Comparative Analysis of Volatile Components in Chi-Nan and Ordinary Agarwood Aromatherapies: Implications for Sleep Improvement
Abstract
1. Introduction
2. Results and Discussions
2.1. Composition Analysis of Agarwood Aromatherapy
2.2. Confirmation of Active Ingredients in Agarwood Aromatherapy and Construction of Target Genealogy Set
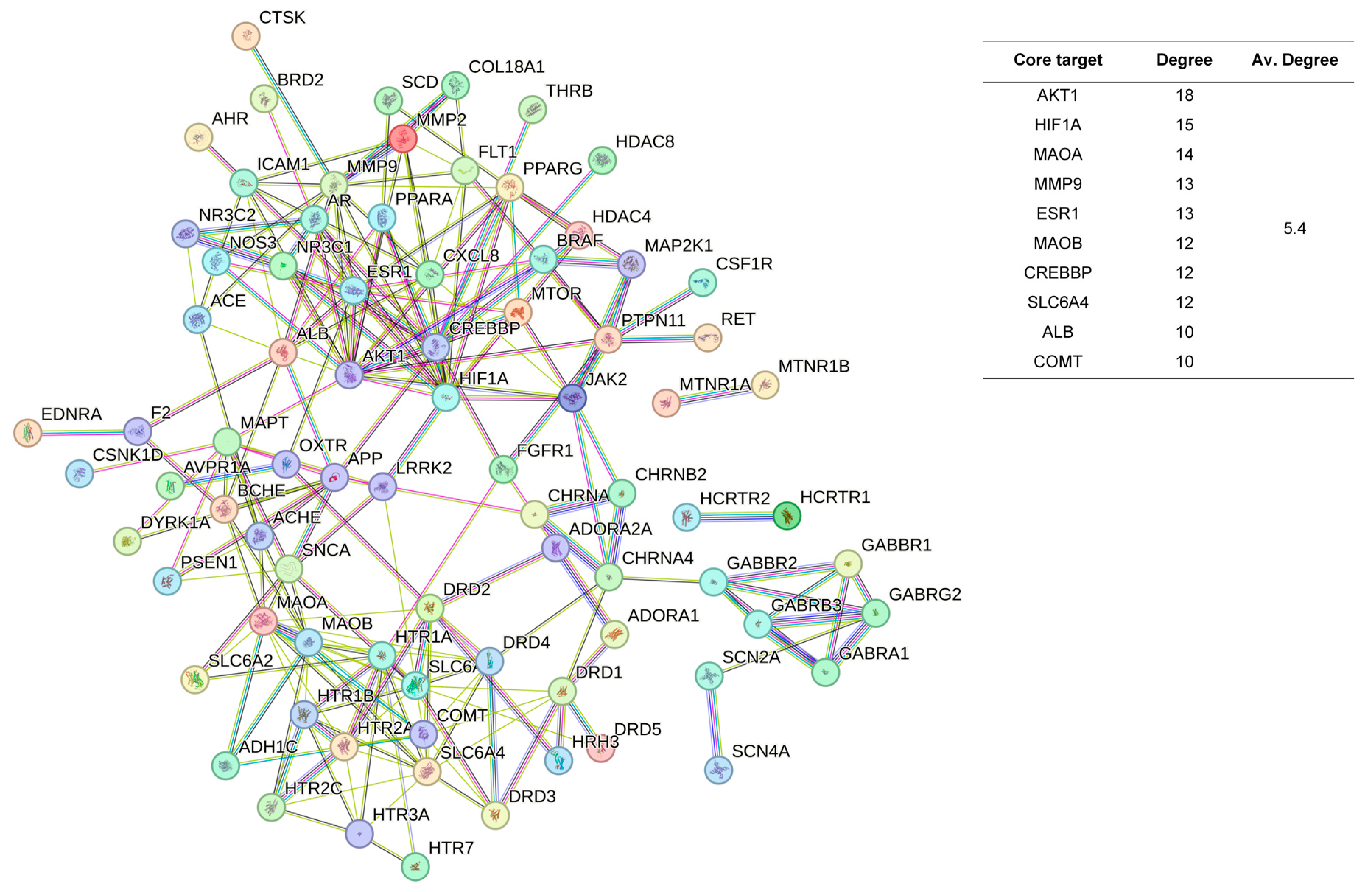
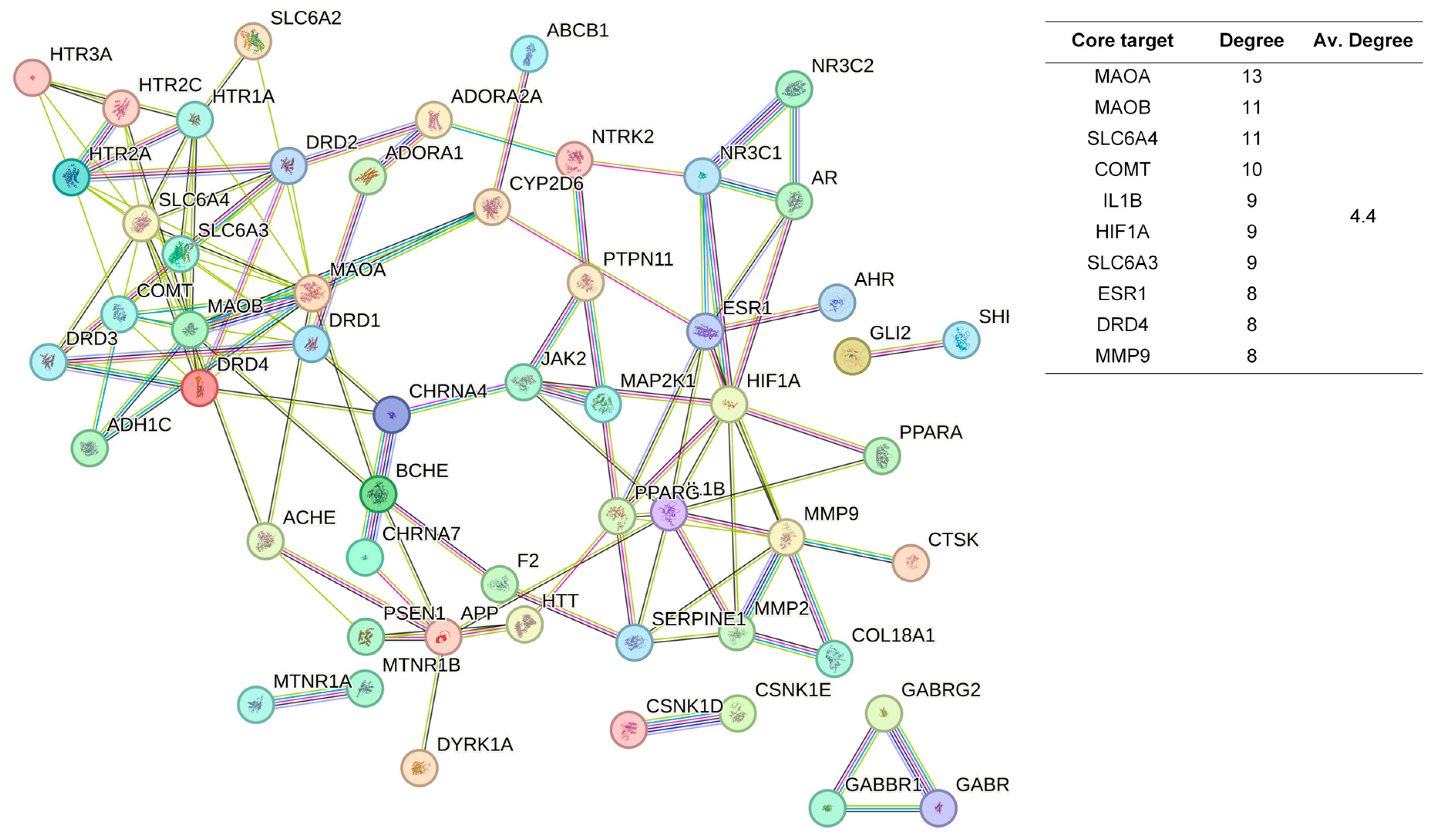
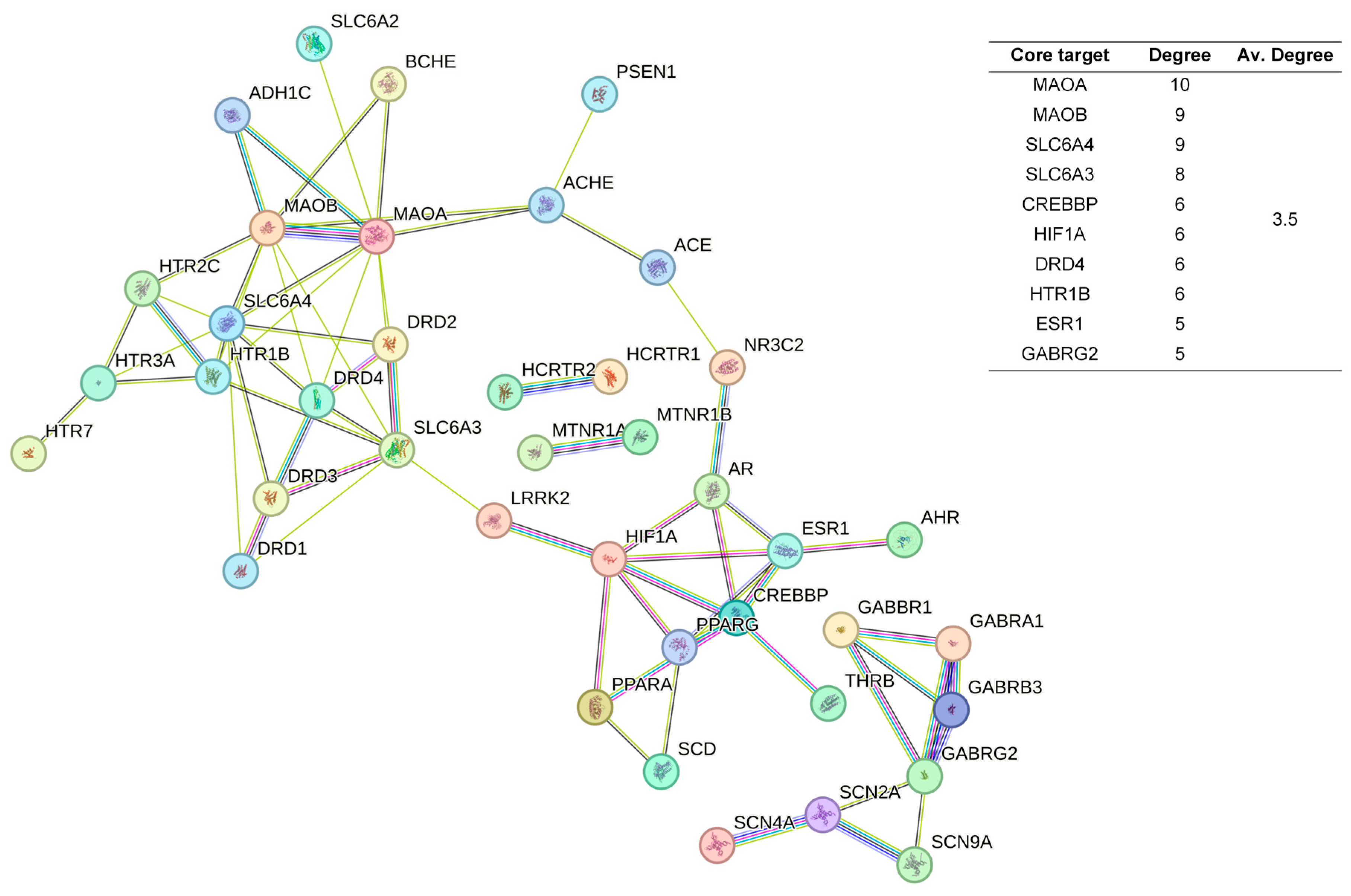
2.3. Protein-Protein Interaction Network Construction and Analysis
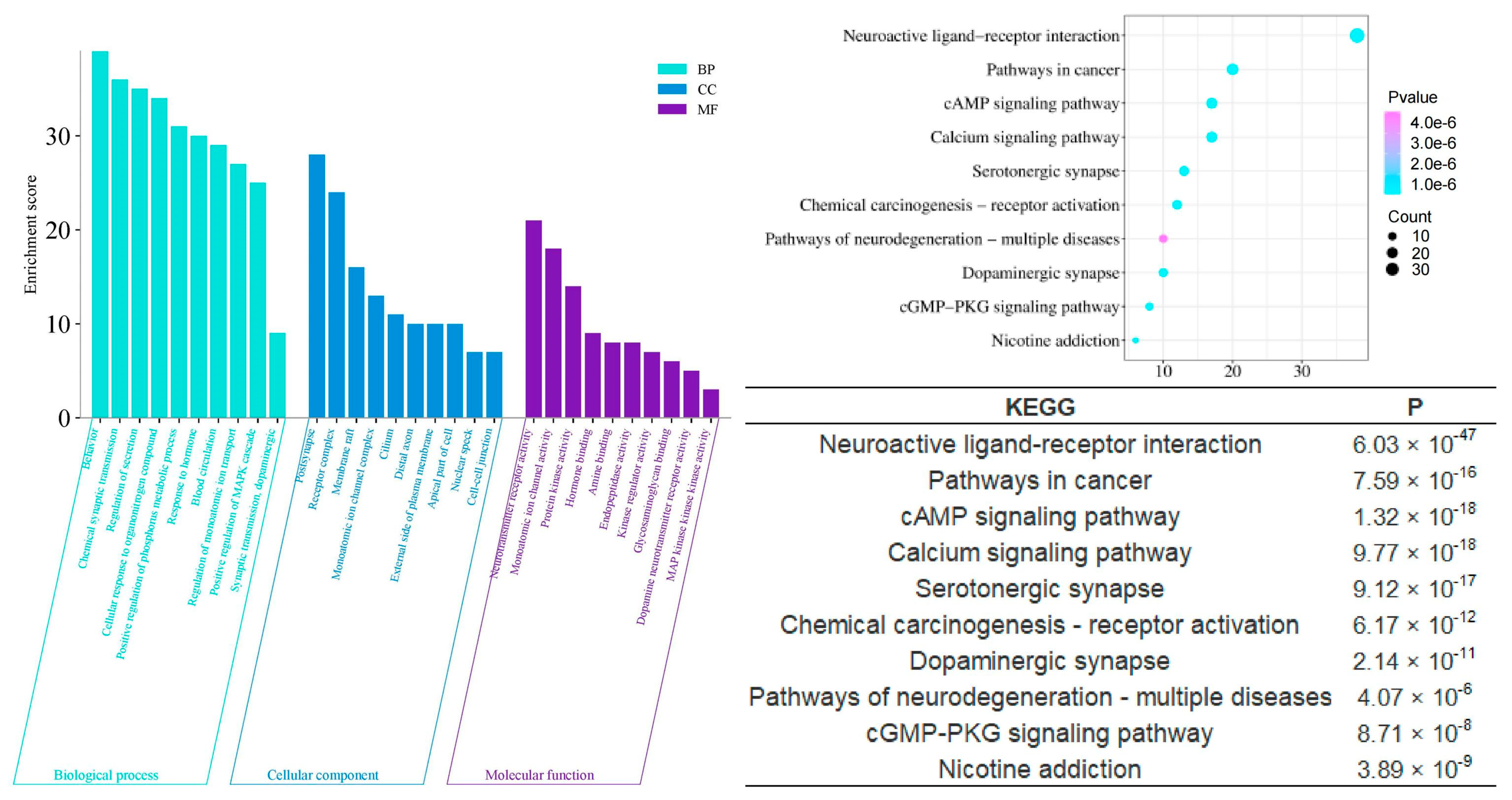
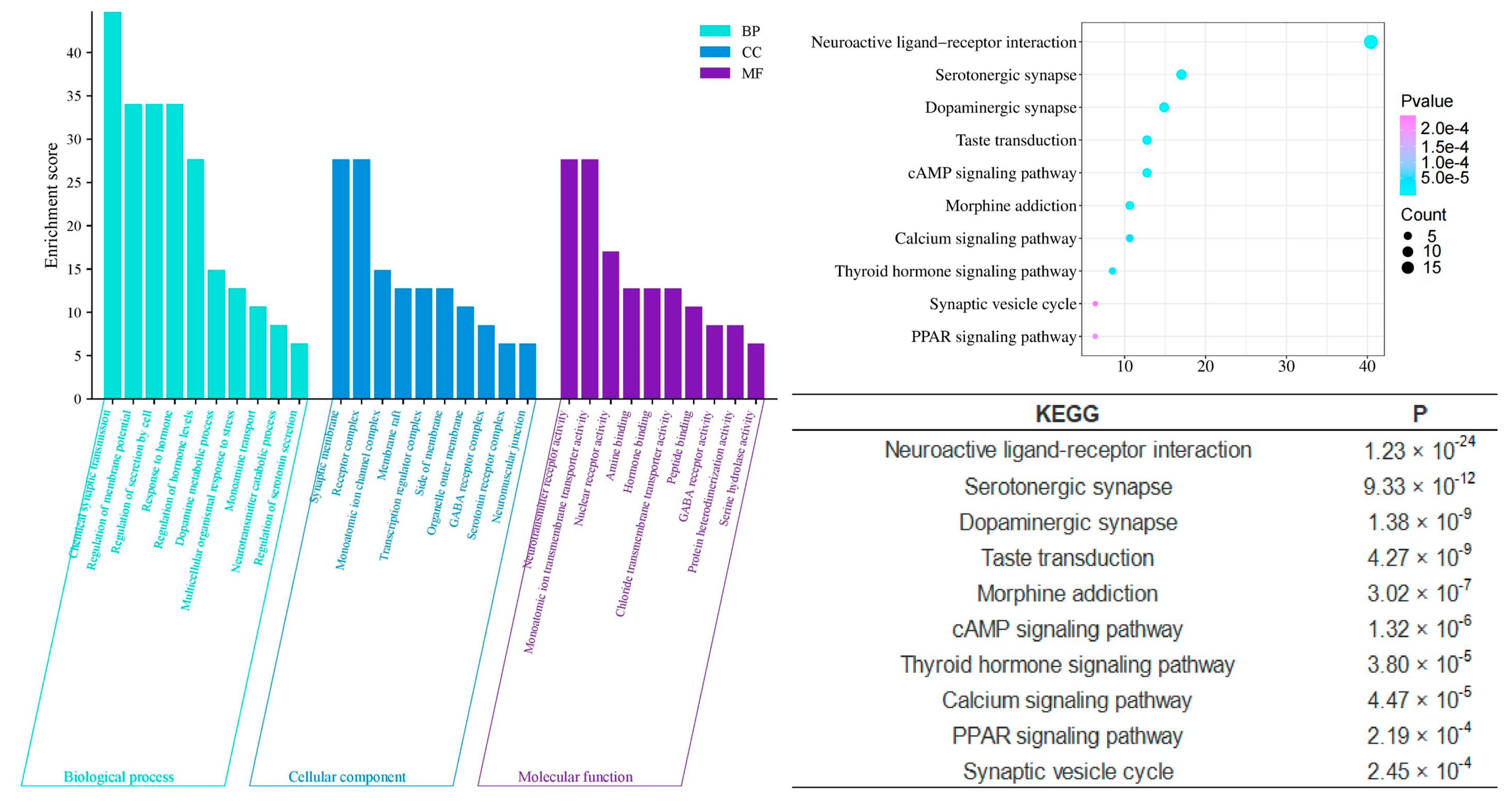
2.4. GO and KEGG Analysis
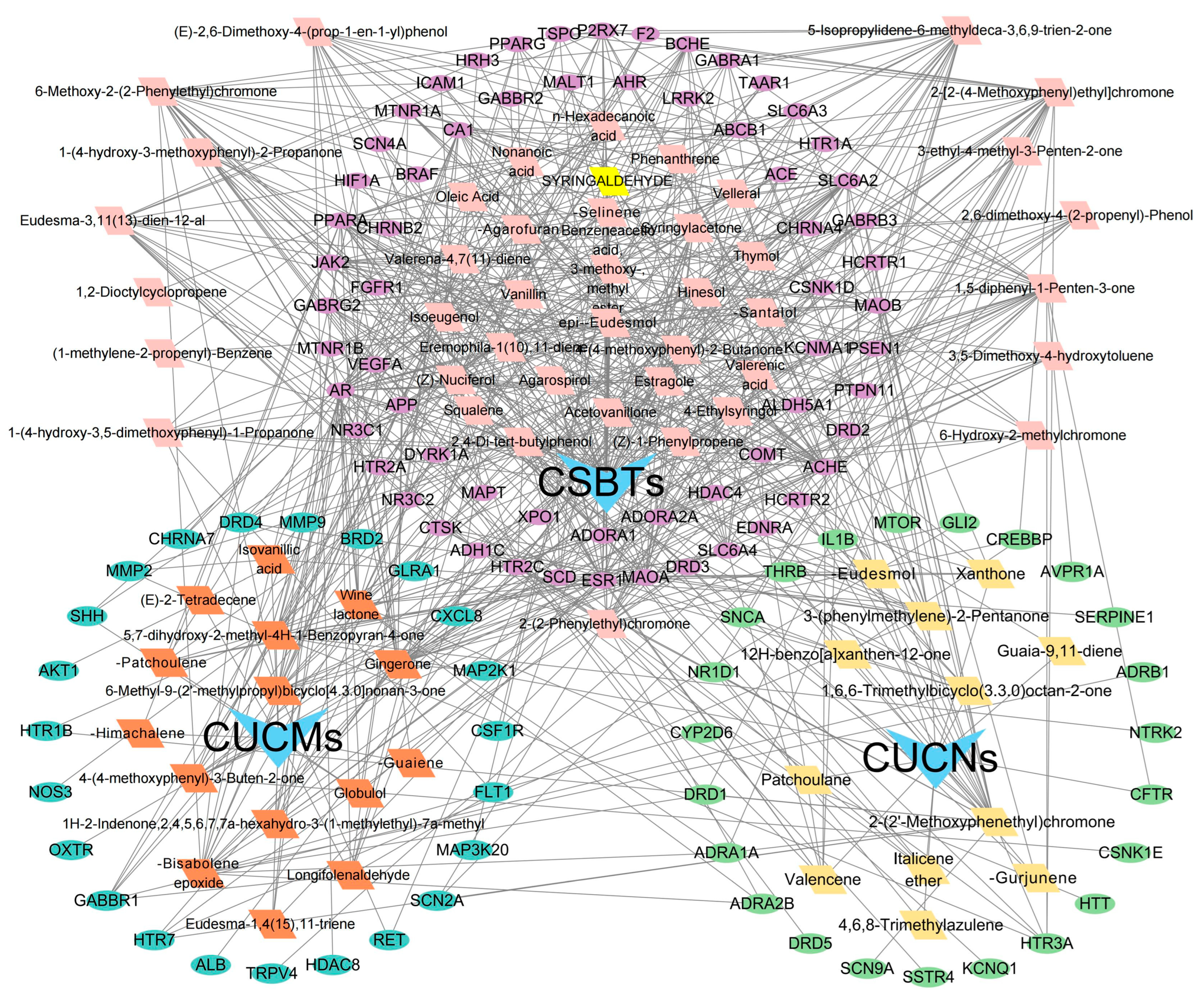
2.5. Construction of the Agarwood Aromatherapy’s ‘Active Components–Efficacy Targets–Action Pathways’ Network
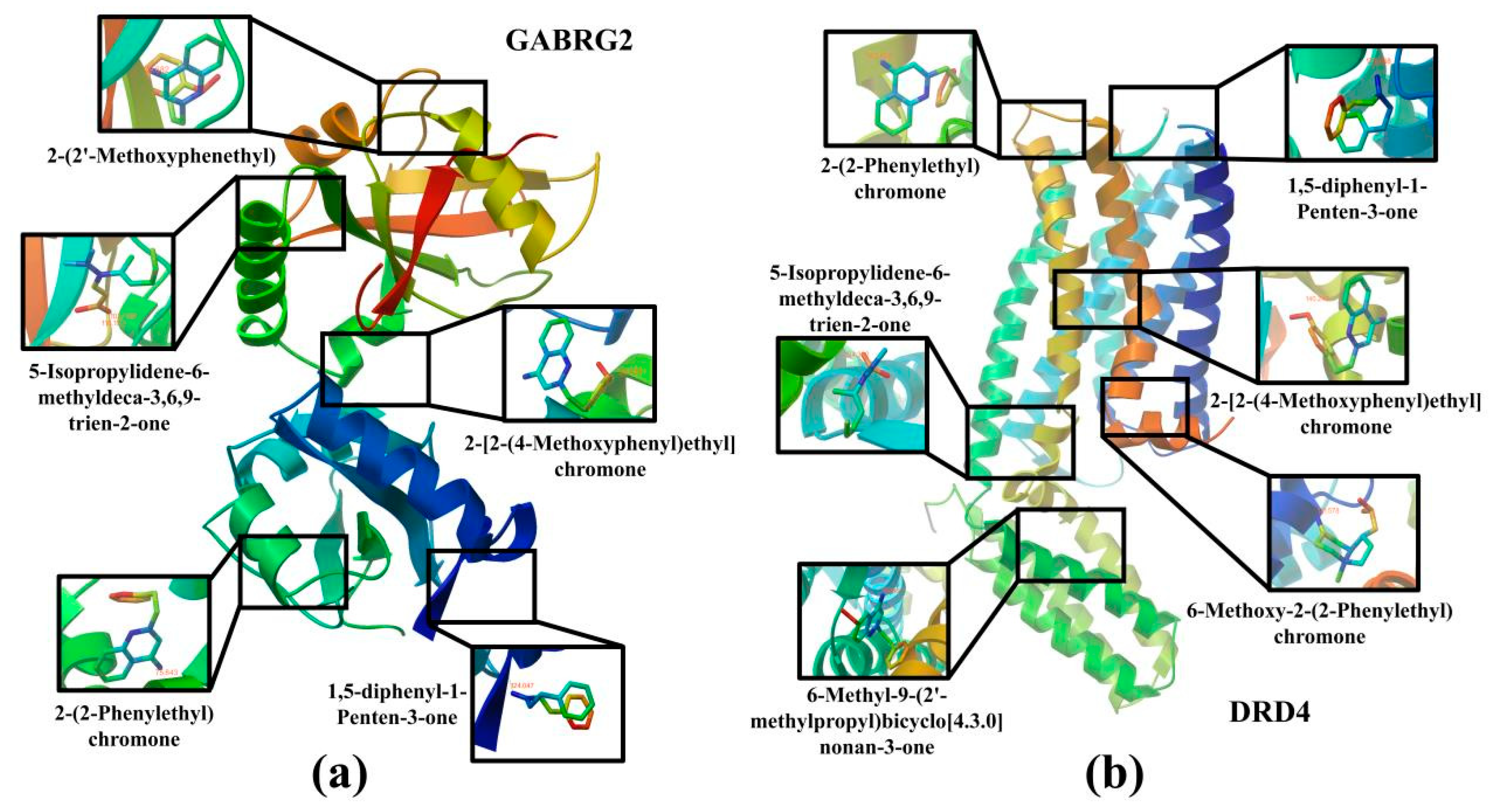
2.6. The Analysis of the Molecular Docking
3. Materials and Methods
3.1. Materials
3.1.1. Experimental Instruments
3.1.2. Experimental Materials
3.2. Activation of Adsorption-Extraction Units
3.3. Detection of Volatile Components in Agarwood Aromatherapy
3.4. GC-MS Analysis
3.4.1. Chromatographic Conditions
3.4.2. Analysis of Sesquiterpene Components
3.5. Network Pharmacology and Analysis
3.5.1. Confirmation of Active Ingredients and Prediction of Targets in Agarwood Aromatherapy
3.5.2. Construction of the Collection of Targets Related to Sleep Disorders
3.5.3. Construction and Analysis of Protein–Protein Interaction Network
3.5.4. Construction of the ‘Active Components–Efficacy Targets–Action Pathways’ Network
3.5.5. GO Functional Analysis and KEGG Pathway Enrichment Analysis
3.5.6. Molecular Docking Validation
4. Conclusions
4.1. Differences in the Chemical Composition of Agarwood Aromatherapy between Ordinary and Chi-Nan Agarwood
4.2. Differences and Similarities in the Mechanisms of Sleep-Improving Effects of Ordinary and Chi-Nan Agarwood Aromatherapies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glidewell, R.N.; Botts, E.M.; Orr, W.C. Insomnia and Anxiety. Sleep Med. Clin. 2015, 10, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, A.C.; Holmes, K.D.; Dale, L.B.; Comps-Agrar, L.; Lee, D.; Yadav, P.N.; Drysdale, L.; Poulter, M.O.; Roth, B.L.; Pin, J.P.; et al. CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat. Neurosci. 2010, 13, 622–629. [Google Scholar] [CrossRef]
- Roth, T.; Drake, C. Evolution of insomnia: Current status and future direction. Sleep Med. 2004, 5, S23–S30. [Google Scholar] [CrossRef]
- López-Sampson, A.; Page, T. History of use and trade of agarwood. Econ. Bot 2018, 72, 1988–1994. [Google Scholar] [CrossRef]
- Albert, K.D.; Landry, K.A.C.; Acafou, Y.T.; Sophie, V.; Jacques, M.; Nicolas, B.; Pierre, T.; Mathieu, P.; Brice, B.J.; Félix, T. Neuropeltis acuminata (P. Beauv.): Investigation of the Chemical Variability and In Vitro Anti-inflammatory Activity of the Leaf Essential Oil from the Ivorian Species. Molecules 2022, 27, 3759. [Google Scholar] [CrossRef]
- Yun, Y.A.N.G.; Peiwei, L.I.U.; Yong, K.A.N.G.; Xuyu, C.H.E.N.; Feifei, L.V.; Liangming, H.U.A.N.G.; Jianhe, W.E.I. Characterisation of the germplasm resources and representative germplasm of Chinese Chi-Nan agarwood in China. Fujian For. Sci. Technol. 2023, 50, 100–106. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, J.; Zhu, X.J.; Liu, Y.Y.; Chen, D.L.; Wei, J.H. Comparison of the quality of incense produced from Chinese and Southeast Asian agarwood trees using the through-body technique of agarwood production. Chin. J. Pharm. 2019, 54, 1988–1994. [Google Scholar]
- Selvi, Y.; Kandeger, A.; Boysan, M.; Akbaba, N.; Sayin, A.A.; Tekinarslan, E.; Koc, B.O.; Uygur, O.F.; Sar, V. The effects of individual biological rhythm differences on sleep quality, daytime sleepiness, and dissociative experiences. Psychiatry Res. 2017, 256, 243–248. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; He, Q.; Feng, J.; Chen, D.; Wei, J.; Liu, Y. Studies on the role of agarwood gas inhalation administration in regulating sleep in mice by affecting neurotransmitters. Pharmacol. Clin. Chin. Med. 2019, 35, 71–77. [Google Scholar] [CrossRef]
