Abstract
In this research, we developed undoped and aluminum-doped zinc oxide for antimicrobial and anticancer activities. This study focuses on the synthesis, characterization, and biological activities of zinc oxide nanoparticles (ZnO NPs) and aluminum-doped zinc oxide nanocomposites (Zn1−xAlxO NCs) at varying concentrations (x = 0, 0.25, 0.5, and 0.75 wt%) using the coprecipitation method. Various characterization techniques such as XRD, UV-Vis, FTIR, EDX, and SEM were performed to analyze the crystal structure, optical properties, functional group identification, elemental composition, and surface morphology. The antimicrobial activity test showed that Zn0.75Al0.25O NCs exhibited the strongest inhibition zone against Bacillus cereus compared to Staphylococcus aureus > Pasteurella multocida > Escherichia coli. Moreover, the cytotoxicity and cell viability of liver cancer (HepG-2), breast cancer (MCF-7), ovarian cancer (SKOV3), and normal liver cell lines) were evaluated using the MTT assay, demonstrating that Zn0.75Al0.25O NCs not only enhance cell destruction but also show low cytotoxicity and high biocompatibility at low concentrations. These results suggest that Zn0.75Al0.25O NCs could be a promising candidate for in vivo anticancer applications and should be further investigated.
1. Introduction
Nanotechnology offers numerous advantages in every field of life, including information technology, medical technology, energy, global security, environmental science, and food safety. However, its impact is particularly effective in the field of nanomedicine, where it holds great potential for cancer diagnosis and treatment. Cancer, a leading cause of mortality and healthcare expenditure, affects millions globally, manifesting in various forms such as breast, liver, lung, and brain cancer [1,2,3].
According to a 2020 WHO report, cancer is the second foremost reason for death globally, resulting in over 10 million deaths annually. Liver cancer (HepG-2) is one of them, and unfortunately, it is on the rise [4,5]. Notably, 85% of cancer cases occur in developing nations. Pakistan has the highest death rate of liver cancer among Asian nations [6,7]. The major risk factors for HepG-2 cancer include infection with human papillomavirus (HPV) and hepatitis (HCV, HBV), as well as exposure to fatty acids, contaminated food, nicotine, and alcohol, which contribute to 25% of liver cancer deaths. However, these factors may vary from country to country [8,9]. Moreover, bacterial infections and their resistance have severely obstructed public health, causing widespread morbidity and mortality [10]. The treatment of bacterial infections often involves traditional medicines, which can have harmful side effects due to overuse and misuse, ultimately impacting patients’ health and well-being [11,12,13].
Metal oxide nanoparticles (MO NPs) and their nanocomposites (especially ZnO NPs, Al2O3 NPs, and their nanocomposites) have demonstrated high efficiency against antimicrobial resistance (AMR), multidrug resistance (MDR), as well as in cancer diagnostics and treatment, and inhibiting both Gram-positive and Gram-negative bacteria [14,15].
A wide range of MO NPs, including ZnO NPs, manganese dioxide, magnesium oxide, aluminum oxide, silver oxide, and selenium oxide nanoparticles, as well as their nanocomposites, have gained significant attention in the biomedical field for applications such as nanomedicine, drug delivery systems (DDS), biosensing, photothermal therapy (PTT), hyperthermia therapy, photodynamic therapy (PDT), diagnostics, and cancer treatment. This interest is driven due to their exceptional physiochemical and biological properties [16,17,18,19,20,21]. For example, Rana et al. [22] described that ZnO NPs and Al-doped ZnO NCs exhibit broad-spectrum antimicrobial activity and promising efficacy against Gram-negative and Gram-positive bacteria. They also exhibit enhanced cytotoxicity toward various cancerous cell lines, including liver, breast, lung, and colon cancer cells. It can also boost cell death by inhibiting cancer growth cells. Yu et al. [23] reported that Al-doped ZnO NCs show great antibacterial activity against various bacterial strains, including E. coli and P. aeruginosa, especially for S. aureus. Moreover, they can also decrease antimicrobial resistance (AMR). Eddy et al. [24] described that Al-doped ZnO NCs exhibited broad-spectrum antimicrobial activity and promising efficacy against Gram-negative and Gram-positive bacteria. These nanocomposites can enhance cytotoxicity, reduce toxicity, and have minimal side effects on various cancerous cell lines, including liver, breast, lung, and colon cancer cells. Also, it was found to boost cell death while inhibiting cancer cell growth [12].
There are several reputable techniques for developing MO NPs, such as auto-combustion, coprecipitation, sol–gel, or hydrothermal methods, etc. [25,26]. These methods are ideal due to their simplicity, ease of use, eco-friendliness, and ability to fabricate nanoparticles with the desired morphology. Furthermore, the size, shape, and properties of nanoparticles can be easily modified by adjusting reaction parameters, including temperature and time [27,28,29].
In this research, undoped and aluminum-doped zinc oxide were successfully fabricated and characterized using various characterization techniques. These nanocomposites not only inhibit bacterial growth and enhance cell destruction but also show low cytotoxicity and high biocompatibility at low concentrations. Additionally, to address the problems of AMR and MDR barriers using these nanomaterials. These findings suggest that Zn0.75Al0.25O NCs could be promising candidates for in vivo anticancer applications.
