Investigating the Therapeutic Mechanisms of Total Saikosaponins in Alzheimer’s Disease: A Metabolomic and Proteomic Approach
Abstract
1. Introduction
2. Results
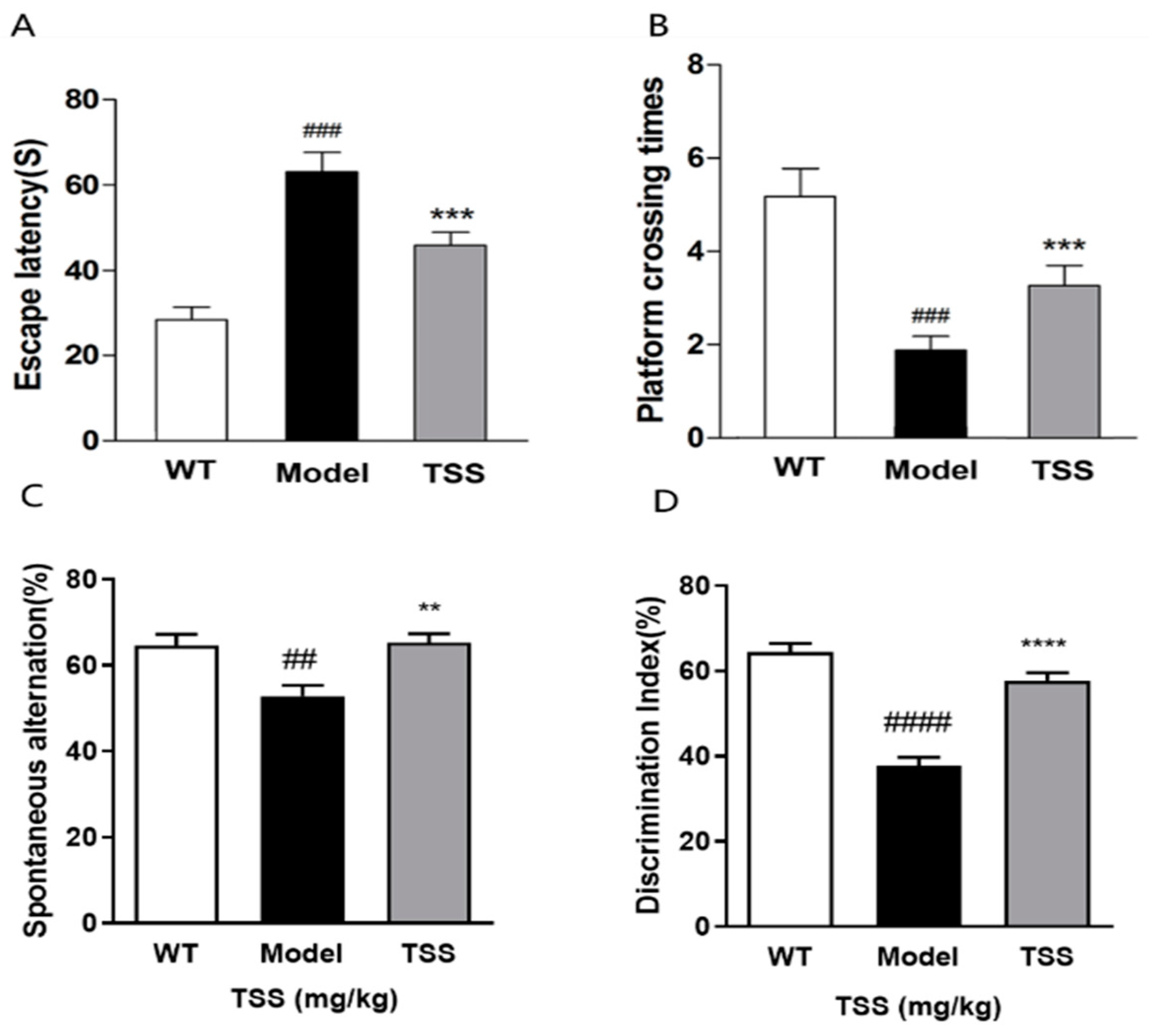
2.1. TSS Treatment Can Alleviate Cognitive Dysfunction in APP/PS1 Mice
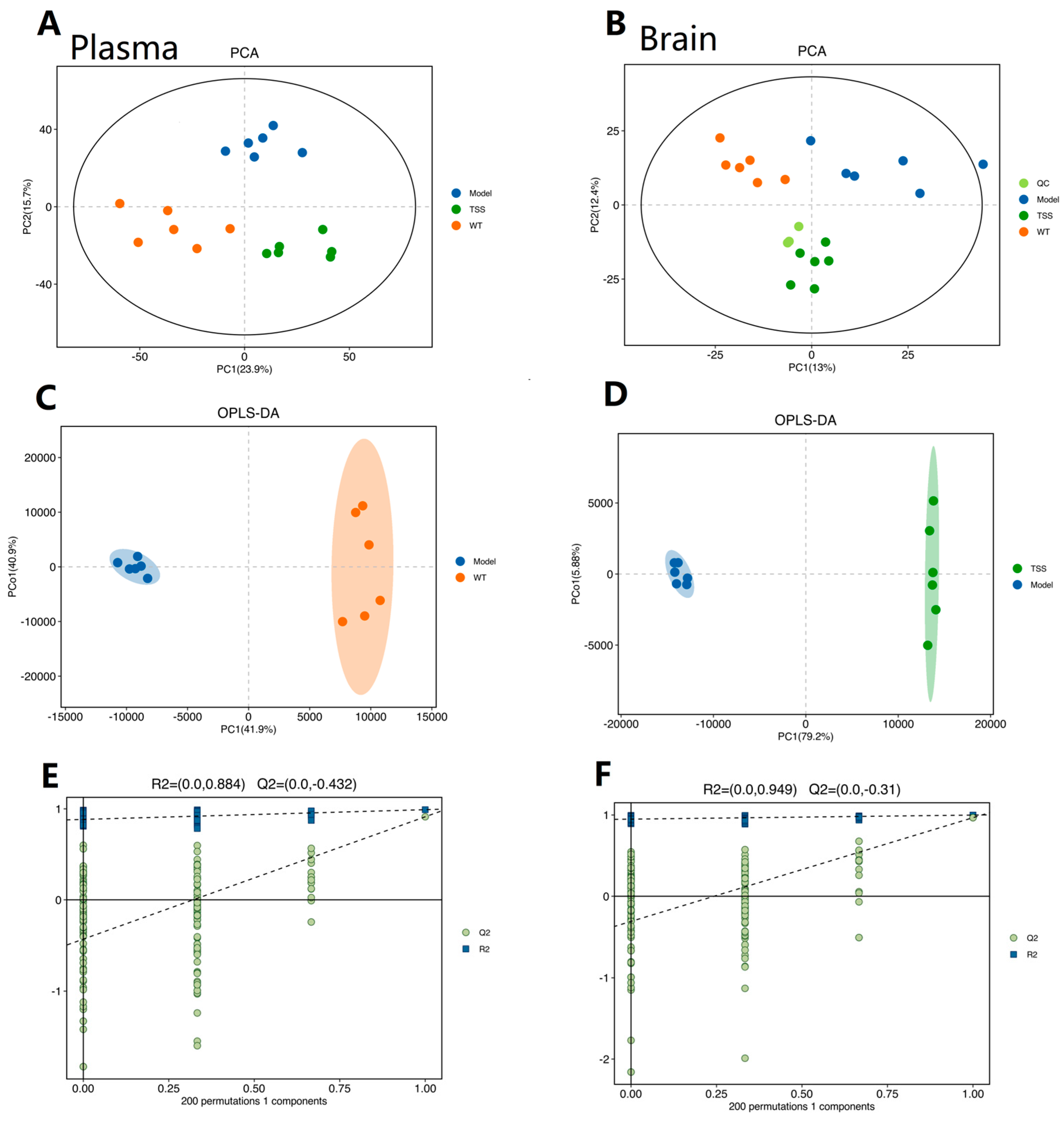
2.2. Metabolomic Analysis
2.2.1. Reliability Assessment of the Analytical Method
2.2.2. Differential Metabolite Screening and Enrichment Analysis
2.3. Proteomic Analysis
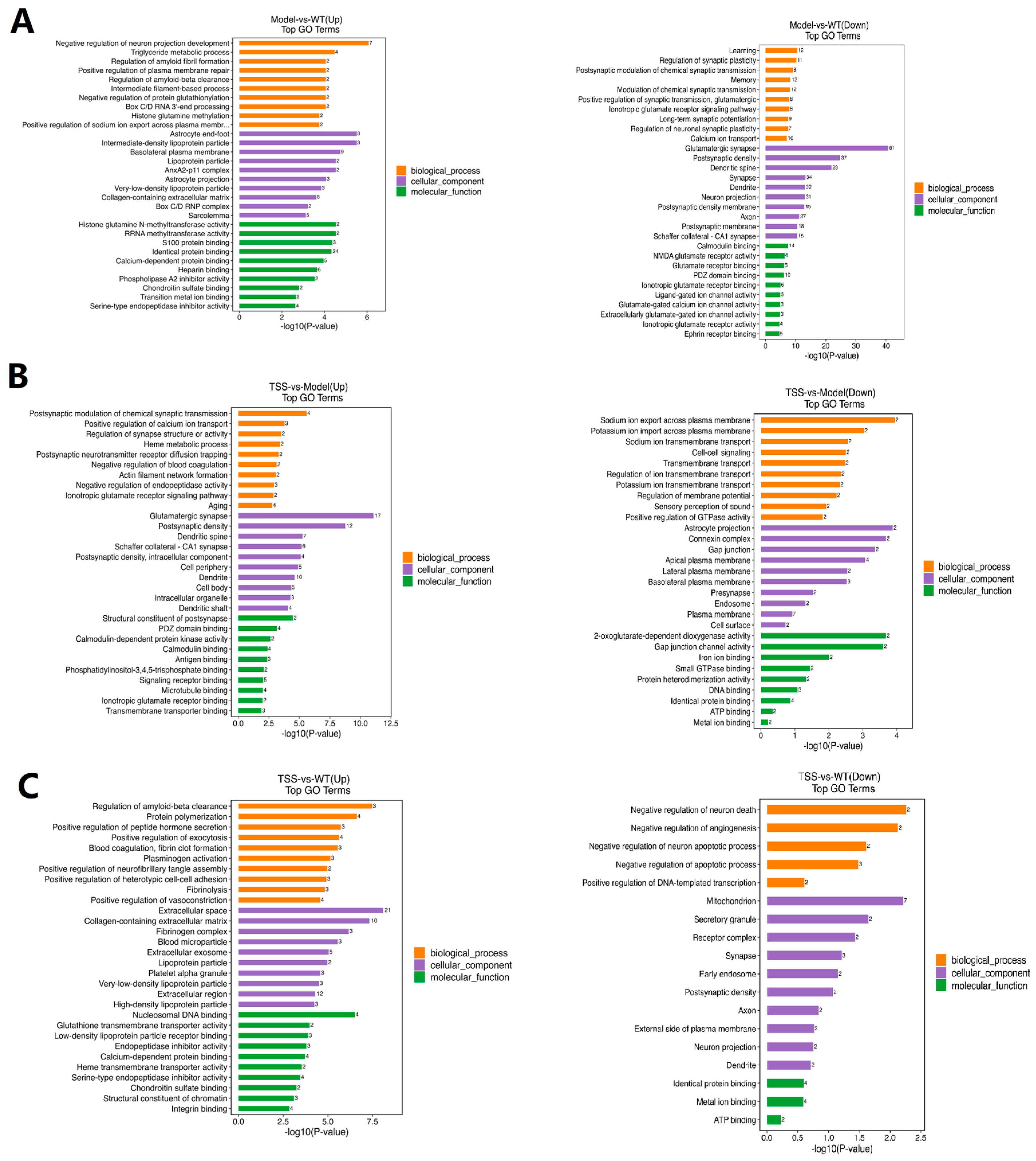
2.4. GO Pathway Analysis of DEPs
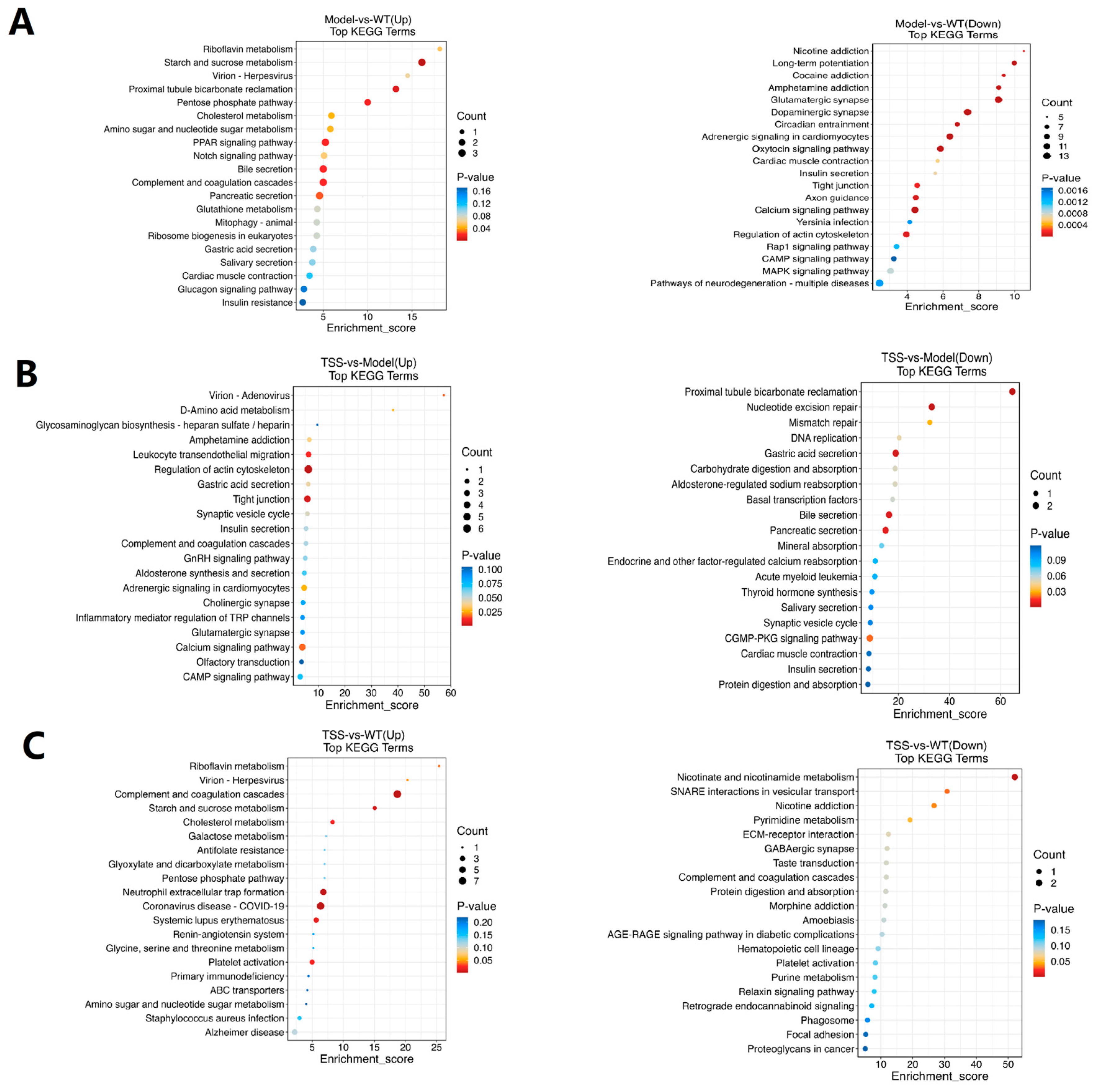
2.5. KEGG Enrichment Analysis of DEPs
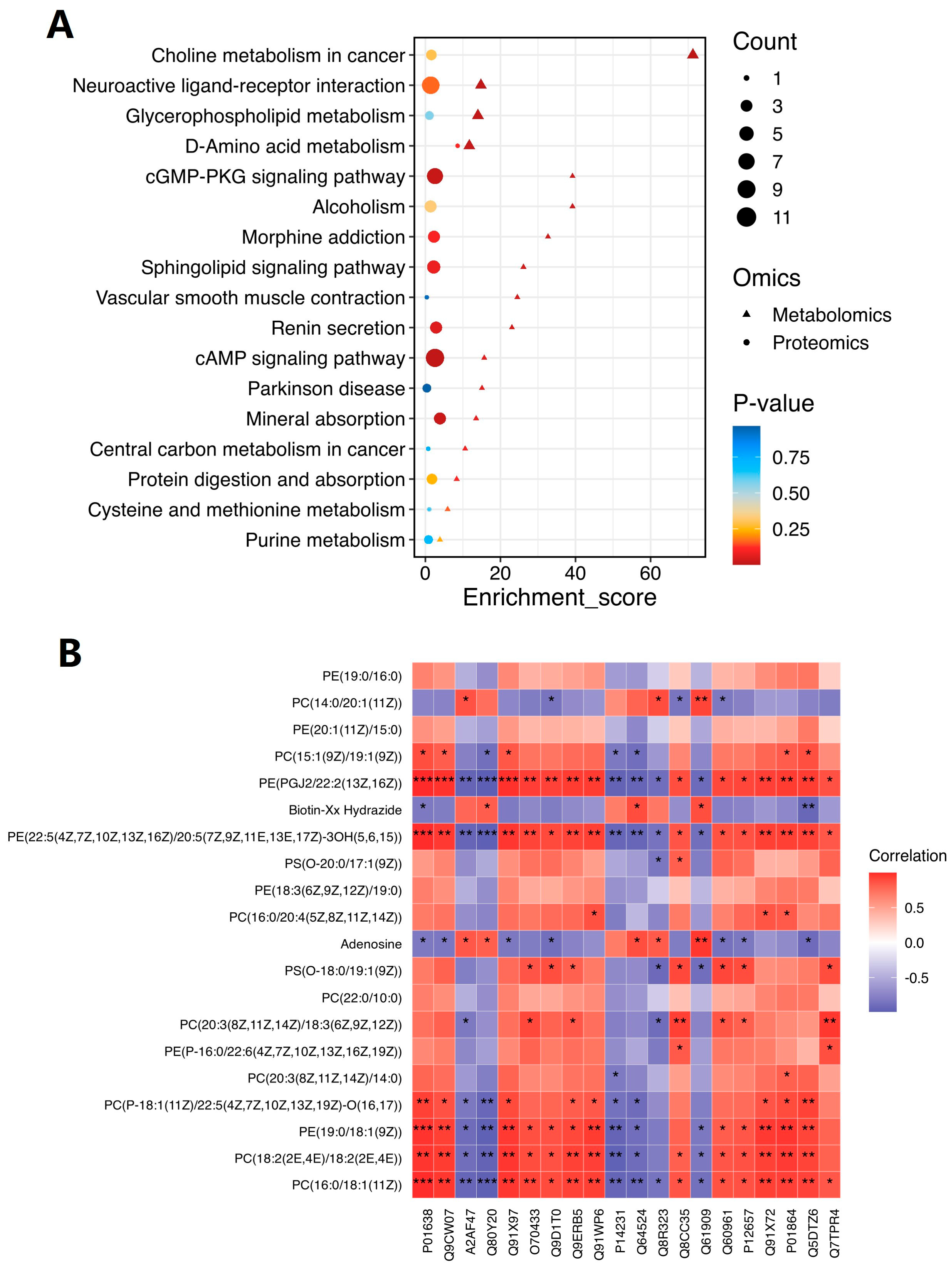
2.6. Comprehensive Analysis of Metabolomic and Proteomic Data
3. Discussion
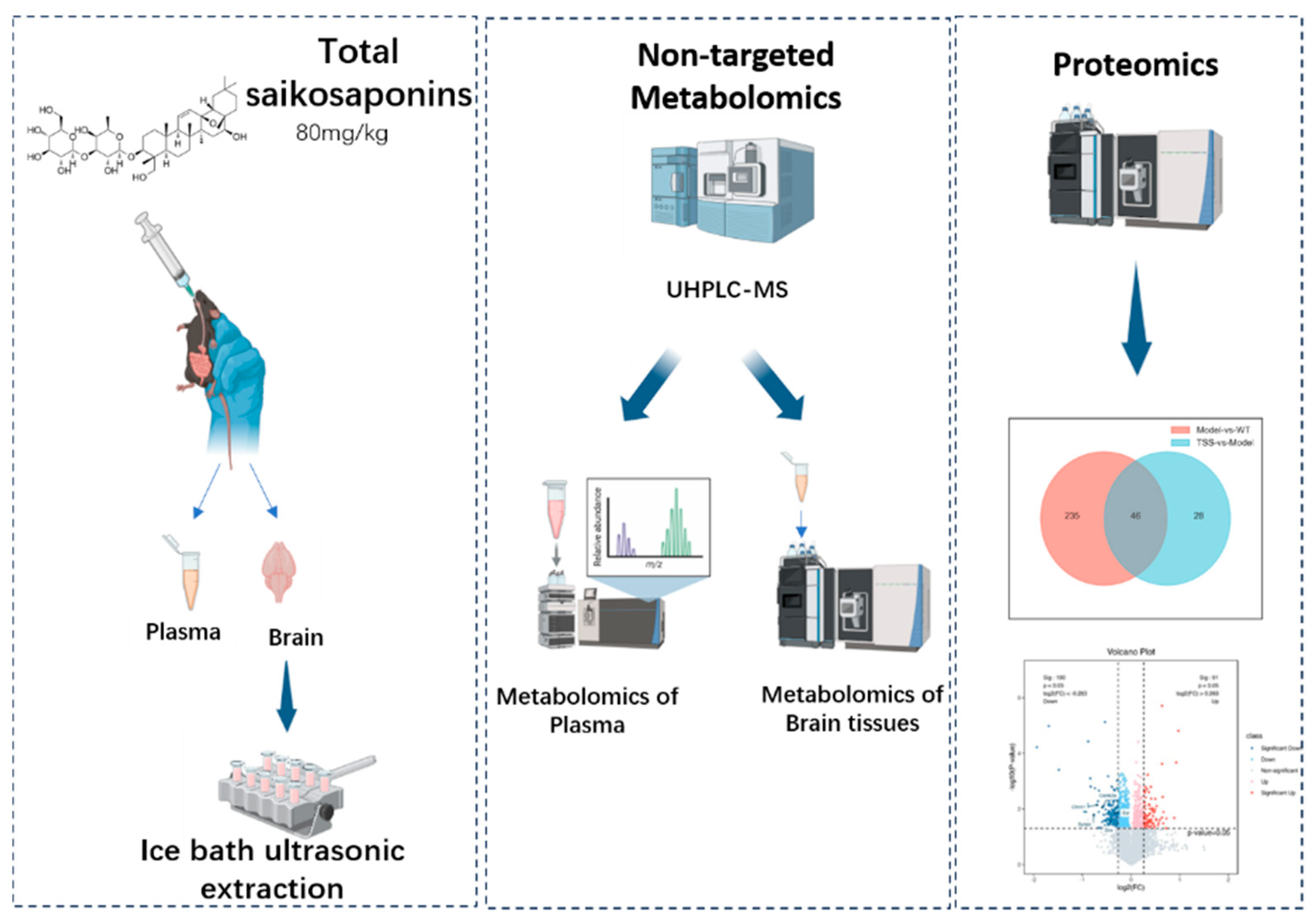
4. Materials and Methods
4.1. Preparation of Total Saikosaponins
4.2. Animals and Experimental Design
4.3. Behavioral Tests
4.4. Sample Collection
4.5. Sample Preparation
4.6. Non-Targeted Metabolomic Experiment
4.7. Proteomic Experiments
4.7.1. Protein Digestion, Peptide Labeling, and High-Performance Liquid Chromatography Separation
4.7.2. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Analysis
4.8. Data Processing
4.8.1. Preprocessing and Statistical Analysis of Non-Targeted Metabolomic Data
4.8.2. Protein Identification, Quantification, and Functional Analysis
4.8.3. Statistical Methods for Behavioral Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Tripathi, T.; Kalita, P. Synergistic Effect of Amyloid-β and Tau Disrupts Neural Circuits. ACS Chem. Neurosci. 2019, 10, 1129–1130. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Adeloye, D.; Asante, K.P.; Calia, C.; Campbell, H.; Danso, S.O.; Juvekar, S.; Luz, S.; Mohan, D.; Muniz-Terrera, G.; et al. Tackling dementia globally: The Global Dementia Prevention Program (GloDePP) collaboration. J. Glob. Health 2020, 9, 020103. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.; Cummings, J.; van der Flier, W. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Niemantsverdriet, E.; Valckx, S.; Bjerke, M.; Engelborghs, S. Alzheimer’s disease CSF biomarkers: Clinical indications and rational use. Acta Neurol. Belg. 2017, 117, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Amft, M.; Ortner, M.; Eichenlaub, U.; Goldhardt, O.; Diehl-Schmid, J.; Hedderich, D.M.; Yakushev, I.; Grimmer, T. The cerebrospinal fluid biomarker ratio Aβ42/40 identifies amyloid positron emission tomography positivity better than Aβ42 alone in a heterogeneous memory clinic cohort. Alzheimers Res. Ther. 2022, 14, 60. [Google Scholar] [CrossRef]
