Anti-Amnesic Effect of Agastache rugosa on Scopolamine-Induced Memory Impairment in Mice
Abstract
1. Introduction
2. Results
2.1. ARE Has Antioxidant and Anti-Inflammatory Activities
2.2. ARE Ameliorates Scopolamine-Induced Learning and Memory Impairments
2.2.1. ARE Attenuates Scopolamine-Induced Impairment of Avoidance Memory
2.2.2. ARE Attenuates Scopolamine-Induced Impairment of Novel Object Recognition Memory
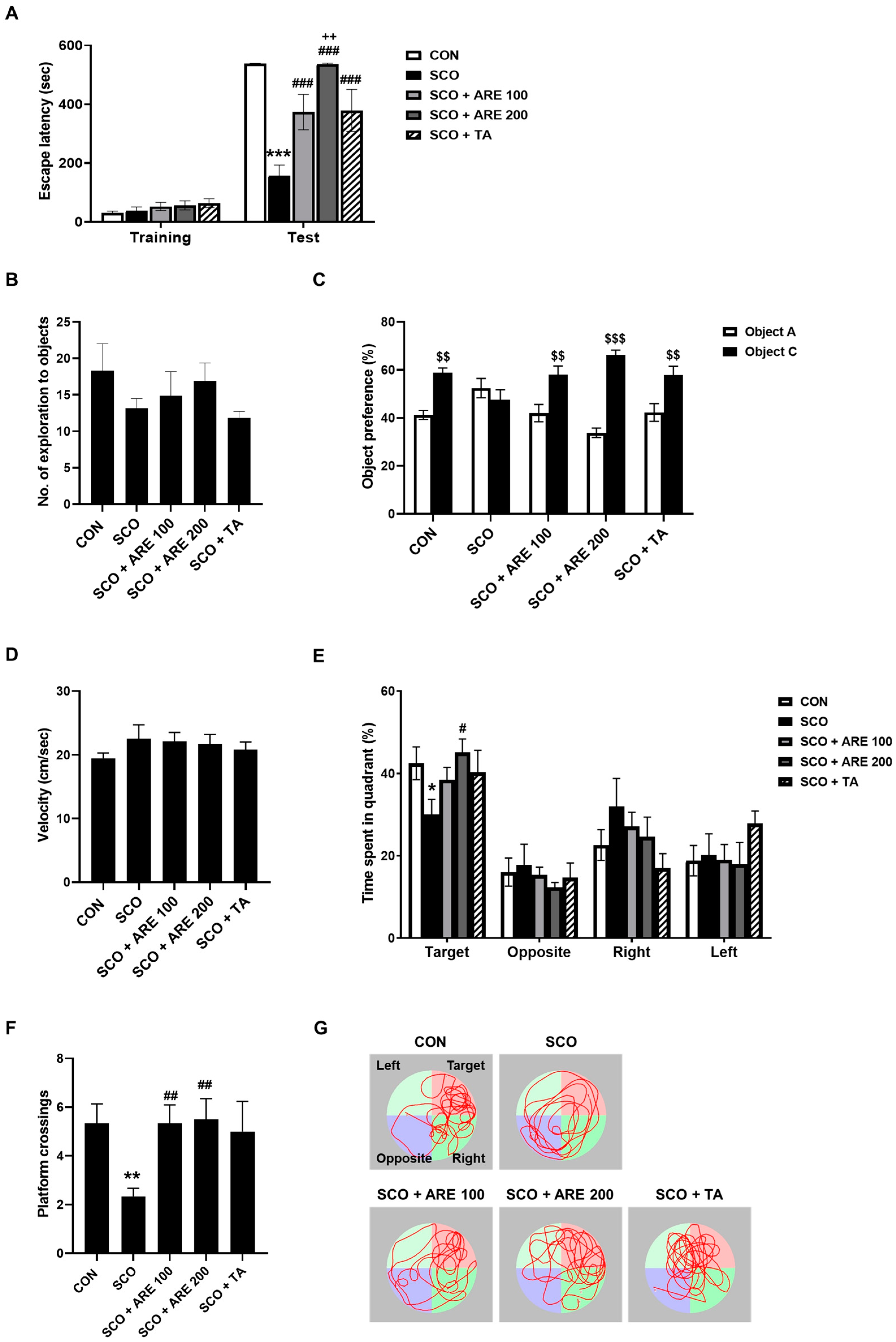
2.2.3. ARE Reduces the Scopolamine-Induced Long-Term Spatial Memory Dysfunction
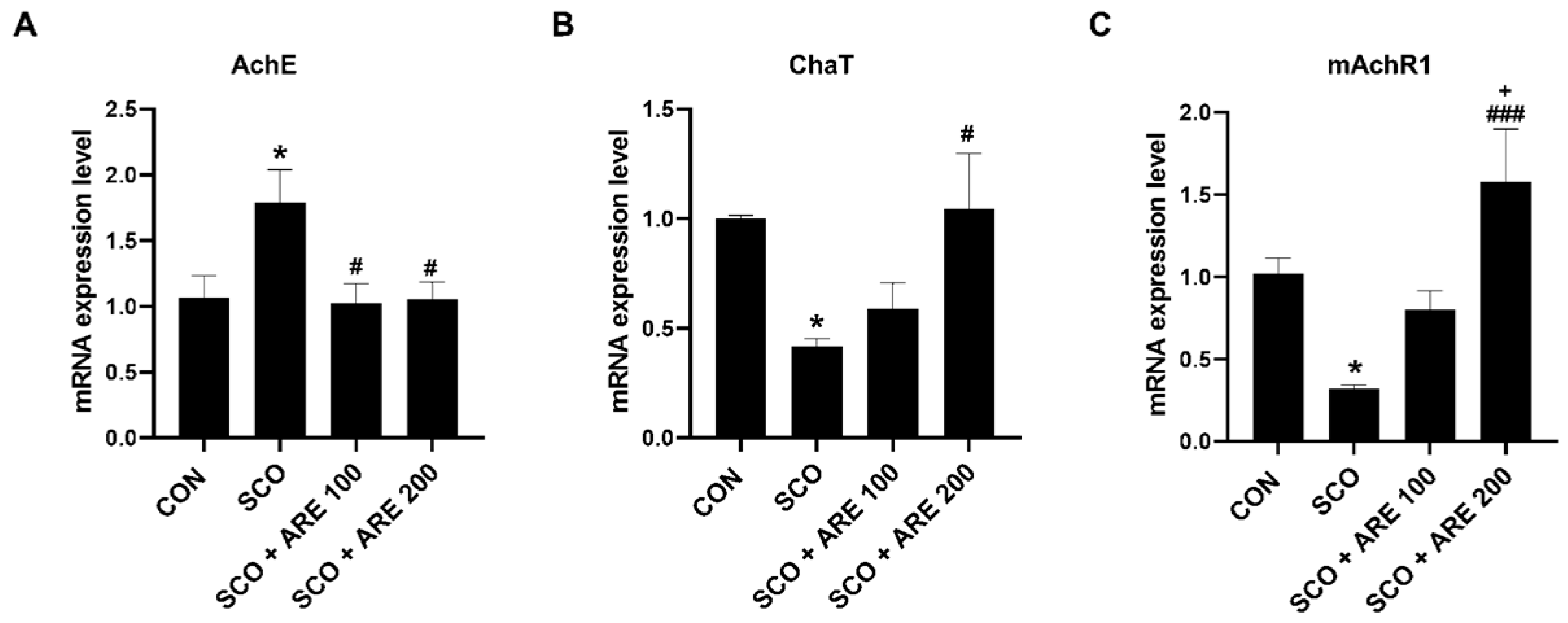
2.3. ARE Modulates Scopolamine-Induced Alteration of Cholinergic Neurotransmitters
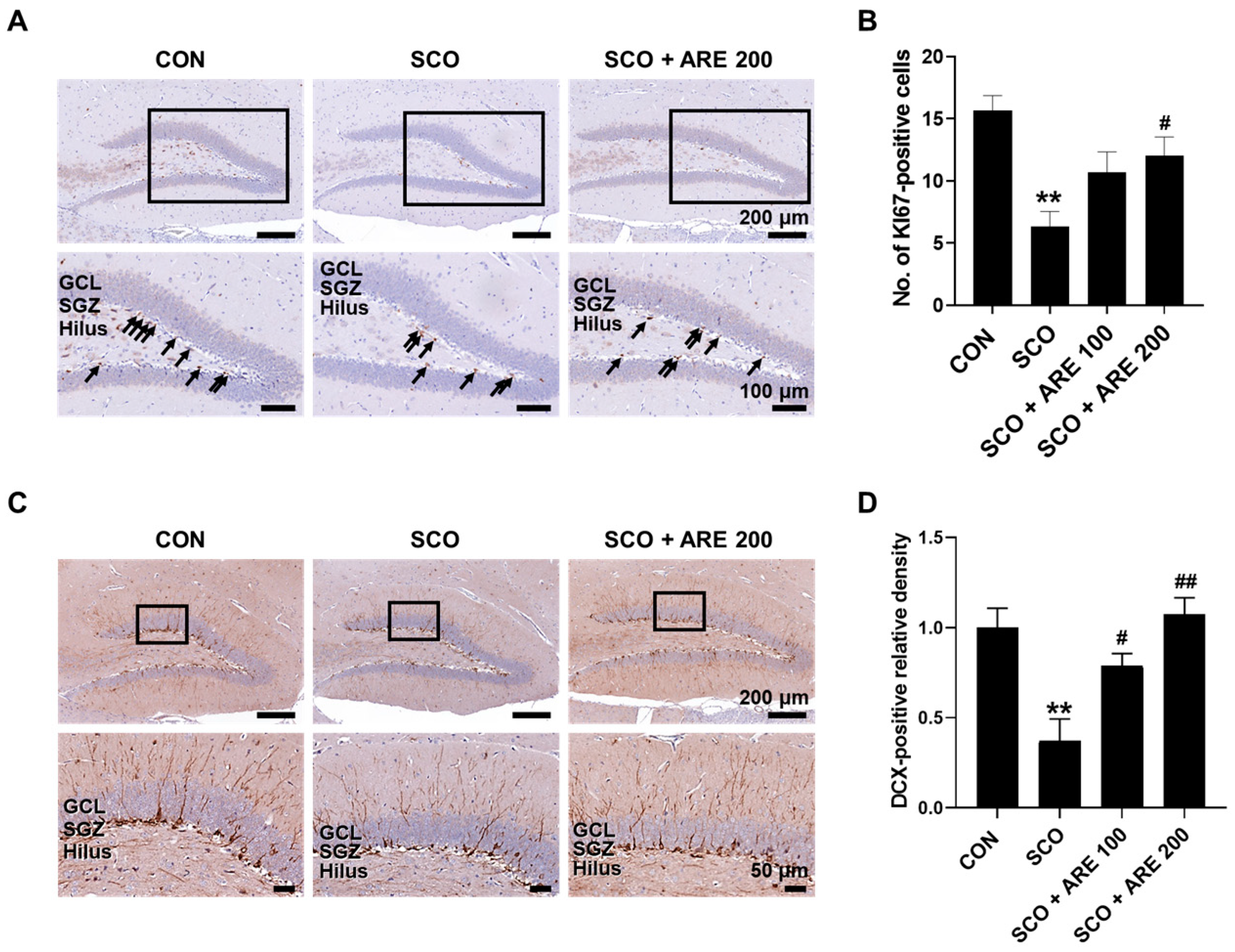
2.4. ARE Attenuates Scopolamine-Induced Impairment of Hippocampal Neurogenesis
3. Discussion
4. Materials and Methods
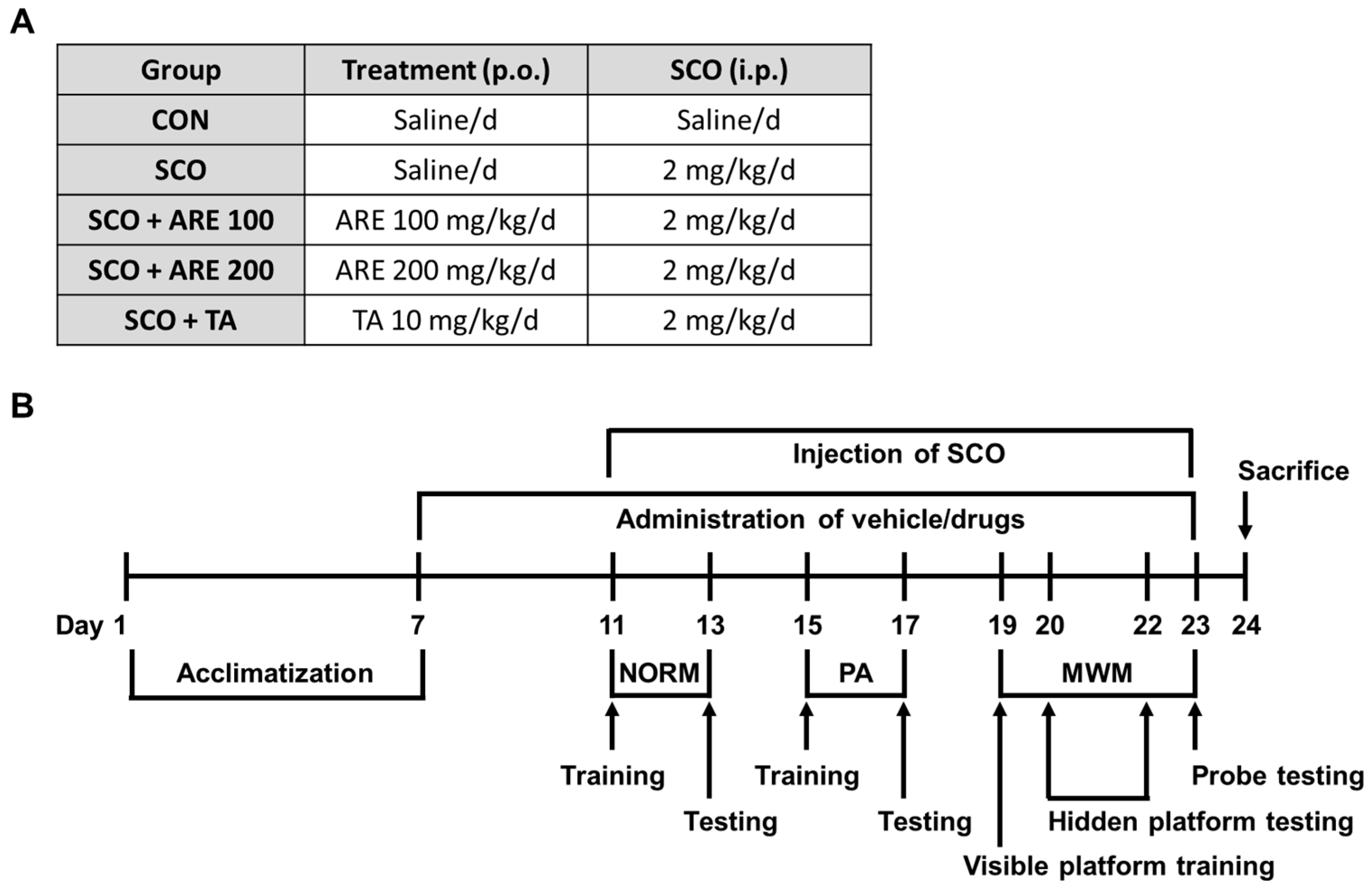
4.1. Animals
4.2. Preparation of ARE
4.3. Drug Administration
4.4. Behavior Measurements
4.4.1. Passive Avoidance Test
4.4.2. Novel Object Recognition Memory Test
4.4.3. Morris Water Maze Test
4.4.4. Immunohistochemistry
4.5. Reverse Transcription Quantitative Polymerase Chain Reaction Analysis
4.6. DPPH Radical Scavenging Assay
4.7. Determination of NO Generation in RAW 264.7 Cells
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wimo, A.; Jönsson, L.; Bond, J.; Prince, M.; Winblad, B. The worldwide economic impact of dementia 2010. Alzheimer’s Dement. 2013, 9, 1–11.e3. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.E.; Harrington, M.O.; Langthorne, D.; Ngo, H.V.; Cairney, S.A. Sleep deprivation induces fragmented memory loss. Learn. Mem. 2020, 27, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Topiwala, A. Alcohol use disorders and the brain. Addiction 2020, 115, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Hammar, Å.; Ronold, E.H.; Rekkedal, G. Cognitive Impairment and Neurocognitive Profiles in Major Depression-A Clinical Perspective. Front. Psychiatry 2022, 13, 764374. [Google Scholar] [CrossRef]
