal Activity of Origanum Syriacum and Origanum Laevigatum on Acanthamoeba Castellanii Cysts and Trophozoites. Exp. Parasitol. 2012, 131, 20–24. [Google Scholar] [CrossRef]
- Basile, A.A.; Mandelli, G.; Cendali, M.; Hufnagel, R. The Lubricating Effect of Eye Drops Containing Hyaluronic Acid and Mallow Extract in Patients with Dry Eye Disease—A Pilot Study. Medicina 2023, 59, 958. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- Lodhi, S.; Vadnere, G.; Sharma, V.; Usman, M. Marrubium Vulgare L.: A Review on Phytochemical and Pharmacological Aspects. J. Intercult. Ethnopharmacol. 2017, 6, 429. [Google Scholar] [CrossRef]
- Jeung, I.C.; Jee, D.; Rho, C.-R.; Kang, S. Melissa Officinalis L. Extracts Protect Human Retinal Pigment Epithelial Cells against Oxidative Stress-Induced Apoptosis. Int. J. Med. Sci. 2016, 13, 139–146. [Google Scholar] [CrossRef]
- Lee, E.K.; Kim, Y.J.; Kim, J.Y.; Song, H.B.; Yu, H.G. Melissa Officinalis Extract Inhibits Laser-Induced Choroidal Neovascularization in a Rat Model. PLoS ONE 2014, 9, e110109. [Google Scholar] [CrossRef]
- Eissa, T.A.F.; Palomino, O.M.; Carretero, M.E.; Gómez-Serranillos, M.P. Ethnopharmacological Study of Medicinal Plants Used in the Treatment of CNS Disorders in Sinai Peninsula, Egypt. J. Ethnopharmacol. 2014, 151, 317–332. [Google Scholar] [CrossRef]
- Amato, R.; Canovai, A.; Melecchi, A.; Maci, S.; Quintela, F.; Fonseca, B.A.; Cammalleri, M.; Dal Monte, M. Efficacy of a Spearmint (Mentha spicata L.) Extract as Nutritional Support in a Rat Model of Hypertensive Glaucoma. Trans. Vis. Sci. Technol. 2023, 12, 6. [Google Scholar] [CrossRef]
- Prashant, D.T.; Marybeth, K.F.; Sanjay, V.P.; Keith, H.B. Oleander-Associated Keratitis and Uveitis. Cornea 2022, 41, 1305–1307. [Google Scholar] [CrossRef]
- Najafian, Y.; Hamedi, S.S.; Kaboli Farshchi, M.; Feyzabadi, Z. Plantago Major in Traditional Persian Medicine and Modern Phytotherapy: A Narrative Review. Electron. Physician 2018, 10, 6390–6399. [Google Scholar] [CrossRef]
- EMA/HMPC/, 437859. Assessment Report on Plantago Lanceolata L., Folium; 2011. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-plantago-lanceolata-l-folium_en.pdf (accessed on 10 October 2024).
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.P.; Mahyari, S.; Hassani, F.V.; Karimi, G. A Review of Traditional Uses, Phytochemistry and Pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef]
- Mahboubi, M. Rosa Damascena as Holy Ancient Herb with Novel Applications. J. Tradit. Complement. Med. 2016, 6, 10–16. [Google Scholar] [CrossRef]
- Biswas, N.R.; Gupta, S.K.; Das, G.K.; Kumar, N.; Mongre, P.K.; Haldar, D.; Beri, S. Evaluation of Ophthacare Eye Drops a Herbal Formulation in the Management of Various Ophthalmic Disorders. Phytother. Res. 2001, 15, 618–620. [Google Scholar] [CrossRef]
- EMA/HMPC/137298/2013 Committee on Herbal Medicinal Products (HMPC). Community herbal monograph on Rosa gallica L., Rosa centifolia L., Rosa damascena Mill., flos EMA/HMPC/137299/2013; 2013.
- Organisciak, D.T.; Darrow, R.M.; Rapp, C.M.; Smuts, J.P.; Armstrong, D.W.; Lang, J.C. Prevention of Retinal Light Damage by Zinc Oxide Combined with Rosemary Extract. Mol. Vis. 2013, 19, 1433–1445. [Google Scholar]
- Cannas, S.; Usai, D.; Pinna, A.; Benvenuti, S.; Roberta, T. Essential Oils in Ocular Pathology: An Experimental Study. J. Infect. Dev. Ctries 2015, 9, 650–654. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Homa, M.; Fekete, I.; Böszörményi, A.; Singh, Y.; Selvam, K. Antifungal Effect of Essential Oils against Fusarium Keratitis Isolates. Planta Med. 2015, 81, 1277–1284. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Hilan, C.; Khater, C. Traditional Uses of Salvia Libanotica (East Mediterranean Sage) and the Effects of Its Essential Oils. J. Ethnopharmacol. 2000, 71, 513–520. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C. Essential Oil of Common Sage (Salvia officinalis L.) from Jordan: Assessment of Safety in Mammalian Cells and Its Antifungal and Anti-Inflammatory Potential. BioMed Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Francés, V.; Hahn, E.; Segundo, R.; Rivera, D.; Roser, V.; Cañigueral, S. Ethnopharmacological and Chemical Characterization of Salvia Species Used in Valencian Traditional Herbal Preparations. Front. Pharmacol. 2017, 8, 467. [Google Scholar] [CrossRef]
- Lin, C.; Li, C.; Liao, P.; Tse, L.; Huang, W.; Cheng, H.; Cheng, Y. Silibinin Inhibits VEGF Secretion and Age-Related Macular Degeneration in a Hypoxia-Dependent Manner through the PI-3 Kinase/Akt/mTOR Pathway: Silibinin Inhibits Retinal Neovascularization. Br. J. Pharmacol. 2013, 168, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Cansever, E.; Turker, A.U. In Vitro Culture and Biological Activity of Solanum Dulcamara, a Medicinal Plant. Planta Med. 2007, 73, P_182. [Google Scholar] [CrossRef]
- Zahara, K.; Ahmad, N.; Bibi, Y.; Bibi, F.; Sadaf, H.M.; Sardar, N. An Insight to Therapeutic Potential and Phytochemical Profile of Solanum villosum (L). Med. Drug Discov. 2019, 2, 100007. [Google Scholar] [CrossRef]
- Egyptian Herbal Monograph; Ismail, S.I.; Abdel-Azim, D.N.S.; Shams, D.K.A.; Batanouny, K.H.; Batanouny, P.K.H. Uriginea maritima (L.), 195-200. EDA, Egypt. 2022. Available online: https://edaegypt.gov.eg/media/4h3gfxr5/urginea-maritima-l-baker-%D8%A8%D8%B5%D9%84-%D8%A7%D9%84%D8%B9%D9%86%D8%B5%D9%84.pdf (accessed on 10 October 2024).