- Hiroaki, T.; Michiho, I.; Tomohiro, S.; Toru, Y.; Gisho, H. Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J. Nat. Med. 2008, 62, 41–46. [Google Scholar]
- Yanyan, W.; Boyan, D.; Qiaoyan, L.; Yingying, L.; Xiping, D.; Mingjing, Z.; Yanbing, Z.; Zedong, J.; Qingbiao, L.; Hui, N.; et al. Fermentation of waste water from agar processing with Bacillus subtilis by metabolomic analysis. Appl. Microbiol. Biotechnol. 2024, 108, 11–15. [Google Scholar]
- YangYang, W.; YaoQi, Z.; JiaXuan, X.; Xiang, Z.; ShuChang, W.; Qing, L.; LiPeng, H.; ShuHeng, J.; ShuangQin, Y.; Jia, X.; et al. MAOA suppresses the growth of gastric cancer by interacting with NDRG1 and regulating the Warburg effect through the PI3K/AKT/mTOR pathway. Cell. Oncol. 2023, 46, 1429–1444. [Google Scholar]
- Maximiliano, J.E.; Ares, I.; Martínez, M.; Torres, B.L.; Larrañaga, M.R.M.; Anadón, A.; Martínez, M.A. Dopaminergic and serotoninergic systems as preferential targets of the pyrethroid tefluthrin exposure in the rat brain. Environ. Res. 2024, 247, 118239. [Google Scholar] [CrossRef]
- Molla, M.H.R.; Asseri, A.H.; Islam, M.S. Integrated structure model-based virtual screening approaches identified anti-cancer agents against prostate cancer by targeting MAOB protein. Egypt. J. Med. Hum. Genet. 2023, 24, 51. [Google Scholar] [CrossRef]
- Ioanna, S.; Charikleia, N.; Georgia, T.; Chryssa, P.; Dimitris, G.; Evanthia, T.; Spiros, A.; Theodosios, P.; Antonis, G. Effect of common OPRM1, COMT, SLC6A4, ABCB1, and CYP2B6 polymorphisms on perioperative analgesic and propofol demands on patients subjected to thyroidectomy surgery. Pharmacol. Rep. 2023, 75, 386–396. [Google Scholar]
- de Oliveira, R.R.; Kuhn, D.; Heidrich, D.; Shansis, F.M.; Ducati, R.G.; Timmers, L.F.S. 5-HTR2B and SLC6A3 as potential molecular targets of sertraline in the treatment of major depressive disorder: The use of bioinformatics and its practical implication. Netw. Model. Anal. Health Inform. Bioinform. 2022, 11, 34. [Google Scholar] [CrossRef]
- Yin, X.; Chen, H.; Chen, S.; Zhang, S. Screening and Validation of a Carvacrol-Targeting Viability-Regulating Protein, SLC6A3, in Liver Hepatocellular Carcinoma. Dis. Markers 2022, 2022, 3736104. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, S.; Ba, S.; Dang, J.; Ren, Q.; Zhu, Y.; Liu, K.; Jin, M. Eucommia ulmoides Olive Male Flower Extracts Ameliorate Alzheimer’s Disease-Like Pathology in Zebrafish via Regulating Autophagy, Acetylcholinesterase, and the Dopamine Transporter. Front. Mol. Neurosci. 2022, 15, 901953. [Google Scholar] [CrossRef]
- May, L.; Chu, C.F.; Zielinski, C.E. Single-Cell RNA Sequencing Reveals HIF1A as a Severity-Sensitive Immunological Scar in Circulating Monocytes of Convalescent Comorbidity-Free COVID-19 Patients. Cells 2024, 13, 300. [Google Scholar] [CrossRef]
- Chaudhary, N.; Chibly, A.M.; Collier, A.; Martinalbo, J.; Moreno, P.P.; Moore, H.M.; Luhn, P.; Metcalfe, C.; Hafner, M. CDK4/6i-treated HR+/HER2− breast cancer tumors show higher ESR1 mutation prevalence and more altered genomic landscape. NPJ Breast Cancer 2024, 10, 15. [Google Scholar] [CrossRef]
- Weidler, C.; Hofhansel, L.; Regenbogen, C.; Müller, D.; Clemens, B.; Montag, C.; Reif, A.; Habel, U. The influence of the COMT Val158Met polymorphism on prefrontal TDCS effects on aggression. Sci. Rep. 2024, 14, 3437. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Tian, X.; Zhao, H.; Li, T.; Lu, L. Genome-wide characterization of COMT family and regulatory role of CsCOMT19 in melatonin synthesis in Camellia sinensis. BMC Plant Biol. 2024, 24, 51. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, L.; Xue, X.; Li, X.; Zhao, K.; Zhang, J.; Li, W.; Yao, W.; Ding, S.; Jia, C.; et al. Captive ERVWE1 triggers impairment of 5-HT neuronal plasticity in the first-episode schizophrenia by post-transcriptional activation of HTR1B in ALKBH5-m6A dependent epigenetic mechanisms. Cell Biosci. 2023, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Mandelenaki, D.; Juvené, E.; Lederer, D.; Aeby, A. Precision medicine: Vinpocetine as a potential treatment for GABRG2-related epilepsy. Epileptic Disord. 2023, 25, 383–389. [Google Scholar] [CrossRef]
