Potential Applications of Rare Earth Metal Nanoparticles in Biomedicine
Abstract
1. Introduction
2. Nanoparticles of the Yttrium Subgroup
2.1. Gadolinium Nanoparticles
2.2. Ytterbium Nanoparticles
2.3. Holmium Nanoparticles
2.4. Lutetium Nanoparticles
2.5. Dysprosium Nanoparticles
2.6. Erbium Nanoparticles
2.7. Terbium Nanoparticles
2.8. Thulium Nanoparticles
2.9. Scandium and Yttrium Nanoparticles
3. Nanoparticles of Metal Oxides of the Cerium Subgroup
3.1. Lanthanum Nanoparticles
3.2. Europium Nanoparticles
3.3. Neodymium Nanoparticles
3.4. Promethium Oxide
3.5. Samarium Nanoparticles
3.6. Praseodymium Nanoparticles
3.7. Cerium Nanoparticles
4. Possible Directions of Modification of Rare Earth Metal Nanoparticles
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uthamacumaran, A. Cancer: A Turbulence Problem. Neoplasia 2020, 22, 759–769. [Google Scholar] [CrossRef]
- Lee, R.J.; Madan, R.A.; Kim, J.; Posadas, E.M.; Yu, E.Y. Disparities in Cancer Care and the Asian American Population. Oncologist 2021, 26, 453–460. [Google Scholar] [CrossRef]
- Baquero, F. Threats of Antibiotic Resistance: An Obliged Reappraisal. Int. Microbiol. 2021, 24, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Hirano, K.; Nakano, M.; Muramatsu, T.; Tsukahara, R.; Ito, Y.; Ishimori, H. Wound Healing and Wound Location in Critical Limb Ischemia Following Endovascular Treatment. Circ. J. 2014, 78, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Cao, Y.; Zhang, R.; Yu, X. Experimental Investigation on the Stability and Heat Transfer Enhancement of Phase Change Materials Composited with Nanoparticles and Metal Foams. J. Energy Storage 2024, 89, 111826. [Google Scholar] [CrossRef]
- Kutlu, A.; Aykut, Y.; Eren, R. Thermal Hysteresis Enhancement and Dispersion Thermal Stability in Paraffin Actuators: Comparison of Pan-Nanofibers vs Metal Oxide Nanoparticles Use. Sens. Actuators A Phys. 2024, 379, 115965. [Google Scholar] [CrossRef]
- Tang, X.; Jiang, Y.; Song, L.; Van Der Eycken, E.V. Metal Nanoparticle-Catalyzed Alkyne Cyclization for the Synthesis of Heterocycles. Adv. Synth. Catal. 2024, 366, 3085–3104. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K. Nanomaterials for Latent Fingerprint Detection: A Review. J. Mater. Res. Technol. 2021, 12, 1856–1885. [Google Scholar] [CrossRef]
- Sharma, V.; Choudhary, S.; Mankotia, P.; Kumari, A.; Sharma, K.; Sehgal, R.; Kumar, V. Nanoparticles as Fingermark Sensors. TrAC Trends Anal. Chem. 2021, 143, 116378. [Google Scholar] [CrossRef]
- Yadav, N. Cerium Oxide Nanostructures: Properties, Biomedical Applications and Surface Coatings. 3 Biotech 2022, 12, 121. [Google Scholar] [CrossRef]
- Jain, A.; Fournier, P.G.J.; Mendoza-Lavaniegos, V.; Sengar, P.; Guerra-Olvera, F.M.; Iñiguez, E.; Kretzschmar, T.G.; Hirata, G.A.; Juárez, P. Functionalized Rare Earth-Doped Nanoparticles for Breast Cancer Nanodiagnostic Using Fluorescence and CT Imaging. J. Nanobiotechnology 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; De Nicola, M.; Mandoli, C.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Ce3+ Ions Determine Redox-Dependent Anti-Apoptotic Effect of Cerium Oxide Nanoparticles. ACS Nano 2011, 5, 4537–4549. [Google Scholar] [CrossRef] [PubMed]
- Placke-Yan, C.; Bendt, G.; Salamon, S.; Landers, J.; Wende, H.; Hagemann, U.; Schulz, S. Versatile Synthesis of Sub-10 Nm Sized Metal-Doped MxCo3−xO4 Nanoparticles and Their Electrocatalytic OER Activity. Mater. Adv. 2024, 5, 3482–3489. [Google Scholar] [CrossRef]
- Alameen, A.S.; Undre, S.B.; Undre, P.B. Synthesis, Dispersion, Functionalization, Biological and Antioxidant Activity of Metal Oxide Nanoparticles: Review. Nano-Struct. Nano-Objects 2024, 39, 101298. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Lee, Y.-H.; Chou, C.-L.; Chang, Y.-S.; Liu, W.-C.; Chiu, H.-W. Oxidative Stress and Potential Effects of Metal Nanoparticles: A Review of Biocompatibility and Toxicity Concerns. Environ. Pollut. 2024, 346, 123617. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cho, W.C.; Jaragh-Alhadad, L.A.; Tarharoudi, R.; Bloukh, S.H.; Edis, Z.; Sari, S.; Falahati, M.; Ten Hagen, T.L.M.; Khan, R.H.; et al. Nano-Bio Interaction: An Overview on the Biochemical Binding of DNA to Inorganic Nanoparticles for the Development of Anticancer and Antibacterial Nano-Platforms. Int. J. Biol. Macromol. 2023, 225, 544–556. [Google Scholar] [CrossRef]
- Oluwasola, I.E.; Ahmad, A.L.; Shoparwe, N.F.; Ismail, S. Gadolinium Based Contrast Agents (GBCAs): Uniqueness, Aquatic Toxicity Concerns, and Prospective Remediation. J. Contam. Hydrol. 2022, 250, 104057. [Google Scholar] [CrossRef] [PubMed]
- Master, A.; Livingston, M.; Sen Gupta, A. Photodynamic Nanomedicine in the Treatment of Solid Tumors: Perspectives and Challenges. J. Control. Release 2013, 168, 88–102. [Google Scholar] [CrossRef]
- Silva, J.M.; Silva, E.; Reis, R.L. Light-Triggered Release of Photocaged Therapeutics—Where Are We Now? J. Control. Release 2019, 298, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tripathy, S.; Singh, O.K.; Singh, S.G. Cerium Oxide Nanofiber Based Electroanalytical Sensor for TNF-α Detection: Improved Interfacial Stability with Nafion. Bioelectrochemistry 2021, 138, 107725. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Cha, B.G.; Lee, J.H.; Yang, W.; Ki, S.K.; Han, J.H.; Cho, H.Y.; Park, E.; Jeon, S.; Lee, S.-H. Ultrasmall Polymer-Coated Cerium Oxide Nanoparticles as a Traumatic Brain Injury Therapy. Nanomed. Nanotechnol. Biol. Med. 2022, 45, 102586. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, T.O.; Vasilyeva, D.N.; Kozlov, D.A.; Kolesnik, I.V.; Teplonogova, M.A.; Tronev, I.V.; Sheichenko, E.D.; Protsenko, M.R.; Kolmanovich, D.D.; Ivanova, O.S.; et al. A Comparative Study of Cerium(III) and Cerium(IV) Phosphates for Sunscreens. Molecules 2024, 29, 2157. [Google Scholar] [CrossRef]
- Yang, J.; Chu, Z.; Jiang, Y.; Zheng, W.; Sun, J.; Xu, L.; Ma, Y.; Wang, W.; Shao, M.; Qian, H. Multifunctional Hyaluronic Acid Microneedle Patch Embedded by Cerium/Zinc-Based Composites for Accelerating Diabetes Wound Healing. Adv. Healthc. Mater. 2023, 12, e2300725. [Google Scholar] [CrossRef]
- De Santis, S.; Sotgiu, G.; Porcelli, F.; Marsotto, M.; Iucci, G.; Orsini, M. A Simple Cerium Coating Strategy for Titanium Oxide Nano-Tubes’ Bioactivity Enhancement. Nanomaterials 2021, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, B.; Stango, A.X.; Balasubramanian, M.; Vijayalakshmi, U. In Situ Fabrication of Cerium-Incorporated Hydroxyapatite/Magnetite Nanocomposite Coatings with Bone Regeneration and Osteosarcoma Potential. Nanoscale Adv. 2023, 5, 5054–5076. [Google Scholar] [CrossRef] [PubMed]
- Cimini, A.; D’Angelo, B.; Das, S.; Gentile, R.; Benedetti, E.; Singh, V.; Monaco, A.M.; Santucci, S.; Seal, S. Antibody-Conjugated PEGylated Cerium Oxide Nanoparticles for Specific Targeting of Aβ Aggregates Modulate Neuronal Survival Pathways. Acta Biomater. 2012, 8, 2056–2067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, Y.; Yu, J.; Ma, C.; Wang, Y.; Wang, Y.; Li, L.; Zhang, L.W. Gadolinium-Bisphosphonate Nanoparticle-Based Low-Dose Radioimmunotherapy for Osteosarcoma. ACS Biomater. Sci. Eng. 2022, 8, 5329–5337. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, B.-I.; Lee, K.S.; Heo, H.; Lee, J.H.; Byeon, S.-H.; Lee, I.S. Fabrication of a Silica Sphere with Fluorescent and MR Contrasting GdPO4 Nanoparticles from Layered Gadolinium Hydroxide. Chem. Commun. 2010, 46, 3654. [Google Scholar] [CrossRef] [PubMed]
- Dey, U.; Chattopadhyay, A. The Potential of Gadolinium Ascorbate Nanoparticles as a Safer Contrast Agent. J. Phys. Chem. B 2023, 127, 346–358. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Z.; Yu, G.; Lu, N.; Liu, Y.; Dai, Y.; Fu, X.; Wang, J.; Chen, X. Gadolinium Metallofullerene-Polypyrrole Nanoparticles for Activatable Dual-Modal Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 28382–28389. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Charmi, J.; Yaray, K.; Ghaffarlou, M.; Balcioglu, E.; Ertas, Y.N. Enhanced In Vivo Radiotherapy of Breast Cancer Using Gadolinium Oxide and Gold Hybrid Nanoparticles. ACS Appl. Bio Mater. 2023, 6, 784–792. [Google Scholar] [CrossRef]
- Park, J.Y.; Baek, M.J.; Choi, E.S.; Woo, S.; Kim, J.H.; Kim, T.J.; Jung, J.C.; Chae, K.S.; Chang, Y.; Lee, G.H. Paramagnetic Ultrasmall Gadolinium Oxide Nanoparticles as Advanced T1 MRI Contrast Agent: Account for Large Longitudinal Relaxivity, Optimal Particle Diameter, and In Vivo T1 MR Images. ACS Nano 2009, 3, 3663–3669. [Google Scholar] [CrossRef]
- Barkhausen, J.; Ebert, W.; Debatin, J.F.; Weinmann, H.-J. Imaging of Myocardial Infarction: Comparison of Magnevist and Gadophrin-3 in Rabbits. J. Am. Coll. Cardiol. 2002, 39, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Pintaske, J.; Martirosian, P.; Graf, H.; Erb, G.; Lodemann, K.-P.; Claussen, C.D.; Schick, F. Relaxivity of Gadopentetate Dimeglumine (Magnevist), Gadobutrol (Gadovist), and Gadobenate Dimeglumine (MultiHance) in Human Blood Plasma at 0. 2, 1.5, and 3 Tesla: Investig. Radiol. 2006, 41, 213–221. [Google Scholar] [CrossRef]
- Takanezawa, Y.; Nakamura, R.; Ohshiro, Y.; Uraguchi, S.; Kiyono, M. Gadolinium-Based Contrast Agents Suppress Adipocyte Differentiation in 3T3-L1 Cells. Toxicol. Lett. 2023, 383, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Bo, R.; Qu, H.; Jia, B.; Xiao, W.; Yuan, Y.; Vapniarsky, N.; Lindstrom, A.; Wu, H.; Zhang, D.; et al. A Nephrotoxicity-Free, Iron-Based Contrast Agent for Magnetic Resonance Imaging of Tumors. Biomaterials 2020, 257, 120234. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Mędrzycka-Dąbrowska, W.; Zorena, K.; Dąbrowski, S.; Ślęzak, D.; Malecka-Dubiela, A.; Rutkowski, P. Nephrogenic Systemic Fibrosis as a Complication after Gadolinium-Containing Contrast Agents: A Rapid Review. Int. J. Environ. Res. Public Health 2021, 18, 3000. [Google Scholar] [CrossRef]
- Bernstein, E.J.; Schmidt-Lauber, C.; Kay, J. Nephrogenic Systemic Fibrosis: A Systemic Fibrosing Disease Resulting from Gadolinium Exposure. Best Pract. Res. Clin. Rheumatol. 2012, 26, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Thanikaivelan, E.; Gokul Raj, S.; Murugakoothan, P. Synthesis and Characterisation of Erbium Doped Gadolinium Oxide (Er:Gd2O3) Nanorods. Mater. Today Proc. 2023, S221478532305054X. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, C.; Wang, S.; Li, Q.; Zhang, M.; Li, J.; Xu, K. Comparative Study on in Vivo Behavior of PEGylated Gadolinium Oxide Nanoparticles and Magnevist as MRI Contrast Agent. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 547–555. [Google Scholar] [CrossRef]
- Abrikossova, N.; Skoglund, C.; Ahrén, M.; Bengtsson, T.; Uvdal, K. Effects of Gadolinium Oxide Nanoparticles on the Oxidative Burst from Human Neutrophil Granulocytes. Nanotechnology 2012, 23, 275101. [Google Scholar] [CrossRef]
- Shalviri, A.; Foltz, W.D.; Cai, P.; Rauth, A.M.; Wu, X.Y. Multifunctional Terpolymeric MRI Contrast Agent with Superior Signal Enhancement in Blood and Tumor. J. Control. Release 2013, 167, 11–20. [Google Scholar] [CrossRef]
- Ashouri, H.; Riyahi Alam, N.; Khoobi, M.; Haghgoo, S.; Rasouli, Z.; Gholami, M. NSF Evaluation of Gadolinium Biodistribution in Renally Impaired Rats: Using Novel Metabolic Gd2O3 Nanoparticles Coated with β-Cyclodextrin (Gd2O3@PCD) in MR Molecular Imaging. Magn. Reson. Imaging 2024, 107, 120–129. [Google Scholar] [CrossRef]
