Use of Ketamine in Patients with Multifactorial Neuropathic Pain: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
Objective
2. Methods
2.1. Literature Search
2.2. Data Extraction and Quality Assessment
2.3. Data Synthesis and Analysis
2.4. Rating the Quality of Evidence
3. Results
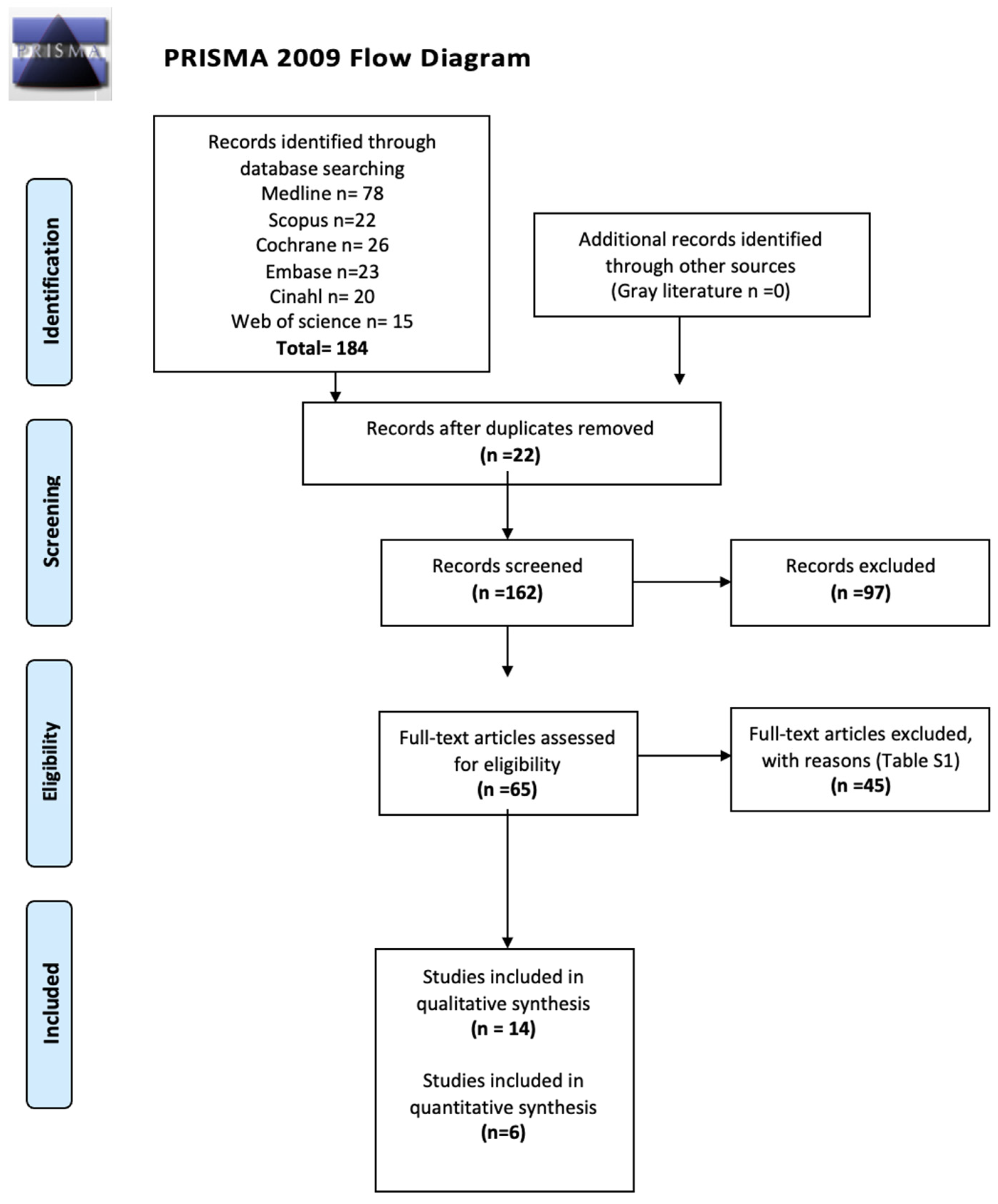
3.1. Study Selection
3.2. Descriptive Analysis of Studies Not Included in the Meta-Analysis
3.3. Study Included Meta-Analysis Characteristics
3.4. Risk of Bias Assessment in Individual Studies
3.5. Synthesis of Results
3.5.1. Scales for Evaluation
NRS LDK First Month
NRS LDK Third Month
VAS LDK First Month
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Neuropathic pain | NP |
| Low doses of ketamine | LDK |
| Intravenous | IV |
| Numeric pain score | NPS |
| Oral morphine equivalent | OME |
| Douleur neuropathique-4 items | DN4 |
| Numeric rating scale | NRS |
| Visual analog scale | VAS |
| Brief pain inventory | BPI |
| Peripheral neuropathy | PN |
References
- Kannan, T.R.; Saxena, A.; Bhatnagar, S.; Barry, A. Oral ketamine as an adjuvant to oral morphine for neuropathic pain in cancer patients. J. Pain Symptom Manag. 2002, 23, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.E.; Clark, A.J.; Sawynok, J.; Sullivan, M.J.L. Topical 2% Amitriptyline and 1% Ketamine in Neuropathic Pain Syndromes: A Randomized, Double-blind, Placebo-controlled Trial. Anesthesiology 2005, 103, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Quinn, S.; Fazekas, B.; Plummer, J.; Eckermann, S.; Agar, M.; Spruyt, O.; Rowett, D.; Currow, D.C. Randomized, double-blind, placebo-controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. J. Clin. Oncol. 2012, 30, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.T.; Wilcock, A.; Kelly, C.A.; Paul, J.; Lewsley, L.-A.; Norrie, J.; Laird, B.J.A. Oral Ketamine vs Placebo in Patients with Cancer-Related Neuropathic Pain: A Randomized, Clinical Trial. JAMA Oncol. 2018, 4, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Hannon, C.P.; Fillingham, Y.A.; Gililland, J.M.; Sporer, S.M.; Casambre, F.D.; Verity, T.J.; Woznica, A.; Nelson, N.; Hamilton, W.G.; Della Valle, C.J. A Systematic Review of the Efficacy and Safety of Ketamine in Total Joint Arthroplasty. J. Arthroplast. 2022, 38, 763–768.e2. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.E.; Mahran, E. Effect of magnesium sulfate with ketamine infusions on intraoperative and postoperative analgesia in cancer breast surgeries: A randomized double-blind trial. Braz. J. Anesthesiol. 2021, 73, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Niesters, M.; Hoitsma, E.; Sarton, E.; Aarts, L.; Dahan, A. Offset Analgesia in Neuropathic Pain Patients and Effect of Treatment with Morphine and Ketamine. Anesthesiology 2011, 115, 1063–1071. [Google Scholar] [CrossRef]