- Fan, W.; Fan, L.; Peng, C.; Zhang, Q.; Wang, L. Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics and Toxicology of Xanthium strumarium L.: A Review. Molecules 2019, 24, 359. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A. A Phytopharmacological Review on the Omani Medicinal Plant: Ziziphus Jujube. J. King Saud Univ.-Sci. 2019, 31, 1352–1357. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J. Ziziphus Spina-Christi (Christ’s Thorn Jujube). CABI Compend. 2017, 57569. [Google Scholar] [CrossRef]
- Ohia, S.E.; Opere, C.A.; LeDay, A.M. Pharmacological Consequences of Oxidative Stress in Ocular Tissues. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 579, 22–36. [Google Scholar] [CrossRef]
- Adelli, G.R. Phytochemicals in Ocular Health: Therapeutic Potential and Delivery Challenges. World J. Pharmacol. 2013, 2, 18. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants 2021, 11, 87. [Google Scholar] [CrossRef]
- Borek, C. Antioxidant Health Effects of Aged Garlic Extract. J. Nutr. 2001, 131, 1010S–1015S. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Maulik, S.K. Effect of Garlic on Cardiovascular Disorders: A Review. Nutr. J. 2002, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Arellano Buendía, A.S.; Tostado González, M.; Sánchez Reyes, O.; García Arroyo, F.E.; Argüello García, R.; Tapia, E.; Sánchez Lozada, L.G.; Osorio Alonso, H. Immunomodulatory Effects of the Nutraceutical Garlic Derivative Allicin in the Progression of Diabetic Nephropathy. Int. J. Mol. Sci. 2018, 19, 3107. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yue, Z.; Nie, L.; Zhao, P.; Zhu, K.; Wang, Q. Biological Functions of Diallyl Disulfide, a Garlic-Derived Natural Organic Sulfur Compound. Evid. Based Complement. Altern. Med. 2021, 2021, 5103626. [Google Scholar] [CrossRef]
- Chai, G.-R.; Liu, S.; Yang, H.-W.; Chen, X.-L. Quercetin Protects against Diabetic Retinopathy in Rats by Inducing Heme Oxygenase-1 Expression. Neural Regen. Res. 2021, 16, 1344. [Google Scholar] [CrossRef] [PubMed]
- Stefek, M. Natural Flavonoids as Potential Multifunctional Agents in Prevention of Diabetic Cataract. Interdiscip. Toxicol. 2011, 4, 69–77. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Du, X. The Therapeutic Use of Quercetin in Ophthalmology: Recent Applications. Biomed. Pharmacother. 2021, 137, 111371. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D.; Jusha, M.; Saroha, K.; Singh, N.; Vashishta, B. Antioxidant and Free Radical Scavenging Potential of Citrullus colocynthis (L.) Schrad. Methanolic Fruit Extract. Acta Pharm. 2008, 58, 215–220. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Hanganu, D.; Vlase, A.-M.; Ielciu, I.; Crișan, G.; Fiţ, N.; Niculae, M.; Bab, T.; Pall, E.; et al. Revealing the Phenolic Composition and the Antioxidant, Antimicrobial and Antiproliferative Activities of Two Euphrasia Sp. Extracts. Plants 2024, 13, 1790. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef]
- Nafees, S.; Akhtar, J.; Kaur, J. Indian Traditional Medicinal Plants in Ophthalmic Diseases. Avicenna J. Phytomed. 2022, 12, 566–575. [Google Scholar] [CrossRef]
- Basgedik, B.; Ugur, A.; Sarac, N. Antimicrobial, Antioxidant, Antimutagenic Activities, and Phenolic Compounds of Iris Germanica. Ind. Crops Prod. 2014, 61, 526–530. [Google Scholar] [CrossRef]
- Kauser, S.; Hussain, A.; Ashraf, S.; Fatima, G.; Ambreen; Javaria, S.; Abideen, Z.U.; Kabir, K.; Yaqub, S.; Akram, S.; et al. Flaxseed (Linum usitatissimum); Phytochemistry, Pharmacological Characteristics and Functional Food Applications. Food Chem. Adv. 2024, 4, 100573. [Google Scholar] [CrossRef]
- Roncone, M.; Bartlett, H.; Eperjesi, F. Essential Fatty Acids for Dry Eye: A Review. Contact Lens Anterior Eye 2010, 33, 49–54. [Google Scholar] [CrossRef]
- Saccà, S.C.; Cutolo, C.A.; Ferrari, D.; Corazza, P.; Traverso, C.E. The Eye, Oxidative Damage and Polyunsaturated Fatty Acids. Nutrients 2018, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Leaves, Flowers, Immature Fruits and Leafy Flowered Stems of Malva Sylvestris: A Comparative Study of the Nutraceutical Potential and Composition. Food Chem. Toxicol. 2010, 48, 1466–1472. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa Officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial Activity and Mechanisms of Salvia Sclarea Essential Oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef]