2. Results and Discussions
2.1. XRD Analysis
XRD analysis was employed to investigate the structural properties of ZnO NPs and Al-doped ZnO nanocomposites fabricated at varying concentrations (0.25%, 0.5%, and 0.75%). The XRD pattern revealed no additional phases, and the intensity of the samples increased by increasing aluminum concentration, as shown in Figure 1. The XRD patterns of ZnO NPs and Al-doped ZnO NCs showed three distinct peaks at 31.5°, 34.08°, and 36.01°, corresponding to the Miller indices (100), (002), and (101), respectively. These peaks matched well with the JCPDS card no. 36-1451, verifying the hexagonal wurtzite phase structure of ZnO NPs and Al-doped ZnO NCs with complete formation and no additional peaks. Moreover, low-intensity peaks were observed at 47.33°, 56.39°, 62.69°, 67.75°, and 68.89°, corresponding to the Miller indices (102), (110), (103), (112), and (201), respectively [16,19]. Table 1 shows that the crystallite size of Al-doped ZnO NPs does not change significantly with increasing Al concentration. The non-significant changes were observed in crystallite size with increasing Al concentration, which can be significant in various applications, such as electronic and optoelectronic devices, energy storage devices, and photocatalytic activities, particularly in biomedical applications. Furthermore, due to their small size, nanoparticles can be effectively used for targeted drug delivery [26,27]. The average crystallite sizes of ZnO NPs and Al-doped ZnO NCs were calculated using Scherrer’s Equation (1).
where,
C.S = crystallite size;
k = shape factor (usually its value is 0.94);
= 0.154 nm, X-ray wavelength;
= FWHM (full-width half maximum) in radian;
= Bragg angle in degrees.

Table 1.
ZnO NPs and Al-doped ZnO NCs at varying concentrations (0.25%, 0.5%, and 0.75%).
Table 1.
ZnO NPs and Al-doped ZnO NCs at varying concentrations (0.25%, 0.5%, and 0.75%).
| (h, k, l) Miller Indices | 2θ (Angle of Diffraction) | C.S (nm) |
|---|---|---|
| ZnO NPs | ||
| 100 | 31.5° | 39.37 |
| 101 | 36.01° | 45.25 |
| 110 | 56.39° | 47.1 |
| 112 | 67.75° | 44.54 |
| Zn0.75Al0.25O NCs | ||
| 100 | 31.5° | 36.67 |
| 101 | 36.01° | 41.65 |
| 110 | 56.39° | 43.71 |
| 112 | 67.75° | 42.64 |
| Zn0.5Al0.5O NCs | ||
| 100 | 31.5° | 35.17 |
| 101 | 36.01° | 39.29 |
| 110 | 56.39° | 42.31 |
| 112 | 67.75° | 40.54 |
| Zn0.25Al0.75O NCs | ||
| 100 | 31.5° | 32.87 |
| 101 | 36.01° | 33.26 |
| 110 | 56.39° | 35.58 |
| 112 | 67.75° | 31.29 |
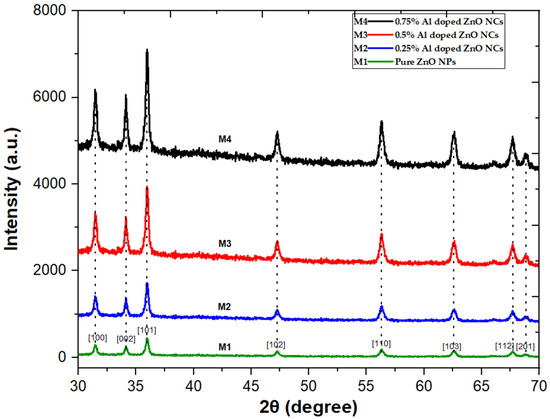
Figure 1.
XRD spectrum of ZnO NPs and Al-doped ZnO NCs at varying concentrations 0.25%, 0.5%, and 0.75%.
2.2. Fourier Transformation Infrared Spectroscopy
Figure 2 displays the FTIR spectra of ZnO NPs and Al-doped ZnO NCs at varying concentrations (0.25%, 0.5%, and 0.75%) over the 400–4000 cm−1 range. The FTIR spectra disclose the Zn-O stretching mode, thereby representing a band between 441 and 665 cm−1, confirming the formation of ZnO NPs. Moreover, the broad band at 3349 cm−1 corresponds to the O-H stretching mode of the hydroxyl group, while peaks at 890 cm−1 and 3349 cm−1 indicate -OH bending and stretching vibrational modes. These peaks originate from the H2O during Zn-O formation and moisture contents in the atmosphere. However, the intensity and magnitude of the –OH emission at 3349 cm−1 vary due to the use of different reducing agents. The peaks at 1422 cm−1 and 1642 cm−1 correspond to C=O and C–H vibrational modes, respectively, which are due to the presence of aldehyde or ketone groups partially substituted by zinc source nitrate ions (Zn(NO3)2). The absorption at 1376 cm−1 suggests the presence of an aromatic ring at a moderate level. The observed peaks at 1642, 1460, 1422, 1376, and 865 cm−1 are associated with the asymmetrical and symmetrical zinc carboxylate [29,30,31].
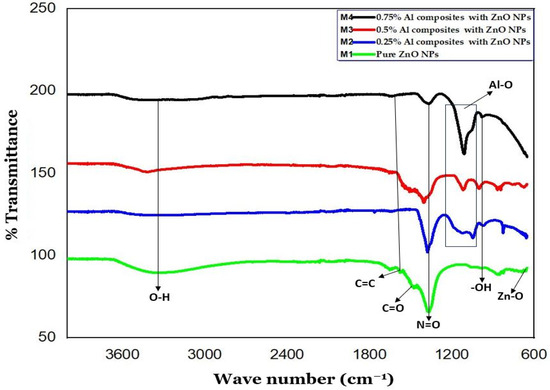
Figure 2.
FTIR spectrum of ZnO NPs and Al-doped ZnO NCs at varying concentrations 0.25%, 0.5%, and 0.75%.
2.3. UV-Visible Spectroscopy
The UV-visible spectra in Figure 3 illustrate the absorption bands of ZnO NPs and Al-doped ZnO NCs at varying concentrations (0.25%, 0.5%, and 0.75%). The presence of aluminum causes a longer wavelength (red shift). Because of their high exciton binding energy (60 mV), ZnO NPs and their Al-doped ZnO NCs exhibit substantial exciton absorption in their spectra, even when synthesized at ambient temperature. Specifically, the absorption peak of ZnO NPs and Al-doped ZnO NCs occurs at 363 nm and ≥363 [19,32,33].