- Brune, N.; Mues, B.; Buhl, E.M.; Hintzen, K.-W.; Jockenhoevel, S.; Cornelissen, C.G.; Slabu, I.; Thiebes, A.L. Dual Labeling of Primary Cells with Fluorescent Gadolinium Oxide Nanoparticles. Nanomaterials 2023, 13, 1869. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Meena, V.K.; Hazari, P.P.; Sharma, S.K.; Sharma, R.K. Rose Bengal Attached and Dextran Coated Gadolinium Oxide Nanoparticles for Potential Diagnostic Imaging Applications. Eur. J. Pharm. Sci. 2018, 117, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Fan, W.; Yang, Z.; Liu, Y.; Bregadze, V.I.; Mandal, S.K.; Yung, B.C.; Lin, L.; Liu, T.; Tang, W.; et al. Exceedingly Small Gadolinium Oxide Nanoparticles with Remarkable Relaxivities for Magnetic Resonance Imaging of Tumors. Small 2019, 15, 1903422. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Y.; Ahmad, M.W.; Yue, H.; Ho, S.L.; Park, J.A.; Jung, K.-H.; Cha, H.; Marasini, S.; Ghazanfari, A.; Liu, S.; et al. In Vivo Positive Magnetic Resonance Imaging Applications of Poly(Methyl Vinyl Ether-Alt-Maleic Acid)-Coated Ultra-Small Paramagnetic Gadolinium Oxide Nanoparticles. Molecules 2020, 25, 1159. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.L.; Choi, G.; Yue, H.; Kim, H.-K.; Jung, K.-H.; Park, J.A.; Kim, M.H.; Lee, Y.J.; Kim, J.Y.; Miao, X.; et al. In Vivo Neutron Capture Therapy of Cancer Using Ultrasmall Gadolinium Oxide Nanoparticles with Cancer-Targeting Ability. RSC Adv. 2020, 10, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Meena, V.K.; Hazari, P.P.; Sharma, R.K. PEG Coated and Doxorubicin Loaded Multimodal Gadolinium Oxide Nanoparticles for Simultaneous Drug Delivery and Imaging Applications. Int. J. Pharm. 2017, 527, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Mortezazadeh, T.; Gholibegloo, E.; Khoobi, M.; Alam, N.R.; Haghgoo, S.; Mesbahi, A. In Vitro and in Vivo Characteristics of Doxorubicin-Loaded Cyclodextrine-Based Polyester Modified Gadolinium Oxide Nanoparticles: A Versatile Targeted Theranostic System for Tumour Chemotherapy and Molecular Resonance Imaging. J. Drug Target. 2020, 28, 533–546. [Google Scholar] [CrossRef]
- Sun, X.; Kou, B. Biocompatibility and Potential Anticancer Activity of Gadolinium Oxide (Gd2O3) Nanoparticles against Nasal Squamous Cell Carcinoma. BMC Biotechnol. 2024, 24, 53. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cai, R.; Zhao, T.; Wu, L.; Zhang, L.; Jin, J.; Xu, L.; Li, P.; Li, T.; Zhang, M.; et al. Hyaluronic Acid-Functionalized Gadolinium Oxide Nanoparticles for Magnetic Resonance Imaging-Guided Radiotherapy of Tumors. Nanoscale Res. Lett. 2020, 15, 94. [Google Scholar] [CrossRef]
- Rajaee, A.; Wang, S.; Zhao, L.; Wang, D.; Liu, Y.; Wang, J.; Ying, K. Multifunction Bismuth Gadolinium Oxide Nanoparticles as Radiosensitizer in Radiation Therapy and Imaging. Phys. Med. Biol. 2019, 64, 195007. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, B.; Shokrani, P.; Hejazi, S.H.; Talebi, A.; Taheri, A. Doxorubicin-Loaded PVP Coated Gd2O3 NPs for Effective Chemoradiotherapy in Melanoma. J. Drug Deliv. Sci. Technol. 2019, 53, 101189. [Google Scholar] [CrossRef]
- Yu, B.; Lu, X.; Feng, X.; Zhao, T.; Li, J.; Lu, Y.; Ye, F.; Liu, X.; Zheng, X.; Shen, Z.; et al. Gadolinium Oxide Nanoparticles Reinforce the Fractionated Radiotherapy-Induced Immune Response in Tri-Negative Breast Cancer via cGAS-STING Pathway. Int. J. Nanomed. 2023, 18, 7713–7728. [Google Scholar] [CrossRef]
- Da Silva, B.A.; Nazarkovsky, M.; Padilla-Chavarría, H.I.; Mendivelso, E.A.C.; Mello, H.L.D.; Nogueira, C.D.S.C.; Carvalho, R.D.S.; Cremona, M.; Zaitsev, V.; Xing, Y.; et al. Novel Scintillating Nanoparticles for Potential Application in Photodynamic Cancer Therapy. Pharmaceutics 2022, 14, 2258. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, T.; Yang, Z.; Zhou, Z.; Tang, W.; Fan, W.; Liu, Y.; Mu, J.; Li, L.; Bregadze, V.I.; et al. Small-Sized Gadolinium Oxide Based Nanoparticles for High-Efficiency Theranostics of Orthotopic Glioblastoma. Biomaterials 2020, 235, 119783. [Google Scholar] [CrossRef] [PubMed]
- Aloy, M.-T.; Sidi Boumedine, J.; Deville, A.; Kryza, D.; Gauthier, A.; Brichart-Vernos, D.; Ollier, G.; La Padula, V.; Lux, F.; Tillement, O.; et al. Proof of Concept of the Radiosensitizing Effect of Gadolinium Oxide Nanoparticles in Cell Spheroids and a Tumor-Implanted Murine Model of Chondrosarcoma. Int. J. Nanomed. 2022, 17, 6655–6673. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. Gadolinium Oxide Nanoparticles Induce Toxicity in Human Endothelial HUVECs via Lipid Peroxidation, Mitochondrial Dysfunction and Autophagy Modulation. Nanomaterials 2020, 10, 1675. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.; Alrokayan, S. Toxicity Mechanism of Gadolinium Oxide Nanoparticles and Gadolinium Ions in Human Breast Cancer Cells. Curr. Drug Metab. 2019, 20, 907–917. [Google Scholar] [CrossRef]
- Liu, S.; Yue, H.; Ho, S.L.; Kim, S.; Park, J.A.; Tegafaw, T.; Ahmad, M.Y.; Kim, S.; Saidi, A.K.A.A.; Zhao, D.; et al. Enhanced Tumor Imaging Using Glucosamine-Conjugated Polyacrylic Acid-Coated Ultrasmall Gadolinium Oxide Nanoparticles in Magnetic Resonance Imaging. Int. J. Mol. Sci. 2022, 23, 1792. [Google Scholar] [CrossRef]
- Shi, X.; Cao, C.; Zhang, Z.; Tian, J.; Hu, Z. Radiopharmaceutical and Eu3+ Doped Gadolinium Oxide Nanoparticles Mediated Triple-Excited Fluorescence Imaging and Image-Guided Surgery. J. Nanobiotechnol. 2021, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Mortezazadeh, T.; Gholibegloo, E.; Alam, N.R.; Dehghani, S.; Haghgoo, S.; Ghanaati, H.; Khoobi, M. Gadolinium (III) Oxide Nanoparticles Coated with Folic Acid-Functionalized Poly(β-Cyclodextrin-Co-Pentetic Acid) as a Biocompatible Targeted Nano-Contrast Agent for Cancer Diagnostic: In Vitro and in Vivo Studies. Magn. Reson. Mater. Phys. 2019, 32, 487–500. [Google Scholar] [CrossRef]
- Cheng, Y.; Tan, X.; Wang, J.; Wang, Y.; Song, Y.; You, Q.; Sun, Q.; Liu, L.; Wang, S.; Tan, F.; et al. Polymer-Based Gadolinium Oxide Nanocomposites for FL/MR/PA Imaging Guided and Photothermal/Photodynamic Combined Anti-Tumor Therapy. J. Control. Release 2018, 277, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Meena, V.K.; Hazari, P.P.; Sharma, R.K. FITC-Dextran Entrapped and Silica Coated Gadolinium Oxide Nanoparticles for Synchronous Optical and Magnetic Resonance Imaging Applications. Int. J. Pharm. 2016, 506, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Aashima; Pandey, S.K.; Singh, S.; Mehta, S.K. Ultrasonication Assisted Fabrication of L-Lysine Functionalized Gadolinium Oxide Nanoparticles and Its Biological Acceptability. Ultrason. Sonochemistry 2018, 49, 53–62. [Google Scholar] [CrossRef]
- Aashima; Pandey, S.K.; Singh, S.; Mehta, S.K. Biocompatible Gadolinium Oxide Nanoparticles as Efficient Agent against Pathogenic Bacteria. J. Colloid Interface Sci. 2018, 529, 496–504. [Google Scholar] [CrossRef]
- Kumar, S.; Chaudhary, S.; Chaudhary, G.R. Effect of Fabrication Strategies on the In-Vitro Antimicrobial and Antifungal Activities of Pr3+ Doped Gb2O3 Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100518. [Google Scholar] [CrossRef]
- Kumar, A.; Sarkar, T.; Solanki, P.R. Amine Functionalized Gadolinium Oxide Nanoparticles-Based Electrochemical Immunosensor for Cholera. Biosensors 2023, 13, 177. [Google Scholar] [CrossRef]
- Kumar, A.; Sarkar, T.; Kumar, R.; Panda, A.K.; Solanki, P.R. Electrochemical Detection of Vibrio Cholerae by Amine Functionalized Biocompatible Gadolinium Oxide Nanoparticles. Micromachines 2023, 14, 995. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Kiran, M.S. Hybrid Nanostructured Gadolinium Oxide-Collagen-Dextran Polymeric Hydrogel for Corneal Repair and Regeneration. Int. J. Biol. Macromol. 2023, 224, 1423–1438. [Google Scholar] [CrossRef]
- Mastrogiacomo, S.; Kownacka, A.E.; Dou, W.; Burke, B.P.; De Rosales, R.T.M.; Heerschap, A.; Jansen, J.A.; Archibald, S.J.; Walboomers, X.F. Bisphosphonate Functionalized Gadolinium Oxide Nanoparticles Allow Long-Term MRI/CT Multimodal Imaging of Calcium Phosphate Bone Cement. Adv. Healthc. Mater. 2018, 7, 1800202. [Google Scholar] [CrossRef] [PubMed]
- Silina, E.V.; Manturova, N.E.; Chuvilina, E.L.; Gasanov, A.A.; Andreeva, O.I.; Pugachevskii, M.A.; Kochura, A.V.; Kryukov, A.A.; Suzdaltseva, Y.G.; Stupin, V.A. Biomedical Application Prospects of Gadolinium Oxide Nanoparticles for Regenerative Medicine. Pharmaceutics 2024, 16, 1627. [Google Scholar] [CrossRef]
- Tcibulnikova, A.V.; Slezhkin, V.A.; Bruykhanov, V.V.; Samusev, I.G.; Kozhevnikov, A.S.; Savin, V.V.; Medvedskaya, P.N.; Lyatun, I.I. Cooperative Luminescence of Yb 3 + Ions of the Ytterbium Oxide Porous Surface. Opt. Commun. 2020, 459, 125006. [Google Scholar] [CrossRef]
- Hannachi, E.; Khan, F.; Slimani, Y.; Rehman, S.; Trabelsi, Z.; Akhtar, S.; Al-Suhaimi, E. In Vitro Antimicrobial and Anticancer Peculiarities of Ytterbium and Cerium Co-Doped Zinc Oxide Nanoparticles. Biology 2022, 11, 1836. [Google Scholar] [CrossRef]
- Peng, H.; Cui, B.; Li, G.; Wang, Y.; Li, N.; Chang, Z.; Wang, Y. A Multifunctional β-CD-Modified Fe3O4@ZnO:Er3+,Yb3+ Nanocarrier for Antitumor Drug Delivery and Microwave-Triggered Drug Release. Mater. Sci. Eng. C 2015, 46, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Kaliyamoorthy, K.; Pillai, A.S.; Alexander, A.; Arivarasu, A.; Enoch, I.V.M.V.; Ramasamy, S. β-Cyclodextrin-Folate Functionalized Poly(Lactic-Co-Glycolide)–Superparamagnetic Ytterbium Ferrite Hybrid Nanocarrier for Targeted Delivery of Camptothecin. Mater. Sci. Eng. C 2021, 122, 111796. [Google Scholar] [CrossRef]
- Muthulakshmi, V.; Kumar, P.; Sundrarajan, M. Green Synthesis of Ionic Liquid Mediated Ytterbium Oxide Nanoparticles by Andrographis Paniculata Leaves Extract for Structural, Morphological and Biomedical Applications. J. Environ. Chem. Eng. 2021, 9, 105270. [Google Scholar] [CrossRef]
- Muthulakshmi, V.; Sundrarajan, M. Green Synthesis of Ionic Liquid Assisted Ytterbium Oxide Nanoparticles by Couroupita Guianensis Abul Leaves Extract for Biological Applications. J. Environ. Chem. Eng. 2020, 8, 103992. [Google Scholar] [CrossRef]
- Almaieli, L.M.A.; Khalaf, M.M.; Gouda, M.; Elmushyakhi, A.; Abou Taleb, M.F.; Abd El-Lateef, H.M. Fabrication of Bio-Based Film Comprising Metal Oxide Nanoparticles Loaded Chitosan for Wound Dressing Applications. Polymers 2022, 15, 211. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Shaker-Ardakani, N.; Farbod, F. Highly Efficient Enzyme Less Glucose Sensor Based on Cross-Linked Nitrogen-Doped Graphene Aerogel Incorporated with Ytterbium Oxide and Decorated with Nickel Nitride Nanoparticles. Sens. Int. 2024, 5, 100290. [Google Scholar] [CrossRef]
- Kumar, S.; Ram Chaudhary, G.; Chaudhary, S. Designing of Surface Engineered Ytterbium Oxide Nanoparticles as Effective Electrochemical Sensing Platform for Dopamine. J. Mol. Liq. 2022, 355, 118929. [Google Scholar] [CrossRef]
- Jafari, H.; Ganjali, M.R.; Dezfuli, A.S.; Faridbod, F. Long Term Determination of Dopamine and Uric Acid in the Presence of Ascorbic Acid Using Ytterbia/Reduced Graphene Oxide Nanocomposite Prepared through a Sonochemical Route. Appl. Surf. Sci. 2018, 427, 496–506. [Google Scholar] [CrossRef]