- Schwabe, L.; Hermans, E.J.; Joëls, M.; Roozendaal, B. Mechanisms of memory under stress. Neuron 2022, 110, 1450–1467. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Benarroch, E.E. Acetylcholine in the cerebral cortex: Effects and clinical implications. Neurology 2010, 75, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimers Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Ruangritchankul, S.; Chantharit, P.; Srisuma, S.; Gray, L.C. Adverse Drug Reactions of Acetylcholinesterase Inhibitors in Older People Living with Dementia: A Comprehensive Literature Review. Ther. Clin. Risk Manag. 2021, 17, 927–949. [Google Scholar] [CrossRef]
- Benninghoff, J.; Perneczky, R. Anti-Dementia Medications and Anti-Alzheimer’s Disease Drugs: Side Effects, Contraindications, and Interactions. In NeuroPsychopharmacotherapy; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–10. [Google Scholar]
- Baek, S.Y.; Li, F.Y.; Kim, D.H.; Kim, S.J.; Kim, M.R. Enteromorpha prolifera Extract Improves Memory in Scopolamine-Treated Mice via Downregulating Amyloid-β Expression and Upregulating BDNF/TrkB Pathway. Antioxidants 2020, 9, 620. [Google Scholar] [CrossRef]
- Sarandol, A.; Sarandol, E.; Eker, S.S.; Erdinc, S.; Vatansever, E.; Kirli, S. Major depressive disorder is accompanied with oxidative stress: Short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum. Psychopharmacol. 2007, 22, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Rahman, S.U.; Ahmad, M.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M.; Alsaiari, A.A.; et al. Synthetic Mono-Carbonyl Curcumin Analogues Attenuate Oxidative Stress in Mouse Models. Biomedicines 2022, 10, 2597. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kang, S.; Tran, M.N.; Lee, S.; Ryu, S.M.; Chae, S.W.; Kim, D.H.; Lee, Y.E.; Jeong, S.; Moon, C.; et al. Antiepileptic and Neuroprotective Effects of Rheum tanguticum Root Extract on Trimethyltin-Induced Epilepsy and Neurodegeneration: In Vivo and in Silico Analyses. J. Integr. Neurosci. 2024, 23, 122. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Hong, S.; Kim, J.-S.; Wang, H.; Moon, C.; Shin, T. Chlorogenic Acid as a Promising Therapeutic for Multiple Sclerosis: Evidence from an Experimental Autoimmune Encephalomyelitis Model. J. Biol. Regul. Homeost. Agents 2024, 38, 3331–3344. [Google Scholar]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Kim, C.; Kim, M.B.; Hwang, J.K. Anti-Photoaging Effect of Korean Mint (Agastache rugosa Kuntze) Extract on UVB-Irradiated Human Dermal Fibroblasts. Prev. Nutr. Food Sci. 2019, 24, 442–448. [Google Scholar] [CrossRef]
- Cao, P.; Xie, P.; Wang, X.; Wang, J.; Wei, J.; Kang, W.Y. Chemical constituents and coagulation activity of Agastache rugosa. BMC Complement. Altern. Med. 2017, 17, 93. [Google Scholar] [CrossRef]
- Song, J.H.; Nam, H.H.; Park, I.; Yang, S.; Chun, J.M.; Seo, Y.S.; Kim, H.Y.; Moon, B.C.; Kang, S.; Moon, C.; et al. Comparative Morphology of Island and Inland Agastache rugosa and Their Gastroprotective Effects in EtOH/HCl-Induced Gastric Mucosal Gastritis. Planta Med. 2024, 90, 4–12. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, J.H.; Yang, W.K.; Geum, J.H.; Kim, H.R.; Choi, S.Y.; Kang, Y.M.; An, H.J.; Lee, Y.C. Herbal Combinational Medication of Glycyrrhiza glabra, Agastache rugosa Containing Glycyrrhizic Acid, Tilianin Inhibits Neutrophilic Lung Inflammation by Affecting CXCL2, Interleukin-17/STAT3 Signal Pathways in a Murine Model of COPD. Nutrients 2020, 12, 926. [Google Scholar] [CrossRef]