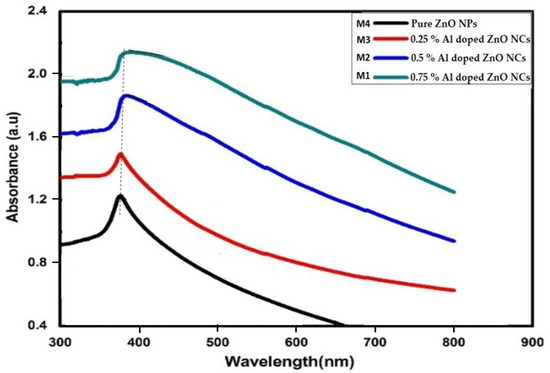
Figure 3.
UV-visible spectrums of ZnO NPs and Al-doped ZnO NCs at varying concentrations 0.25%, 0.5%, and 0.75%.
2.4. Scanning Electron Microscopy and Zeta Potential Analysis
SEM analysis was conducted to investigate the surface morphology of the ZnO NPs and Al-doped ZnO NCs at varying concentrations (0.25%, 0.5%, and 0.75%), as shown in Figure 4a–d. With increasing Al3+ concentration, prominent changes in particle shape and size were observed. The SEM images demonstrate irregular shapes with small and large spherical grains, also observed in the Al-doped ZnO NCs. The spherical grains were circulated with red circles. In particular, the grain size increased with the increase in aluminum content. All the SEM images were taken at a resolution of 3 µm. Figure 4a–d displays SEM images of ZnO NPs, Zn0.75Al0.25O, Zn0.5Al0.5O, and Zn0.25Al0.75O NCs, revealing average crystallite sizes of 43.5, 40.5, 39, and 33 nm, respectively. Notably, these values are in excellent agreement with the corresponding XRD results, confirming the consistency of the crystallite size measurements. The zeta potential analysis results of ZnO NPs and Al-doped ZnO NCs are shown in Figure 5. The zeta potential values of ZnO NPs, Zn0.75Al0.25O, Zn0.5Al0.5O, and Zn0.25Al0.75O NCs dispersions were found to be +20, ±15, −10, and 13.7 mV, respectively. These results show the high stability of the prepared nanomaterials. These results are very similar to previously published articles [34,35,36].
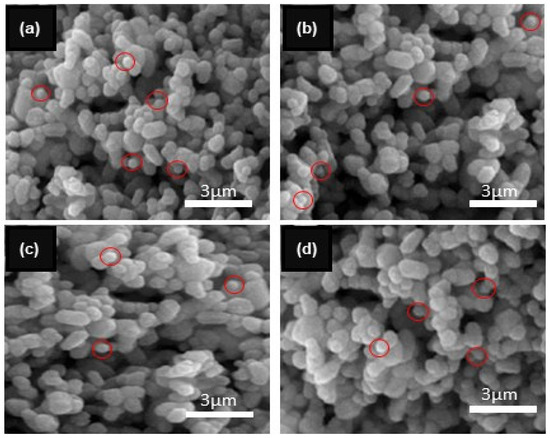
Figure 4.
SEM results of (a) ZnO NPs and Al-doped ZnO NCs at varying concentrations (b) 0.25%, (c) 0.5%, and (d) 0.75%.
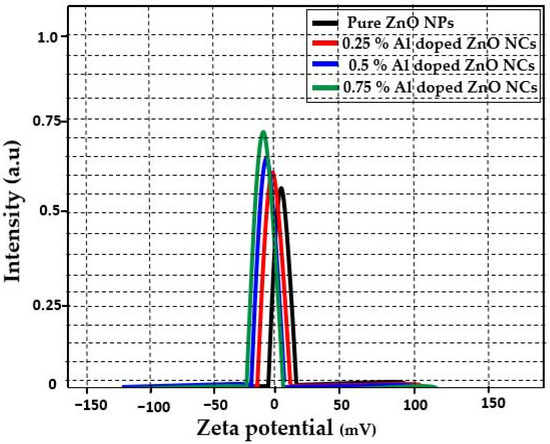
Figure 5.
Zeta potential of ZnO NPs and Al-doped ZnO NCs at varying concentrations 0.25%, 0.5%, and 0.75%.
2.5. Energy Dispersive X-ray Spectroscopy
The EDX analysis was employed to measure the elemental composition of the prepared ZnO NPs and Al-doped ZnO NCs. The outcomes of the EDX analysis confirmed the presence of Zn and Al as primary elements and O as a secondary element in the prepared nanomaterials, as shown in Figure 6a–d. For example, Musleh et al. [37] and Aldalbahi et al. [38] stated comparable outcomes where zinc and aluminum elements were detected in the EDX spectrum, confirming the reduction in zinc and aluminum-doped zinc ions to Zn and Al elements.
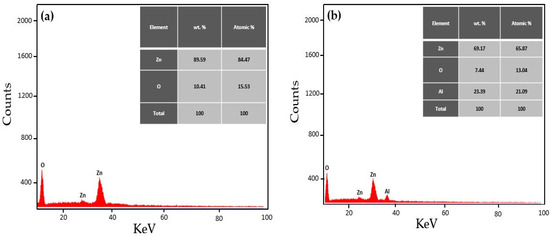
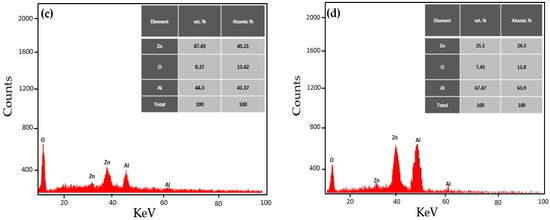
Figure 6.
EDX spectrum of (a) ZnO NPs and Al-doped ZnO NCs at varying concentrations (b) 0.25%, (c) 0.5%, and (d) 0.75%.
2.6. Antimicrobial Activity
In this research work, the antimicrobial potential of ZnO NPs and Al-doped ZnO NCs at varying concentrations (0.25%, 0.5%, and 0.75%) was evaluated against four bacterial strains, including two Gram-positive strains, Staphylococcus aureus and Bacillus cereus, and two Gram-negative strains, Escherichia coli, and Pasteurella multocida. The results showed that ZnO NPs and Al-doped NCs exhibited the highest antimicrobial potential against the tested bacterial strains at a high-dose concentration of 40 mg/mL. Notably, the zones of inhibition were clear and significant, except for the Zn0.75Al0.25O NCs sample, which showed very high inhibition against all bacterial strains (indicated by the symbol “#”), as shown in Figure 7 and Figure 8.