- Hosseini, M.; Pur, M.R.K.; Norouzi, P.; Moghaddam, M.R.; Ganjali, M.R. An Enhanced Electrochemiluminescence Sensor Modified with a Ru(Bpy)32+/Yb2O3 Nanoparticle/Nafion Composite for the Analysis of Methadone Samples. Mater. Sci. Eng. C 2017, 76, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Wysokińska, E.; Cichos, J.; Zioło, E.; Bednarkiewicz, A.; Strządała, L.; Karbowiak, M.; Hreniak, D.; Kałas, W. Cytotoxic Interactions of Bare and Coated NaGdF4:Yb3+:Er3+ Nanoparticles with Macrophage and Fibroblast Cells. Toxicol. Vitr. 2016, 32, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, M.; Chen, B.; Hu, B. Study on Cytotoxicity, Cellular Uptake and Elimination of Rare-Earth-Doped Upconversion Nanoparticles in Human Hepatocellular Carcinoma Cells. Ecotoxicol. Environ. Saf. 2020, 203, 110951. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Tingting, J.; Hussain, T.; He, X.; Ahmad, M.A.; Irshad, M.K.; Shakoor, N.; Zhang, P.; Changjian, X.; Hao, Y.; et al. Bioaccumulation of Ytterbium Oxide Nanoparticles Insinuate Oxidative Stress, Inflammatory, and Pathological Lesions in ICR Mice. Environ. Sci. Pollut. Res. 2020, 27, 32944–32953. [Google Scholar] [CrossRef]
- Gómez-González, E.; Caro, C.; Martínez-Gutiérrez, D.; García-Martín, M.L.; Ocaña, M.; Becerro, A.I. Holmium Phosphate Nanoparticles as Negative Contrast Agents for High-Field Magnetic Resonance Imaging: Synthesis, Magnetic Relaxivity Study and in Vivo Evaluation. J. Colloid Interface Sci. 2021, 587, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bult, W.; Varkevisser, R.; Soulimani, F.; Seevinck, P.R.; De Leeuw, H.; Bakker, C.J.G.; Luijten, P.R.; Van Het Schip, A.D.; Hennink, W.E.; Nijsen, J.F.W. Holmium Nanoparticles: Preparation and In Vitro Characterization of a New Device for Radioablation of Solid Malignancies. Pharm. Res. 2010, 27, 2205–2212. [Google Scholar] [CrossRef]
- Marasini, S.; Yue, H.; Ho, S.L.; Park, J.A.; Kim, S.; Jung, K.-H.; Cha, H.; Liu, S.; Tegafaw, T.; Ahmad, M.Y.; et al. Synthesis, Characterizations, and 9.4 Tesla T2 MR Images of Polyacrylic Acid-Coated Terbium(III) and Holmium(III) Oxide Nanoparticles. Nanomaterials 2021, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi-Ahodashti, M.; Mortazavi-Derazkola, S.; Ebrahimzadeh, M.A. Nanostructures of Rare Earth Oxides (Ho2O3 and Nd2O3): Synthesis Methods, Properties, and Comparative Analysis. J. Mater. Res. Technol. 2023, 27, 1843–1856. [Google Scholar] [CrossRef]
- Cerqueira-Coutinho, C.; Vidal, L.P.; Pinto, S.R.; Santos-Oliveira, R. Drug Metabolism: Comparison of Biodistribution Profile of Holmium in Three Different Compositions in Healthy Wistar Rats. Appl. Radiat. Isot. 2016, 112, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shao, X.-J.; Li, Z.; Lin, C.-H.; Wang, C.-W.-Q.; Jiao, K.; Xu, J.; Pan, H.-X.; Wu, Y. Synthesis of Holmium-Oxide Nanoparticles for Near-Infrared Imaging and Dye-Photodegradation. Molecules 2022, 27, 3522. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, Y.; Chen, Z.; Mo, M.; Xie, J.; Zhou, X.; Wu, Y.; Yang, Q.; Zheng, M.; Hu, X.; et al. Proangiogenic Effect and Underlying Mechanism of Holmium Oxide Nanoparticles: A New Biomaterial for Tissue Engineering. J. Nanobiotechnol. 2024, 22, 357. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Mohamed, H.E.; Khalil, A.T.; Hkiri, K.; Ayaz, M.; Usman, A.; Sadiq, A.; Ullah, F.; Hussain, I.; Maaza, M. Phyto-Fabrication of Ultrafine Nanoscale Holmium Oxide HT-Ho2 O3 NPs and Their Biomedical Potential. RSC Adv. 2023, 13, 27912–27922. [Google Scholar] [CrossRef] [PubMed]
- Munaweera, I.; Levesque-Bishop, D.; Shi, Y.; Di Pasqua, A.J.; Balkus, K.J. Radiotherapeutic Bandage Based on Electrospun Polyacrylonitrile Containing Holmium-166 Iron Garnet Nanoparticles for the Treatment of Skin Cancer. ACS Appl. Mater. Interfaces 2014, 6, 22250–22256. [Google Scholar] [CrossRef]
- Lee, J.D.; Park, K.K.; Lee, M.G.; Kim, E.H.; Rhim, K.J.; Lee, J.T.; Yoo, H.S.; Kim, Y.M.; Park, K.B.; Kim, J.R. Radionuclide Therapy of Skin Cancers and Bowen’s Disease Using a Specially Designed Skin Patch. J. Nucl. Med. 1997, 38, 697–702. [Google Scholar] [PubMed]
- Osipitan, O.O.; Sun, M.; Gordish-Dressman, H.; Wendt, R.; Wight-Carter, M.; Balkus, K.J.; Di Pasqua, A.J. Laminated Holmium-166-Containing Electrospun Bandages for Use against Skin Cancer. Nucl. Med. Biol. 2022, 114–115, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yue, H.; Ho, S.L.; Kim, S.; Park, J.A.; Tegafaw, T.; Ahmad, M.Y.; Kim, S.; Saidi, A.K.A.A.; Zhao, D.; et al. Polyethylenimine-Coated Ultrasmall Holmium Oxide Nanoparticles: Synthesis, Characterization, Cytotoxicities, and Water Proton Spin Relaxivities. Nanomaterials 2022, 12, 1588. [Google Scholar] [CrossRef]
- Atabaev, T.; Shin, Y.; Song, S.-J.; Han, D.-W.; Hong, N. Toxicity and T2-Weighted Magnetic Resonance Imaging Potentials of Holmium Oxide Nanoparticles. Nanomaterials 2017, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoon, J.; Kim, H.K.; Lee, W.T.; Nguyen, N.T.; Le, X.T.; Lee, E.-H.; Lee, E.S.; Oh, K.T.; Choi, H.-G.; et al. Upconverting Nanoparticle-Containing Erythrocyte-Sized Hemoglobin Microgels That Generate Heat, Oxygen and Reactive Oxygen Species for Suppressing Hypoxic Tumors. Bioact. Mater. 2023, 22, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Jiménez, T.; Cruz-Nova, P.; Ancira-Cortez, A.; Gibbens-Bandala, B.; Lara-Almazán, N.; Ocampo-García, B.; Santos-Cuevas, C.; Morales-Avila, E.; Ferro-Flores, G. Toxicity Assessment of [177Lu]Lu−iFAP/iPSMA Nanoparticles Prepared under GMP-Compliant Radiopharmaceutical Processes. Nanomaterials 2022, 12, 4181. [Google Scholar] [CrossRef]
- Tran, T.A.; Kappelhoff, J.; Jüstel, T.; Anderson, R.R.; Purschke, M. UV Emitting Nanoparticles Enhance the Effect of Ionizing Radiation in 3D Lung Cancer Spheroids. Int. J. Radiat. Biol. 2022, 98, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Luna-Gutiérrez, M.; Ocampo-García, B.; Jiménez-Mancilla, N.; Ancira-Cortez, A.; Trujillo-Benítez, D.; Hernández-Jiménez, T.; Ramírez-Nava, G.; Hernández-Ramírez, R.; Santos-Cuevas, C.; Ferro-Flores, G. Targeted Endoradiotherapy with Lu2O3-iPSMA/-iFAP Nanoparticles Activated by Neutron Irradiation: Preclinical Evaluation and First Patient Image. Pharmaceutics 2022, 14, 720. [Google Scholar] [CrossRef] [PubMed]
- Ancira-Cortez, A.; Ferro-Flores, G.; Jiménez-Mancilla, N.; Morales-Avila, E.; Trujillo-Benítez, D.; Ocampo-García, B.; Santos-Cuevas, C.; Escudero-Castellanos, A.; Luna-Gutiérrez, M. Synthesis, Chemical and Biochemical Characterization of Lu2O3-iPSMA Nanoparticles Activated by Neutron Irradiation. Mater. Sci. Eng. C 2020, 117, 111335. [Google Scholar] [CrossRef]
- Locardi, F.; Gianotti, E.; Nelli, I.; Caratto, V.; Martinelli, A.; Ferretti, M.; Costa, G.A.; Canesi, L.; Balbi, T.; Fasoli, M.; et al. Facile Synthesis of NIR and Visible Luminescent Sm3+ Doped Lutetium Oxide Nanoparticles. Mater. Res. Bull. 2017, 86, 220–227. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, K.; Liu, J.; Han, X.; Ren, J.; Qu, X. Anti-Biofouling Polymer-Decorated Lutetium-Based Nanoparticulate Contrast Agents for In Vivo High-Resolution Trimodal Imaging. Small 2014, 10, 2429–2438. [Google Scholar] [CrossRef]
- Gómez-González, E.; Caro, C.; García-Martín, M.L.; Becerro, A.I.; Ocaña, M. Outstanding MRI Contrast with Dysprosium Phosphate Nanoparticles of Tuneable Size. Nanoscale 2022, 14, 11461–11470. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gónzalez, E.; Núñez, N.O.; Caro, C.; García-Martín, M.L.; Fernández-Afonso, Y.; De La Fuente, J.M.; Balcerzyk, M.; Ocaña, M. Dysprosium and Holmium Vanadate Nanoprobes as High-Performance Contrast Agents for High-Field Magnetic Resonance and Computed Tomography Imaging. Inorg. Chem. 2021, 60, 152–160. [Google Scholar] [CrossRef]
- Yue, H.; Park, J.Y.; Chang, Y.; Lee, G.H. Ultrasmall Europium, Gadolinium, and Dysprosium Oxide Nanoparticles: Polyol Synthesis, Properties, and Biomedical Imaging Applications. Mini-Rev. Med. Chem. 2020, 20, 1767–1780. [Google Scholar] [CrossRef]
- Yue, H.; Park, J.A.; Ho, S.L.; Ahmad, M.Y.; Cha, H.; Liu, S.; Tegafaw, T.; Marasini, S.; Ghazanfari, A.; Kim, S.; et al. New Class of Efficient T2 Magnetic Resonance Imaging Contrast Agent: Carbon-Coated Paramagnetic Dysprosium Oxide Nanoparticles. Pharmaceuticals 2020, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Kattel, K.; Park, J.Y.; Xu, W.; Kim, H.G.; Lee, E.J.; Bony, B.A.; Heo, W.C.; Jin, S.; Baeck, J.S.; Chang, Y.; et al. Paramagnetic Dysprosium Oxide Nanoparticles and Dysprosium Hydroxide Nanorods as T2 MRI Contrast Agents. Biomaterials 2012, 33, 3254–3261. [Google Scholar] [CrossRef] [PubMed]
- Tegafaw, T.; Xu, W.; Ahmad, M.W.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Kim, T.J.; Lee, G.H. Dual-Mode T1 and T2 Magnetic Resonance Imaging Contrast Agent Based on Ultrasmall Mixed Gadolinium-Dysprosium Oxide Nanoparticles: Synthesis, Characterization, and in Vivo Application. Nanotechnology 2015, 26, 365102. [Google Scholar] [CrossRef]
- Balachandran, Y.L.; Wang, W.; Yang, H.; Tong, H.; Wang, L.; Liu, F.; Chen, H.; Zhong, K.; Liu, Y.; Jiang, X. Heterogeneous Iron Oxide/Dysprosium Oxide Nanoparticles Target Liver for Precise Magnetic Resonance Imaging of Liver Fibrosis. ACS Nano 2022, 16, 5647–5659. [Google Scholar] [CrossRef]
- Vignesh, K.; Senthil Kumar, A.; Arumugam Napoleon, A.; Govindasamy, M.; Yusuf, K. Ag-La(OH)3@Dy2O3 Hybrid Composite Modified Laser-Induced Graphene Surface for Simultaneous Electrochemical Detection of Bisphenol A and Tartrazine. Appl. Surf. Sci. 2024, 676, 160901. [Google Scholar] [CrossRef]
- Baytak, A.K.; Akbaş, E.; Aslanoglu, M. A Novel Voltammetric Platform Based on Dysprosium Oxide for the Sensitive Determination of Sunset Yellow in the Presence of Tartrazine. Anal. Chim. Acta 2019, 1087, 93–103. [Google Scholar] [CrossRef]
- Que, W.; Buddhudu, S.; Zhou, Y.; Lam, Y.L.; Zhou, J.; Chan, Y.C.; Kam, C.H.; Gan, L.H.; Roshan Deen, G. Preparation and Characterization of Erbium Oxalate and Erbium Oxide Nanoparticles by Microemulsion Technique. Mater. Sci. Eng. C 2001, 16, 51–54. [Google Scholar] [CrossRef]
- Tse, J.J.; Dunmore-Buyze, P.J.; Drangova, M.; Holdsworth, D.W. Erbium-Based Perfusion Contrast Agent for Small-Animal Microvessel Imaging. Contrast Media Mol. Imaging 2017, 2017, 7368384. [Google Scholar] [CrossRef]
- Azhagar, S.; Kumar Chinnalagu, D.; Mayakrishnan, A.; Sundrarajan, M. Study on the Therapeutic Activity of [BMIM]-PF6-IL Assisted Er2O3 NPs Synthesized by Polyol Method against Pathogenic Bacterium as Well as MCF-7 Breast Tumor Cells. Inorg. Chem. Commun. 2023, 155, 110988. [Google Scholar] [CrossRef]
- Dědková, K.; Kuzníková, Ľ.; Pavelek, L.; Matějová, K.; Kupková, J.; Čech Barabaszová, K.; Váňa, R.; Burda, J.; Vlček, J.; Cvejn, D.; et al. Daylight Induced Antibacterial Activity of Gadolinium Oxide, Samarium Oxide and Erbium Oxide Nanoparticles and Their Aquatic Toxicity. Mater. Chem. Phys. 2017, 197, 226–235. [Google Scholar] [CrossRef]
- Mohamed, H.E.A.; Khalil, A.T.; Hkiri, K.; Ayaz, M.; Abbasi, J.A.; Sadiq, A.; Ullah, F.; Nawaz, A.; Ullah, I.; Maaza, M. Physicochemical and Nanomedicine Applications of Phyto-Reduced Erbium Oxide (Er2O3) Nanoparticles. AMB Expr. 2023, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Safwat, G.; Soliman, E.S.M.; Mohamed, H.R.H. Induction of ROS Mediated Genomic Instability, Apoptosis and G0/G1 Cell Cycle Arrest by Erbium Oxide Nanoparticles in Human Hepatic Hep-G2 Cancer Cells. Sci. Rep. 2022, 12, 16333. [Google Scholar] [CrossRef] [PubMed]
- Saifullah, A.; Ul Hassan, S.M.; Khurshid, A. Carbon-Coated (Eu/Dy-Enabled) Erbium Hydroxide Nanostructures for Biomedical Imaging. Materialia 2024, 35, 102108. [Google Scholar] [CrossRef]