- Morris, E.; Chalkidou, A.; Hammers, A.; Peacock, J.; Summers, J.; Keevil, S. Diagnostic accuracy of 18F amyloid PET tracers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 374–385. [Google Scholar] [CrossRef]
- Rufino-Ramos, D.; Albuquerque, P.R.; Carmona, V.; Perfeito, R.; Nobre, R.J.; de Almeida, L.P. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J. Control. Release 2017, 262, 247–258. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; García, A.; García-Barrera, T.; Barbas, C.; Gómez-Ariza, J.L. Metabolomic profiling of serum in the progression of Alzheimer’s disease by capillary electrophoresis-mass spectrometry. Electrophoresis 2014, 35, 3321–3330. [Google Scholar] [CrossRef]
- Padala, S.P.; Newhouse, P.A. Blood-based biomarkers in Alzheimer’s disease: A mini-review. Metab. Brain Dis. 2022, 38, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhao, G.; Shuang, R.; Wang, H.; Zeng, N. Saikosaponin a activates tet1/dll3/notch1 signalling and promotes hippocampal neurogenesis to improve depression-like behavior in mice. J. Ethnopharmacol. 2024, 319, 117289. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, L.; Lian, G.; Wang, X.; Zhang, H.; Yao, X.; Yang, J.; Wu, C. Sildenafil attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-κB signaling pathways in N9 microglia. Int. Immunopharmacol. 2011, 11, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, L.F.; Siu, W.S.; Jin, Y.; Cao, M.Q.; Li, W.F.; Chen, J.; Cong, W.H.; Ma, M.; Chen, K.J.; et al. A combined molecular biology and network pharmacology approach to investigate the multi-target mechanisms of Chaihu Shugan San on Alzheimer’s disease. Biomed. Pharmacother. 2019, 120, 109370. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Q.; Hu, S.; Liu, Z.; Wang, S.; Jin, Y.; Li, L.; Ou, H.; Wu, Z. Chaihu Shugan San ameliorated cognitive deficits through regulating gut microbiota in senescence-accelerated mouse prone 8. Front. Pharmacol. 2023, 14, 1181226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Guo, Q.; Cheng, Z.B.; Zeng, K.W.; Liang, H.; Tu, P.F.; Chen, S.Z.; Zhang, Q.Y. New saikosaponins from the roots of Bupleurum chinense. Phytochem. Lett. 2017, 21, 183–189. [Google Scholar] [CrossRef]
- Li, J.; Zou, B.; Cheng, X.Y.; Yang, X.H.; Li, J.; Zhao, C.H.; Ma, R.X.; Tian, J.X.; Yao, Y. Therapeutic effects of total saikosaponins from Radix bupleuri against Alzheimer’s disease. Front. Pharmacol. 2022, 13, 940999. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M. The Role of Saikosaponins in Therapeutic Strategies for Age-Related Diseases. Oxidative Med. Cell. Longev. 2018, 2018, 8275256. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Park, S.; You, M.H.; Lim, J.H.; Min, S.H.; Kim, B.M. A potential therapeutic effect of saikosaponin C as a novel dual-target anti-Alzheimer agent. J. Neurochem. 2016, 136, 1232–1245. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.H.; Gao, Y.P.; Zhan, S.H.; Xiong, F.; Qiu, W.Y.; Qian, X.J.; Wang, T.; Wang, N.L.; Zhang, D.; Yang, Q.; et al. Quantitative protein profiling of hippocampus during human aging. Neurobiol. Aging 2016, 39, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Mubeen, S.; Khan, A.; Ibrahim, S.; Meer, B. Plasma Biomarkers: Potent Screeners of Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Demen. 2019, 34, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Ogishima, S.; Kitatani, K.; Kikuchi, M.; Tanaka, H.; Yaegashi, N.; Nakaya, J. Network Analysis of a Comprehensive Knowledge Repository Reveals a Dual Role for Ceramide in Alzheimer’s Disease. PLoS ONE 2016, 11, e0148431. [Google Scholar] [CrossRef]
- Coelho, D.; Schwartz, S.; Merino, M.; Hauert, B.; Topfel, B.; Tieche, C.; Rhiner, C.; Moreno, E. Culling Less Fit Neurons Protects against Amyloid-β-Induced Brain Damage and Cognitive and Motor Decline. Cell Rep. 2018, 25, 3661–3673.e3. [Google Scholar] [CrossRef] [PubMed]
- Soto-Faguás, C.M.; Sanchez-Molina, P.; Saura, C.A. Loss of presenilin function enhances tau phosphorylation and aggregation in mice. Acta Neuropathol. Commun. 2021, 9, 162. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Kuntz, T.; Shaik, S.M.; Baufeld, C.; Leibowitz, J.; Zhang, X.L.; Gottel, N.; Zhang, X.Q.; Butovsky, O.; Gilbert, J.A.; et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med. 2019, 216, 1542–1560. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Hu, T.; Su, S.J.; Liu, J.M.; Ma, Y.Z.; Zhuo, Y.; Fang, S.H.; Wang, Q.; Mo, Z.Z.; Pan, H.F.; et al. Comparative Metabolomics Analysis Reveals Key Metabolic Mechanisms and Protein Biomarkers in Alzheimer’s Disease. Front. Pharmacol. 2022, 13, 904857. [Google Scholar] [CrossRef]
- Ma, X.Y.; Huang, M.; Zheng, M.N.; Dai, C.X.; Song, Q.X.; Zhang, Q.; Li, Q.; Gu, X.; Chen, H.; Jiang, G.; et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease. J. Control. Release 2020, 327, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Song, D.; Li, Y.; Liu, J.; Huang, X.; Li, B. Saikosaponin-D Mitigates Oxidation in SH-SY5Y Cells Stimulated by Glutamate Through Activation of Nrf2 Pathway: Involvement of PI3K. Neurotox Res. 2022, 40, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, C.; Gao, Y.; Zhou, Y.; Ma, B.; Jiang, J.; Long, X.; Yimamu, N.; Zhong, K.; Li, Y.; et al. HLH-30/TFEB modulates autophagy to improve proteostasis in Aβ transgenic Caenorhabditis elegans. Front. Pharmacol. 2024, 15, 1433030. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, X.; Zhang, Y.-W.; Rockenstein, E.; Bu, G.; Golde, T.E.; Masliah, E.; Xu, H. Alzheimer’s β-secretase (BACE1) regulates the cAMP/PKA/CREB pathway independently of β-amyloid. J. Neurosci. 2012, 32, 11390–11395. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Lim, W.L.F.; Shui, G.; Gupta, V.B.; James, I.; Fagan, A.M.; Xiong, C.; Sohrabi, H.R.; Taddei, K.; Brown, B.M.; et al. Plasma Phospholipid and Sphingolipid Alterations in Presenilin1 Mutation Carriers: A Pilot Study. J. Alzheimers Dis. 2016, 50, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Lonze, B.E.; Ginty, D.D. Function and regulation of CREB family transcription factors in the nervous system. Neuron 2002, 35, 605–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Tang, X.L.; Li, M.T.; Marshall, J.; Mao, Z.X. Regulation of neuroprotective activity of myocyte-enhancer factor 2 by cAMP-protein kinase A signaling pathway in neuronal survival. J. Biol. Chem. 2005, 280, 16705–16713. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Fedele, E. cAMP, cGMP and Amyloid β: Three Ideal Partners for Memory Formation. Trends Neurosci. 2018, 41, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Thornton, P.; Balazs, R.; Cotman, C. Beta-amyloid-(1-42) impairs activity-dependent cAMP-response element-binding protein signaling in neurons at concentrations in which cell survival Is not compromised. J. Biol. Chem. 2001, 276, 17301–17306. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, S.; Wu, J.; Ji, L.; Zhu, L.; Cao, L.; Huang, J.; Jiang, Q.; Wei, J.; Liu, M.; et al. cAMP/PKA signaling pathway contributes to neuronal apoptosis via regulating IDE expression in a mixed model of type 2 diabetes and Alzheimer’s disease. J. Cell Biochem. 2017, 119, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cheng, Y.F.; Wang, H.T.; Wang, C.; Wilson, S.P.; Xu, J.P.; Zhang, H.T. RNA Interference-Mediated Knockdown of Long-Form Phosphodiesterase-4D (PDE4D) Enzyme Reverses Amyloid-β42-Induced Memory Deficits in Mice. J. Alzheimer’s Dis. 2014, 38, 269–280. [Google Scholar] [CrossRef]
- Shi, J.; Li, Y.Y.; Zhang, Y.; Chen, J.; Gao, J.Q.; Zhang, T.Y.; Shang, X.G.; Zhang, X.N. Baicalein Ameliorates Aβ-Induced Memory Deficits and Neuronal Atrophy via Inhibition of PDE2 and PDE4. Front. Pharmacol. 2021, 12, 794458. [Google Scholar] [CrossRef]