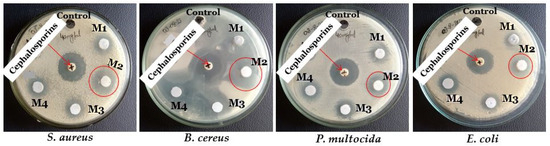
Figure 7.
The antimicrobial activity of samples DMSO (control), standard antibiotic (cephalosporin), M1 (ZnO NPs), M2 (Zn0.75Al0.25O), M3 (Zn0.5Al0.5O), and M4 (Zn0.25Al0.75O NCs) against S. aureus, B. cereus, P. multocida, and E. coli.
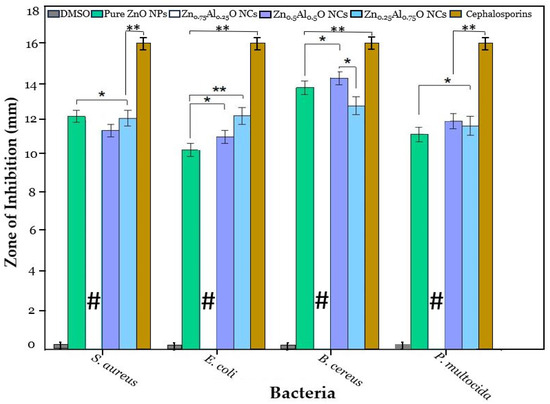
Figure 8.
The antimicrobial activity of DMSO (control), standard antibiotic (cephalosporin), ZnO NPs, Zn0.75Al0.25O NCs, Zn0.5Al0.5O NCs, and Zn0.25Al0.75O NCs at 40 mg/mL, where * is significance for p ≤ 0.05 and ** is highly significance for p ≤ 0.01.
Hence, the antimicrobial activity of Zn0.75Al0.25O NCs was repeated at lower concentrations, and even at 150 µg/mL, it showed a significant zone of inhibition against each bacterial strain, which showed its highest antimicrobial potential against these bacterial strains. A standard antibiotic, cephalosporin, was used as a positive control, and dimethyl sulfoxide (DMSO) was used as a negative control and did not show any antimicrobial activity against any of the bacterial strains to determine the assay validity. ZnO NPs, Zn0.75Al0.25O, Zn0.5Al0.5O, and Zn0.25Al0.75O NCs showed significant antimicrobial activity against all bacterial strains. Notably, Zn0.75Al0.25O NCs exhibited the highest antibacterial efficiency, especially against B. cereus. Moreover, Zn0.75Al0.25O NCs demonstrated the biggest inhibition zone against S. aureus, B. cereus, E. coli, and P. multocida, with 12 ± 0.09 mm, 15.5 ± 0.11 mm, 12.5 ± 0.07 mm, and 13.3 ± 0.11 mm, respectively, as shown in Figure 9 and Table 2. In this discussion, Yu et al. [23] described that Al-doped ZnO NCs showed great antibacterial activity against numerous bacterial strains, including E. coli and P. aeruginosa, especially against S. aureus. Also, it can decrease antimicrobial resistance (AMR). For instance, the antimicrobial activity of ZnO NPs can induce Zn2+ and ROS, such as hydroxyl radicals and superoxides, which can disrupt bacterial cell membrane integrity and DNA and inhibit enzyme activity, causing structural damage and leakage of cellular contents. These nanomaterials can prevent biofilm formation, which is important for bacterial survival and antibiotic resistance [38,39,40,41].
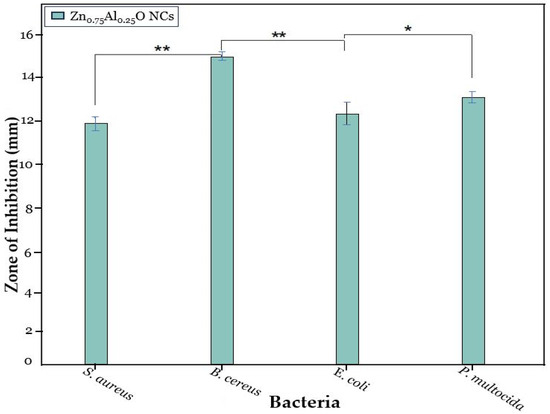
Figure 9.
The antimicrobial activity of Zn0.75Al0.25O NCs at 150 mg/mL, where * is significance for p ≤ 0.05 and ** is highly significance for p ≤ 0.01.

Table 2.
Inhibition of ZnO NPs and Al-doped ZnO NCs at varying concentrations (0.25%, 0.5%, and 0.75%) against S. aureus, E. coli, B. cereus, and P. multocida.
2.7. Anticancer Activity
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium-bromide (MTT) assay was used to examine the cytotoxicity of ZnO NPs, Zn0.75Al0.25O, Zn0.5Al0.5O, and Zn0.25Al0.75O NCs toward HepG-2, MCF-7, SKOV3, and normal cells. In the initial screening, the cell viability of ZnO NPs, Zn0.75Al0.25O, Zn0.5Al0.5O, and Zn0.25Al0.75O NCs were tested against HepG-2, MCF-7, SKOV3, and normal cells at 100 μg/mL, and substantial cell viability was noticed for ZnO NPs and Zn0.75Al0.25O NCs compared to Zn0.5Al0.5O and Zn0.25Al0.75O NCs (see Figure 10). Notably, Zn0.75Al0.25O NCs exhibited > 70% cell viability toward HepG-2 cells. Zn0.75Al0.25O NCs showed reasonably higher % cell viability. For further validation, the antiproliferative activity of Zn0.75Al0.25O NCs was performed using a dose-dependent method (0, 3.125, 6.25, 12.5, 25, 50, and 100 µg/mL) toward all mentioned cell lines; then, dose-dependent curves were attained, as shown in Figure 11. The IC50 value was calculated as 20.47 µg/mL, indicating significant cell viability of Zn0.75Al0.25O NCs. The cell viability results showed that Zn0.75Al0.25O NCs not only enhanced HepG-2 cell destruction but also exhibited low cytotoxicity and high biocompatibility at low concentrations. Furthermore, Zn0.75Al0.25O NCs have reduced toxicity toward normal liver cells compared to HepG-2, MCF-7, and SKOV3 cells. These results suggest that Zn0.75Al0.25O NCs can serve as a promising preliminary point for further identification and isolation of compounds that inhibit HepG-2 cells or for the development of anticancer functional foods [34].