- Hong, S.; Cha, K.H.; Kwon, D.Y.; Son, Y.J.; Kim, S.M.; Choi, J.H.; Yoo, G.; Nho, C.W. Agastache rugosa ethanol extract suppresses bone loss via induction of osteoblast differentiation with alteration of gut microbiota. Phytomedicine 2021, 84, 153517. [Google Scholar] [CrossRef]
- Tuan, P.A.; Park, W.T.; Xu, H.; Park, N.I.; Park, S.U. Accumulation of tilianin and rosmarinic acid and expression of phenylpropanoid biosynthetic genes in Agastache rugosa. J. Agric. Food Chem. 2012, 60, 5945–5951. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; He, L.; Li, S.; Chen, Z.; Ashraf, M.A. Antimicrobial, antibiofilm and antitumor activities of essential oil of Agastache rugosa from Xinjiang, China. Saudi J. Biol. Sci. 2016, 23, 524–530. [Google Scholar]
- Lee, Y.; Lim, H.W.; Ryu, I.W.; Huang, Y.H.; Park, M.; Chi, Y.M.; Lim, C.J. Anti-Inflammatory, Barrier-Protective, and Antiwrinkle Properties of Agastache rugosa Kuntze in Human Epidermal Keratinocytes. BioMed Res. Int. 2020, 2020, 1759067. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.M.; Wang, X.C.; Xu, T.T.; Li, H.Y.; Hei, S.Y.; Luo, N.C.; Wang, H.; Zhao, W.; Fang, S.H.; Chen, Y.B.; et al. Kai Xin San ameliorates scopolamine-induced cognitive dysfunction. Neural Regen. Res. 2019, 14, 794–804. [Google Scholar]
- Savi, F.F.; de Oliveira, A.; de Medeiros, G.F.; Bozza, F.A.; Michels, M.; Sharshar, T.; Dal-Pizzol, F.; Ritter, C. What animal models can tell us about long-term cognitive dysfunction following sepsis: A systematic review. Neurosci. Biobehav. Rev. 2021, 124, 386–404. [Google Scholar] [CrossRef]
- Kang, S.; Lee, S.; Kim, J.; Kim, J.C.; Kim, S.H.; Son, Y.; Shin, T.; Youn, B.; Kim, J.S.; Wang, H.; et al. Chronic Treatment with Combined Chemotherapeutic Agents Affects Hippocampal Micromorphometry and Function in Mice, Independently of Neuroinflammation. Exp. Neurobiol. 2018, 27, 419–436. [Google Scholar] [CrossRef]