- Sierksma, A.; Rutten, K.; Sydlik, S.; Rostamian, S.; Steinbusch, H.; van den Hove, D.; Prickaerts, J. Chronic phosphodiesterase type 2 inhibition improves memory in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Neuropharmacology 2013, 64, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Barger, S.W.; Fiscus, R.R.; Ruth, P.; Hofmann, F.; Mattson, M.P. Role of cyclic-GMP in the regulation of neuronal calcium and survival by secreted forms of beta-amyloid precursor. J. Neurochem. 1995, 64, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, L.L.; Liu, Y.Y.; Liu, Y.; Yu, W.P.; Zhao, Y.F. CircRNA-ceRNA Network Revealing the Potential Regulatory Roles of CircRNA in Alzheimer’s Disease Involved the cGMP-PKG Signal Pathway. Front. Mol. Neurosci. 2021, 14, 665788. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Tang, M.; Zeidler, C.; Höhfeld, J.; Johnson, G.V. BAG3 and SYNPO (synaptopodin) facilitate phospho-MAPT/Tau degradation via autophagy in neuronal processes. Autophagy 2019, 15, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-F.; Fan, Y.; Wang, D.; Bi, R.; Zhang, C.; Fang, Y.; Yao, Y.-G. PLD3 in Alzheimer’s Disease: A Modest Effect as Revealed by Updated Association and Expression Analyses. Mol. Neurobiol. 2015, 53, 4034–4045. [Google Scholar] [CrossRef]
- Pala, S.; Atilgan, R.; Kuloglu, T.; Yalçın, E.; Kaya, N.; Etem, E. The decrease in hippocampal transient receptor potential M2 (TRPM2) channel and muscarinic acetylcholine receptor 1 (CHRM1) is associated with memory loss in a surgical menopause rat model. Arch. Med. Sci. 2021, 17, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Scarr, E.; Craig, J.M.; Cairns, M.J.; Seo, M.S.; Galati, J.C.; Beveridge, N.J.; Gibbons, A.; Juzva, S.; Weinrich, B.; Parkinson-Bates, M.; et al. Decreased cortical muscarinic M1 receptors in schizophrenia are associated with changes in gene promoter methylation, mRNA and gene targeting microRNA. Transl. Psychiaty 2013, 3, e230. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, Y.; Shin, J.; Lee, W.; Wie, J.; Kim, K.; Lee, M.; Hwang, D.; Ratan, R.; Pae, A.; et al. Epigenetic regulation of cholinergic receptor M1 (CHRM1) by histone H3K9me3 impairs Ca(2+) signaling in Huntington’s disease. Acta Neuropathol. 2013, 125, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Fang, Y.; Cui, X.; Jia, R.; Kang, X.; Zhao, R. Magnolol upregulates CHRM1 to attenuate Amyloid-β-triggered neuronal injury through regulating the cAMP/PKA/CREB pathway. J. Nat. Med. 2022, 76, 188–199. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, Y.; Wang, Y.; Qi, S.; Wang, Y.; Ma, C.; Li, S.; Jiang, B.; Cheng, X.; Wang, Z. Anti-amnesic effect of extract and alkaloid fraction from aerial parts of Peganum harmala on scopolamine-induced memory deficits in mice. J. Ethnopharmacol. 2017, 204, 95–106. [Google Scholar] [CrossRef]