- Delage, N.; Morel, V.; Picard, P.; Marcaillou, F.; Pereira, B.; Pickering, G. Effect of ketamine combined with magnesium sulfate in neuropathic pain patients (KETAPAIN): Study protocol for a randomized controlled trial. Trials 2017, 18, 517. [Google Scholar] [CrossRef] [PubMed]
- Kvarnström, A.; Karlsten, R.; Quiding, H.; Emanuelsson, B.; Gordh, T. The effectiveness of intravenous ketamine and lidocaine on peripheral neuropathic pain. Acta Anaesthesiol. Scand. 2003, 47, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Aveline, C.; Le Roux, A.; Le Hetet, H.; Gautier, J.F.; Vautier, P.; Cognet, F.; Bonnet, F. Pain and recovery after total knee arthroplasty: A 12-month follow-up after a prospective randomized study evaluating nefopam and ketamine for early rehabilitation. Clin. J. Pain 2014, 30, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Monks, D.T.; Palanisamy, A.; Jaffer, D.; Singh, P.M.; Carter, E.; Lenze, S. A randomized feasibility pilot-study of intravenous and subcutaneous administration of ketamine to prevent postpartum depression after planned cesarean delivery under neuraxial anesthesia. BMC Pregnancy Childbirth 2022, 22, 786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, D.; He, A.; Xu, R.; Xiu, X.; Wei, Y. Efficacy of Pain Relief in Different Postherpetic Neuralgia Therapies: A Network Me-ta-Analysis. Pain Physician 2018, 21, 19–32. [Google Scholar] [PubMed]
- Timm, C.; Linstedt, U.; Weiss, T.; Zenz, M.; Maier, C. Sympathomimetic effects of low-dose S(+)-ketamine: Effect of propofol dosage. Anaesthesist 2008, 57, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Chumbley, G.M.; Thompson, L.; Swatman, J.E.; Urch, C. Ketamine infusion for 96 hr after thoracotomy: Effects on acute and persistent pain. Eur. J. Pain 2019, 23, 985–993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.; Campbell, M.K.; Campbell, M.J. Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB2). Additional Considerations for Cluster-Randomized Trials (RoB 2 CRT). Available online: https://www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-cluster-randomized-trials (accessed on 30 April 2024).