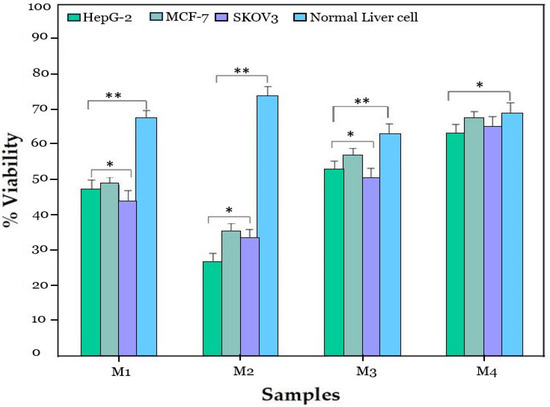
Figure 10.
The anticancer activity of samples M1 (ZnO NPs), M2 (Zn0.75Al0.25O), M3 (Zn0.5Al0.5O), and M4 (Zn0.25Al0.75O NCs) against HepG-2, MCF-7, SKOV3 cancer cell lines, and normal liver cell line, where * is significance for p ≤ 0.05 and ** is highly significance for p ≤ 0.01.
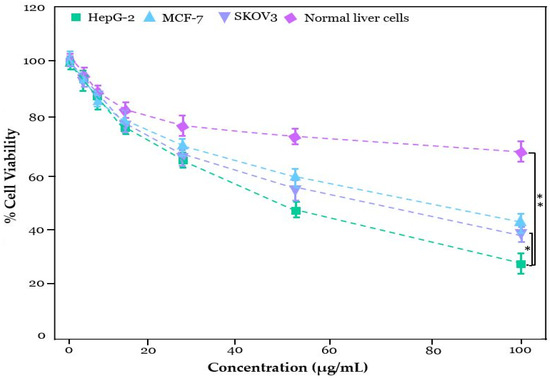
Figure 11.
% cell viability of Zn0.75Al0.25O NCs at varying concentration (μg/mL) against HepG-2, MCF-7, SKOV3, and normal liver cell line, where * is significance for p ≤ 0.05 and ** is highly significance for p ≤ 0.01.
Allayeith et al. [41] described that ZnO NPs and their nanocomposites could be used for targeted cancer therapy, reducing side effects and improving the efficiency of cancer treatment. In these mechanisms, Al doping increases the surface area to volume ratio of ZnO and enhances the release of Zn2+ ions, improving interaction with cancer cells and bacterial strains (pathogens) and causing cell death and damage. Particularly, Al-doped ZnO NCs produce reactive oxygen species (ROS) and oxidative stress, leading to damage to cancer cell DNA, proteins, and lipids, as well as microbial damage. Anjum et al. [42] described that the anticancer activity of ZnO nanoparticles could induce programmed cell death (apoptosis) in cancer cells through mitochondrial dysfunction and activation of pro-apoptotic proteins. These nanoparticles can also inhibit cancer cell proliferation by arresting the cell cycle at specific stages (e.g., G2/M phase) and prevent the formation of new blood vessels, which is essential for cancer growth and metastasis. Notably, Al-doping can improve ROS generation, leading to enhanced antimicrobial and anticancer activity. Al-doping can facilitate the uptake of ZnO NPs by bacterial and cancer cells, enhancing their efficiency. Moreover, Al-doping can enhance the interaction between ZnO NPs and cell membranes, leading to superior structural damage.
3. Materials and Methods
3.1. Chemicals
Zinc nitrate [Zn(NO3)2, 98%], aluminum nitrate monohydrate [Al(NO3)3, 99.9%], sodium hydroxide (NaOH, 98%), MTT ≥ 98%, Dulbecco’s Modified Eagles Medium (DMEM), dimethyl sulfoxide (DMSO), fetal bovine serum (FBS), and nutrient agar were used as chemical reagents without any further purification. All chemicals were purchased from the Punjab Scientific store, Sigma Aldrich (St. Louis, MO, USA).
3.2. Synthesis of ZnO NPs and Al-Doped ZnO Nanocomposites
A 0.5 M zinc nitrate solution was prepared using 100 mL of deionized water. In the next step, the solution was constantly stirred on a magnetic stirrer at 80 °C for 30 min. Furthermore, the pH was maintained between 10 and 11 by dropwise adding of NaOH solution. After 2 h of continuous stirring, a milky white precipitate formed. The precipitate was then centrifuged, washed, and dried. Finally, the ZnO NPs powder was annealed for 3 h at 300 °C in a muffle furnace. Similarly, Al-doped ZnO NCs were prepared by preparing a solution of 0.25% aluminum nitrate in 0.5 mM zinc nitrate. The solution was constantly stirred at 80 °C for 30 min. Finally, the same procedure was repeated twice with varying concentrations (0.5 and 0.75 wt%) [19,20]. The synthesis process of ZnO NPs and Al-doped ZnO NPs is shown in Scheme 1.
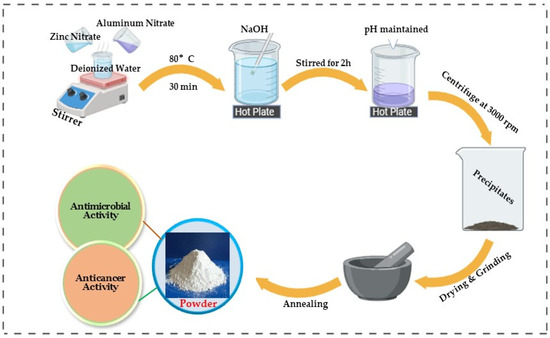
Scheme 1.