- Mohamed, H.R.H.; Ibrahim, M.M.H.; Soliman, E.S.M.; Safwat, G.; Diab, A. Estimation of Calcium Titanate or Erbium Oxide Nanoparticles Induced Cytotoxicity and Genotoxicity in Normal HSF Cells. Biol. Trace Elem. Res. 2023, 201, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Dos Santos, M.; Goetz, J.; Bartenlian, H.; Wong, K.-L.; Charbonnière, L.J.; Hildebrandt, N. Autofluorescence-Free Live-Cell Imaging Using Terbium Nanoparticles. Bioconjugate Chem. 2018, 29, 1327–1334. [Google Scholar] [CrossRef]
- Charpentier, C.; Cifliku, V.; Goetz, J.; Nonat, A.; Cheignon, C.; Cardoso Dos Santos, M.; Francés-Soriano, L.; Wong, K.; Charbonnière, L.J.; Hildebrandt, N. Ultrabright Terbium Nanoparticles for FRET Biosensing and in Situ Imaging of Epidermal Growth Factor Receptors**. Chem. A Eur. J. 2020, 26, 14602–14611. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chi, Y.; Wen, H.; Lu, Z. Sensitized Luminescent Terbium Nanoparticles: Preparation and Time-Resolved Fluorescence Assay for DNA. Anal. Chem. 2007, 79, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Mohanto, S.; Biswas, A.; Gholap, A.D.; Wahab, S.; Bhunia, A.; Nag, S.; Ahmed, M.G. Potential Biomedical Applications of Terbium-Based Nanoparticles (TbNPs): A Review on Recent Advancement. ACS Biomater. Sci. Eng. 2024, 10, 2703–2724. [Google Scholar] [CrossRef]
- Pandi, P.S.; Krishnan, P.; Sathish, S.; Sudhahar, S. Biomedical Applications of Terbium Oxide Nanoparticles by Couroupita Guianensis Aubl Leaves Extract: A Greener Approach. Nano-Struct. Nano-Objects 2024, 40, 101341. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Li, X.; Fan, S.; Liu, Y.; Jiang, X.; Boudreau, M.D.; Pan, Y.; Tian, X.; Yin, J.-J. Bactericidal Effects and Accelerated Wound Healing Using Tb4O7 Nanoparticles with Intrinsic Oxidase-like Activity. J. Nanobiotechnol. 2019, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Iram, S.; Khan, S.; Ansary, A.A.; Arshad, M.; Siddiqui, S.; Ahmad, E.; Khan, R.H.; Khan, M.S. Biogenic Terbium Oxide Nanoparticles as the Vanguard against Osteosarcoma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 123–131. [Google Scholar] [CrossRef]
- Khalaf, E.I.; El-Shafai, N.M.; Nassar, A.M.; Assem, E.E.; Yahia, I.S.; El-Mehasseb, I.M. Enhancing the Photoinduced via a Novel Nano-Combination of Terbium Oxide and Nickel Oxide on Graphene Oxide Surface: Cytotoxicity and Water Treatment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 121949. [Google Scholar] [CrossRef] [PubMed]
- Fetouh, H.A.; El-Mossalamy, E.H.; El Desouky, J.M.; Batouti, M.E. Synthesis and Characterization of New Organometallic Lanthanides Metal Complexes for Photodynamic Therapy. Sci. Rep. 2024, 14, 26184. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Wu, W.; Yang, K.; Mao, R.; Ahmad, F.; Chen, X.; Li, W. CT/MRI-Guided Synergistic Radiotherapy and X-ray Inducible Photodynamic Therapy Using Tb-Doped Gd-W-Nanoscintillators. Angew. Chem. Int. Ed. 2019, 58, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Daouk, J.; Iltis, M.; Dhaini, B.; Béchet, D.; Arnoux, P.; Rocchi, P.; Delconte, A.; Habermeyer, B.; Lux, F.; Frochot, C.; et al. Terbium-Based AGuIX-Design Nanoparticle to Mediate X-Ray-Induced Photodynamic Therapy. Pharmaceuticals 2021, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Minaei, E.; Engels, E.; Ashford, B.G.; McAlary, L.; Clark, J.R.; Gupta, R.; Tehei, M.; Corde, S.; Carolan, M.; et al. Thulium Oxide Nanoparticles as Radioenhancers for the Treatment of Metastatic Cutaneous Squamous Cell Carcinoma. Phys. Med. Biol. 2020, 65, 215018. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zheng, J.; Sheng, Q.; Zheng, X. In Situ Synthesis of Thulium(III) Hexacyanoferrate(II) Nanoparticles and Its Application for Glucose Detection. Anal. Chim. Acta 2011, 689, 47–51. [Google Scholar] [CrossRef]
- Juliebø-Jones, P.; Somani, B.K.; Gjengstø, P.; Æsøy, M.S.; Beisland, C.; Ulvik, Ø. Holmium and Thulium Fiber Laser Safety in Endourological Practice: What Does the Clinician Need to Know? Curr. Urol. Rep. 2023, 24, 409–415. [Google Scholar] [CrossRef]
- Gismondi, J.P.M.; Bento, A.D.S.A.; Mazzucchi, E.; Nahas, W.C. Thulium Fiber Laser in Cystine Calculi. Int. Braz. J. Urol. 2023, 49, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Spinos, T.; Tatanis, V.; Peteinaris, A.; Somani, B.; Kartalas Goumas, I.; Liatsikos, E.; Kallidonis, P. Thulium Fiber Laser Enucleation of the Prostate: A Systematic Review of the Current Outcomes. Minerva Urol. Nephrol. 2024, 76, 157–165. [Google Scholar] [CrossRef]
- Forlini, V.; Pellegrino, C.; Lena, F.; Capitanucci, M.L.; Van Uitert, A.; Mosiello, G. Thulium Laser for the Treatment of Posterior Urethral Valves in Infants. J. Endourol. 2023, 37, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Pu, R.; Wang, B.; Zhu, Z.; Liu, K.; Wang, F.; Wei, W.; Liu, H.; Zhan, Q. The Spectroscopic Properties and Microscopic Imaging of Thulium-Doped Upconversion Nanoparticles Excited at Different NIR-II Light. Biosensors 2021, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Guryev, E.L.; Smyshlyaeva, A.S.; Shilyagina, N.Y.; Shanwar, S.; Kostyuk, A.B.; Shulga, A.A.; Konovalova, E.V.; Zvyagin, A.V.; Deyev, S.M.; Petrov, R.V. Multifunctional Complexes Based on Photoluminescent Upconversion Nanoparticles for Theranostics of the HER2-Positive Tumors. Dokl. Biochem. Biophys. 2020, 491, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Pei, P.; Fan, Y.; Wang, S.; Zhang, H.; Zhao, D.; Qian, B.-Z.; Zhang, F. Fluorescence-Amplified Nanocrystals in the Second near-Infrared Window for in Vivo Real-Time Dynamic Multiplexed Imaging. Nat. Nanotechnol. 2023, 18, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Kantamneni, H.; Barkund, S.; Donzanti, M.; Martin, D.; Zhao, X.; He, S.; Riman, R.E.; Tan, M.C.; Pierce, M.C.; Roth, C.M.; et al. Shortwave Infrared Emitting Multicolored Nanoprobes for Biomarker-Specific Cancer Imaging in Vivo. BMC Cancer 2020, 20, 1082. [Google Scholar] [CrossRef] [PubMed]
- Guryev, E.L.; Smyshlyaeva, A.S.; Shilyagina, N.Y.; Sokolova, E.A.; Shanwar, S.; Kostyuk, A.B.; Lyubeshkin, A.V.; Schulga, A.A.; Konovalova, E.V.; Lin, Q.; et al. UCNP-Based Photoluminescent Nanomedicines for Targeted Imaging and Theranostics of Cancer. Molecules 2020, 25, 4302. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, T.A.; Mitroshina, E.V.; Smyshlyaeva, A.S.; Guryev, E.L.; Vedunova, M.V. Comparative Analysis of the Effects of Upconversion Nanoparticles on Normal and Tumor Brain Cells. Acta Naturae 2020, 12, 86–94. [Google Scholar] [CrossRef]
- Duosiken, D.; Yang, R.; Dai, Y.; Marfavi, Z.; Lv, Q.; Li, H.; Sun, K.; Tao, K. Near-Infrared Light-Excited Reactive Oxygen Species Generation by Thulium Oxide Nanoparticles. J. Am. Chem. Soc. 2022, 144, 2455–2459. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Huo, C.; Chen, Y.; Ding, P.; Tong, S.; Xue, W.; Zhu, J. Cancer-Thylakoid Hybrid Membrane Camouflaged Thulium Oxide Nanoparticles with Oxygen Self-Supply Capability for Tumor-Homing Phototherapy. Adv. Healthc. Mater. 2024, 13, 2303779. [Google Scholar] [CrossRef]
- Zhou, K.; Qiu, X.; Xu, L.; Li, G.; Rao, B.; Guo, B.; Pei, D.; Li, A.; He, G. Poly(Selenoviologen)-Assembled Upconversion Nanoparticles for Low-Power Single-NIR Light-Triggered Synergistic Photodynamic and Photothermal Antibacterial Therapy. ACS Appl. Mater. Interfaces 2020, 12, 26432–26443. [Google Scholar] [CrossRef]
- Wu, J.; Wei, W.; Ahmad, W.; Li, S.; Ouyang, Q.; Chen, Q. Enhanced Detection of Endocrine Disrupting Chemicals in On-Chip Microfluidic Biosensors Using Aptamer-Mediated Bridging Flocculation and Upconversion Luminescence. J. Hazard. Mater. 2023, 458, 132025. [Google Scholar] [CrossRef]
- Soares, A.C.C.; Sales, T.O.; Ximendes, E.C.; Jaque, D.; Jacinto, C. Lanthanide Doped Nanoparticles for Reliable and Precise Luminescence Nanothermometry in the Third Biological Window. Nanoscale Adv. 2023, 5, 3664–3670. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, D.; Ekner-Grzyb, A.; Grześkowiak, B.F.; Grzyb, T. Upconverting SrF2 Nanoparticles Doped with Yb3+/Ho3+, Yb3+/Er3+ and Yb3+/Tm3+ Ions—Optimisation of Synthesis Method, Structural, Spectroscopic and Cytotoxicity Studies. Sci. Rep. 2019, 9, 8669. [Google Scholar] [CrossRef]
- Shwetabh, K.; Banerjee, A.; Poddar, R.; Kumar, K. Assessment of NIR-Triggered PEG-Coated NaGdF4:Tm3+/Yb3+ Bio-Compatible Upconversion Nanoparticles for Contrast Enhancement in OCT Imaging and Optical Thermometry. Biomed. Mater. 2024, 19, 055001. [Google Scholar] [CrossRef]
- Zhao, J.; Ellis-Davies, G.C.R. Intracellular Photoswitchable Neuropharmacology Driven by Luminescence from Upconverting Nanoparticles. Chem. Commun. 2020, 56, 9445–9448. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Chen, Y.; Zhang, G. Elemental Bio-Imaging of PEGylated NaYF4:Yb/Tm/Gd Upconversion Nanoparticles in Mice by Laser Ablation Inductively Coupled Plasma Mass Spectrometry to Study Toxic Side Effects on the Spleen, Liver and Kidneys. Metallomics 2017, 9, 1150–1156. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liu, Y.; Huang, Y.; Wang, F.; Qian, Y.; Wang, Y. Preparation and Properties of (Sc2O3-MgO)/Pcl/Pvp Electrospun Nanofiber Membranes for the Inhibition of Escherichia Coli Infections. Int. J. Mol. Sci. 2023, 24, 7649. [Google Scholar] [CrossRef]
- Chu, B.H.; Kang, B.S.; Hung, S.C.; Chen, K.H.; Ren, F.; Sciullo, A.; Gila, B.P.; Pearton, S.J. Aluminum Gallium Nitride (GaN)/GaN High Electron Mobility Transistor-Based Sensors for Glucose Detection in Exhaled Breath Condensate. J. Diabetes Sci. Technol. 2010, 4, 171–179. [Google Scholar] [CrossRef]
- Herath, H.M.T.U.; Silvio, L.D.; Evans, J.R.G. Scandia—A Potential Biomaterial? J. Mater. Sci Mater. Med. 2005, 16, 1061–1065. [Google Scholar] [CrossRef]
- Nnomo Assene, A.; Dieme, D.; Jomaa, M.; Côté, J.; Bouchard, M. Toxicokinetic Study of Scandium Oxide in Rats. Toxicol. Lett. 2024, 392, 56–63. [Google Scholar] [CrossRef]
- Shaker, T.M.; Chung, C.; Varma, M.K.; Doherty, M.G.; Wolf, A.M.; Chung, M.H.; Assifi, M.M. Is There a Role for Ytrrium-90 in the Treatment of Unresectable and Metastatic Intrahepatic Cholangiocarcinoma? Am. J. Surg. 2018, 215, 467–470. [Google Scholar] [CrossRef]
- Ferreira, C.; Johnson, D.; Rasmussen, K.; Leinweber, C.; Ahmad, S.; Jung, J.W. A Novel Conformal Superficial High-Dose-Rate Brachytherapy Device for the Treatment of Nonmelanoma Skin Cancer and Keloids. Brachytherapy 2017, 16, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Pashazadeh, A.; Landes, R.; Boese, A.; Kreissl, M.C.; Klopfleisch, M.; Friebe, M. Superficial Skin Cancer Therapy with Y-90 Microspheres: A Feasibility Study on Patch Preparation. Ski. Res. Technol. 2020, 26, 25–29. [Google Scholar] [CrossRef]
- Zeki, K.; Yabaş, E.; Erden, F.; Salih, B.; Canlıca, M. Lu, Sm, and Y-Based Double-Decker Phthalocyanines with Enhanced Photodynamic Therapy Performance. Polyhedron 2024, 261, 117138. [Google Scholar] [CrossRef]
- Kassem, S.; Mohamed, M.; Sayour, H.; Canfarotta, F.; Piletsky, S.; Soliman, M.A.M. Functionalized Core-Shell Yttrium Oxide Nanoparticles as Antioxidants Agents in Heat Stressed Rats. Biol. Trace Elem. Res. 2020, 198, 189–197. [Google Scholar] [CrossRef]
- Kassem, S.; Arafa, M.M.; Yehya, M.M.; Soliman, M.A.M. In Vivo Study of Dose-Dependent Antioxidant Efficacy of Functionalized Core–Shell Yttrium Oxide Nanoparticles. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 593–606. [Google Scholar] [CrossRef]
- Mohamed, H.R.H.; Farouk, A.H.; Elbasiouni, S.H.; Nasif, K.A.; Safwat, G.; Diab, A. Genotoxicity and Oxidative Stress Induction by Calcium Hydroxide, Calcium Titanate or/and Yttrium Oxide Nanoparticles in Mice. Sci. Rep. 2023, 13, 19633. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Shang, P.; Sun, Z.; Lu, M.; You, G.; Yan, S.; Chen, G.; Zhou, H. Therapeutic Effect of Yttrium Oxide Nanoparticles for the Treatment of Fulminant Hepatic Failure. Nanomedicine 2019, 14, 2519–2533. [Google Scholar] [CrossRef]