- Maaloul, S.; Ghzaiel, I.; Mahmoudi, M.; Mighri, H.; Pires, V.; Vejux, A.; Martine, L.; De Barros, J.-P.P.; Prost-Camus, E.; Boughalleb, F.; et al. Characterization of Silybum Marianum and Silybum Eburneum Seed Oils: Phytochemical Profiles and Antioxidant Properties Supporting Important Nutritional Interests. PLoS ONE 2024, 19, e0304021. [Google Scholar] [CrossRef]
- Fani, M.; Kohanteb, J. In Vitro Antimicrobial Activity of Thymus Vulgaris Essential Oil Against Major Oral Pathogens. J. Evid.Based Complement. Altern. Med. 2017, 22, 660–666. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Čmiková, N.; Kačániová, M. Thymus Vulgaris Essential Oil and Its Biological Activity. Plants 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Carović-Stanko, K.; Petek, M.; Grdiša, M.; Pintar, J.; Bedeković, D.; Herak Ćustić, M. Medicinal Plants of the Family Lamiaceae as Functional Foods—A Review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Alagar Yadav, S.; Koshi, F.S. Phytochemicals from Solanaceae Family and Their Anticancer Properties. In Medicinal Plants; Kumar, S., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-80356-032-8. [Google Scholar]
- Joo, J.-H. Effects and Pharmacological Use of Alkaloids on the Eyes. In Drug Repurposing—Advances, Scopes and Opportunities in Drug Discovery; Rudrapal, M., Ed.; IntechOpen: London, UK, 2023; ISBN 978-1-83768-776-3. [Google Scholar]
- Sharifi-Rad, J.; Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Taheri, Y.; Shabnum, S. Malva Species: Insights on Its Chemical Composition towards Pharmacological Applications. Phytother. Res. 2020, 34, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Liu, X.; Li, J.; Zhang, J.; Liu, D. Chemical Constituents and Pharmacological Activities of Medicinal Plants from Rosa Genus. Chin. Herb. Med. 2022, 14, 187–209. [Google Scholar] [CrossRef]
- Sakna, S.T.; Maghraby, Y.R.; Abdelfattah, M.S.; Farag, M.A. Phytochemical Diversity and Pharmacological Effects of Triterpenes from Genus Ziziphus: A Comprehensive Review. Phytochem. Rev. 2022, 22, 1611–1636. [Google Scholar] [CrossRef]
- El Beyrouthy, M.; Arnold, N.; Delelis-Dusollier, A.; Dupont, F. Plants Used as Remedies Antirheumatic and Antineuralgic in the Traditional Medicine of Lebanon. J. Ethnopharmacol. 2008, 120, 315–334. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Pokkalath, A.S.; Sawant, A.; Sawarkar, S.P. Herbal Medicine for Ocular Diseases: An Age Old Therapy and Its Future Perspective. J. Drug Deliv. Sci. Technol. 2022, 68, 102979. [Google Scholar] [CrossRef]
- Ye, X.; Fung, N.S.K.; Lam, W.C.; Lo, A.C.Y. Nutraceuticals for Diabetic Retinopathy: Recent Advances and Novel Delivery Systems. Nutrients 2024, 16, 1715. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Diego, M. Use of Strawberry Tree (Arbutus Unedo) as a Source of Functional Fractions with Biological Activities. Foods 2022, 11, 3838. [Google Scholar] [CrossRef]
- Raúl, D.-P.; Lourdes, G.-G.; Dámaso, H.-M. Carotenoid Composition of Strawberry Tree (Arbutus unedo L.) Fruits. Food Chem. 2016, 199, 165–175. [Google Scholar] [CrossRef]
- Mariela, P.; Rubén, B.; José Luis, B.; Patricia, P. Allium Sativum Produces Terpenes with Fungistatic Properties in Response to Infection with Sclerotium Cepivorum. Phytochemistry 2015, 115, 152–160. [Google Scholar] [CrossRef]
- Omidi, M.; Khandan-Mirkohi, A.; Kafi, M.; Rasouli, O.; Shaghaghi, A.; Kiani, M.; Zamani, Z. Comparative Study of Phytochemical Profiles and Morphological Properties of Some Damask Roses from Iran. Chem. Biol. Technol. Agric. 2022, 9, 51. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus Officinalis L.: An Update Review of Its Phytochemistry and Biological Activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A Comprehensive Insight on Ocular Pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Pubchem—Recherche Google. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 12 January 2025).