Synthesis process of ZnO NPs and Al-doped ZnO NCs.
3.3. Characterization of Nanomaterials
SEM analysis was performed using the “Cube 10 Emcraft, Hanam, Republic of Korea” to analyze the surface morphology of the prepared nanomaterials. The UV-Vis spectrometer was employed to calculate the optical density on a “BK-UV1800PC double beam Bio-base, Jinan, China”. The XRD was performed using the “D8 advance Bruker, Karlsruhe, Germany” to examine the crystal structure using Cu Kα radiation (λ = 0.154). The FTIR analysis was performed on a “Spectrum 2, Perkin Elmer, Waltham, MA, USA” to analyze the functional groups. The EDX analysis was used to measure the elemental composition of the prepared nanomaterials on a “Zetasizer Nano ZS, Malvern, UK”.
3.4. Well Diffusion Assay
The Staphylococcus aureus (S. aureus), Bacillus cereus (B. cereus), Pasteurella multocida (P. multocida), and Escherichia coli (E. coli) strains were obtained from the laboratory of Muhammad Ali, Department of Zoology, Government College University Faisalabad, Pakistan. The antimicrobial potential of ZnO NPs and Al-doped ZnO NCs was evaluated using a modified well diffusion method [21]. Nutrient agar powder (2.8 g) was dissolved in distilled water (100 mL). The media was then poured into sterile petri dishes in a biosafety cabinet and allowed to solidify for 2 hours. The plates were stacked and sealed, then incubated overnight at 37 °C to check for pre-experimental contamination. After 24 h, 100 µL of each strain (E. coli, B. cereus, S. aureus, and P. multocida) was inoculated onto agar plates at a concentration of 0.6 McFarland scale. Solutions of ZnO NPs and Al-doped ZnO NPs (40 mg/mL) were dispensed into separate plates in the agar medium and incubated at 37 °C for 24 h. Cephalosporin antibiotics were used as a positive control, while DMSO was used as a negative control to determine the validity of the assay. After 24 h, the inhibition zones were measured using a vernier caliper.
3.5. Cell Culturing
Liver cancer (HepG-2), breast cancer (MCF-7), ovarian cancer (SKOV3), and normal liver cell lines were gifted from the laboratory of Muhammad Ali, Department of Zoology, Government College University Faisalabad (GCUF), Pakistan. HepG-2, MCF-7, SKOV3, and normal liver cells were cultured in a 96-well plate separately, using a culture medium supplemented with FBS (10%), DMEM (2 mM), penicillin and streptomycin (100 IU mL−1), as well as HEPES (20 mM) as a buffering medium. The mixture was maintained under optimal conditions (37 °C, 5% CO2) in a humidified atmosphere. The standard protocol was adopted as recommended by the Ethics Committee of GCUF. For more details, see our latest published paper [5,20].
3.6. MTT Assay
Using the MTT assay, the in vitro cytotoxicity of ZnO NPs and Al-doped ZnO NCs on the HepG-2, MCF-7, SKOV3, and normal liver cell lines was evaluated. HepG-2, MCF-7, SKOV3, and normal liver cells were separately cultured in a 96-well plate and incubated at 37 °C for 24 h. Then, the cells were incubated for 24 h in varying concentrations of nanocomposites for treatment. After 24 h, 10 microliters (µL) of MTT reagent was added, and the mixture was incubated for an additional 4 h. Subsequently, 150 microliters (µL) of DMSO was used to dissolve the formazan crystals, and the absorbance was determined using a microplate reader [28].
Equation (2) was used to calculate cell viability.
3.7. Statistical Analysis
The statistical analysis was performed using GraphPad Prism (version 8.0). The mean ± standard deviation of each experiment is shown, with * p < 0.05 indicating statistical significance, and ** p < 0.01 indicating high statistical significance.
4. Conclusions
Zinc oxide nanoparticles and aluminum-doped zinc oxide nanocomposites were prepared using the coprecipitation method. The XRD outcomes revealed a hexagonal wurtzite phase structure. UV-Vis results determined maximum absorption spectra at 363 nm and ≥363, respectively, confirming the complete formation of ZnO NPs and Al-doped ZnO NCs. Zeta potential values of ZnO NPs, Zn0.75Al0.25O, Zn0.5Al0.5O, and Zn0.25Al0.75O NCs were found to be +20, ±15, −10, and 13.7 mV, respectively. The SEM micrograph results revealed nonuniform, agglomerated, irregularly shaped particles with small and large spherical grains. With increasing Al3+ concentration, prominent changes in particle shape and size were observed. Additionally, an in vitro MTT assay of ZnO NPs and Al-doped ZnO NCs disclosed that they possessed significant anticancer activity toward HepG-2, MCF-7, SKOV3, and normal liver cells, respectively. The microbial activity of ZnO NPs and Al-doped ZnO NCs was also examined by well diffusion assay toward S. aureus, B. cereus, E. coli, and P. multocida. However, the Zn0.75Al0.25O NCs exhibited the highest inhibition zone against B. cereus. Additionally, Zn0.75Al0.25O NCs not only enhanced the destruction of cancerous cells but also showed low toxicity and high biocompatibility at low concentrations. These findings indicate that Zn0.75Al0.25O NCs could serve as a new inhibitor for cytotoxic and antimicrobial agent, which suggests that it could be a promising candidate for in vivo anticancer and antimicrobial applications.