- Khurana, A.; Saifi, M.A.; Godugu, C. Yttrium Oxide Nanoparticles Attenuate L-Arginine Induced Chronic Pancreatitis. Biol. Trace Elem. Res. 2023, 201, 3404–3417. [Google Scholar] [CrossRef]
- Khurana, A.; Anchi, P.; Allawadhi, P.; Kumar, V.; Sayed, N.; Packirisamy, G.; Godugu, C. Yttrium Oxide Nanoparticles Reduce the Severity of Acute Pancreatitis Caused by Cerulein Hyperstimulation. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 54–65. [Google Scholar] [CrossRef]
- Hosseini, A.; Abdollahi, M. Through a Mechanism-Based Approach, Nanoparticles of Cerium and Yttrium May Improve the Outcome of Pancreatic Islet Isolation. J. Med. Hypotheses Ideas 2012, 6, 4–6. [Google Scholar] [CrossRef][Green Version]
- Hosseini, A.; Baeeri, M.; Rahimifard, M.; Navaei-Nigjeh, M.; Mohammadirad, A.; Pourkhalili, N.; Hassani, S.; Kamali, M.; Abdollahi, M. Antiapoptotic Effects of Cerium Oxide and Yttrium Oxide Nanoparticles in Isolated Rat Pancreatic Islets. Hum. Exp. Toxicol. 2013, 32, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Tavoosi, S.; Baghsheikhi, A.H.; Shetab-Boushehri, S.V.; Navaei-Nigjeh, M.; Sarvestani, N.N.; Karimi, M.Y.; Ranjbar, A.; Ebadollahi-Natanzi, A.; Hosseini, A. Cerium and Yttrium Oxide Nanoparticles and Nano-Selenium Produce Protective Effects Against H2O2-Induced Oxidative Stress in Pancreatic Beta Cells by Modulating Mitochondrial Dysfunction. Pharm. Nanotechnol. 2020, 8, 63–75. [Google Scholar] [CrossRef]
- Khaksar, M.R.; Rahimifard, M.; Baeeri, M.; Maqbool, F.; Navaei-Nigjeh, M.; Hassani, S.; Moeini-Nodeh, S.; Kebriaeezadeh, A.; Abdollahi, M. Protective Effects of Cerium Oxide and Yttrium Oxide Nanoparticles on Reduction of Oxidative Stress Induced by Sub-Acute Exposure to Diazinon in the Rat Pancreas. J. Trace Elem. Med. Biol. 2017, 41, 79–90. [Google Scholar] [CrossRef]
- Navaei-Nigjeh, M.; Daniali, M.; Rahimifard, M.; Khaksar, M.R. Multi-Organ Toxicity Attenuation by Cerium Oxide and Yttrium Oxide Nanoparticles: Comparing the Beneficial Effects on Tissues Oxidative Damage Induced by Sub-Acute Exposure to Diazinon. Pharm. Nanotechnol. 2020, 8, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.; Dargusch, R.; Raitano, J.; Chan, S.-W. Cerium and Yttrium Oxide Nanoparticles Are Neuroprotective. Biochem. Biophys. Res. Commun. 2006, 342, 86–91. [Google Scholar] [CrossRef]
- Ghaznavi, H.; Najafi, R.; Mehrzadi, S.; Hosseini, A.; Tekyemaroof, N.; Shakeri-zadeh, A.; Rezayat, M.; Sharifi, A.M. Neuro-Protective Effects of Cerium and Yttrium Oxide Nanoparticles on High Glucose-Induced Oxidative Stress and Apoptosis in Undifferentiated PC12 Cells. Neurol. Res. 2015, 37, 624–632. [Google Scholar] [CrossRef]
- Baghaee, P.; Yoonesi, M.; Esfahani, D.E.; Beirami, E.; Dargahi, L.; Rashidi, F.S.; Valian, N. Yttrium Oxide Nanoparticles Alleviate Cognitive Deficits, Neuroinflammation, and Mitochondrial Biogenesis Impairment Induced by Streptozotocin. Neurosci. Lett. 2024, 837, 137895. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Sharifi, A.M.; Abdollahi, M.; Najafi, R.; Baeeri, M.; Rayegan, S.; Cheshmehnour, J.; Hassani, S.; Bayrami, Z.; Safa, M. Cerium and Yttrium Oxide Nanoparticles Against Lead-Induced Oxidative Stress and Apoptosis in Rat Hippocampus. Biol. Trace Elem. Res. 2015, 164, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Abu-Taweel, G.M.; Al-Mutary, M.G.; Albetran, H.M. Yttrium Oxide Nanoparticles Moderate the Abnormal Cognitive Behaviors in Male Mice Induced by Silver Nanoparticles. Oxidative Med. Cell. Longev. 2022, 2022, 9059371. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M.; Albetran, H.M.; Al-Mutary, M.G.; Ahmad, M.; Low, I.M. Alleviation of Silver Nanoparticle-Induced Sexual Behavior and Testicular Parameters Dysfunction in Male Mice by Yttrium Oxide Nanoparticles. Toxicol. Rep. 2021, 8, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.N.; Merwin, M.J.; Han, Z.; Conley, S.M.; Al-Ubaidi, M.R.; Naash, M.I. Yttrium Oxide Nanoparticles Prevent Photoreceptor Death in a Light-Damage Model of Retinal Degeneration. Free Radic. Biol. Med. 2014, 75, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Emad, B.; WalyEldeen, A.A.; Hassan, H.; Sharaky, M.; Abdelhamid, I.A.; Ibrahim, S.A.; Mohamed, H.R. Yttrium Oxide Nanoparticles Induce Cytotoxicity, Genotoxicity, Apoptosis, and Ferroptosis in the Human Triple-Negative Breast Cancer MDA-MB-231 Cells. BMC Cancer 2023, 23, 1151. [Google Scholar] [CrossRef] [PubMed]
- Zako, T.; Yoshimoto, M.; Hyodo, H.; Kishimoto, H.; Ito, M.; Kaneko, K.; Soga, K.; Maeda, M. Cancer-Targeted near Infrared Imaging Using Rare Earth Ion-Doped Ceramic Nanoparticles. Biomater. Sci. 2015, 3, 59–64. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alrokayan, S.A.; Ramamoorthy, M.M.; Alaizeri, Z.M. High Surface Reactivity and Biocompatibility of Y2O3 NPs in Human MCF-7 Epithelial and HT-1080 Fibro-Blast Cells. Molecules 2020, 25, 1137. [Google Scholar] [CrossRef]
- Sayour, H.; Kassem, S.; Canfarotta, F.; Czulak, J.; Mohamed, M.; Piletsky, S. Biocompatibility and Biodistribution of Surface-Modified Yttrium Oxide Nanoparticles for Potential Theranostic Applications. Environ. Sci. Pollut. Res. 2020, 27, 19095–19107. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Bodapati, S.; Murray, E.; Blough, E.; Winston, N.; Rice, K.; Shokufar, T.; Zhao, Y. Cytotoxicity and Genotoxicity Caused by Yttrium Oxide Nanoparticles in HEK293 Cells. Int. J. Nanomed. 2014, 9, 1379–1391. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Zheng, M.; Mo, M.; Hu, X.; Liu, C.; Pathak, J.L.; Wang, L.; Chen, L. TRIM24-DTNBP1-ATP7A Mediated Astrocyte Cuproptosis in Cognition and Memory Dysfunction Caused by Y2O3 NPs. Sci. Total Environ. 2024, 954, 176353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, X.; Feng, L.; Yu, Y.; Hu, J.; Liu, X.; Wu, H. Structural Insight into the Alginate Derived Nano-La(OH)3/Porous Carbon Composites for Highly Selective Adsorption of Phosphate. Int. J. Biol. Macromol. 2022, 200, 172–181. [Google Scholar] [CrossRef]
- Wei, Y.; Yuan, P.; Zhou, J.; Liu, J.; Losic, D.; Wu, H.; Bu, H.; Tan, X.; Li, Z. Direct Atomic-Scale Insight into the Precipitation Formation at the Lanthanum Hydroxide Nanoparticle/Solution Interface. J. Phys. Chem. Lett. 2023, 14, 3995–4003. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.-H.; Chen, D.-H. LaB6 Nanoparticles with Carbon-Doped Silica Coating for Fluorescence Imaging and near-IR Photothermal Therapy of Cancer Cells. Acta Biomater. 2013, 9, 7556–7563. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Leong, Y.-K.; Saunders, M.; Martyniuk, M.; Faraone, L.; Keating, A.; Dell, J.M. Uniform Dispersion of Lanthanum Hexaboride Nanoparticles in a Silica Thin Film: Synthesis and Optical Properties. ACS Appl. Mater. Interfaces 2012, 4, 5833–5838. [Google Scholar] [CrossRef] [PubMed]
- Sobhani-Nasab, A.; Zahraei, Z.; Akbari, M.; Maddahfar, M.; Hosseinpour-Mashkani, S.M. Synthesis, Characterization, and Antibacterial Activities of ZnLaFe 2 O 4 /NiTiO 3 Nanocomposite. J. Mol. Struct. 2017, 1139, 430–435. [Google Scholar] [CrossRef]
- Dinakaran, D.; Sengupta, J.; Pink, D.; Raturi, A.; Chen, H.; Usmani, N.; Kumar, P.; Lewis, J.D.; Narain, R.; Moore, R.B. PEG-PLGA Nanospheres Loaded with Nanoscintillators and Photosensitizers for Radiation-Activated Photodynamic Therapy. Acta Biomater. 2020, 117, 335–348. [Google Scholar] [CrossRef]
- Lu, V.M.; Jue, T.R.; McDonald, K.L. Cytotoxic Lanthanum Oxide Nanoparticles Sensitize Glioblastoma Cells to Radiation Therapy and Temozolomide: An in Vitro Rationale for Translational Studies. Sci. Rep. 2020, 10, 18156. [Google Scholar] [CrossRef]
- Zheng, G.; Wu, S.; Deng, X.; Wang, A.; Ying, Y.; Li, S.; Wang, F.; Liu, X.; Wang, P.; Wei, D. Lanthanum-Based Dendritic Mesoporous Nanoplatform for Tumor Microenvironment Activating Synergistic Anti-Glioma Efficacy. Mater. Today Bio 2024, 28, 101223. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.V.; Woodward, J.D.; Chen, N.; Rondinone, A.J.; Castano, C.H.; Mirzadeh, S. Synthesis and Characterization of Lanthanum Phosphate Nanoparticles as Carriers for 223Ra and 225Ra for Targeted Alpha Therapy. Nucl. Med. Biol. 2015, 42, 614–620. [Google Scholar] [CrossRef]
- Muthulakshmi, V.; Dhilip Kumar, C.; Sundrarajan, M. Biological Applications of Green Synthesized Lanthanum Oxide Nanoparticles via Couroupita Guianensis Abul Leaves Extract. Anal. Biochem. 2022, 638, 114482. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, L.; Li, Q.X.; Liang, H.; Qin, H.; Masutani, S.; Yoza, B. Toxicity of Lanthanum Oxide Nanoparticles to the Fungus Moniliella Wahieum Y12T Isolated from Biodiesel. Chemosphere 2018, 199, 495–501. [Google Scholar] [CrossRef]
- Tiwari, S.; Gupta, P.K.; Bagbi, Y.; Sarkar, T.; Solanki, P.R. L-Cysteine Capped Lanthanum Hydroxide Nanostructures for Non-Invasive Detection of Oral Cancer Biomarker. Biosens. Bioelectron. 2017, 89, 1042–1052. [Google Scholar] [CrossRef]
- Almukhlafi, H.; Ali, D.; Almutairi, B.; Yaseen, K.N.; Alyami, N.; Almeer, R.; Alkahtani, S.; Alarifi, S. Role of Oxidative Stress in La2O3 Nanoparticle-Induced Cytotoxicity and Apoptosis in CHANG and HuH-7 Cells. Int. J. Nanomed. 2021, 16, 3487–3496. [Google Scholar] [CrossRef]
- Alsubaie, S.M.; Ali, D.; Almutairi, B.O.; Almeer, R.; Alarifi, S. Evaluation of Cyto—And Genotoxic Influence of Lanthanum Dioxide Nanoparticles on Human Liver Cells. Dose-Response 2022, 20, 15593258221128428. [Google Scholar] [CrossRef]
- Alyami, N.M.; Alobadi, H.; Maodaa, S.; Alothman, N.S.; Almukhlafi, H.; Yaseen, K.N.; Alnakhli, Z.A.; Alshiban, N.M.; Elnagar, D.M.; Rady, A.; et al. Determination of Dose- and Time-Dependent Hepatotoxicity and Apoptosis of Lanthanum Oxide Nanoparticles in Female Swiss Albino Mice. Environ. Sci. Pollut. Res. 2024, 31, 17124–17139. [Google Scholar] [CrossRef]
- Dressler, V.L.; Ogunmodede, O.T.; Heidrich, G.M.; Neves, V.M.; Schetinger, M.R.C.; Morsch, V.M. Investigative Analysis of Lanthanum Oxide Nanoparticles on Elements in Bone of Wistar Rats After 30 Days of Repeated Oral Administration. Biol. Trace Elem. Res. 2020, 196, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Sreekumar, S.; Ahina, K.M.; Lakra, R.; Kiran, M.S. Lanthanum Oxide Nanoparticles Reinforced Collagen ƙ-Carrageenan Hydroxyapatite Biocomposite as Angio-Osteogenic Biomaterial for In Vivo Osseointegration and Bone Repair. Adv. Biol. 2023, 7, 2300039. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Li, P.; Bian, Y. Assessment the Exposure Level of Rare Earth Elements in Workers Producing Cerium, Lanthanum Oxide Ultrafine and Nanoparticles. Biol. Trace Elem. Res. 2017, 175, 298–305. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Zheng, S.; Miao, Y.; Yin, S.; Li, P.; Bian, Y. Direct Quantification of Rare Earth Elements Concentrations in Urine of Workers Manufacturing Cerium, Lanthanum Oxide Ultrafine and Nanoparticles by a Developed and Validated ICP-MS. Int. J. Environ. Res. Public Health 2016, 13, 350. [Google Scholar] [CrossRef]
- Xiao, X.; Yong, L.; Jiao, B.; Yang, H.; Liang, C.; Jia, X.; Liu, Z.; Sang, Y.; Song, Y. Postweaning Exposure to Lanthanum Alters Neurological Behavior during Early Adulthood in Rats. NeuroToxicology 2021, 83, 40–50. [Google Scholar] [CrossRef]