- Al-Ghadeer, H.; Al-Amry, M. Ocular Complications Resulting from the Use of Traditional Herbal Medicine in Central Saudi Arabia: A Review. Middle East Afr. J. Ophthalmol. 2021, 28, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Miraldi, E.; Masti, A.; Ferri, S.; Barni Comparini, I. Distribution of Hyoscyamine and Scopolamine in Datura Stramonium. Fitoterapia 2001, 72, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Dong, X.; Yan, F.; Guo, H.; Yang, J. Oleandrin: A Systematic Review of Its Natural Sources, Structural Properties, Detection Methods, Pharmacokinetics and Toxicology. Front. Pharmacol. 2022, 13, 822726. [Google Scholar] [CrossRef] [PubMed]
- Calf, O.W.; Huber, H.; Peters, J.L.; Weinhold, A.; Van Dam, N.M. Glycoalkaloid Composition Explains Variation in Slug Resistance in Solanum Dulcamara. Oecologia 2018, 187, 495–506. [Google Scholar] [CrossRef] [PubMed]



| Scientific Name | Local Name | Family | Part Used | Preparation | Ocular Treatments | Category | Type of Article | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Althaea officinalis | El Khaimah | Malvaceae | Flower | NA | Eye inflammation | Anterior part | Traditional medicine | [24] |
| Leaves | Puffy and swollen eyelids | Review | [25] | ||||||
| Conjunctivitis | |||||||||
| Eye discharge | |||||||||
| Hordeolum | |||||||||
| 2 | Alhagi maurorum | Chawk El Jamal | Papilionaceae | Flower | Ground flowers powdered with sugar | Cleaning the eye | Anterior part | Ethno-medicine | [26] |
| Improving eyesight | |||||||||
| 3 | Allium sativum | Sin El Thoum | Liliaceae | Bulb | Methanolic extract | Preventing/delaying cataracts | Anterior and posterior part | Research on the eye using in vivo study | [27] |
| Tablet | Diabetic retinopathy | Research on the eye using in vivo study | [28] | ||||||
| Lowering IOP | |||||||||
| Improving visual acuity | |||||||||
| Adjuvant treatment in diabetic macular edema | |||||||||
| 4 | Anagallis arvensis | Ain El Jamal | Primulaceae | NA | Plant juice | Ophthalmia Kertistis | Anterior part | Literature survey and fieldwork | [29] |
| Improving eyesight | |||||||||
| 5 | Arbutus unedo | Quotlob | Ericaceae | Fruit | Edible | Preventing/delaying cataracts | Posterior part | Review (Zeaxanthin occurrence) | [30] |
| Age-related macular degeneration | |||||||||
| 6 | Bidens pilosa | Hsseiki | Asteraceae | Plant juice | Plant juice | Eye irritation | Anterior part | Review | [31] |
| Conjunctivitis | |||||||||
| Leaves | Infusion | Improving aqueous tear quantity | Anterior part | Research on the eye using in vivo study | [32] | ||||
| Maintaining tear film stability | |||||||||
| Inhibiting the inflammation of the lacrimal gland | |||||||||
| Maintaining tear film stability | |||||||||
| 7 | Borago officinalis | Lissan El Thawr | Boraginaceae | Leaves | Infusion | Conjunctivitis | Anterior part | Ethnobotanical survey | [33] |
| Flower | |||||||||
| Flower | Infusion | Reduce retinal venous pressure | Posterior part | Research on the eye using in vivo study | [34] | ||||
| 8 | Capparis spinosa | El Quoubar | Capparidaceae | Leaves | Infusion | Eye infection | Anterior part | Review | [35] |
| Bud | Orally with a glass of water | ||||||||
| 9 | Centaurea cyanus | El Quantarioun | Asteraceae | Flower | Eyewash with cornflower | Eye inflammation | Anterior part | Review | [36] |
| Infused blossoms | Conjunctivitis | ||||||||
| Blepharitis | |||||||||
| Relieve strained tired and puffy eyes | |||||||||
| 10 | Chenopodium opulifolium | Sarmouc | Chenopodiaceae | Leaves | Ointment | Eye diseases | Unspecified eye diseases | Research | [37] |
| 11 | Cichorioum intybus | El Hindbeh | Asteraceae | Flower | Infusion | Eye inflammation | Anterior part | Review | [38] |
| Periorbital puffiness | |||||||||
| Symptoms of eye tiredness | |||||||||
| Leaves | External application | Eye infection | Review | [39] | |||||
| Root | |||||||||
| Juice | |||||||||
| 12 | Citrullus colocynthis | El Hanzal | Cucurbitaceae | Fruit | Methanolic extracts | Eye redness | Anterior part | Research on the eye using in vitro study | [40] |
| Preventing/delaying cataracts | |||||||||
| 13 | Crepis robertioides | Saraghat Robertieih | Asteraceae | Flowering part | Infusion | Eye infection | Anterior part | Ethnopharmacological survey | [41] |
| 14 | Crepis libanotica | Saraghat Lebnen | Asteraceae | Flowering part | Infusion | Eye infection | Anterior part | Ethnopharmacological survey | [41] |
| 15 | Cyperus rotundus | El Saad | Cyperaceae | Rhizome | Decoction | Eye disease | Review | [14] | |
| Ocular discharges | Anterior part | Review | [42] | ||||||
| 16 | Datura stramonium | El Khawkhara | Solanaceae | Seeds | Extraction | Mydriasis | Toxicity | Research | [43] |
| Leaves | Smoking | Photophobia | |||||||