- Othman, M.Z.; Hassan, Z.; Che Has, A.T. Morris water maze: A versatile and pertinent tool for assessing spatial learning and memory. Exp. Anim. 2022, 71, 264–280. [Google Scholar] [CrossRef]
- Prado, M.A.; Reis, R.A.; Prado, V.F.; de Mello, M.C.; Gomez, M.V.; de Mello, F.G. Regulation of acetylcholine synthesis and storage. Neurochem. Int. 2002, 41, 291–299. [Google Scholar] [CrossRef]
- Deiana, S.; Platt, B.; Riedel, G. The cholinergic system and spatial learning. Behav. Brain Res. 2011, 221, 389–411. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Mostafa, A.M.; Ashour, M.L.; Elhady, S.S. Neuroprotective Effects of Black Pepper Cold-Pressed Oil on Scopolamine-Induced Oxidative Stress and Memory Impairment in Rats. Antioxidants 2021, 10, 1993. [Google Scholar] [CrossRef]
- Choi, J.S.; Song, B.-M.; Park, H.-J. Gas chromatographic analysis and cholinesterase activity of the essential oil from Korean Agastache rugosa. Korean J. Pharmacogn. 2016, 47, 192–196. [Google Scholar]
- Ang, M.J.; Kang, S.; Moon, C. Melatonin alters neuronal architecture and increases cysteine-rich protein 1 signaling in the male mouse hippocampus. J. Neurosci. Res. 2020, 98, 2333–2348. [Google Scholar] [CrossRef]
- Üçel, U.; Can, Ö.D.; Demir Özkay, Ü.; Ulupinar, E. Antiamnesic effects of tofisopam against scopolamine-induced cognitive impairments in rats. Pharmacol. Biochem. Behav. 2020, 190, 172858. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Subedi, L.; Yeo, E.J.; Kim, S.Y. Lactucopicrin ameliorates oxidative stress mediated by scopolamine-induced neurotoxicity through activation of the NRF2 pathway. Neurochem. Int. 2016, 99, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.A.; Adlimoghaddam, A.; Albensi, B.C. Role of Nrf2 in Synaptic Plasticity and Memory in Alzheimer’s Disease. Cells 2021, 10, 1884. [Google Scholar] [CrossRef]