- Scott, K.A.; Ida, M.; Peterson, V.L.; Prenderville, J.A.; Moloney, G.M.; Izumo, T.; Murphy, K.; Murphy, A.; Ross, R.P.; Stanton, C.; et al. Revisiting Metchnikoff: Age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav. Immun. 2017, 65, 20–32. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, C.; Zhang, Z.; Yi, P.; Xu, P.; Huang, J.; Peng, W. β Integrated Metabolomic and Lipidomic Analysis Reveals the Neuroprotective Mechanisms of Bushen Tiansui Formula in an A1-42-Induced Rat Model of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2020, 2020, 5243453. [Google Scholar] [CrossRef]
- Hair, K.; Macleod, M.R.; Sena, E.S.; on behalf of the IICARus Collaboration. A randomised controlled trial of an Intervention to Improve Compliance with the ARRIVE guidelines (IICARus). Res. Integ. Peer Rev. 2019, 4, 12. [Google Scholar] [CrossRef]
- Tihanyi, D.K.; Szijarto, A.; Fülöp, A.; Denecke, B.; Lurje, G.; Neumann, U.P.; Czigany, Z.; Tolba, R. Systematic Review on Characteristics and Reporting Quality of Animal Studies in Liver Regeneration Triggered by Portal Vein Occlusion and Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy: Adherence to the ARRIVE Guidelines. J. Surg. Res. 2019, 235, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Jiang, Y.; Zhang, T.; Shang, Z.; Zhang, W.; Hu, K.; Chen, F.; Mei, F.; Gao, Q.; Zhao, L.; et al. Quality of interventional animal experiments in Chinese journals: Compliance with ARRIVE guidelines. BMC Vet Res. 2020, 16, 460. [Google Scholar] [CrossRef] [PubMed]
- Han, S. A checklist is associated with increased quality of reporting preclinical biomedical research: A systematic review. PLoS ONE 2017, 12, e0183591. [Google Scholar] [CrossRef]
- Chatzimanouil, M.K.T.; Wilkens, L.; Anders, H.-J. Quantity and Reporting Quality of Kidney Research. J. Am. Soc. Nephrol. 2019, 30, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Rousseau-Blass, F.; Beauchamp, G.; Pang, D.S.J. ARRIVE has not ARRIVEd: Support for the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines does not improve the reporting quality of papers in animal welfare, analgesia or anesthesia. PLoS ONE 2018, 13, e0197882. [Google Scholar] [CrossRef]
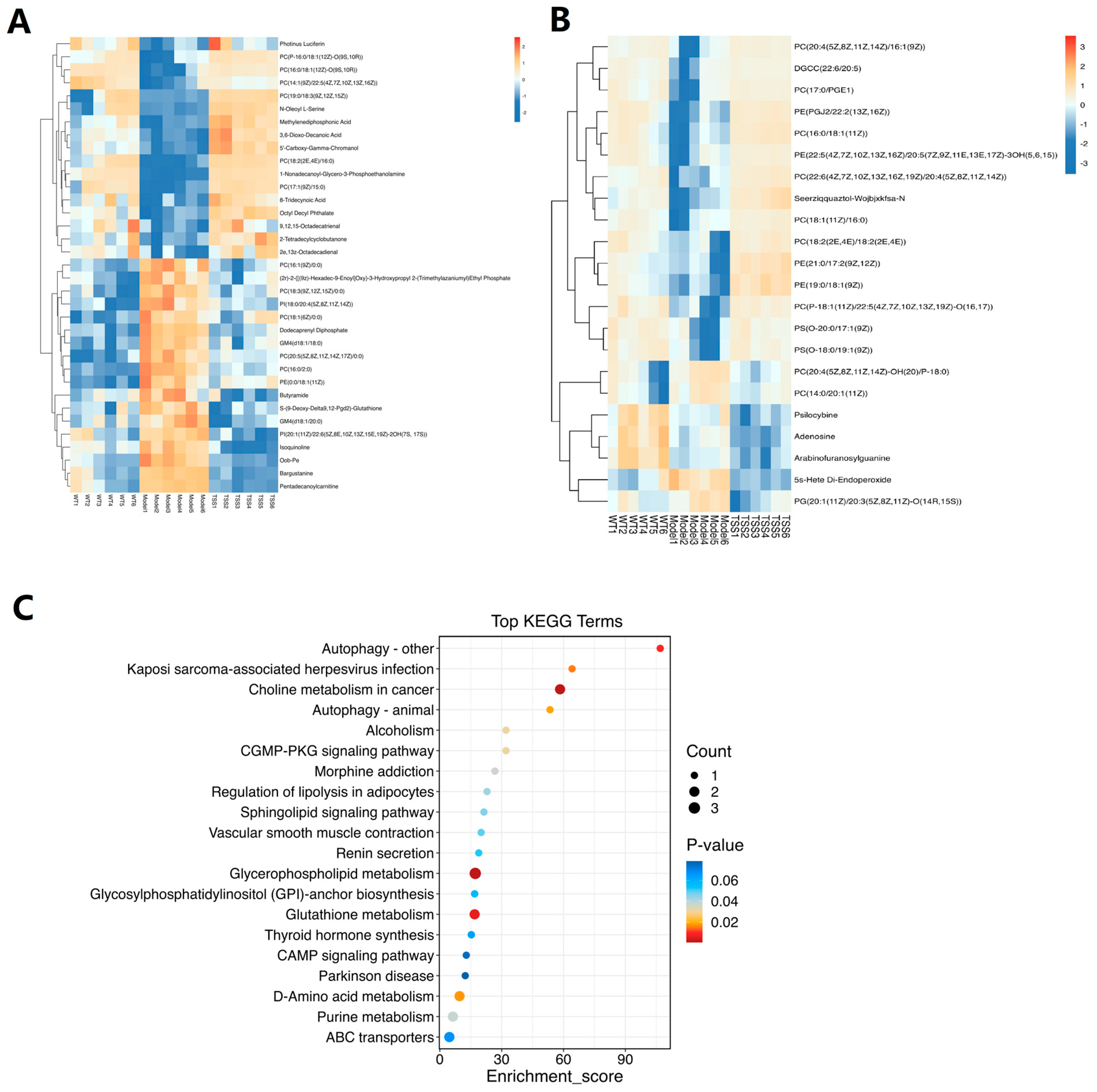


| No. | Metabolites | Model vs. WT | TSS vs. Model |
|---|---|---|---|
| 1 | PC(16:0/2:0) | down | up |
| 2 | PC(18:3(9Z,12Z,15Z)/0:0) | up | down |
| 3 | 8-Tridecynoic Acid | down | up |
| 4 | S-(9-Deoxy-Delta9, 12-Pgd2)-Glutathione | up | down |
| 5 | Oob-Pe | up | down |
| 6 | 1-Nonadecanoyl-Glycero-3-Phosphoethanolamine | down | up |
| 7 | Photinus Luciferin | down | up |
| 8 | PC(20:5(5Z,8Z,11Z,14Z,17Z)/0:0) | up | down |
| 9 | PC(16:0/18:1(12Z)-O(9S,10R)) | down | up |
| 10 | 3,6-Dioxo-Decanoic Acid | down | up |