- Yuan, L.; Qu, Y.; Li, Q.; An, T.; Chen, Z.; Chen, Y.; Deng, X.; Bai, D. Protective Effect of Astaxanthin against La2O3 Nanoparticles Induced Neurotoxicity by Activating PI3K/AKT/Nrf-2 Signaling in Mice. Food Chem. Toxicol. 2020, 144, 111582. [Google Scholar] [CrossRef]
- Dundar, B.; Alsawas, M.; Masaadeh, A.; Conway, K.; Snow, A.N.; Sompallae, R.R.; Bossler, A.D.; Ma, D.; Lopes Abath Neto, O. Molecular Characterization and Survival Analysis of a Cohort of Glioblastoma, IDH-Wildtype. Pathol.—Res. Pract. 2024, 257, 155272. [Google Scholar] [CrossRef]
- Jing, Y.; Kang, T.; Hu, P.; Fan, H.; Teng, F.; Zhao, X.; Song, J. Surfactant-Induced Photocatalytic Performance Enhancement of Europium Oxide Nanoparticles. Process Saf. Environ. Prot. 2023, 171, 888–894. [Google Scholar] [CrossRef]
- Teng, M.; Liang, X.; Liu, H.; Li, Z.; Gao, X.; Zhang, C.; Cheng, H.; Chen, H.; Liu, G. Cerenkov Radiation Shining a Light for Cancer Theranostics. Nano Today 2024, 55, 102174. [Google Scholar] [CrossRef]
- Hu, Z.; Chi, C.; Liu, M.; Guo, H.; Zhang, Z.; Zeng, C.; Ye, J.; Wang, J.; Tian, J.; Yang, W.; et al. Nanoparticle-Mediated Radiopharmaceutical-Excited Fluorescence Molecular Imaging Allows Precise Image-Guided Tumor-Removal Surgery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, J.; Yao, L.; Zhang, L.; Li, D.; Li, Z.; Wu, Q.; Wang, S.; Ding, J.; Liu, Y.; et al. Determining Toxicity of Europium Oxide Nanoparticles in Immune Cell Components and Hematopoiesis in Dominant Organs in Mice: Role of Lysosomal Fluid Interaction. Sci. Total Environ. 2024, 937, 173482. [Google Scholar] [CrossRef] [PubMed]
- Chávez-García, D.; Juarez-Moreno, K.; Calderón-Osuna, I.; Navarro, P.; Hirata, G.A. Nanotoxicological Study of Downconversion Y2O3:Eu3+ Luminescent Nanoparticles Functionalized with Folic Acid for Cancer Cells Bioimaging. J. Biomed. Mater. Res. 2020, 108, 2396–2406. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, S.; Wang, Y.; Liu, Y.; Wu, T.; Chen, T. Ligand-Functionalization of BPEI-Coated YVO4:Bi3+,Eu3+ Nanophosphors for Tumor-Cell-Targeted Imaging Applications. Chem. Asian J. 2013, 8, 2652–2659. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, Y.; Zheng, M.; Shan, C.; Yan, H.; Wu, W.; Gao, X.; Cheng, B.; Liu, W.; Tang, Y. Functionalized Eu(III)-Based Nanoscale Metal–Organic Framework To Achieve Near-IR-Triggered and -Targeted Two-Photon Absorption Photodynamic Therapy. Inorg. Chem. 2018, 57, 300–310. [Google Scholar] [CrossRef]
- Gadzhimagomedova, Z.; Polyakov, V.; Pankin, I.; Butova, V.; Kirsanova, D.; Soldatov, M.; Khodakova, D.; Goncharova, A.; Mukhanova, E.; Belanova, A.; et al. BaGdF5 Nanophosphors Doped with Different Concentrations of Eu3+ for Application in X-Ray Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 13040. [Google Scholar] [CrossRef]
- Abd Elkader, M.F.; Almogbel, M.S.; Elabbasy, M.T. Survival and Reduction in Foodborne Bacteria Using Methyl Cellulose Film Doped with Europium Oxide Nanoparticles. Food Sci. Nutr. 2020, 8, 291–298. [Google Scholar] [CrossRef]
- Fu, M.; Zhao, Y.; Wang, Y.; Li, Y.; Wu, M.; Liu, Q.; Hou, Z.; Lu, Z.; Wu, K.; Guo, J. On-Demand Removable Self-Healing and pH-Responsive Europium-Releasing Adhesive Dressing Enables Inflammatory Microenvironment Modulation and Angiogenesis for Diabetic Wound Healing. Small 2023, 19, e2205489. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Villa, D.; Aguilar, M.R.; Rojo, L. Europium-Tannic Acid Nanocomplexes Devised for Bone Regeneration under Oxidative or Inflammatory Environments. J. Mater. Chem. B 2024, 12, 7153–7170. [Google Scholar] [CrossRef]
- Wiatrak, B.; Sobierajska, P.; Szandruk-Bender, M.; Jawien, P.; Janeczek, M.; Dobrzynski, M.; Pistor, P.; Szelag, A.; Wiglusz, R.J. Nanohydroxyapatite as a Biomaterial for Peripheral Nerve Regeneration after Mechanical Damage-In Vitro Study. Int. J. Mol. Sci. 2021, 22, 4454. [Google Scholar] [CrossRef] [PubMed]
- Teker, T.; Aslanoglu, M. Development of a Dy2O3@Eu2O3-Carbon Nanofiber Based Electrode for Highly Sensitive Detection of Papaverine. Anal. Chim. Acta 2021, 1183, 338972. [Google Scholar] [CrossRef] [PubMed]
- Narasimharao, K.; Ali, T.T. Influence of Synthesis Conditions on Physico-Chemical and Photocatalytic Properties of Rare Earth (Ho, Nd and Sm) Oxides. J. Mater. Res. Technol. 2020, 9, 1819–1830. [Google Scholar] [CrossRef]
- Zawadzki, M.; Kępiński, L. Synthesis and Characterization of Neodymium Oxide Nanoparticles. J. Alloys Compd. 2004, 380, 255–259. [Google Scholar] [CrossRef]
- Dorris, A.; Sicard, C.; Chen, M.C.; McDonald, A.B.; Barrett, C.J. Stabilization of Neodymium Oxide Nanoparticles via Soft Adsorption of Charged Polymers. ACS Appl. Mater. Interfaces 2011, 3, 3357–3365. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Park, S.-Y.; Sung, A.-Y. Characterization of Biocompatible Hydrogel Lenses Using Methacrylic Acid with Neodymium Oxide Nanoparticles. Polymers 2021, 13, 1575. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Naskar, H.; Pradhan, S.; Wang, Y.; Bandyopadhyay, R.; Pramanik, P. Simultaneous Voltammetric Determination of Adrenaline and Tyrosine in Real Samples by Neodymium Oxide Nanoparticles Grafted Graphene. Talanta 2020, 206, 120176. [Google Scholar] [CrossRef]
- Muthulakshmi, V.; Dhilip Kumar, C.; Sundrarajan, M. Green Synthesis of Ionic Liquid Mediated Neodymium Oxide Nanoparticles via Couroupita Guianensis Abul Leaves Extract with Its Biological Applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 1063–1082. [Google Scholar] [CrossRef] [PubMed]
- Sundrarajan, M.; Muthulakshmi, V. Green Synthesis of Ionic Liquid Mediated Neodymium Oxide Nanoparticles by Andrographis Paniculata Leaves Extract for Effective Bio-Medical Applications. J. Environ. Chem. Eng. 2021, 9, 104716. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, X.; Gao, L.; Xue, H.; Liu, L.; Wang, S.; Chen, S.; Huang, L. LncRNA Loc105377478 Promotes NPs-Nd2O3-Induced Inflammation in Human Bronchial Epithelial Cells through the ADIPOR1/NF-κB Axis. Ecotoxicol. Environ. Saf. 2021, 208, 111609. [Google Scholar] [CrossRef]
- Liu, L.; Cui, J.; Chen, S.; Zhang, X.; Wang, S.; Huang, L. Circ_002363 Is Regulated by the RNA Binding Protein BCAS2 and Inhibits Neodymium Oxide Nanoparticle-Induced DNA Damage by Non-Homologous End-Joining Repair. Sci. Total Environ. 2023, 863, 160819. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, X.; Cui, J.; Liu, L.; Tai, D.; Wang, S.; Huang, L. Transcription Factor TP63 Mediates LncRNA CNTFR-AS1 to Promote DNA Damage Induced by Neodymium Oxide Nanoparticles via Homologous Recombination Repair. Environ. Pollut. 2023, 334, 122191. [Google Scholar] [CrossRef]
- May, E.; Thoennessen, M. Discovery of Cesium, Lanthanum, Praseodymium and Promethium Isotopes. At. Data Nucl. Data Tables 2012, 98, 960–982. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, S.-H.; Jeong, J.; Lee, D.-K.; Lee, S.; Kim, S.; Kim, G.; Maruthupandy, M.; Cho, W.-S. ABCG1 and ABCG4 as Key Transporters in the Development of Pulmonary Alveolar Proteinosis by Nanoparticles. J. Hazard. Mater. 2021, 420, 126595. [Google Scholar] [CrossRef]
- Bartenbach, D.; Popescu, R.; Gerthsen, D.; Feldmann, C. [Sm6O4(Cbz)10(Thf)6]·2C7H8: A Polynuclear Samarium Oxo Cluster Obtained from Carbazole-Driven Oxidation of Samarium Nanoparticles. Inorg. Chem. 2022, 61, 3072–3077. [Google Scholar] [CrossRef] [PubMed]
- Damirchi, Z.; Firoozbakhtian, A.; Hosseini, M.; Ganjali, M.R. Ti3C2/Ni/Sm-Based Electrochemical Glucose Sensor for Sweat Analysis Using Bipolar Electrochemistry. Microchim. Acta 2024, 191, 137. [Google Scholar] [CrossRef] [PubMed]
- Gilnezhad, J.; Firoozbakhtian, A.; Hosseini, M.; Adel, S.; Xu, G.; Ganjali, M.R. An Enzyme-Free Ti3C2/Ni/Sm-LDH-Based Screen-Printed-Electrode for Real-Time Sweat Detection of Glucose. Anal. Chim. Acta 2023, 1250, 340981. [Google Scholar] [CrossRef]
- Muthulakshmi, V.; Balaji, M.; Sundrarajan, M. Biomedical Applications of Ionic Liquid Mediated Samarium Oxide Nanoparticles by Andrographis Paniculata Leaves Extract. Mater. Chem. Phys. 2020, 242, 122483. [Google Scholar] [CrossRef]
- Alshahrani, A.A.; Alqarni, L.S.; Alghamdi, M.D.; Alotaibi, N.F.; Moustafa, S.M.N.; Nassar, A.M. Phytosynthesis via Wasted Onion Peel Extract of Samarium Oxide/Silver Core/Shell Nanoparticles for Excellent Inhibition of Microbes. Heliyon 2024, 10, e24815. [Google Scholar] [CrossRef]
- Zahmatkesh, H.; Mirpour, M.; Zamani, H.; Rasti, B.; Rahmani, F.A.; Padasht, N. Effect of Samarium Oxide Nanoparticles on Virulence Factors and Motility of Multi-Drug Resistant Pseudomonas Aeruginosa. World J. Microbiol. Biotechnol. 2022, 38, 209. [Google Scholar] [CrossRef] [PubMed]
- Chanket, W.; Pipatthana, M.; Sangphukieo, A.; Harnvoravongchai, P.; Chankhamhaengdecha, S.; Janvilisri, T.; Phanchana, M. The Complete Catalog of Antimicrobial Resistance Secondary Active Transporters in Clostridioides Difficile: Evolution and Drug Resistance Perspective. Comput. Struct. Biotechnol. J. 2024, 23, 2358–2374. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.E.A.; Khalil, A.T.; Hkiri, K.; Ayaz, M.; Usman, A.; Sadiq, A.; Ullah, F.; Khan, M.A.; Ullah, I.; Maaza, M. Potential Nanomedicinal Applications and Physicochemical Nature of Hyphaene thebaica-reduced Nano-samaria. Microsc. Res. Tech. 2024, 87, 2829–2841. [Google Scholar] [CrossRef]
- Smith, A.W.; Greenberger, B.A.; Den, R.B.; Stock, R.G. Radiopharmaceuticals for Bone Metastases. Semin. Radiat. Oncol. 2021, 31, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, N.; Faridbod, F. ALS Genosensing Using DNA-Hybridization Electrochemical Biosensor Based on Label-Free Immobilization of ssDNA on Sm2O3 NPs-rGO/PANI Composite. Sens. Actuators B Chem. 2018, 275, 432–438. [Google Scholar] [CrossRef]
- Kujur, M.S.; Manakari, V.; Parande, G.; Prasadh, S.; Wong, R.; Mallick, A.; Gupta, M. Effect of Samarium Oxide Nanoparticles on Degradation and Invitro Biocompatibility of Magnesium. Mater. Today Commun. 2021, 26, 102171. [Google Scholar] [CrossRef]
- Shrestha, S.; Yeung, C.M.Y.; Nunnerley, C.; Tsang, S.C. Comparison of Morphology and Electrical Conductivity of Various Thin Films Containing Nano-Crystalline Praseodymium Oxide Particles. Sens. Actuators A Phys. 2007, 136, 191–198. [Google Scholar] [CrossRef]
- Bakht, M.K.; Sadeghi, M.; Ahmadi, S.J.; Sadjadi, S.S.; Tenreiro, C. Preparation of Radioactive Praseodymium Oxide as a Multifunctional Agent in Nuclear Medicine: Expanding the Horizons of Cancer Therapy Using Nanosized Neodymium Oxide. Nucl. Med. Commun. 2013, 34, 5–12. [Google Scholar] [CrossRef]
- Jang, H.; Choi, M.; Yim, Y.; Kim, Y.; Min, D. Dual-Wavelength Irradiation and Dox Delivery for Cancer Cell Ablation with Photocatalytic Pr Doped TiO2 /NGO Hybrid Nanocomposite. Adv. Healthc. Mater. 2015, 4, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Manikantan, V.; Varalakshmi, G.S.; Pillai, A.S.; Alexander, A.; Lucas, A.; Kathiravan, E.; Akash, B.A.; Enoch, I.V.M.V. 5-Fluorouracil-Loaded Designed Praseodymium Oxide—Poly-β-Cyclodextrin Nanorods for Effectively Inhibiting Breast Cancer Cells. Inorg. Chem. Commun. 2023, 153, 110830. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Yan, W.-L.; Jiang, L.; Zheng, Y.-G.; Wang, J.-X.; Zhang, R.-K. Synthesis of Nano-Praseodymium Oxide for Cataluminescence Sensing of Acetophenone in Exhaled Breath. Molecules 2019, 24, 4275. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kaur, S.; Chaudhary, S.; Umar, A.; Kumar, R. Bare and Nonionic Surfactant-Functionalized Praseodymium Oxide Nanoparticles: Toxicological Studies. Chemosphere 2018, 209, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Sreekumar, S.; Singh, F.; Srivatsan, K.V.; Lakra, R.; Sai, K.P.; Kiran, M.S. Nanotized Praseodymium Oxide Collagen 3-D pro-Vasculogenic Biomatrix for Soft Tissue Engineering. Nanomed. Nanotechnol. Biol. Med. 2021, 33, 102364. [Google Scholar] [CrossRef]