| 17 | Daucus carota | El Jazar | Umbelliferae | Root | Edible | Protecting against chronic eye defects and vision loss | Anterior part | Review | [44,45] |
| Protection against UVB Improving eyesight | |||||||||
| Seeds | Extraction | Lower internal ocular pressure (IOP) | Research on the eye using in vivo study | [46] | |||||
| 18 | Euphrasia officinalis | Arkoun Toubi | Orobanchaceae | Whole plant | Tincture Extraction with ethanol Herbal tea | Conjunctivitis | Anterior part | Assessment report | [47] |
| Blepharitis | |||||||||
| Eye fatigue | |||||||||
| Ocular inflammation | |||||||||
| Styes | |||||||||
| Ocular allergies | |||||||||
| Commercial eye drops | Protecting corneal epithelial cells from UVB exposure | Research on the eye using in vitro study | [48] | ||||||
| Methanol and ethanol extract | Anti-inflammatory Reducing pro-inflammatory cytokine expression | Research on the eye using in vitro study | [49] | ||||||
| 19 | Ficus carica | El Tin | Moraceae | Stem | Sap of the plant | Used in eye irritation | Toxicity | Case study | [50,51] |
| Fruit | Edible fruit | Improving eyesight | Anterior part | Review | [52] | ||||
| Powder of dry fruits and sugar taken orally with water twice a day | |||||||||
| 20 | Foeniculum vulgare | El Choumar | Apiaceae | Seeds | Water seed extract | Reducing intraocular pressure (IOP) | Anterior and posterior part | Research on the eye using in vivo study | [53] |
| Anti-glaucoma | |||||||||
| Protective and therapeutic effects against induced cataracts | Research on the eye using in vivo study | [54] | |||||||
| Raw or with a sweetener | Improving eyesight | Review | [55] | ||||||
| 21 | Fumaria officinalis | Bakleht El Malak | Papaveraceae | Stem | Eye lotion | Red eye | Toxicity | Book chapter | [56] |
| Flower | NA | Conjunctivitis | |||||||
| Heaviness in the eyes, | |||||||||
| Stinging pain in the eyes | |||||||||
| Swelling and puffiness of the eyes | |||||||||
| Photophobia and tired eyes | |||||||||
| 22 | Hyoscyamus niger | El Binij El Aswad | Solanaceae | Seeds | Extraction | Insecticidal activity (Lucilia sericata) | Anterior part | Research | [57] |
| Red eye | |||||||||
| Itching in the eye | |||||||||
| Mydriasis | Toxicity | Review | [58] | ||||||
| Blurred vision | |||||||||
| Photophobia | |||||||||
| 23 | Iris germanica | El Sawsan | Iridaceae | Leaves | Methanol extract | Conjunctivitis | Anterior part | Research on the eye using in vitro study | [59] |
| Eye infection | |||||||||
| 24 | Juniperus excelsa | El Charbin | Cupressaceae | Seeds | Seed extract | Eye diseases | Unspecified eye diseases | Review | [14] |
| 25 | Linum usitatissimum | El Kittein | Linaceae | Seeds | Seed mucilage | Removing foreign material from the eye | Anterior part | Assessment report | [60] |
| Eye irritation | |||||||||
| Oral flaxseed oil capsules | Dry-eye Sjögren’s syndrome patients | Research on the eye using in vivo study | [61] | ||||||
| 26 | Ocimum basilicum | El Rihan | Lamiaceae | Seeds | Aqueous extract | Lower internal ocular pressure (IOP) | Anterior part | Research on the eye using in vivo study | [62] |
| 27 | El Zaytoun | Oleaceae | Leaves | Infusions used as ointment | Eye infections | Anterior part | Research | [63] | |
| Management of dry-eye syndrome | Review | [64] | |||||||
| 28 | Origanum syriacum | Zaatar souri | Lamiaceae | Arial parts | Methanol extract | Treatment for amoebic keratitis | Anterior part | Research | [65] |
| 29 | Origanum laevigatum | Mardakouch | Lamiaceae | Arial parts | Methanol extract | Treatment for amoebic keratitis | Anterior part | Research | [65] |
| 30 | Malvae sylvestris | Khibeizi | Malvaceae | Whole plant | Mucilaginous extract | Dry-eye disease | Anterior part | Research on the eye using in vivo study | [66] |
| 31 | Matricaria chamomilla | Asteraceae | Flower | Infusion | Protecting against UVB exposure | Anterior part | Research on commercial eye drops (Dacriovis™) | [48] | |
| Leaves | Decoction | Eye irritation or eye infection | Review | [67] | |||||
| Eyewash | |||||||||
| Eye care | |||||||||
| Swollen eyes | |||||||||
| Tired eyes | |||||||||
| Ameliorating wound healing | |||||||||
| 32 | Marrubium vulgare | Kourrat Jabali | Lamiaceae | Leaves | Extraction | Eye inflammation | Anterior part | Review | [68] |
| Sore eyes | |||||||||
| Leaf juice with honey | Night blindness | ||||||||
| Clean eyesight | |||||||||
| 33 | Melissa officinalis | El Mleissi | Lamiaceae | Leaves | Aqueous ethanol extraction | Dry age-related macular degeneration (AMD) | Posterior part | Research on the eye using in vitro study | [69] |
| Exudative AMD | Research | [70] | |||||||
| 34 | Mentha longifolia | Alnaenae al Tawil | Lamiaceae | Aerial part Leaves | Infusion | Eye diseases | Anterior part | Ethnopharmacological survey | [71] |