| 11 | PC(18:2(2E,4E)/16:0) | down | up |
| 12 | Dodecaprenyl Diphosphate | up | down |
| 13 | PC(P-16:0/18:1(12Z)-O(9S,10R)) | up | down |
| 14 | GM4(d18:1/18:0) | up | down |
| 15 | PC(17:1(9Z)/15:0) | down | up |
| 16 | PI(18:0/20:4(5Z,8Z,11Z,14Z)) | up | down |
| 17 | PC(16:1(9Z)/0:0) | up | down |
| 18 | 2-Tetradecylcyclobutanone | down | up |
| 19 | 2e,13z-Octadecadienal | down | up |
| 20 | Bargustanine | up | down |
| 21 | Pentadecanoylcarnitine | up | down |
| 22 | PC(19:0/18:3(9Z,12Z,15Z)) | down | up |
| 23 | Isoquinoline | up | down |
| 24 | 9,12,15-Octadecatrienal | down | up |
| 25 | 5′-Carboxy-Gamma-Chromanol | down | up |
| 26 | PI(20:1(11Z)/22:6 (5Z,8E,10Z,13Z,15E,19Z)-2OH(7S,17S)) | up | down |
| 27 | Methylenediphosphonic Acid | down | up |
| 28 | GM4(d18:1/20:0) | up | down |
| 29 | Octyl Decyl Phthalate | down | up |
| 30 | N-Oleoyl L-Serine | up | down |
| 31 | Butyramide | up | down |
| No. | Metabolites | Model vs. WT | TSS vs. Model |
|---|---|---|---|
| 1 | PE(PGJ2/22:2(13Z,16Z)) | up | down |
| 2 | PS(O-20:0/17:1(9Z)) | up | down |
| 3 | PC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/20:4(5Z,8Z,11Z,14Z)) | up | down |
| 4 | DGCC(22:6/20:5) | up | down |
| 5 | PE(21:0/17:2(9Z,12Z)) | up | down |
| 6 | PC(18:2(2E,4E)/18:2(2E,4E)) | up | down |
| 7 | PC(20:4(5Z,8Z,11Z,14Z)-OH(20)/P-18:0) | up | down |
| 8 | Seerziqquaztol-Wojbjxkfsa-N | up | down |
| 9 | 5s-Hete Di-Endoperoxide | down | up |
| 10 | PC(16:0/18:1(11Z)) | up | down |
| 11 | PE(19:0/18:1(9Z)) | up | down |
| 12 | PC(17:0/PGE1) | up | down |
| 13 | PE(22:5(4Z,7Z,10Z,13Z,16Z)/20:5(7Z,9Z,11E,13E,17Z)3OH(5,6,15)) | up | down |
| 14 | PS(O-18:0/19:1(9Z)) | up | down |
| 15 | PC(P-18:1(11Z)/22:5(4Z,7Z,10Z,13Z,19Z)-O(16,17)) | up | down |
| 16 | PC(20:4(5Z,8Z,11Z,14Z)/16:1(9Z)) | down | down |
| 17 | PC(14:0/20:1(11Z)) | down | up |
| 18 | PC(18:1(11Z)/16:0) | up | down |
| 19 | PG(20:1(11Z)/20:3(5Z,8Z,11Z)-O(14R,15S)) | down | up |
| Accession | Gene Name | Regulated Type | log2FC | p-Value |
|---|---|---|---|---|
| P11798 | Camk2a | up | 0.415196667 | 0.00623 |
| Q9Z2Y3 | Homer1 | up | 0.380023333 | 0.027427 |
| Q3U214 | Mast3 | up | 0.28294 | 0.017301 |
| Q8CC35 | Synpo | up | 0.58669 | 0.005549 |
| Q3UQ44 | Iqgap2 | up | 0.301723333 | 0.015091 |
| P70207 | Plxna2 | up | 0.36823 | 0.034991 |
| Q9QVP9 | Ptk2b | up | 0.34146 | 0.031864 |
| Q9JJV5 | Cacng3 | up | 0.278546667 | 0.018327 |
| Q8C3Q5 | Shisa7 | up | 0.333286667 | 0.026621 |
| O35405 | Pld3 | up | 0.366233333 | 0.021005 |
| Q61909 | Runx1t1 | down | −0.547753333 | 0.005818 |
| F6SEU4 | Syngap1 | up | 0.3196 | 0.025881 |
| A2AF47 | Dock11 | down | −0.322023333 | 0.000447 |