- Zou, S.; Guo, F.; Wu, L.; Ju, H.; Sun, M.; Cai, R.; Xu, L.; Gong, Y.; Gong, A.; Zhang, M.; et al. One-Pot Synthesis of Cerium and Praseodymium Co-Doped Carbon Quantum Dots as Enhanced Antioxidant for Hydroxyl Radical Scavenging. Nanotechnology 2020, 31, 165101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Tang, J.; Wei, Y.; Deng, X.; Zhang, Y.; Ma, X.; Jiang, X.; Xu, Z.P.; Liao, H. Antibacterial Properties of Cerium Oxide Nanoparticles: Recent Progresses and Future Challenges. Particuology 2024, 93, 264–283. [Google Scholar] [CrossRef]
- Yadav, S.; Chamoli, S.; Kumar, P.; Maurya, P.K. Structural and Functional Insights in Polysaccharides Coated Cerium Oxide Nanoparticles and Their Potential Biomedical Applications: A Review. Int. J. Biol. Macromol. 2023, 246, 125673. [Google Scholar] [CrossRef]
- Nosrati, H.; Heydari, M.; Khodaei, M. Cerium Oxide Nanoparticles: Synthesis Methods and Applications in Wound Healing. Mater. Today Bio 2023, 23, 100823. [Google Scholar] [CrossRef] [PubMed]
- S Jairam, L.; Chandrashekar, A.; Prabhu, T.N.; Kotha, S.B.; Girish, M.S.; Devraj, I.M.; Dhanya Shri, M.; Prashantha, K. A Review on Biomedical and Dental Applications of Cerium Oxide Nanoparticles ― Unearthing the Potential of This Rare Earth Metal. J. Rare Earths 2023, 41, 1645–1661. [Google Scholar] [CrossRef]
- Chai, W.F.; Tang, K.S. Protective Potential of Cerium Oxide Nanoparticles in Diabetes Mellitus. J. Trace Elem. Med. Biol. 2021, 66, 126742. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Naik, P. Synthesis and Biomedical Applications of Cerium Oxide Nanoparticles—A Review. Biotechnol. Rep. 2018, 17, 1–5. [Google Scholar] [CrossRef]
- Meng, X.; Wang, W.-D.; Li, S.-R.; Sun, Z.-J.; Zhang, L. Harnessing Cerium-Based Biomaterials for the Treatment of Bone Diseases. Acta Biomater. 2024, 183, 30–49. [Google Scholar] [CrossRef] [PubMed]
- Zandi, M.; Hosseini, F.; Adli, A.H.; Salmanzadeh, S.; Behboudi, E.; Halvaei, P.; Khosravi, A.; Abbasi, S. State-of-the-Art Cerium Nanoparticles as Promising Agents against Human Viral Infections. Biomed. Pharmacother. 2022, 156, 113868. [Google Scholar] [CrossRef]
- Hashem, R.M.; Rashd, L.A.; Hashem, K.S.; Soliman, H.M. Cerium Oxide Nanoparticles Alleviate Oxidative Stress and Decreases Nrf-2/HO-1 in D-GALN/LPS Induced Hepatotoxicity. Biomed. Pharmacother. 2015, 73, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Vafaei, S.A.; Naseri, N.; Darini, A.; Azandaryani, M.T.; Ara, F.K.; Mirzaei, F. Protective Effects of Cerium Oxide Nanoparticles in Non-Alcoholic Fatty Liver Disease (NAFLD) and Carbon Tetrachloride-Induced Liver Damage in Rats: Study on Intestine and Liver. Metab. Open 2021, 12, 100151. [Google Scholar] [CrossRef] [PubMed]
- Oró, D.; Yudina, T.; Fernández-Varo, G.; Casals, E.; Reichenbach, V.; Casals, G.; González De La Presa, B.; Sandalinas, S.; Carvajal, S.; Puntes, V.; et al. Cerium Oxide Nanoparticles Reduce Steatosis, Portal Hypertension and Display Anti-Inflammatory Properties in Rats with Liver Fibrosis. J. Hepatol. 2016, 64, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Virchenko, O.; Falalyeyeva, T.; Kondro, M.; Beregova, T.; Bodnar, P.; Shcherbakov, O.; Bubnov, R.; Caprnda, M.; Delev, D.; et al. Cerium Dioxide Nanoparticles Possess Anti-Inflammatory Properties in the Conditions of the Obesity-Associated NAFLD in Rats. Biomed. Pharmacother. 2017, 90, 608–614. [Google Scholar] [CrossRef]
- Ibrahim, H.G.; Attia, N.; Hashem, F.E.Z.A.; El Heneidy, M.A.R. Cerium Oxide Nanoparticles: In Pursuit of Liver Protection against Doxorubicin-Induced Injury in Rats. Biomed. Pharmacother. 2018, 103, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Nassar, S.Z.; Hassaan, P.S.; Abdelmonsif, D.A.; ElAchy, S.N. Cardioprotective Effect of Cerium Oxide Nanoparticles in Monocrotaline Rat Model of Pulmonary Hypertension: A Possible Implication of Endothelin-1. Life Sci. 2018, 201, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Tisi, A.; Passacantando, M.; Lozzi, L.; Riccitelli, S.; Bisti, S.; Maccarone, R. Retinal Long Term Neuroprotection by Cerium Oxide Nanoparticles after an Acute Damage Induced by High Intensity Light Exposure. Exp. Eye Res. 2019, 182, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.Y.; Pritchard, S.; Harper, K.; Aston, J.W.; Lynch, A.; Lucky, J.J.; Ludington, J.S.; Chatani, P.; Mosenthal, W.P.; Leiter, J.C.; et al. Neuroprotective Mechanisms of Cerium Oxide Nanoparticles in a Mouse Hippocampal Brain Slice Model of Ischemia. Free Radic. Biol. Med. 2011, 51, 1155–1163. [Google Scholar] [CrossRef]
- Kadivar, F.; Haddadi, G.; Mosleh-Shirazi, M.A.; Khajeh, F.; Tavasoli, A. Protection Effect of Cerium Oxide Nanoparticles against Radiation-Induced Acute Lung Injuries in Rats. Rep. Pract. Oncol. Radiother. 2020, 25, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Silina, E.V.; Manturova, N.E.; Ivanova, O.S.; Baranchikov, A.E.; Artyushkova, E.B.; Medvedeva, O.A.; Kryukov, A.A.; Dodonova, S.A.; Gladchenko, M.P.; Vorsina, E.S.; et al. Cerium Dioxide–Dextran Nanocomposites in the Development of a Medical Product for Wound Healing: Physical, Chemical and Biomedical Characteristics. Molecules 2024, 29, 2853. [Google Scholar] [CrossRef] [PubMed]
- Silina, E.V.; Stupin, V.A.; Manturova, N.E.; Ivanova, O.S.; Popov, A.L.; Mysina, E.A.; Artyushkova, E.B.; Kryukov, A.A.; Dodonova, S.A.; Kruglova, M.P.; et al. Influence of the Synthesis Scheme of Nanocrystalline Cerium Oxide and Its Concentration on the Biological Activity of Cells Providing Wound Regeneration. Int. J. Mol. Sci. 2023, 24, 14501. [Google Scholar] [CrossRef]
- Fang, X.; Song, H. Synthesis of Cerium Oxide Nanoparticles Loaded on Chitosan for Enhanced Auto-Catalytic Regenerative Ability and Biocompatibility for the Spinal Cord Injury Repair. J. Photochem. Photobiol. B Biol. 2019, 191, 83–87. [Google Scholar] [CrossRef]
- Silina, E.V.; Manturova, N.E.; Vasin, V.I.; Artyushkova, E.B.; Khokhlov, N.V.; Ivanov, A.V.; Stupin, V.A. Efficacy of A Novel Smart Polymeric Nanodrug in the Treatment of Experimental Wounds in Rats. Polymers 2020, 12, 1126. [Google Scholar] [CrossRef]
- Wei, F.; Neal, C.J.; Sakthivel, T.S.; Kean, T.; Seal, S.; Coathup, M.J. Multi-Functional Cerium Oxide Nanoparticles Regulate Inflammation and Enhance Osteogenesis. Mater. Sci. Eng. C 2021, 124, 112041. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhou, Y.; Zheng, K.; Xu, X.; Yang, J.; Wang, X.; Miao, L.; Wei, H.; Xu, Y. Cerium Oxide Nanoparticles Loaded Nanofibrous Membranes Promote Bone Regeneration for Periodontal Tissue Engineering. Bioact. Mater. 2022, 7, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Nilawar, S.; Yadav, P.; Jain, N.; Saini, D.K.; Chatterjee, K. Protective Role of Nanoceria-Infused Nanofibrous Scaffold toward Bone Tissue Regeneration with Senescent Cells. Biomacromolecules 2024, 25, 4074–4086. [Google Scholar] [CrossRef]
- Foroutan, Z.; Afshari, A.R.; Sabouri, Z.; Mostafapour, A.; Far, B.F.; Jalili-Nik, M.; Darroudi, M. Plant-Based Synthesis of Cerium Oxide Nanoparticles as a Drug Delivery System in Improving the Anticancer Effects of Free Temozolomide in Glioblastoma (U87) Cells. Ceram. Int. 2022, 48, 30441–30450. [Google Scholar] [CrossRef]
- Parvathy, S.; Manjula, G.; Balachandar, R.; Subbaiya, R. Green Synthesis and Characterization of Cerium Oxide Nanoparticles from Artabotrys Hexapetalus Leaf Extract and Its Antibacterial and Anticancer Properties. Mater. Lett. 2022, 314, 131811. [Google Scholar] [CrossRef]
- Sridharan, M.; Kamaraj, P.; Vennilaraj; Arockiaselvi, J.; Pushpamalini, T.; Vivekanand, P.A.; Hari Kumar, S. Synthesis, Characterization and Evaluation of Biosynthesized Cerium Oxide Nanoparticle for Its Anticancer Activity on Breast Cancer Cell (MCF 7). Mater. Today Proc. 2021, 36, 914–919. [Google Scholar] [CrossRef]
- Mousaiyan, S.; Baharara, J.; Es-haghi, A. Biopreparation of Cerium Oxide Nanoparticles Using Alginate: Characterization and Estimation of Antioxidant and Its Activity against Breast Cancer Cell Lines (MCF7). Results Chem. 2024, 7, 101468. [Google Scholar] [CrossRef]
- Rasouli, Z.; Yousefi, M.; Torbati, M.B.; Samadi, S.; Kalateh, K. Synthesis and Characterization of Nanoceria-Based Composites and in Vitro Evaluation of Their Cytotoxicity against Colon Cancer. Polyhedron 2020, 176, 114297. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Khorsandi, K.; Esfahani, H.S.; Habibi, M.; Hosseinzadeh, G. Preparation of Cerium-Curcumin and Cerium-Quercetin Complexes and Their LEDs Irradiation Assisted Anticancer Effects on MDA-MB-231 and A375 Cancer Cell Lines. Photodiagnosis Photodyn. Ther. 2021, 34, 102326. [Google Scholar] [CrossRef]
- Wason, M.S.; Colon, J.; Das, S.; Seal, S.; Turkson, J.; Zhao, J.; Baker, C.H. Sensitization of Pancreatic Cancer Cells to Radiation by Cerium Oxide Nanoparticle-Induced ROS Production. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 558–569. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, S.; Zheng, H.; Yang, S.; Zhou, L.; Liu, K.; Zhang, Q.; Zhang, H. Cu-Doped Cerium Oxide-Based Nanomedicine for Tumor Microenvironment-Stimulative Chemo-Chemodynamic Therapy with Minimal Side Effects. Colloids Surf. B Biointerfaces 2021, 205, 111878. [Google Scholar] [CrossRef] [PubMed]
- Pop, O.L.; Mesaros, A.; Vodnar, D.C.; Suharoschi, R.; Tăbăran, F.; Magerușan, L.; Tódor, I.S.; Diaconeasa, Z.; Balint, A.; Ciontea, L.; et al. Cerium Oxide Nanoparticles and Their Efficient Antibacterial Application In Vitro against Gram-Positive and Gram-Negative Pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Abid, S.A.; Taha, A.A.; Ismail, R.A.; Mohsin, M.H. Antibacterial and Cytotoxic Activities of Cerium Oxide Nanoparticles Prepared by Laser Ablation in Liquid. Environ. Sci. Pollut. Res. 2020, 27, 30479–30489. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, R.P.; Bhuvaneshwari, V.; Ranjithkumar, R.; Sathiyavimal, S.; Malayaman, V.; Chandarshekar, B. Synthesis, Characterization and Antibacterial Activity of Hybrid Chitosan-Cerium Oxide Nanoparticles: As a Bionanomaterials. Int. J. Biol. Macromol. 2017, 104, 1746–1752. [Google Scholar] [CrossRef]
- Zamani, K.; Allah-Bakhshi, N.; Akhavan, F.; Yousefi, M.; Golmoradi, R.; Ramezani, M.; Bach, H.; Razavi, S.; Irajian, G.-R.; Gerami, M.; et al. Antibacterial Effect of Cerium Oxide Nanoparticle against Pseudomonas Aeruginosa. BMC Biotechnol. 2021, 21, 68. [Google Scholar] [CrossRef]
- Silina, E.V.; Stupin, V.A.; Manturova, N.E.; Chuvilina, E.L.; Gasanov, A.A.; Ostrovskaya, A.A.; Andreeva, O.I.; Tabachkova, N.Y.; Abakumov, M.A.; Nikitin, A.A.; et al. Development of Technology for the Synthesis of Nanocrystalline Cerium Oxide Under Production Conditions with the Best Regenerative Activity and Biocompatibility for Further Creation of Wound-Healing Agents. Pharmaceutics 2024, 16, 1365. [Google Scholar] [CrossRef] [PubMed]