| 35 | Mentha spicata | Alnaenae al Akhdar | Lamiaceae | Extract | Marketed as Neumentix | Nutritional support in a rat model of hypertensive glaucoma | Posterior part | Research on the eye using in vivo study | [72] |
| 36 | Nerium oleander | El Delfi | Apocynaceae | Leaves | Sap of the plant | Eye inflammation | Toxicity | Case study | [73] |
| Light sensitivity | |||||||||
| Keratitis and uveitis | |||||||||
| Corneal edema | |||||||||
| 37 | Plantago lanceolata | Lissan El Hamal | Plantaginaceae | Whole plant | Lotion | Eye illness wound repair | Anterior part | Report | [74] |
| Leaves | Ointment Eye drops | Eye irritation | Review | [75] | |||||
| Eye choroid diseases | |||||||||
| Day blindness | |||||||||
| Conjunctivitis | |||||||||
| Eyes sores | |||||||||
| 38 | Portulaca oleracea | Bakleh Barrieh | Portulacaceae | Leaves | Edible plant | Inflammation of the eyes | Anterior part | Review | [76] |
| Seeds | Decoction | ||||||||
| 39 | Rosa damascena | Ward Dimachqy | Rosaceae | Flower | Rose water | Eye wash | Anterior and posterior part | Review | [77] |
| Eye inflammation | Research on the eye using in vivo study on eye drop preparation (Ophthacare®) | [78] | |||||||
| Degenerative ophthalmic disorders | |||||||||
| 40 | Rosa centifolia | Ward Outri | Rosaceae | Flower | Infusion | Eye wash | Anterior part | Assessment report | [79] |
| Eye inflammation | |||||||||
| 41 | Rosemarinus officinalis | Eklil El Jabal | Lamiaceae | Whole plant | Essential oil | Prevention of retinal light damage | Anterior and posterior part | Research on the eye using in vivo study | [80] |
| Antibacterial activity | Research on the eye using in vitro study | [81] | |||||||
| Antimicrobial activity | |||||||||
| 42 | Salvia sclarea | Qasein | Lamiaceae | Leaves | Essential oil | Antimicrobial activity | Anterior part | Research on the eye using in vitro study | [81] |
| Antifungal effect (fusarium keratitis) | Research on the eye using in vitro study | [82] | |||||||
| 43 | Salvia Libanotica fruticosa | Mariamia Loubnenieh | Lamiaceae | Leaves | Infusion | Eye inflammation | Anterior part | Short communication | [83] |
| 44 | Salvia officinalis | Qasein Toubi | Lamiaceae | Arial parts | Essential oil | Eye disease | Anterior part | Research | [84] |
| Arial parts | Vapor inhalation | Ophthalmic anti-inflammatory | Ethnopharmacological and chemical characterization | [85] | |||||
| Leaves | NA | ||||||||
| 45 | Silybum marianum | Kharfeich | Asteraceae | Whole plant | Isolation of Silibinin | Age-related macular degeneration | Posterior part | Research on the eye using in vitro study | [86] |
| Neovascular AMD | |||||||||
| 46 | Solanum dulcamara | Bathenjein Aswad | Solanaceae | Stem | Lipstick | Mydriasis | Toxicity | Case report | [87] |
| 47 | Solanum villosum | Bathenjein Ahmar | Solanaceae | Whole plant | Edible | Sore eyes | Anterior part | Review | [88] |
| 48 | Thymus vulgaris | Zaatar Bari | Lamiaceae | Whole plant | Essential oil extraction Steam distillation | Antimicrobial activity | Anterior part | Research on the eye using in vitro study | [81] |
| 49 | Urginea maritima | Basal Bari | Asparagaceae | Bulb | Fresh | Eye illness | Unspecified eye diseases | Report | [89] |
| 50 | Xanthium strumarium | El Lizeiq | Asteraceae | Leaves | NA | Eye diseases | Anterior part | Review | [90] |
| Seeds | Improving eyesight | ||||||||
| 51 | Ziziphus jujuba | El Inab | Rhamnaceae | Seeds | Extraction | Eye inflammation | Anterior part | Review | [91] |
| 52 | Ziziphus spina-christi | El Sidir | Rhamnaceae | Leaves | Extraction | Eye inflammation | Anterior part | Review | [92] |
| Plant Source | Family | Disease or Target | Phytochemical(s) | Structure | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|
| Allium sativum | Liliaceae | Cataracts | Allicin | 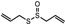 | Antioxidant activity prevents protein modifications in cataractous lenses | [95] |
| S-allyl cysteine | 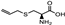 | Antioxidant and anti-inflammatory properties Protection against neurodegenerative diseases | [96] | |||
| Diabetic retinopathy | S-allyl cysteine | An antidiabetic mechanism inhibits angiogenesis by downregulating vascular endothelial growth factor (VEGF) expression | [97] | |||
| Allicin | Allicin delays the progression of diabetic nephropathy through antioxidant and anti-inflammatory mechanisms | [98] | ||||
| Diallyl disulfide (DADS) |  | Anti-inflammatory and antioxidant mechanisms | [99] | |||
| Flavonoids | 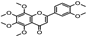 | Anti-oxidative, anti-inflammatory, and anti-apoptotic mechanisms Inducing heme oxygenase-1 expression | [100] | |||