- Irawan, C.; Putri, I.D.; Sukiman, M.; Utami, A.; Putri, R.K.; Lisandi, A.; Pratama, A.N. Antioxidant activity of DPPH, CUPRAC, and FRAP methods, as well as activity of alpha-glucosidase inhibiting enzymes from Tinospora crispa (L.) stem ultrasonic extract. Pharmacogn. J. 2022, 14, 511–520. [Google Scholar] [CrossRef]
- Olayinka, J.; Eduviere, A.; Adeoluwa, O.; Fafure, A.; Adebanjo, A.; Ozolua, R. Quercetin mitigates memory deficits in scopolamine mice model via protection against neuroinflammation and neurodegeneration. Life Sci. 2022, 292, 120326. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.H.; Kang, S.Y.; Shin, J.S.; Ryu, S.M.; Lee, A.Y.; Choi, G.; Moon, B.C.; Jang, D.S.; Shim, S.H.; Lee, D.; et al. Chemical Constituents from the Aerial Parts of Agastache rugosa and Their Inhibitory Activities on Prostaglandin E(2) Production in Lipopolysaccharide-Treated RAW 264.7 Macrophages. J. Nat. Prod. 2019, 82, 3379–3385. [Google Scholar] [CrossRef]
- Nam, H.H.; Kim, J.S.; Lee, J.; Seo, Y.H.; Kim, H.S.; Ryu, S.M.; Choi, G.; Moon, B.C.; Lee, A.Y. Pharmacological Effects of Agastache rugosa against Gastritis Using a Network Pharmacology Approach. Biomolecules 2020, 10, 1298. [Google Scholar] [CrossRef]
- Sun, T.; Tan, L.; Liu, M.; Zeng, L.; Zhao, K.; Cai, Z.; Sun, S.; Li, Z.; Liu, R. Tilianin improves cognition in a vascular dementia rodent model by targeting miR-193b-3p/CaM- and miR-152-3p/CaMKIIα-mediated inflammatory and apoptotic pathways. Front. Immunol. 2023, 14, 1118808. [Google Scholar] [CrossRef]
- Barman, D.; Dey, N.; Sen, S.; Kakoti, B.B.; Vanlalhriatpuii, C. Neuromodulatory effect of plant metabolites. Sci. Phytochem. 2022, 1, 47–69. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, L.; Chen, X.; Zhou, X.; Cao, L. Acacetin alleviates neuroinflammation and oxidative stress injury via the Nrf2/HO-1 pathway in a mouse model of spinal cord injury. Transl. Neurosci. 