- Chatzimentor, I.; Tsamesidis, I.; Ioannou, M.-E.; Pouroutzidou, G.K.; Beketova, A.; Giourieva, V.; Papi, R.; Kontonasaki, E. Study of Biological Behavior and Antimicrobial Properties of Cerium Oxide Nanoparticles. Pharmaceutics 2023, 15, 2509. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Luo, J.; Li, J.; Fu, S.; Ran, Y.; Li, J.; Zhao, Y.; Hao, Y. Fluorescent Pirfenidone-Cerium(III) Nanocomplexes Protect against Radiation-Induced Pulmonary Fibrosis and Inhibit Tumor Cell Growth. J. Drug Deliv. Sci. Technol. 2023, 86, 104651. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A. Glycols Functionalized Fluorescent Eu2O3 Nanoparticles: Functionalization Effect on the Structural and Optical Properties. J. Alloys Compd. 2016, 682, 160–169. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Jia, H.; Liu, Z.; Liu, C.; Qi, Y.; Wang, Z. PEG Coated Nano-Phosphor GdPO4•H·H2O:12%Eu3+ and Its Performance Analysis. J. Alloys Compd. 2022, 905, 164223. [Google Scholar] [CrossRef]
- Paulis, L.E.; Jacobs, I.; Van Den Akker, N.M.; Geelen, T.; Molin, D.G.; Starmans, L.W.; Nicolay, K.; Strijkers, G.J. Targeting of ICAM-1 on Vascular Endothelium under Static and Shear Stress Conditions Using a Liposomal Gd-Based MRI Contrast Agent. J. Nanobiotechnol. 2012, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, L.; Li, J.; Thu, H.E.; Hussain, Z. Nanomedicines Guided Nanoimaging Probes and Nanotherapeutics for Early Detection of Lung Cancer and Abolishing Pulmonary Metastasis: Critical Appraisal of Newer Developments and Challenges to Clinical Transition. J. Control. Release 2018, 292, 29–57. [Google Scholar] [CrossRef] [PubMed]
- Kukowska-Latallo, J. Targeted Gadolinium-Loaded Dendrimer Nanoparticles for Tumor-Specific Magnetic Resonance Contrast Enhancement. Int. J. Nanomed. 2008, 3, 201–210. [Google Scholar] [CrossRef]
- Sikder, A.; Vambhurkar, G.; Amulya, E.; Bagasariya, D.; Famta, P.; Shah, S.; Khatri, D.K.; Singh, S.B.; Sinha, V.R.; Srivastava, S. Advancements in Redox-Sensitive Micelles as Nanotheranostics: A New Horizon in Cancer Management. J. Control. Release 2022, 349, 1009–1030. [Google Scholar] [CrossRef]
- Idée, J.-M.; Port, M.; Medina, C.; Lancelot, E.; Fayoux, E.; Ballet, S.; Corot, C. Possible Involvement of Gadolinium Chelates in the Pathophysiology of Nephrogenic Systemic Fibrosis: A Critical Review. Toxicology 2008, 248, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Sun, Z.; Chu, H.; Wang, Y. Self-Assembled Nanoplatforms for Chemodynamic Therapy. Chem. Eng. J. 2024, 479, 147702. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft Copolymerized Chitosan—Present Status and Applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, Properties and Application of Alginic Acid: A Review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef]
- Sehgal, V.; Pandey, S.P.; Singh, P.K. Prospects of Charged Cyclodextrins in Biomedical Applications. Carbohydr. Polym. 2024, 323, 121348. [Google Scholar] [CrossRef]
- Petrova, V.A.; Dubashynskaya, N.V.; Gofman, I.V.; Golovkin, A.S.; Mishanin, A.I.; Aquino, A.D.; Mukhametdinova, D.V.; Nikolaeva, A.L.; Ivan’kova, E.M.; Baranchikov, A.E.; et al. Biocomposite Films Based on Chitosan and Cerium Oxide Nanoparticles with Promising Regenerative Potential. Int. J. Biol. Macromol. 2023, 229, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Shurfa, M.K.; Girigoswami, A.; Sakthi Devi, R.; Harini, K.; Agraharam, G.; Deepika, B.; Pallavi, P.; Girigoswami, K. Combinatorial Effect of Doxorubicin Entrapped in Alginate-Chitosan Hybrid Polymer and Cerium Oxide Nanocomposites on Skin Cancer Management in Mice. J. Pharm. Sci. 2023, 112, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Farrugia, B.L.; Yan, C.M.Y.; Vassie, J.A.; Whitelock, J.M. Hyaluronan Coated Cerium Oxide Nanoparticles Modulate CD44 and Reactive Oxygen Species Expression in Human Fibroblasts. J. Biomed. Mater. Res. 2016, 104, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Orhan, B.; Karadeniz, D.; Kalaycıoğlu, Z.; Kaygusuz, H.; Torlak, E.; Erim, F.B. Foam-Based Antibacterial Hydrogel Composed of Carboxymethyl Cellulose/Polyvinyl Alcohol/Cerium Oxide Nanoparticles for Potential Wound Dressing. Int. J. Biol. Macromol. 2025, 291, 138924. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, L.; Li, M.; Peng, H.; Qiu, X.; Li, Y.; Zhu, B.; Liu, C.; Ren, S.; Miao, L. Injectable CNPs/DMP1-Loaded Self-Assembly Hydrogel Regulating Inflammation of Dental Pulp Stem Cells for Dentin Regeneration. Mater. Today Bio 2024, 24, 100907. [Google Scholar] [CrossRef]
- Marchianò, V.; Matos, M.; Marcet, I.; Carmen Blanco-López, M.; Gutiérrez, G.; Cioffi, N.; Ditaranto, N. Preparation and Characterization of Biodegradable Gelatine and Starch Films Embedding Cerium Oxide Nanoparticles Stabilized by PLGA Micelles for Antibiofilm Applications. J. Mol. Liq. 2024, 398, 124215. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Gudkov, S.V.; Turovsky, E.A. Differential Effect of Cerium Nanoparticles on the Viability, Redox-Status and Ca2+-Signaling System of Cancer Cells of Various Origins. Arch. Biochem. Biophys. 2025, 764, 110261. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Sharma, P.; Kumar, S.; Alex, S.A.; Kumar, R.; Mehta, S.K.; Mukherjee, A.; Umar, A. A Comparative Multi-Assay Approach to Study the Toxicity Behaviour of Eu2O3 Nanoparticles. J. Mol. Liq. 2018, 269, 783–795. [Google Scholar] [CrossRef]
- Hekmatimoghaddam, S.; Iman, M.; Shahdadi Sardo, H.; Jebali, A. Gelatin Hydrogel Containing Cerium Oxide Nanoparticles Covered by Interleukin-17 Aptamar as an Anti- Inflammatory Agent for Brain Inflammation. J. Neuroimmunol. 2019, 326, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Murphy, S.; Kiselev, B.; Bakshi, K.S.; Zhang, J.; Eltahir, A.; Zhang, Y.; Chen, Y.; Zhu, J.; Davis, R.M.; et al. A New Interleukin-13 Amino-Coated Gadolinium Metallofullerene Nanoparticle for Targeted MRI Detection of Glioblastoma Tumor Cells. J. Am. Chem. Soc. 2015, 137, 7881–7888. [Google Scholar] [CrossRef]
- Bulmahn, J.C.; Kutscher, H.L.; Cwiklinski, K.; Schwartz, S.A.; Prasad, P.N.; Aalinkeel, R. A Multimodal Theranostic Nanoformulation That Dramatically Enhances Docetaxel Efficacy Against Castration Resistant Prostate Cancer. J. Pharm. Sci. 2020, 109, 2874–2883. [Google Scholar] [CrossRef]
- Zgheib, C.; Hilton, S.A.; Dewberry, L.C.; Hodges, M.M.; Ghatak, S.; Xu, J.; Singh, S.; Roy, S.; Sen, C.K.; Seal, S.; et al. Use of Cerium Oxide Nanoparticles Conjugated with MicroRNA-146a to Correct the Diabetic Wound Healing Impairment. J. Am. Coll. Surg. 2019, 228, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-P.; Xie, X.-L.; Wei, Y.-P.; Mao, C.-J.; Chen, J.-S.; Niu, H.-L.; Song, J.-M.; Jin, B.-K. Photoelectrochemical Immunoassay for Human Interleukin 6 Based on the Use of Perovskite-Type LaFeO3 Nanoparticles on Fluorine-Doped Tin Oxide Glass. Mikrochim. Acta 2017, 185, 52. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ying, H.; Jiang, D.; Liu, F.; Tian, Y.; Du, C.; Zhang, L.; Pu, X. Rapid and Sensitive Detection of Interleukin-6 in Serum via Time-Resolved Lateral Flow Immunoassay. Anal. Biochem. 2020, 588, 113468. [Google Scholar] [CrossRef]
- Peng, J.; Guan, J.; Yao, H.; Jin, X. Magnetic Colorimetric Immunoassay for Human Interleukin-6 Based on the Oxidase Activity of Ceria Spheres. Anal. Biochem. 2016, 492, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-E.; Ni, X.-M.; Zheng, H.-G.; Zhang, X.-J.; Song, J.-M. Fabrication of Rod-like CeO2: Characterization, Optical and Electrochemical Properties. Solid. State Sci. 2006, 8, 1290–1293. [Google Scholar] [CrossRef]
- Clavijo-Chaparro, S.L.; Hernández-Gordillo, A.; Camposeco-Solis, R.; Rodríguez-González, V. Water Splitting Behavior of Copper-Cerium Oxide Nanorods and Nanocubes Using Hydrazine as a Scavenging Agent. J. Mol. Catal. A Chem. 2016, 423, 143–150. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Tao, Z.; Gao, X.; Wang, S.; Liu, Y. A Asp/Ce Nanotube-Based Colorimetric Nanosensor for H2O2-Free and Enzyme-Free Detection of Cysteine. Talanta 2019, 196, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Kruglova, M.P.; Ivanov, A.V.; Fedoseev, A.N.; Virus, E.D.; Stupin, V.A.; Parfenov, V.A.; Titova, S.A.; Lazareva, P.I.; Kubatiev, A.A.; Silina, E.V. The Diagnostic and Prognostic Roles Played by Homocysteine and Other Aminothiols in Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 5653. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.d.P.; Costa, K.R.B.d.S.; de Lima, I.S.; Barreto, H.M.; Garcia, R.R.P.; Triboni, E.R.; Silva-Filho, E.C.; Viana, B.C.; Cecilia, J.A.; Osajima, J.A. Unveiling the Bacterial Photoinactivation through Cerium-Gallium Titanate Nanotubes. J. Photochem. Photobiol. A Chem. 2024, 451, 115483. [Google Scholar] [CrossRef]
- Vanasundari, K.; Ponnarasi, P.; Mahalakshmi, G. A Green Approach to Synthesis of Ag-Doped CeO2 Nanorods Embedded Reduced Graphene Oxide Nanocomposite for Excellent Photocatalytic and Antimicrobial Activity. Inorg. Chem. Commun. 2024, 165, 112523. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Singhwane, A.; Jaiswal, A.; Mili, M.; Tilwari, A.; Mohapatra, R.K.; Srivastava, A.K.; Verma, S. Robust Synthesis and Characteristics of Novel One-Dimensional Gadolinium Oxide Nanorods Decorated Multiwalled Carbon Nanotubes Based Antibacterial Nanocomposites for Health Care Applications. Inorg. Chem. Commun. 2023, 157, 111324. [Google Scholar] [CrossRef]
- Patra, C.R.; Abdel Moneim, S.S.; Wang, E.; Dutta, S.; Patra, S.; Eshed, M.; Mukherjee, P.; Gedanken, A.; Shah, V.H.; Mukhopadhyay, D. In Vivo Toxicity Studies of Europium Hydroxide Nanorods in Mice. Toxicol. Appl. Pharmacol. 2009, 240, 88–98. [Google Scholar] [CrossRef]
- He, Q.; Zhao, H.; Teng, Z.; Wang, Y.; Li, M.; Hoffmann, M.R. Phosphate Removal and Recovery by Lanthanum-Based Adsorbents: A Review for Current Advances. Chemosphere 2022, 303, 134987. [Google Scholar] [CrossRef]
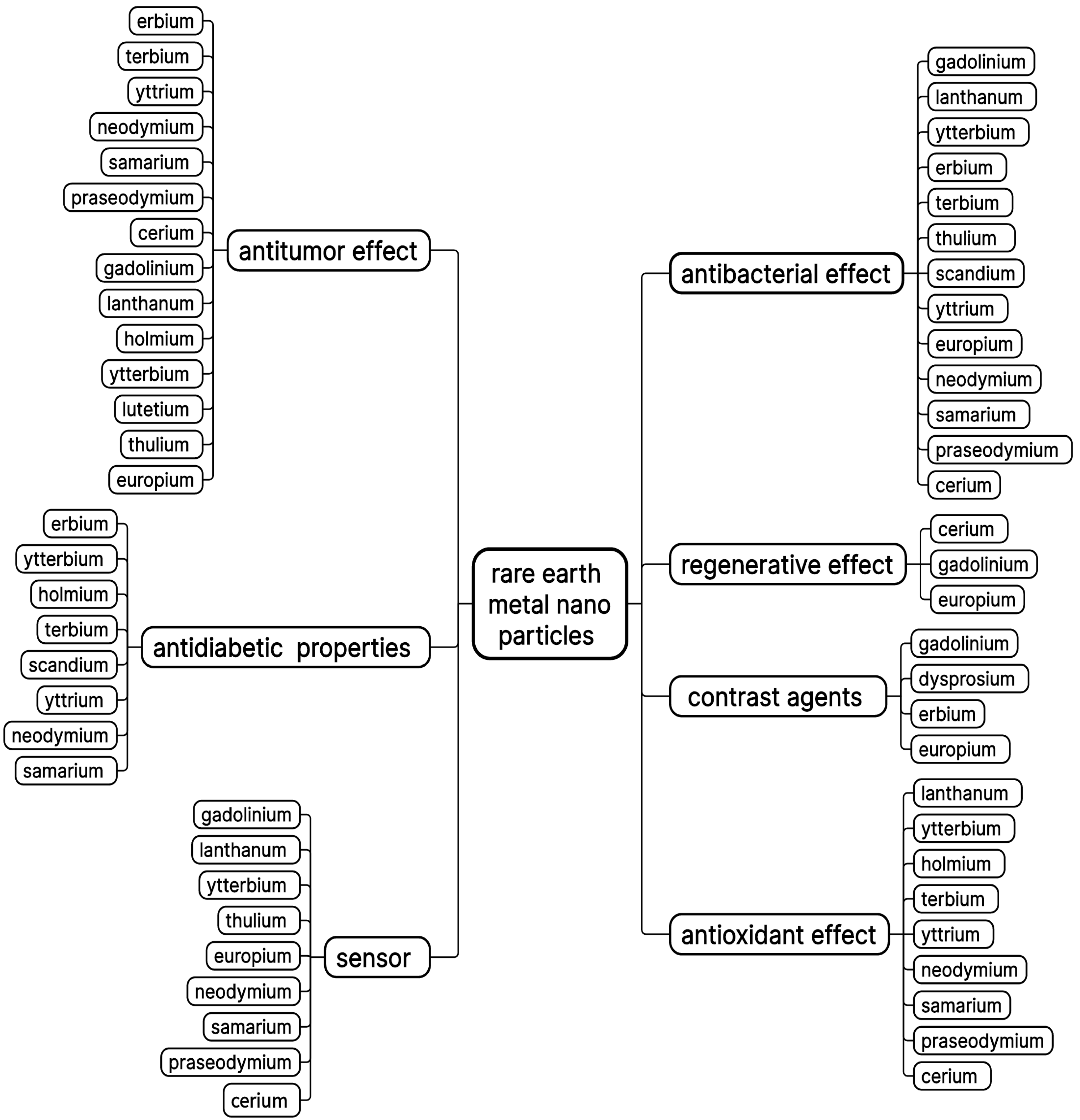
| Metal | Excipient | Class of Compound | Effect | Type of Study | Sources |
|---|---|---|---|---|---|
| Gadolinium | Polyethyleneglycol | Biopolymer | Solubilization | In vitro | [296] |
| Gadolinium | Liposomes | Fatty substance | Targeted action on pathologically altered tissue | bEnd.5, RAW | [297] |
| Gadolinium | Polyamidoamine dendrimer | Biopolymer | Increased accumulation in the tumor microenvironment | In vivo | [298,299] |
| Gadolinium | Cyclen | Hybrid micelles | Increased accumulation in tumor cells | HeLa, in vivo | [300] |
| Ytterbium | Cyclen | Hybrid micelles | Increased accumulation in tumor cells | HeLa, in vivo | [300] |
| Ytterbium | Ferrocene and mannose | Metallocene and monosaccharide | Targeted antitumor | In vitro HepG2 | [302] |
| Ytterbium | Anionic cyclodextrins | Oligomer | High strength of the complex | In vitro | [305] |
| Europium | Cyclen | Hybrid micelles | No effect | HeLa, in vivo | [300] |
| Europium | Chitosan and mannose | Biopolymer and monosaccharide | Low strength of the complex | In vitro | [303] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titova, S.A.; Kruglova, M.P.; Stupin, V.A.; Manturova, N.E.; Silina, E.V. Potential Applications of Rare Earth Metal Nanoparticles in Biomedicine. Pharmaceuticals 2025, 18, 154. https://doi.org/10.3390/ph18020154
Titova SA, Kruglova MP, Stupin VA, Manturova NE, Silina EV. Potential Applications of Rare Earth Metal Nanoparticles in Biomedicine. Pharmaceuticals. 2025; 18(2):154. https://doi.org/10.3390/ph18020154
Chicago/Turabian StyleTitova, Svetlana A., Maria P. Kruglova, Victor A. Stupin, Natalia E. Manturova, and Ekaterina V. Silina. 2025. "Potential Applications of Rare Earth Metal Nanoparticles in Biomedicine" Pharmaceuticals 18, no. 2: 154. https://doi.org/10.3390/ph18020154
APA StyleTitova, S. A., Kruglova, M. P., Stupin, V. A., Manturova, N. E., & Silina, E. V. (2025). Potential Applications of Rare Earth Metal Nanoparticles in Biomedicine. Pharmaceuticals, 18(2), 154. https://doi.org/10.3390/ph18020154