| Borago officinalis | Boraginaceae | Reducing retinal venous pressure | Gamma-linolenic acid |  | Intermediate of PGE1, an endogenous vasodilator that enhances blood flow | [34] |
| Phenolic Compounds |  Phenol Phenol | Improving blood flow | ||||
| Citrullus colocynthis | Cucurbitaceae | Preventing/delaying cataracts | Flavonoids (Quercetin) |  | Oxidative stress leads to protein aggregation in the lens, which causes cataracts Quercetin’s antioxidant activity can help protect lens proteins from oxidation | [101,102] |
| Phenolic compounds |  Phenol Phenol | Antioxidant and free radical scavenging | [103] | |||
| Daucus carota | Umbelliferae | Protecting against chronic defects and vision loss | Carotenoids (beta-carotene, lutein, and zeaxanthin) | 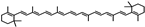 Beta-carotene Beta-carotene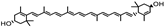 Lutein Lutein | Protecting the eye from oxidative stress, apoptosis, mitochondrial dysfunction, and inflammation | [104] |
| Euphrasia officinalis | Orobanchaceae | Protecting corneal epithelial cells against UVB exposure Anti-inflammatory effects | Phenolic compounds gentisic, caftaric, vanillic and rosmarinic acid, hyperoside (quercetin-3-O-galactoside), and quercitrin (quercetin 3-O-rhamnoside) | 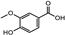 Vanillic acid Vanillic acid Gentisic acid Gentisic acid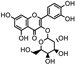 Quercetin-3-O-galactoside Quercetin-3-O-galactoside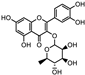 Quercetin 3-O-rhamnoside Quercetin 3-O-rhamnoside | Antioxidant, antimicrobial and antiproliferative activities | [105] |
| Foeniculum vulgare | Apiaceae | Reducing intraocular pressure (IOP) | NA | Anticholinesterase activity, which may help lower IOP | [53] | |
| Protective and therapeutic effects against induced cataracts | Anethole Fenchone Flavonoids | 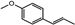 Anethole Anethole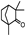 Fenchone Fenchone | Inhibiting oxidative stress and preventing lens opacity Antioxidant and anti-inflammatory activities | [54,106,107] | ||
| Iris germanica | Iridacea | Eye infection | Phenolic compounds: Protocatechuic acid, Catechin, p-Hydroxy benzoic acid, and caffeic and Ferulic acid | 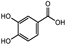 Protocatechuic acid Protocatechuic acid Catechin Catechin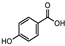 p-HydroxyBenzoicacid p-HydroxyBenzoicacid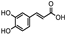 Caffeic acid Caffeic acid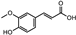 Ferulic acid Ferulic acid | Antimicrobial, antioxidant, and antimutagenic activities | [108] |
| Linum usitatissimum | Linaceae | Dry-eye Sjögren’s syndrome patients | Essential fatty acids (EFAs), particularly omega-3 |  α-linolenic acid α-linolenic acid | Improving tear production and reducing inflammation | [109,110] |
| Ocimum basilicum | Lamiaceae | Lower internal ocular pressure (IOP) | Unsaturated fatty acids including α-linolenic, linoleic, and oleic acids Saturated fatty acids (palmitic and stearic acid) | 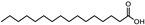 Palmitic acid Palmitic acid Stearic acid Stearic acid Oleic acid Oleic acid Linoleic acid Linoleic acid α-linolenic acid α-linolenic acid | Anti-inflammatory effects Reduction in aqueous humor production | [111] |
| Malvae sylvestris | Malvaceae | Dry-eye disease | Plant mucus | Mucilaginous substances have the potential to support the lubricating effect | [66,112] | |
| Phenols, flavonoids, carotenoids, unsaturated fatty acids | See above structures | Antioxidants | ||||
| Melissa officinalis | Lamiaceae | Age-related macular degeneration (AMD) | Volatile compounds (geranial, neral, citronellal, and geraniol) Phenolic acids (rosmarinic and caffeic acid) Flavonoids (quercetin, rhamnocitrin, and luteolin) | 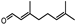 Geranial Geranial Citronellal Citronellal Geraniol Geraniol | Reducing apoptosis and oxidative damage Potent antioxidant properties and ability to act as a radical scavenger | [69,113] |
| Mentha spicata | Lamiaceae | Glaucoma | Phenolic compounds | Improving levels of neurotrophins, along with reducing oxidative stress and inflammation markers | [72] | |
| Rosemarinus officinalis | Lamiaceae | Prevention of retinal light damage Antibacterial activity | Essential oil components including monoterpene, diterpene, and sesquiterpene hydrocarbons, azulene, alcohols, aldehydes, and ketones | 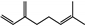 Monoterpene myrcene Monoterpene myrcene Diterpene retiol Diterpene retiol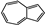 Azulene Azulene | Antimicrobial activity of EO | [80,81] |
| Carnosol, carnosic, rosmanol, rosmarinic and ursolic acid | 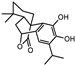 Carnosol Carnosol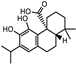 Carnosic acid Carnosic acid | Antioxidants | ||||