- Sukrit, P.; Chairat, T.; Mon, O.E.; Ladawan, K.; Panapat, U.; Supin, C. Germinated brown rice protects against glutamate toxicity in HT22 hippocampal neurons through the jnk-mediated apoptotic pathway via the GABAA receptor. IBRO Neurosci. Rep. 2023, 14, 38–49. [Google Scholar]
- Zhao, Y.; Kehui, S.; Shaobo, H. Plasticity mechanism and memory formation in the chemical synapse. Nonlinear Dyn. 2023, 111, 19411–19423. [Google Scholar]
- Taotao, L.; Wenfei, W.; Qiuting, G.; Jia, L.; Tiantian, T.; Yujiao, W.; Ding, L.; Kai, Y.; Jiayi, L.; Kaixue, D.; et al. Rosemary (Rosmarinus officinalis L.) hydrosol based on serotonergic synapse for insomnia. J. Ethnopharmacol. 2023, 318, 116984. [Google Scholar]
- Huang, S.; Zhang, C.; Xie, X.; Zhu, Y.; Song, Q.; Ye, L.; Hu, Y. GRID2 aberration leads to disturbance in neuroactive ligand-receptor interactions via changes to the species richness and composition of gut microbes. Biochem. Biophys. Res. Commun. 2022, 631, 9–17. [Google Scholar] [CrossRef]
- Kuschke, S.; Thon, S.; Sattler, C.; Schwabe, T.; Benndorf, K.; Schmauder, R. cAMP binding to closed pacemaker ion channels is cooperative. Proc. Natl. Acad. Sci. USA 2024, 121, e2315132121. [Google Scholar] [CrossRef]
- Li, W.; He, H.; Du, M.; Gao, M.; Sun, Q.; Wang, Y.; Lu, H.; Ou, S.; Xia, C.; Xu, C.; et al. Quercetin as a promising intervention for rat osteoarthritis by decreasing M1-polarized macrophages via blocking the TRPV1-mediated P2X7/NLRP3 signaling pathway. Phytother. Res. 2024, 38, 1990–2006. [Google Scholar] [CrossRef]
- Gorobets, O.; Gorobets, S.; Polyakova, T.; Zablotskii, V. Modulation of calcium signaling and metabolic pathways in endothelial cells with magnetic fields. Nanoscale Adv. 2024, 6, 1163–1182. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Li, P.; Shen, Y.; Manaenko, A.; Yang, W.; Wang, P.; Li, X.; Liu, F.; Xie, P.; Li, Q. Multi-time point metabolomics reveals key metabolic features from the ultra-early stage of intracerebral hemorrhage in mice. Exp. Neurol. 2023, 368, 114507. [Google Scholar] [CrossRef] [PubMed]
- Shishkov, A.G.; Nifantova, N.V.; Korenkova, O.M.; Sopova, E.S.; Brodin, L.; Shupliakov, O. BAR Domain Proteins as Putative Regulators of the Protein Liquid Phase in Nerve Terminals in the Central Nervous System. Biochem. Suppl. Ser. A Membr. Cell Biol. 2023, 17, 69–82. [Google Scholar]
- Wang, B.; Zhang, X.; Li, Z.S.; Wei, C.; Yu, R.Z.; Du, X.Z.; He, Y.J.; Ren, Y.; Zhen, Y.W.; Han, L. Polo-like kinase 4 promotes tumorigenesis and glucose metabolism in glioma by activating AKT1 signaling. Cancer Lett. 2024, 585, 216665. [Google Scholar] [CrossRef]
- Shinde, D.; Bhat, S.K.; Ganesh, C.B. The opioid peptide β-endorphin interferes with the pituitary-testis axis in the Mozambique tilapia Oreochromis mossambicus. Fish Physiol. Biochem. 2024, 50, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Bühler, J.; Messmer, F.; Bruckmaier, R.; Aybek, S. Cortisol in functional neurological disorders: State, trait and prognostic biomarkers. J. Psychosom. Res. 2024, 179, 111615. [Google Scholar] [CrossRef]
- Malkani, R. Rapid Eye Movement Sleep Behavior Disorder: Management and Prognostic Counseling. Sleep Med. Clin. 2024, 19, 83–92. [Google Scholar] [CrossRef]
- Qiu, C.W.; Richmond, M.; Ma, Y.; Zhang, S.; Liu, W.; Feng, X.; Ahmed, I.M.; Wu, F. Melatonin enhances cadmium tolerance in rice via long non-coding RNA-mediated modulation of cell wall and photosynthesis. J. Hazard. Mater. 2024, 465, 133251. [Google Scholar] [CrossRef]
- Ting, L.; Juan, W.; Ronghao, Z.; Can, G.; Xiao, L.; Lixiang, L.; Yingwen, L.; Zhencong, G.; Weijia, W.; Ming, H. Therapeutic targets and molecular mechanisms of Huangqin decoction in liver cancer: A network pharmacology and molecular docking approach. J. Herb. Med. 2024, 43, 100822. [Google Scholar]