2022, 13, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, S.; Ang, M.J.; Kim, J.; Kim, J.C.; Kim, S.H.; Jeon, T.I.; Jung, C.; Im, S.S.; Moon, C. Deficiency of sterol regulatory element-binding protein-1c induces schizophrenia-like behavior in mice. Genes Brain Behav. 2019, 18, e12540. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cheng, Z.; Moore, J.; Yu, L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef]
- Nam, H.H.; Yang, S.; Kim, H.S.; Kim, M.J.; Kim, J.S.; Lee, J.H. Role of Semisulcospira gottschei extract as medicinal food on reflux esophagitis in rats. Food Sci. Nutr. 2021, 9, 3114–3122. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Lee, N.; Jung, B.; Jeong, H.; Moon, C.; Park, S.-I.; Yun, S.; Yim, T.; Oh, J.M.; Kim, J.-W.; et al. Anti-Amnesic Effect of Agastache rugosa on Scopolamine-Induced Memory Impairment in Mice. Pharmaceuticals 2024, 17, 1173. https://doi.org/10.3390/ph17091173
Kang S, Lee N, Jung B, Jeong H, Moon C, Park S-I, Yun S, Yim T, Oh JM, Kim J-W, et al. Anti-Amnesic Effect of Agastache rugosa on Scopolamine-Induced Memory Impairment in Mice. Pharmaceuticals. 2024; 17(9):1173. https://doi.org/10.3390/ph17091173
Chicago/Turabian StyleKang, Sohi, Nari Lee, Bokyung Jung, Huiyeong Jeong, Changjong Moon, Sang-Ik Park, Seungpil Yun, Teresa Yim, Jung Min Oh, Jae-Won Kim, and et al. 2024. "Anti-Amnesic Effect of Agastache rugosa on Scopolamine-Induced Memory Impairment in Mice" Pharmaceuticals 17, no. 9: 1173. https://doi.org/10.3390/ph17091173
APA StyleKang, S., Lee, N., Jung, B., Jeong, H., Moon, C., Park, S.-I., Yun, S., Yim, T., Oh, J. M., Kim, J.-W., Song, J. H., Chae, S., & Kim, J. S. (2024). Anti-Amnesic Effect of Agastache rugosa on Scopolamine-Induced Memory Impairment in Mice. Pharmaceuticals, 17(9), 1173. https://doi.org/10.3390/ph17091173