| Q64314 | Cd34 | up | 0.51789 | 0.041513 |
| P49135 | Ercc3 | down | −0.280473333 | 0.047553 |
| Q9QZX7 | Srr | up | 0.34103 | 0.030063 |
| Q9ERB5 | Slco1c1 | up | 0.26938 | 0.002302 |
| Q8BIZ1 | Anks1b | up | 0.372903333 | 0.033418 |
| P60853 | Lzts1 | up | 0.317206667 | 0.024648 |
| Q64336 | Tbr1 | up | 0.273523333 | 0.046988 |
| P14231 | Atp1b2 | down | −0.487503333 | 0.004089 |
| Q91XU3 | Pip4k2c | up | 0.301373333 | 0.010448 |
| O88343 | Slc4a4 | down | −0.297656667 | 0.01442 |
| Q5DTZ6 | Rnf150 | up | 0.289186667 | 0.007382 |
| Q8BLQ9 | Cadm2 | up | 0.317206667 | 0.024648 |
| Q9D1T0 | Lingo1 | up | 0.26856 | 0.002194 |
| Q8BG58 | P4htm | down | −0.31637 | 0.036054 |
| Q76HP3 | Tmem132d | up | 0.366693333 | 0.046749 |
| P60761 | Nrgn | up | 0.46404 | 0.041644 |
| O70433 | Fhl2 | up | 0.35065 | 0.001092 |
| P97441 | Slc30a3 | up | 0.364163333 | 0.044378 |
| Q60987 | Foxg1 | up | 0.337033333 | 0.031355 |
| Q5NBX1 | Cobl | up | 0.28674 | 0.010194 |
| Q6ZPQ6 | Pitpnm2 | up | 0.289453333 | 0.01947 |
| O88809 | Dcx | up | 0.303026667 | 0.013699 |
| Q60625 | Icam5 | up | 0.283706667 | 0.044947 |
| Q64524 | H2bc21 | down | −0.51608 | 0.005131 |
| P97792 | Cxadr | up | 0.30167 | 0.03135 |
| Q7TPR4 | Actn1 | up | 0.416243333 | 0.00801 |
| Q9R257 | Hebp1 | up | 0.301436667 | 0.035898 |
| P35282 | Rab21 | down | −0.295966667 | 0.024016 |
| G5E829 | Atp2b1 | up | 0.278036667 | 0.04069 |
| P26040 | Ezr | up | 0.35864 | 0.048959 |
| P12657 | Chrm1 | up | 0.415196667 | 0.00623 |
| O35526 | Stx1a | up | 0.454896667 | 0.035476 |
| Q9CW07 | Ppp1r3g | up | 0.448413333 | 0.000286 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Du, T.; Zhang, W.; Ma, W.; Yao, Y.; Li, J. Investigating the Therapeutic Mechanisms of Total Saikosaponins in Alzheimer’s Disease: A Metabolomic and Proteomic Approach. Pharmaceuticals 2025, 18, 100. https://doi.org/10.3390/ph18010100
Wei H, Du T, Zhang W, Ma W, Yao Y, Li J. Investigating the Therapeutic Mechanisms of Total Saikosaponins in Alzheimer’s Disease: A Metabolomic and Proteomic Approach. Pharmaceuticals. 2025; 18(1):100. https://doi.org/10.3390/ph18010100
Chicago/Turabian StyleWei, Huiling, Tianyi Du, Weiwei Zhang, Wei Ma, Yao Yao, and Juan Li. 2025. "Investigating the Therapeutic Mechanisms of Total Saikosaponins in Alzheimer’s Disease: A Metabolomic and Proteomic Approach" Pharmaceuticals 18, no. 1: 100. https://doi.org/10.3390/ph18010100
APA StyleWei, H., Du, T., Zhang, W., Ma, W., Yao, Y., & Li, J. (2025). Investigating the Therapeutic Mechanisms of Total Saikosaponins in Alzheimer’s Disease: A Metabolomic and Proteomic Approach. Pharmaceuticals, 18(1), 100. https://doi.org/10.3390/ph18010100