Author Contributions
Conceptualization, M.A. and M.F.-e.-A.; Writing—original draft, M.A.; Methodology, M.A. and M.T.; Software, M.A., M.T. and F.J.; Validation, M.F.-e.-A. and K.A.D.; Formal analysis, F.J., H.S. and J.R.; Investigation, M.A., M.F.-e.-A. and J.R.; Resources, M.T., H.S. and F.J.; Data curation, M.A.; Writing—review & editing, M.A., M.F.-e.-A., J.R. and K.A.D.; Visualization, M.A. and J.R.; Supervision, M.F.-e.-A. and K.A.D.; Project administration, M.F.-e.-A.; Funding acquisition, K.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by researchers Supporting Project Number (RSP2024R388) King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
This work was funded by researchers Supporting Project Number (RSP2024R388) King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Fakhar-e-Alam, M.; Hassan, M.; Sardar, H.; Zulqarnian, M.; Li, L.; Alothman, A.A.; Alangary, A.B.; Mohammad, S. Synergistic response of PEG coated manganese dioxide nanoparticles conjugated with doxorubicin for breast cancer treatment and MRI application. Arab. J. Chem. 2024, 17, 105958. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Iqbal, W.; Fakhar-e-Alam, M.; Hussain, Z.; Saadullah, M.; Hassan, M.; Rehman, J.; Dahlous, K.A.; Al-Qahtani, N.H. Synthesis and Characterization of Chemically and Green-Synthesized Silver Oxide Particles for Evaluation of Antiviral and Anticancer Activity. Pharmaceuticals 2024, 17, 908. [Google Scholar] [CrossRef]
- Tahir, M.; Fakhar-e-Alam, M.; Asif, M.; Iqbal, M.J.; Abbas, A.; Hassan, M.; Rehman, J.; Bhatti, Q.A.; Mustafa, G.; Alothman, A.A.; et al. Investigation of gadolinium doped manganese nano spinel ferrites via magnetic hypothermia therapy effect towards MCF-7 breast cancer. Heliyon 2024, 10, e24792. [Google Scholar] [CrossRef]
- Hashem, A.H.; El-Sayyad, G.S. Antimicrobial and anticancer activities of biosynthesized bimetallic silver-zinc oxide nanoparticles (Ag-ZnO NPs) using pomegranate peel extract. Biomass Conv. Bioref. 2023, 14, 20345–20357. [Google Scholar] [CrossRef]
- Hassan, S.E.; Fouda, A.; Radwan, A.A.; Salem, S.S.; Barghoth, M.G.; Awad, M.A.; Abdo, A.M.; El-Gamal, M.S. Endophytic Actinomycetes Streptomyces spp. mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J. Biol. Inorg. Chem. 2019, 24, 377–393. [Google Scholar] [CrossRef]
- Menazea, A.A.; Awwad, N.S. Antibacterial activity of TiO2 doped ZnO composite synthesized via laser ablation route for antimicrobial application. J. Mater. Res. Technol. 2020, 9, 9434–9441. [Google Scholar] [CrossRef]
- Oves, M.; Aslam, M.; Rauf, M.A.; Qayyum, S.; Qari, H.A.; Khan, M.S.; Alam, M.Z.; Tabrez, S.; Pugazhendhi, A.; Ismail, I.M. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater. Sci. Eng. C 2018, 89, 429–443. [Google Scholar] [CrossRef]
- Lallo da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef]
- Seshadri, V.D. Zinc oxide nanoparticles from Cassia auriculata flowers showed the potent antimicrobial and in vitro anticancer activity against the osteosarcoma MG-63 cells. Saudi J. Biol. Sci. 2021, 28, 4046–4054. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.R.; Sharif, S.; Shaheen, F.; Khalid, M.; Iqbal, Y.; Faisal, A.; Aziz, M.H.; Atif, M.; Ahmad, S.; Fakhar-e-Alam, M.; et al. Green synthesis of RGO-ZnO mediated Ocimum basilicum leaves extract nanocomposite for antioxidant, antibacterial, antidiabetic and photocatalytic activity. J. Saudi Chem. Soc. 2022, 26, 101438. [Google Scholar] [CrossRef]
- Geffers, C.; Gastmeier, P. Nosocomial infections and multidrug-resistant organisms in Germany: Epidemiological data from KISS (the Hospital Infection Surveillance System). Dtsch. Ärzteblatt Int. 2011, 108, 87. [Google Scholar]
- Waktole, G.; Chala, B. The Role of Biosynthesized Metallic and Metal Oxide Nanoparticles in Combating Anti-Microbial Drug Resilient Pathogens. J. Biomater. Nanobiotechnol. 2023, 14, 1–22. [Google Scholar] [CrossRef]
- Sruthi, S.; Ashtami, J.; Mohanan, P.V. Biomedical application and hidden toxicity of Zinc oxide nanoparticles, Mater. Today Chem. 2018, 10, 175–186. [Google Scholar]
- Baghdadi, N.; Salah, N.; Alshahrie, A.; Koumoto, K. Microwave irradiation to produce high performance thermoelectric material based on Al doped ZnO nanostructures. Crystals 2020, 10, 610. [Google Scholar] [CrossRef]
- Ghotekar, S. Plant extract mediated biosynthesis of Al2O3 nanoparticles-a review on plant parts involved, characterization and applications. Nanochemistry Res. 2019, 4, 163–169. [Google Scholar]
- Zhang, B.; Yang, K.; Zhang, K.; Wang, Q.; Wu, N. Migration transformation, prevention, and control of typical heavy metal lead in coal gangue: A review. Int. J. Coal Sci. Technol. 2023, 10, 85. [Google Scholar] [CrossRef]
- Mahmood, A.; Munir, T.; Fakhar-e-Alam, M.; Atif, M.; Shahzad, K.; Alimgeer, K.S.; Gia, T.N.; Ahmad, H.; Ahmad, S. Analyses of structural and electrical properties of aluminium doped ZnO-NPs by experimental and mathematical approaches. J. King Saud Univ.-Sci. 2022, 34, 101796. [Google Scholar] [CrossRef]
- Fakhar-e-Alam, M.; Amjad, I.; Saadullah, M.; Tahir, M.; Jawad, M.; Asif, M.; Atif, M.; Zara, S.; Rashad, M. Antitumor activity of zinc oxide nanoparticles fused with green extract of Nigella sativa. J. Saudi Chem. Soc. 2024, 28, 101814. [Google Scholar] [CrossRef]