| Salvia sclarea | Lamiaceae | Antimicrobial and antifungal effects | Essential oil components include monoterpene, diterpene, and sesquiterpene hydrocarbons and azulene, | See above structures | Antimicrobial activity of EO | [81,114] |
| Silybum marianum | Asteraceae | Age-related macular degeneration | Flavonolignans, silymarin (silybin, isosilybin, silychristin, dihydrosilybin, and silydrianin) | 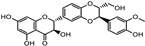 silybin silybin Isosilybin Isosilybin | Antioxidant properties | [86,115] |
| Thymus vulgaris | Lamiaceae | Antimicrobial activity | Essential oil components including monoterpene, diterpene, and sesquiterpene hydrocarbons, azulene, alcohols, aldehydes, ketones, α-thujene, α-pinene, and camphene |  α-thujene α-thujene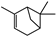 α-pinene α-pinene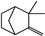 Camphene Camphene | Antimicrobial activity of EO | [81,116,117] |
| Medicinal Plant | Compound | Solubility | Molecular Weight (Daltons) | Functional Groups | Pubchem CID |
|---|---|---|---|---|---|
Allium sativum [132]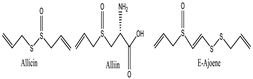 | Allicin | Soluble in alcohol and organic solvents; sparingly soluble in water | 162.19 | Thioester, alkene (C=C), eher | CID: 6391 |
| Allin | Soluble in water and organic solvents | 177.21 | Sulfide, amine | CID: 6320 | |
| E-Ajoene | Soluble in alcohol and organic solvents, low solubility in water | 206.33 | Disulfide, alkene (C=C) | CID: 10632 | |
Foeniculum vulgare [55]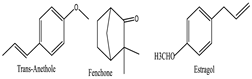 | Trans-Anethole | Soluble in alcohol and lipids, sparingly soluble in water | 148.21 | Ether (-OCH₃), vinyl group (C=C) | CID: 10203 |
| Fenchone | Soluble in organic solvents, practically insoluble in water | 150.22 | Ketone (C=O) | CID: 6395 | |
| Estragole | Soluble in organic solvents, low solubility in water | 148.21 | Ether (-OCH₃), alkene (C=C) | CID: 9887 | |
Rosa damascene [133]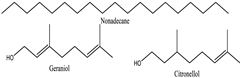 | Nonadecane | Insoluble in water but soluble in organic solvents | 270.45 | Alkane | CID: 6295 |
| Geraniol | Soluble in alcohol and lipids, low solubility in water | 154.25 | Alcohol (-OH), alkene (C=C) | CID: 10445 | |
| Citronellol | Soluble in alcohol and oils, low solubility in water | 154.25 | Alcohol (-OH), alkene (C=C) | CID: 10242 | |
Rosemarinus officinalis [134] | Carnosic Acid | Soluble in organic solvents, insoluble in water | 330.46 | Carboxylic acid (-COOH) | CID: 5281642 |
| Carnosol | Soluble in organic solvents, insoluble in water | 316.45 | Alcohol (-OH), ketone (C=O) | CID: 5281643 | |
| Rosmarinic Acid | Soluble in water and organic solvents | 360.36 | Ester, carboxylic acid (-COOH) | CID: 442800 |
| Plant Source | Disease | Phytochemical(s) | Mechanism of Action | Reference |
|---|---|---|---|---|
Datura stramonium 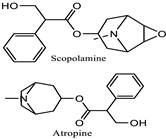 | Mydriasis, photophobia | Alkaloids: atropine, scopolamine, and hyoscyamine | Alkaloids work by blocking the muscarinic acetylcholine receptors in the iris sphincter muscle, which results in prolonged pupil dilation | [43,138] |
Ficus carica | Eye irritation | Furocoumarins: psoralen and bergapten. | Can cause skin and eye irritation Photosensitizing properties, which can lead to photodermatitis | [51] |
Hyoscyamus niger 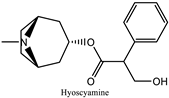 | Red eye Mydriasis | Alkaloids, primarily hyoscyamine, atropine, and scopolamine | Anticholinergic activities | [43,138] |
Nerium oleander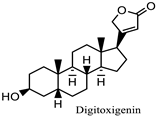 | Eye inflammation, Light sensitivity, keratitis, and uveitis Corneal edema | Cardiac glycosides oleandrin, nerin, and digitoxigenin | Can irritate skin and mucous membranes, including the eyes | [73,139] |
Solanum dulcamara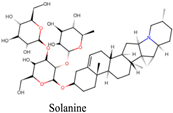 | Mydriasis | Tropane alkaloids, such as solanine and other glycoalkaloids | Anticholinergics can cause pupil dilation by blocking the action of acetylcholine | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andary, J.; El Ballouz, H.; Abou-Khalil, R. Lebanese Medicinal Plants with Ophthalmic Properties. Pharmaceuticals 2025, 18, 155. https://doi.org/10.3390/ph18020155
Andary J, El Ballouz H, Abou-Khalil R. Lebanese Medicinal Plants with Ophthalmic Properties. Pharmaceuticals. 2025; 18(2):155. https://doi.org/10.3390/ph18020155
Chicago/Turabian StyleAndary, Jeanne, Haitham El Ballouz, and Rony Abou-Khalil. 2025. "Lebanese Medicinal Plants with Ophthalmic Properties" Pharmaceuticals 18, no. 2: 155. https://doi.org/10.3390/ph18020155
APA StyleAndary, J., El Ballouz, H., & Abou-Khalil, R. (2025). Lebanese Medicinal Plants with Ophthalmic Properties. Pharmaceuticals, 18(2), 155. https://doi.org/10.3390/ph18020155