- Ryuta, J.; ShinIchi, A.; Masanori, T.; KenIchi, H. SLC6A and SLC16A family of transporters: Contribution to transport of creatine and creatine precursors in creatine biosynthesis and distribution. Biochim. Et Biophys. Acta. Biomembr. 2021, 1864, 183840. [Google Scholar]
- Zhang, B.; Shen, J. Dexmedetomidine activates the PKA/CREB pathway and inhibits proinflammatory factor expression through β2 adrenergic receptors. Immun. Inflamm. Dis. 2024, 12, e1176. [Google Scholar] [CrossRef]
- Ren, P.; Wang, J.Y.; Chen, H.L.; Wang, Y.; Cui, L.Y.; Duan, J.Y.; Guo, W.Z.; Zhao, Y.Q.; Li, Y.F. Activation of σ-1 receptor mitigates estrogen withdrawal-induced anxiety/depressive-like behavior in mice via restoration of GABA/glutamate signaling and neuroplasticity in the hippocampus. J. Pharmacol. Sci. 2024, 154, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bansal, P.; Saini, L.; Sharma, N.; Dhingra, R. Zuranolone, a neuroactive drug, used in the treatment of postpartum depression by modulation of GABAA receptors. Pharmacol. Biochem. Behav. 2024, 238, 173734. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-X.; Fan, J.-J.; Xu, L.-L.; Yu, R.; Kuang, Y.; Chai, Y.; Zheng, R.; Zhang, X.-Y.; Shang, H.-C.; Qiao, X.; et al. Network Pharmacology-Based Dissection of the Bioactive Compounds and Pharmacological Mechanisms of Yiqi Fumai Lyophilized Injection for the Treatment of Heart Failure. World J. Tradit. Chin. Med. 2024, 10, 75–82. [Google Scholar] [CrossRef]
- Girdhar, N.; Yadav, V.; Kumari, N.; Subbarao, N.; Krishnamachari, A. Insilico screening to identify novel inhibitors targeting 30S ribosomal protein S12 in meningitis-causing organism ‘Elizabethkingia meningoseptica’. J. Biomol. Struct. Dyn. 2024, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Li, S.; Xu, C.; XiaoLi, Z. Exploring the mechanism underlying the therapeutic effects of butein in colorectal cancer using network pharmacology and single-cell RNA sequencing data. J. Gene Med. 2023, 26, e3628. [Google Scholar]
- Tanner, A.; Sagoo, M.S.; Mahroo, O.A.; Pulido, J.S. Genetic analysis of ocular tumour-associated genes using large genomic datasets: Insights into selection constraints and variant representation in the population. BMJ Open Ophthalmol. 2024, 9, e001565. [Google Scholar] [CrossRef]
- Yong, L.; Yong, F.; Taifa, T.; Jun, H.; Fang, L.; Zhanchen, L.; Jian, P. Screening of lncRNA-miRNA-mRNA Coexpression Regulatory Networks Involved in Acute Traumatic Coagulation Dysfunction Based on CTD, GeneCards, and PharmGKB Databases. Oxidative Med. Cell. Longev. 2022, 2022, 7280312. [Google Scholar]
- David, O.; Andrew, H.; Miguel, C.; Daniel, S.; Jarrod, B.; Cinzia, M.; Irene, L.; Alfredo, M.; Carlos, C.; Luca, F.; et al. The next-generation Open Targets Platform: Reimagined, redesigned, rebuilt. Nucleic Acids Res. 2022, 51, D1353–D1359. [Google Scholar]
- Wang, L.; Wang, C.; Li, L.; Zhou, X.; Hua, X.; Yuan, X. Analysis of the Molecular Mechanism of Xueshuantong in the Treatment of Wet Age-Related Macular Degeneration (AMD) Using GEO Datasets, Network Pharmacology, and Molecular Docking. Biochem. Genet. 2024, 1–18. [Google Scholar] [CrossRef]
- Dawkins, M.; Cheung, N.; Rozenblit, G.; Wolf, D.C. Intrahepatic Arterioportal Fistula With Subsequent Portal Hypertension After Percutaneous Liver Biopsy. ACG Case Rep. J. 2024, 11, e01287. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Liang, W.; Mo, C.; Hou, X.; Ou, M. Multi-dimensional analysis reveals NCKAP5L is a promising biomarker for the diagnosis and prognosis of human cancers, especially colorectal cancer. Oncol. Lett. 2024, 27, 53. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, S.E.; Vaishampayan, P.; Jandova, J.; Ai, Y.E.; Kirschnerova, V.; Zhang, T.; Calvert, V.; Petricoin, E.; Chow, H.H.S.; Hu, C.; et al. Inhibition of UV-Induced Stress Signaling and Inflammatory Responses in SKH-1 Mouse Skin by Topical Small-Molecule PD-L1 Blockade. JID Innov. Ski. Sci. Mol. Popul. Health 2024, 4, 100255. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yuan, N.; Chen, Y.; Ma, Y.; Chen, A.; Wang, F.; Yan, S.; He, Z.e.; He, J.; Zhang, C.; et al. SKP alleviates the ferroptosis in diabetic kidney disease through suppression of HIF-1α/HO-1 pathway based on network pharmacology analysis and experimental validation. Chin. Med. 2024, 19, 31. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, L.; Lin, H.; Cui, W.; Jiao, Y.; Wang, B.; Li, H.; Wang, X.; Wu, J. Network pharmacology and experimental validation to investigate the mechanism of Nao-Ling-Su capsule in the treatment of Ischemia/reperfusion-induced acute kidney injury. J. Ethnopharmacol. 2024, 326, 117958. [Google Scholar] [CrossRef]