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine 2009, 5, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Rana, G.; Dhiman, P.; Sharma, A. Advances on ZnO hetro-structure as nanoadsorbant for heavy metal removals. In ZnO and Their Hybrid Nano-Structures: Potential Candidates for Diverse Applications; Materials Research Forum LLC: Millersville, PA, USA, 2023; Volume 146, pp. 173–201. [Google Scholar]
- Yu, Q.; Wang, C.; Zhang, X.; Chen, H.; Wu, M.X.; Lu, M. Photochemical Strategies toward Precision Targeting against Multidrug-Resistant Bacterial Infections. ACS Nano 2024, 18, 14085–14122. [Google Scholar] [CrossRef] [PubMed]
- Eddy, N.O.; Ukpe, R.A.; Garg, R.; Garg, R.; Odiongenyi, A.; Ameh, P.; Akpet, I.N.; Udo, S.E. Review of in-depth knowledge on the application of oxides nanoparticles and nanocomposites of Al, Si and Ca as photocatalyst and antimicrobial agents in the treatment of contaminants in water. Clean Technol. Environ. Policy 2023, 1–32. [Google Scholar]
- Guo, M.Y.; Ng, A.M.C.; Liu, F.; Djurisic, A.B.; Chan, W.K.; Su, H.; Wong, K.S. Effect of native defects on photocatalytic properties of ZnO. J. Phys. Chem. C 2011, 115, 11095–11101. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Smirnova, V.V.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of Al2O3 nanoparticles. Nanomaterials 2022, 12, 2635. [Google Scholar] [CrossRef]
- Fatima, N.; ul Hassan, S.M.; Fakhar-e-Alam, M.; Asif, M.; Imtiaz, S.; Anwar, S.; Arooj, H.; Imran, M. Development of a novel fluorescence spectroscopy-based method using layered double hydroxides to study degradation of E. coli in water. J. Mol. Struct. 2024, 1310, 138248. [Google Scholar] [CrossRef]
- Suresh, J.; Pradheesh, G.; Alexramani, V.; Sundrarajan, M.; Hong, S.I. Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015008. [Google Scholar] [CrossRef]
- Thongam, D.D.; Gupta, J.; Sahu, N.K.; Bahadur, D. Investigating the role of different reducing agents, molar ratios, and synthesis medium over the formation of ZnO nanostructures and their photo-catalytic activity. J. Mater. Sci. 2018, 53, 1110–1122. [Google Scholar] [CrossRef]
- Srivastava, V.; Choubey, A.K. Kinetic and isothermal study of effect of transition metal doping on adsorptive property of zinc oxide nanoparticles synthesized via green route using Moringa oleifera leaf extract. Mater. Res. Express 2020, 6, 1250i7. [Google Scholar] [CrossRef]
- Dhamodharan, P.; Manoharan, C.; Bououdina, M.; Venkadachalapathy, R.; Ramalingam, S. Al-doped ZnO thin films grown onto ITO substrates as photoanode in dye sensitized solar cell. Sol. Energy 2017, 141, 127–144. [Google Scholar] [CrossRef]
- Sanguanprang, S.; Phuruangrat, A.; Thongtem, T.; Thongtem, S. Preparation of visible-light-driven Al-doped ZnO nanoparticles used for photodegradation of methylene blue. J. Electron. Mater. 2020, 49, 1841–1848. [Google Scholar] [CrossRef]
- Sadiqa, A.; Rasul, A.; Hassan, M.; Sultana, S.; Jabeen, F. Identification of Novel Natural Inhibitors to Human 3-Phosphoglycerate Dehydrogenase (PHGDH) for Cancer Treatment. Molecules 2022, 27, 6108. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Verma, Y.; Rana, K.; Rana, S.V.S. Zinc oxide nanoparticles inhibit dimethylnitrosamine induced liver injury in rat. Chem.-Biol. Interact. 2018, 295, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.R.; Mandal, B.K. Synthesis, Characterization of ZnO and Al2O3 Nanoparticles and Its Application in Chromium Remediation Studies. Asian J. Chem. 2017, 29, 2459–2462. [Google Scholar] [CrossRef]
- Musleh, H.; Zayed, H.; Shaat, S.; Al-Kahlout, A.; Tamous, H.; Issa, A.; Asad, J.; AlDahoudi, N. Enhancement of the performance of dye-sensitized solar cells using sensitized zinc oxide nanoparticles by rhodamine B dye. Egypt. J. Chem. 2019, 62, 111–123. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Alterary, S.; Ali Abdullrahman Almoghim, R.; Awad, M.A.; Aldosari, N.S.; Fahad Alghannam, S.; Alabdan, A.N.; Alharbi, S.; Alateeq, B.A.M.; Al Mohsen, A.A.; et al. Greener synthesis of zinc oxide nanoparticles: Characterization and multifaceted applications. Molecules 2020, 25, 4198. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Athinarayanan, J.; Periyasamy, V.S.; Alshuniaber, M.A.; Alshammari, G.; Hakeem, M.J.; Ahmed, M.A.; Alshatwi, A.A. Antibacterial mechanisms of zinc oxide nanoparticle against bacterial food pathogens resistant to beta-lactam antibiotics. Molecules 2022, 27, 2489. [Google Scholar] [CrossRef]
- Ahmed, B.; Solanki, B.; Zaidi, A.; Khan, M.S.; Musarrat, J. Bacterial toxicity of biomimetic green zinc oxide nanoantibiotic: Insights into ZnONP uptake and nanocolloid–bacteria interface. Toxicol. Res. 2019, 8, 246–261. [Google Scholar] [CrossRef]
- Allayeith, H.K. Zinc-Based Nanoparticles Prepared by a Top-Down Method Exhibit Extraordinary Antibacterial Activity Against Both Pseudomonas aeruginosa and Staphylococcus aureus. Ph.D. Thesis, Kent State University, Kent, OH, USA, 2020. [Google Scholar]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent advances in zinc oxide nanoparticles (ZnO NPs) for cancer diagnosis, target drug delivery, and treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).