- Aso, K.; Gohda, Y.; Hotta, M.; Minamimoto, R.; Shimizu, Y.; Uemura, Y.; Yano, H. Clinical Effectiveness of Preoperative 18F-FDG PET/CT in Predicting Pathological Tumor Grade in Patients with Pseudomyxoma Peritonei Originating from Appendix: A Retrospective Cohort Study. Ann. Surg. Oncol. 2024, 31, 1990–1995. [Google Scholar] [CrossRef]
- Xie, D.; Quan, J.; Yu, X.; Liang, Z.; Chen, Y.; Wu, L.; Lin, L.; Fan, L. Molecular mechanism of Jianpiyifei II granules in the treatment of chronic obstructive pulmonary disease: Network pharmacology analysis, molecular docking, and experimental assessment. Phytomedicine 2024, 126, 155273. [Google Scholar] [CrossRef]
- Kleywegt, G.J.; Adams, P.D.; Butcher, S.J.; Lawson, C.L.; Rohou, A.; Rosenthal, P.B.; Subramaniam, S.; Topf, M.; Abbott, S.; Baldwin, P.R.; et al. Community recommendations on cryoEM data archiving and validation. IUCrJ 2024, 11, 140–151. [Google Scholar] [CrossRef]
- Mboto, C.I.; Edet, U.O.; Mbim, E.N.; Ndifon, W.O.; Ebenso, E.E.; Egharevba, H.O.; George, U.E.; Nwaokorie, F.O.; Udo, S.I. In- silico evaluation of bioactive compounds from selected medicinal plants from Southern Nigeria against hepatitis C virus genotype 1 RNA-directed RNA polymerase. Sci. Afr. 2023, 22, e01919. [Google Scholar] [CrossRef]
- Siripoltangman, N.; Chickos, J. Vapor pressure and vaporization enthalpy studies of the major components of ginger, α-zingiberene, β-sesquiphellandrene and (−) ar curcumene by correlation gas chromatography. J. Chem. Thermodyn. 2019, 138, 107–115. [Google Scholar] [CrossRef]
- Narjara, S.d.S.G.; Patricia, P.; Fabiane, R.; Rosmari, H.; Sydney, H.A.; Augusto, M.C.; Maria, H.B. Antimicrobial evaluation of sesquiterpene alpha-curcumene and its synergism with imipenem. J. Microbiol. Biotechnol. Food Sci. 2015, 04, 434–436. [Google Scholar] [CrossRef]
- Tu, H.; Wang, W.; Feng, Y.; Zhang, L.; Zhou, H.; Cheng, C.; Ji, L.; Cai, Q.; Feng, Y. β-Patchoulene represses hypoxia-induced proliferation and epithelial-mesenchymal transition of liver cancer cells. Bioengineered 2022, 13, 11907–11922. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, Y.; Li, Z.; Zhang, Y.; He, S.; Zhang, Z.; Leong, S.; Wong, A.; Zhang, C.Y. Combining Metabolic Engineering and Lipid Droplet Storage Engineering for Improved α-Bisabolene Production in Yarrowia Lipolytica. J. Agric. Food Chem. 2023, 71, 11534–11543. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, S. In Vitro Antithrombotic, Hematological Toxicity, and Inhibitor Studies of Protocatechuic, Isovanillic, and p-Hydroxybenzoic Acids from Maclura tricuspidata (Carr.) Bur. Molecules 2022, 27, 3496. [Google Scholar] [CrossRef]
- Mileski, K.S.; Trifunović, S.S.; Ciric, A.D.; Sakic, Z.M.; Ristic, M.S.; Todorović, N.M.; Matevski, V.S.; Marin, P.D.; Tešević, V.V.; Dzamic, A.M. Research on Chemical Composition and Biological Properties Including Antiquorum Sensing Activity of Angelica pancicii Vandas Aerial Parts and Roots. J. Agric. Food Chem. 2017, 65, 10933–10949. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, X.; Luo, X.; Gao, Y.; Zhao, R.; Li, J.; Cai, H.; Dang, X.; Ye, X.; Bai, R.; et al. Discovery and bioassay of disubstituted β-elemene-NO donor conjugates: Synergistic enhancement in the treatment of leukemia. Chin. J. Nat. Med. 2023, 21, 916–926. [Google Scholar] [CrossRef]
- Kai, T.; Changhui, Z.; Zuomei, H.; Puhua, Z. Construction of an anoikis-associated lncRNA-miRNA-mRNA network reveals the prognostic role of β-elemene in non-small cell lung cancer. Sci. Rep. 2023, 13, 20185. [Google Scholar]
- Hiroyuki, M.; YuanE, L.; ShePo, S. Identification of a diarylpentanoid-producing polyketide synthase in the biosynthesis of 2-(2-phenylethyl)chromones in agarwood. J. Nat. Med. 2023, 77, 667–676. [Google Scholar]
- Meng, Y.; QingQin, H.; XiQin, C.; Jian, F.; JianHe, W.; Yangyang, L. Chemical and bioactivity diversity of 2-(2-phenylethyl)chromones in agarwood: A review. Chem. Biodivers. 2022, 19, e202200490. [Google Scholar]
- Faingold, I.I.; Smolina, A.V.; Soldatova, Y.V.; Poletaeva, D.A.; Balakina, A.A.; Sashenkova, T.E.; Allayarova, U.Y.; Prikhodchenko, T.R.; Blokhina, S.V.; Makartseva, L.A.; et al. Cardioprotective Effect of 2-Ethyl-3-Hydroxy-6-Methylpyridinium 2-Nitroxysuccinate Against Adrenaline/Hydrocortisone-Induced Myocardial Ischemia in Mice: Modulation of Free-Radical Processes in Biomembranes and Monoamine Oxidase A Activity. Cell Biochem. Biophys. 2024, 82, 235–245. [Google Scholar] [CrossRef]
- Bringas, T.I.N.; Manrique, C.M.J.; Huerta, N.C.G.; Hernández, E.M.; Duarte, A.M. COMT and SCN9A gene variants do not contribute to chronic low back pain in Mexican-Mestizo patients. Acta Neurochir. 2024, 166, 73. [Google Scholar] [CrossRef] [PubMed]
- Kiros, H.; Won, Y.J. Dopamine receptor D4 (DRD4) negatively regulates UCP1- and ATP-dependent thermogenesis in 3T3-L1 adipocytes and C2C12 muscle cells. Pflug. Arch. Eur. J. Physiol. 2023, 475, 757–773. [Google Scholar]
- My, T.T.A.; Loan, H.T.P.; Hai, N.T.T.; Hieu, L.T.; Hoa, T.T.; Thuy, B.T.P.; Quang, D.T.; Triet, N.T.; Anh, T.T.V.; Dieu, N.T.X.; et al. Evaluation of the Inhibitory Activities of COVID-19 of Melaleuca cajuputi Oil Using Docking Simulation. ChemistrySelect 2020, 5, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Chen, M.; Yi, H.; Hanqing, X.; Yunkun, Q.; Yingguang, W.; Yunhui, F.; Xiaojian, H.; Hongbo, Y. An in vitro and in vivo study: Valencene protects cartilage and alleviates the progression of osteoarthritis by anti-oxidative stress and anti-inflammatory effects. Int. Immunopharmacol. 2023, 123, 110726. [Google Scholar]
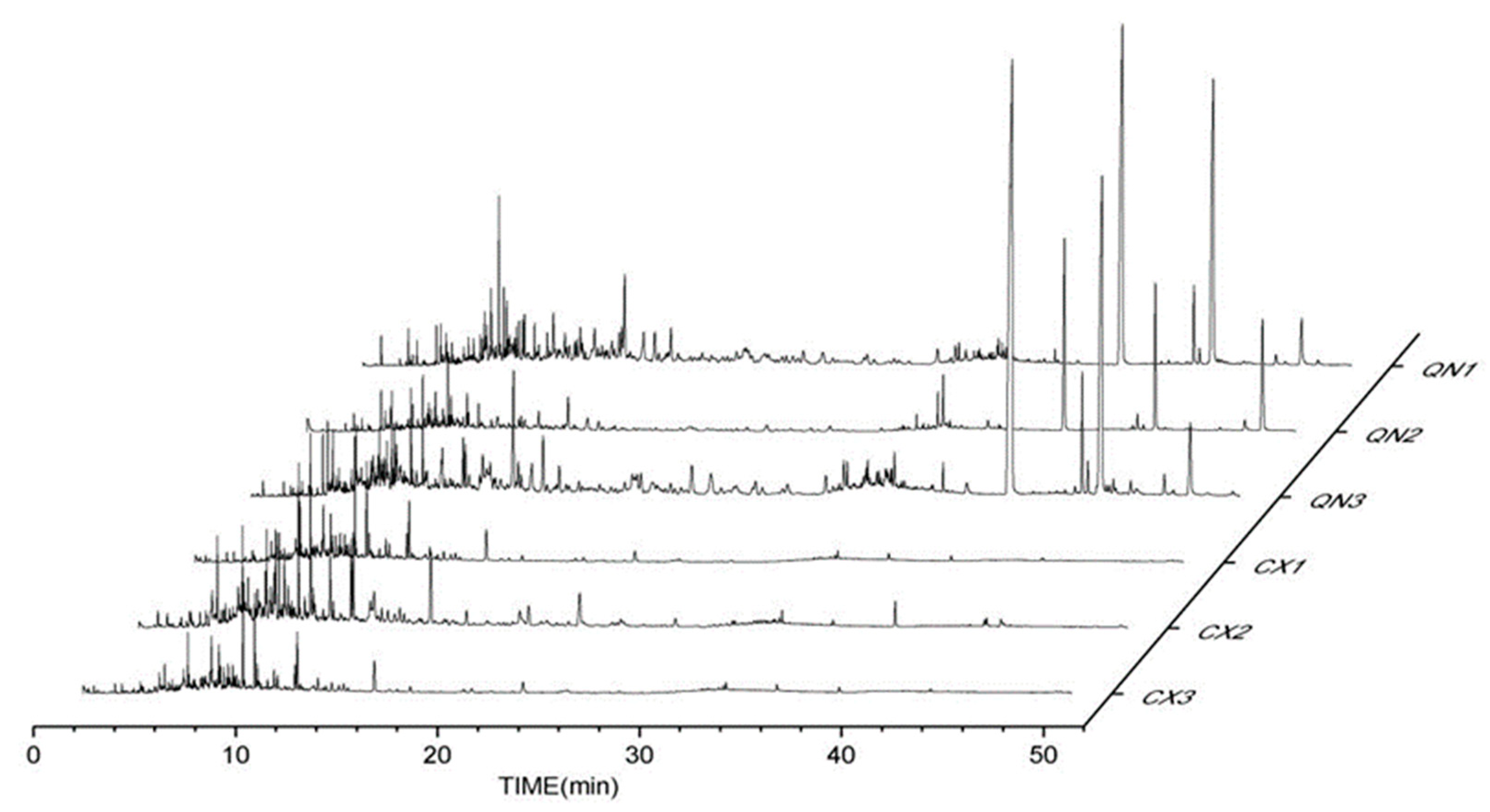
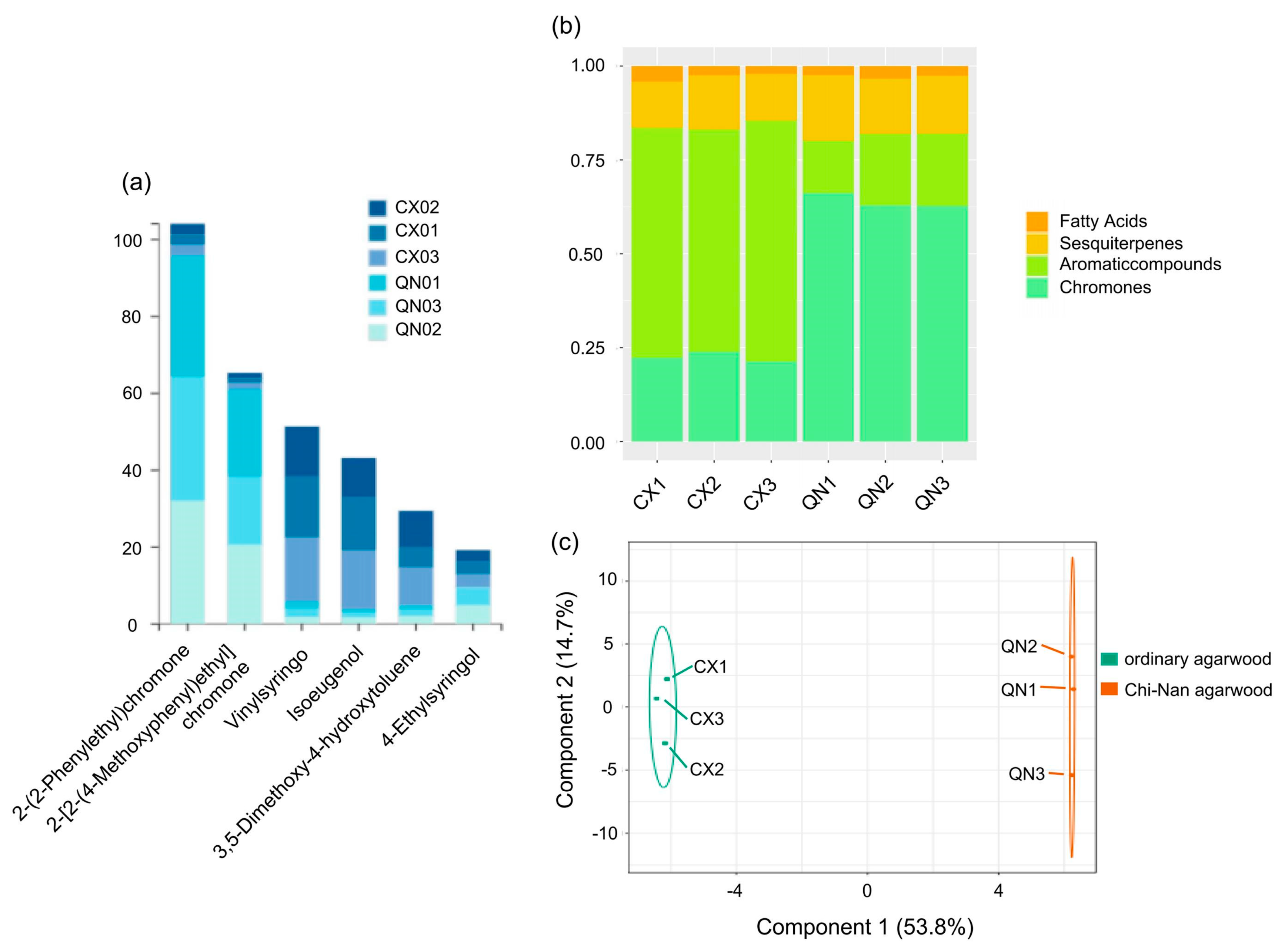

| No. | Compounds | Chemical Formula | MW | Relative Percentage of Agarwood Aromatherapy Compounds (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| CX01 | CX02 | CX03 | QN01 | QN02 | QN03 | ||||
| 1 | α-Agarofuran | C15H24O | 220.35 | 1.24 | 1.53 | 1.37 | 2.57 | 2.52 | 1.88 |
| 2 | α-Santalol | C15H24O | 220.35 | 1.27 | 1.13 | 1.27 | 1.83 | 0.81 | 1.11 |
| 3 | epi-γ-Eudesmol | C15H26O | 222.37 | 1.18 | 0.96 | 0.81 | 0.41 | 0.83 | 0.63 |
| 4 | Agarospirol | C15H26O | 222.37 | 0.34 | 0.65 | 0.26 | 0.46 | 0.23 | 0.30 |
| 5 | Hinesol | C15H26O | 222.37 | 0.12 | 0.19 | 0.17 | 0.39 | 0.28 | 0.28 |
| 6 | β-Gurjunene | C15H24 | 204.35 | 1.53 | 2.19 | 1.07 | 1.05 | 0.58 | 0.65 |
| 7 | Eudesma-3,11(13)-dien-12-al | C15H22O | 218.33 | 0.96 | - | - | 0.57 | - | 0.30 |
| 8 | α-Selinene | C15H24 | 204.35 | 0.46 | 0.54 | 0.44 | 0.31 | 0.44 | 0.56 |
| 9 | Longifolene | C15H24 | 204.35 | 0.90 | 1.11 | 0.94 | 0.65 | 0.37 | 0.54 |
| 10 | Aromandendrene | C15H24 | 204.35 | 0.40 | 0.46 | 0.64 | 0.28 | 0.37 | 0.60 |
| 11 | Alloaromadendrene | C15H24 | 204.35 | 1.27 | 0.96 | 0.47 | 0.98 | 0.49 | 0.75 |
| 12 | Valerenic acid | C15H22O2 | 234.33 | 0.68 | 0.69 | 0.68 | 0.41 | 0.49 | 0.81 |
| 13 | Velleral | C15H20O2 | 232.32 | 1.61 | 0.19 | - | 0.78 | - | 0.42 |
| 14 | (Z)-Nuciferol | C15H22O2 | 260.39 | 0.40 | 1.30 | - | 0.39 | 0.56 | 0.51 |
| 15 | Eremophila-1(10),11-diene | C15H24 | 204.35 | 0.86 | 1.21 | 1.12 | 0.79 | 0.76 | 1.44 |
| 16 | Valerena-4,7(11)-diene | C15H24 | 204.35 | 0.65 | 1.07 | 1.46 | 0.17 | 0.19 | 0.11 |
| 17 | (4ar-cis)-2(3H)-Naphthalenone 4,4a,5,6,7,8-hexahydro-4a,5-dimethyl-3-(1-methylethylidene) | C15H22O | 218.33 | - | 0.38 | 0.51 | 0.54 | 0.62 | 0.84 |
| 18 | Hydroquinone | C6H6O2 | 110.11 | 1.18 | 0.69 | 1.11 | 0.65 | - | 0.74 |
| 19 | (1-methylene-2-propenyl)-Benzene | C10H10 | 130.19 | 0.56 | 0.84 | 0.72 | 1.38 | 1.39 | 0.54 |
| 20 | Vanillin | C8H8O3 | 152.15 | 3.84 | - | 4.10 | 2.64 | 2.85 | 2.21 |
| 21 | (Z)-1-Phenylpropene | C9H10 | 118.18 | - | 0.84 | - | - | - | 0.25 |
| 22 | Estragole | C10H12O | 148.20 | - | 0.42 | - | - | - | 0.93 |
| 23 | 3,5-Dimethoxy-4-hydroxytoluene | C9H12O3 | 168.19 | 5.14 | 9.55 | 9.79 | 1.22 | 2.04 | 1.67 |
| 24 | Isoeugenol | C10H12O2 | 164.20 | 13.99 | 10.12 | 15.15 | 1.01 | 1.71 | 1.23 |
| 25 | Acetovanillone | C9H10O3 | 166.17 | 4.80 | 7.44 | 5.22 | 0.78 | 0.72 | 1.81 |
| 26 | 4-(4-methoxyphenyl)-2-Butanone | C11H14O2 | 178.23 | 2.35 | 2.42 | 2.65 | - | - | 0.46 |
| 27 | 2,4-Di-tert-butylphenol | C14H22O | 206.32 | 1.65 | - | 2.53 | 1.68 | 1.09 | 1.39 |
| 28 | 4-Ethylsyringol | C10H14O3 | 182.22 | 3.31 | 2.91 | 3.93 | - | 4.88 | 4.69 |
| 29 | 1-(4-hydroxy-3-methoxyphenyl)-2-Propanone | C10H12O3 | 180.20 | 1.42 | 1.92 | 1.62 | 0.42 | - | 0.42 |
| 30 | Nonanoic acid | C9H18O2 | 158.24 | 1.23 | 0.96 | - | 0.35 | - | - |
| 31 | Vinylsyringo | C10H12O3 | 180.20 | 15.86 | 13.04 | 16.55 | 1.94 | 1.85 | 2.16 |
| 32 | 5-Isopropylidene-6-methyldeca-3,6,9-trien-2-one | C14H20O | 204.31 | 0.27 | - | 0.49 | 0.54 | 0.60 | - |
| 33 | Benzeneacetic acid, 3-methoxy-, methyl ester | C10H12O3 | 180.20 | 0.10 | - | - | 0.50 | 0.51 | 0.39 |
| 34 | 2,6-dimethoxy-4-(2-propenyl)-Phenol | C11H14O3 | 194.23 | 2.77 | 2.88 | 2.06 | 0.41 | 0.79 | 0.81 |
| 35 | (E)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol | C11H14O3 | 194.23 | - | 5.18 | 1.97 | 1.18 | - | 0.91 |
| 36 | SYRINGALDEHYDE | C7H6O2 | 182.17 | 3.72 | 4.87 | 2.99 | 0.61 | - | 0.98 |
| 37 | Thymol | C10H14O | 150.22 | 0.47 | 0.33 | 0.36 | 0.74 | 0.74 | 0.65 |
| 38 | Phenanthrene | C14H10 | 178.23 | - | 0.11 | 0.13 | 0.44 | - | - |
| 39 | Syringylacetone | C11H14O4 | 224.25 | 1.27 | 2.22 | 1.17 | 0.37 | 0.21 | 0.63 |
| 40 | 3-ethyl-4-methyl-3-Penten-2-one | C8H14O | 126.20 | 0.40 | - | - | 0.87 | 0.49 | 1.19 |
| 41 | 1-(4-hydroxy-3,5-dimethoxyphenyl)-1-Propanone | C11H14O4 | 210.23 | 0.65 | 0.77 | 0.34 | - | 0.28 | - |
| 42 | 6-Hydroxy-2-methylchromone | C10H8O3 | 176.17 | 0.11 | - | 0.21 | 0.52 | 0.44 | 0.37 |
| 43 | 1,2-Dioctylcyclopropene | C19H36 | 264.50 | 0.25 | 0.46 | - | - | 0.51 | - |
| 44 | n-Hexadecanoic acid | C16H32O3 | 256.42 | 1.58 | 0.84 | 1.41 | 1.07 | 2.20 | 1.26 |
| 45 | Oleic Acid | C18H34O2 | 282.50 | 0.15 | - | - | 0.11 | 0.21 | 0.16 |
| 46 | 1,5-diphenyl-1-Penten-3-one | C17H16O | 236.31 | 0.12 | 0.15 | - | 0.39 | 0.39 | 0.54 |
| 47 | 2-(2-Phenylethyl)chromone | C17H14O2 | 250.29 | 2.54 | 2.86 | 2.82 | 31.65 | 32.04 | 32.20 |
| 48 | 6-Methoxy-2-(2-Phenylethyl)chromone | C18H16O3 | 280.30 | 1.33 | 0.84 | 0.65 | 0.72 | 0.67 | 0.21 |
| 49 | 2-[2-(4-Methoxyphenyl)ethyl]chromone | C18H16O3 | 280.30 | 1.20 | 1.34 | 1.58 | 22.83 | 20.56 | 17.78 |
| 50 | 2-hydroxy-1,2-bis(4-methoxyphenyl)-Ethanone | C16H16O4 | 272.29 | 0.87 | - | 0.41 | 0.20 | 0.49 | 0.19 |
| 51 | Squalene | C30H50 | 410.70 | 0.68 | 0.46 | 0.77 | 0.33 | 1.23 | 0.19 |
| 52 | β-Patchoulene | C15H24 | 204.35 | 0.22 | 0.35 | 0.41 | - | - | - |
| 53 | α-Himachalene | C15H24 | 204.35 | 0.55 | 0.63 | 0.45 | - | - | - |
| 54 | α-Curcumene | C15H22 | 202.33 | 4.33 | 5.02 | 2.01 | - | - | - |
| 55 | α-Bisabolene epoxide | C15H24 | 220.35 | 0.43 | 0.34 | 0.22 | - | - | - |
| 56 | α-Guaiene | C15H24 | 204.35 | 0.31 | 0.81 | 0.73 | - | - | - |
| 57 | Longifolenaldehyde | C15H22O | 220.35 | 0.20 | - | - | - | - | - |
| 58 | Globulol | C15H26O | 222.37 | 0.56 | 0.31 | 0.62 | - | - | - |
| 59 | Eudesma-1,4(15),11-triene | C15H22 | 202.33 | 0.32 | - | - | - | - | - |
| 60 | Isovanillic acid | C8H8O4 | 168.15 | 0.46 | - | - | - | - | - |
| 61 | 9-hydroxy-Eremophila-7(11),9-dien-8-one | C15H22O2 | 238.35 | 0.06 | - | 0.12 | - | - | - |
| 62 | Gingerone | C11H14O3 | 194.23 | 0.42 | - | 0.11 | - | - | - |
| 63 | 1H-2-Indenone,2,4,5,6,7,7a-hexahydro-3-(1-methylethyl)-7a-methyl | C13H20O | 192.30 | 0.62 | 0.58 | 0.31 | - | - | - |
| 64 | Di(2-furyl)ketone | C9H6O3 | 162.14 | - | 0.26 | 0.22 | - | - | - |
| 65 | 5,7-dihydroxy-2-methyl-4H-1-Benzopyran-4-one | C10H12O3 | 192.17 | 0.31 | - | 0.13 | - | - | - |
| 66 | Wine lactone | C10H14O2 | 166.22 | - | 0.30 | - | - | - | - |
| 67 | (E)-2-Tetradecene | C14H28 | 196.37 | 1.97 | 1.50 | 1.80 | - | - | - |
| 68 | 4-(4-methoxyphenyl)-3-Buten-2-one | C11H12O2 | 176.21 | 0.84 | 0.11 | 0.23 | - | - | - |
| 69 | 6-Methyl-9-(2′-methylpropyl)bicyclo [4.3.0]nonan-3-one | C14H24O | 208.34 | 1.08 | 0.76 | 0.69 | - | - | - |
| 70 | 5-[(4-Hydroxy-3-methoxyphenyl)methyl]-1,3-dimethyl-1,3-diazinane-2,4,6-trione | C14H14N4O5 | 292.29 | - | - | - | 3.28 | 2.38 | 3.18 |
| 71 | 12H-benzo[a]xanthen-12-one | C17H10O2 | 246.26 | - | - | - | 0.15 | 0.05 | 0.09 |
| 72 | 2-(2′-Methoxyphenethyl) chromone | C18H16O3 | 280.30 | - | - | - | - | 0.71 | 0.86 |
| 73 | Xanthone | C13H8O2 | 196.20 | - | - | - | 0.66 | 0.51 | 0.53 |
| 74 | 1,6,6-Trimethylbicyclo(3.3.0)octan-2-one | C11H18O | 166.26 | - | - | - | 0.18 | 0.11 | - |
| 75 | 3-(phenylmethylene)-2-Pentanone | C12H14O | 174.24 | - | - | - | - | 0.23 | 0.39 |
| 76 | 4,6,8-Trimethylazulene | C13H14 | 170.25 | - | - | - | - | 0.79 | - |
| 77 | [1S-(1R*,9S*)]-10,10-dimethyl-2,6-bis(methylene)-Bicyclo [7.2.0]undecane | C15H24 | 204.35 | - | - | - | 0.41 | 0.51 | 0.77 |
| 78 | Guaia-9,11-diene | C15H24 | 204.35 | - | - | - | 0.72 | 0.69 | 0.72 |
| 79 | Italicene ether | C15H24O | 220.35 | - | - | - | 2.33 | 3.01 | 1.98 |
| 80 | Eremophila-9(10),11(12)-diene | C15H24 | 204.35 | - | - | - | 1.05 | 0.51 | 0.72 |
| 81 | Eudesma-4(14),(11)-diene | C15H24 | 204.35 | - | - | - | - | - | 0.28 |
| 82 | Patchoulane | C15H26 | 206.37 | - | - | - | - | - | 0.30 |
| 83 | γ-Gurjunene | C15H24 | 204.35 | - | - | - | 0.87 | 0.42 | - |
| 84 | γ-Eudesmol | C15H26O | 222.37 | - | - | - | 0.61 | 0.42 | 0.53 |
| 85 | β-Elemene | C15H24 | 204.35 | - | - | - | 0.46 | - | 0.33 |
| 86 | Valencene | C15H24 | 204.35 | - | - | - | 0.54 | 0.42 | - |
| 87 | Kessane | C15H26O | 222.37 | - | - | - | 0.66 | 0.86 | 1.04 |
| Main Active Ingredients | Core Targets | Binding Energy kcal·mol−1 | Main Active Ingredients | Core Targets | Binding Energy kcal·mol−1 |
|---|---|---|---|---|---|
| 5-Isopropylidene-6-methyldeca-3,6,9-trien-2-one | MAOB | −5.32 | 2-[2-(4-Methoxyphenyl)ethyl] chromone | DRD4 | −5.10 |
| GABRG2 | −4.80 | CREBBP | −6.94 | ||
| DRD4 | −4.02 | Gingerone | ESR1 | −5.88 | |
| 1,5-diphenyl-1- Penten-3-one | GABRG2 | −5.74 | MAOB | −4.37 | |
| DRD4 | −6.59 | COMT | −4.53 | ||
| 2-(2-Phenylethyl) chromone | DRD4 | −5.94 | 5,7-dihydroxy-2-methyl-4H-1-Benzopyran-4-one | ESR1 | −4.98 |
| GABRG2 | −5.73 | 6-Methyl-9-(2′-methylpropyl)bicyclo [4.3.0]nonan-3-one | DRD4 | −5.97 | |
| 6-Methoxy-2-(2-Phenylethyl) chromone | MAOB | −5.42 | 2-(2′-Methoxyphenethyl) chromone | HTR1B | −5.32 |
| DRD4 | −4.93 | GABRG2 | −5.24 | ||
| 2-[2-(4-Methoxyphenyl)ethyl] chromone | HIF1A | −7.08 | γ-Eudesmol | ESR1 | −6.76 |
| GABRG2 | −6.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Mou, J.; Feng, J.; Zhang, S.; Li, D.; Liu, Y. Comparative Analysis of Volatile Components in Chi-Nan and Ordinary Agarwood Aromatherapies: Implications for Sleep Improvement. Pharmaceuticals 2024, 17, 1196. https://doi.org/10.3390/ph17091196
Jiang Z, Mou J, Feng J, Zhang S, Li D, Liu Y. Comparative Analysis of Volatile Components in Chi-Nan and Ordinary Agarwood Aromatherapies: Implications for Sleep Improvement. Pharmaceuticals. 2024; 17(9):1196. https://doi.org/10.3390/ph17091196
Chicago/Turabian StyleJiang, Zixiao, Junyu Mou, Jian Feng, Shunan Zhang, Dan Li, and Yangyang Liu. 2024. "Comparative Analysis of Volatile Components in Chi-Nan and Ordinary Agarwood Aromatherapies: Implications for Sleep Improvement" Pharmaceuticals 17, no. 9: 1196. https://doi.org/10.3390/ph17091196
APA StyleJiang, Z., Mou, J., Feng, J., Zhang, S., Li, D., & Liu, Y. (2024). Comparative Analysis of Volatile Components in Chi-Nan and Ordinary Agarwood Aromatherapies: Implications for Sleep Improvement. Pharmaceuticals, 17(9), 1196. https://doi.org/10.3390/ph17091196