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Gaillat, F.; Duponq, R.; Lieven, R.; Baumstarck, K.; Thomas, P.; Penot-Ragon, C.; Kerbaul, F. Is there any benefit to adding intravenous ketamine to patient-controlled epidural analgesia after thoracic surgery? A randomized double-blind study. Eur. J. Cardio-Thorac. Surg. 2012, 42, e58–e65. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Cho, A.-R.; Kim, K.-H.; Lee, E.-A.; Lee, H.-J.; Kwon, J.-Y.; Kim, H.; Kim, E.; Baik, J.-S.; Kim, C. Effects of Intraoperative Low-Dose Ketamine on Persistent Postsurgical Pain after Breast Cancer Surgery: A Prospective, Randomized, Controlled, Double-Blind Study. Pain Physician 2020, 23, 37–47. [Google Scholar] [PubMed]
- Peyton, P.J.; Wu, C.; Jacobson, T.; Hogg, M.; Zia, F.; Leslie, K. The effect of a perioperative ketamine infusion on the incidence of chronic postsurgical pain—A pilot study. Anaesth. Intensiv. Care 2017, 45, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Remérand, F.; Le Tendre, C.; Baud, A.; Couvret, C.; Pourrat, X.; Favard, L.; Laffon, M.; Fusciardi, J. The Early and delayed analgesic effects of ketamine after total hip arthroplasty: A prospective, randomized, controlled, double-blind study. Anesth. Analg. 2009, 109, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Loftus, R.W.; Yeager, M.P.; Clark, J.A.; Brown, J.R.; Abdu, W.A.; Sengupta, D.K.; Beach, M.L. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology 2010, 113, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, H.-P.; Jeong, M.-H.; Son, J.-D.; Kim, H.-C. Efficacy of ketamine for postoperative pain following robotic thyroidectomy: A prospective randomised study. J. Int. Med Res. 2017, 46, 1109–1120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carver, T.W.; Kugler, N.W.; Juul, J.; Peppard, W.J.; Drescher, K.M.; Somberg, L.B.; Szabo, A.; Yin, Z.; Paul, J.S. Ketamine infusion for pain control in adult patients with multiple rib fractures: Results of a randomized control trial. J. Trauma Inj. Infect. Crit. Care 2019, 86, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Czarnetzki, C.; Desmeules, J.; Tessitore, E.; Faundez, A.; Chabert, J.; Daali, Y.; Fournier, R.; Dupuis-Lozeron, E.; Cedraschi, C.; Tramèr, M.R. Perioperative intravenous low-dose ketamine for neuropathic pain after major lower back surgery: A randomized, placebo-controlled study. Eur. J. Pain 2020, 24, 555–567. [Google Scholar] [CrossRef]
- Jafarinia, M.; Afarideh, M.; Tafakhori, A.; Arbabi, M.; Ghajar, A.; Noorbala, A.A.; Saravi, M.A.; Agah, E.; Akhondzadeh, S. Efficacy and safety of oral ketamine versus diclofenac to alleviate mild to moderate depression in chronic pain patients: A double-blind, randomized, controlled trial. J. Affect. Disord. 2016, 204, 1–8. [Google Scholar] [CrossRef]
- Jain, S.; Nazir, N.; Mustafi, S. Preemptive low-dose intravenous ketamine in the management of acute and chronic postoperative pain following laparoscopic cholecystectomy: A prospective randomized control study. Med. Gas Res. 2022, 12, 141–145. [Google Scholar] [CrossRef]
- Lauretti, G.R.; Gomes, J.M.; Reis, M.P.; Pereira, N.L. Low doses of epidural ketamine or neostigmine, but not midazolam, improve morphine analgesia in epidural terminal cancer pain therapy. J. Clin. Anesth. 1999, 11, 663–668. [Google Scholar] [CrossRef]
- Lumanauw, D.D.; Youn, S.; Horeczko, T.; Yadav, K.; Tanen, D.A. Subdissociative-dose ketamine is effective for treating acute exacerbations of chronic Pain. Acad. Emerg. Med. 2019, 26, 1044–1051. [Google Scholar] [CrossRef]
- Nielsen, R.V.; Fomsgaard, J.S.; Siegel, H.; Martusevicius, R.; Nikolajsen, L.; Dahl, J.B.; Mathiesen, O. Intraoperative ketamine reduces immediate postoperative opioid consumption after spinal fusion surgery in chronic pain patients with opioid dependency: A randomized, blinded trial. Pain 2017, 158, 463–470. [Google Scholar] [CrossRef]
- Rakhman, E.; Shmain, D.; White, I.; Ekstein, M.P.; Kollender, Y.; Chazan, S.; Dadia, S.; Bickels, J.; Amar, E.; Weinbroum, A.A. Repeated and escalating preoperative subanesthetic doses of ketamine for postoperative pain control in patients undergoing tumor resection: A randomized, placebo-controlled, double-blind trial. Clin. Ther. 2011, 33, 863–873. [Google Scholar] [CrossRef]
- Rigo, F.K.; Trevisan, G.; Godoy, M.C.; Rossato, M.F.; Dalmolin, G.D.; A Silva, M.; Menezes, M.S.; Caumo, W.; Ferreira, J. Management of Neuropathic Chronic Pain with Methadone Combined with Ketamine: A Randomized, Double Blind, Active-Controlled Clinical Trial. Pain Physician 2017, 20, 207–215. [Google Scholar] [PubMed]
- Sigtermans, M.J.; van Hilten, J.J.; Bauer, M.C.; Arbous, S.M.; Marinus, J.; Sarton, E.Y.; Dahan, A. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain 2009, 145, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Yazigi, A.; Abou-Zeid, H.; Srouji, T.; Madi-Jebara, S.; Haddad, F.; Jabbour, K. The effect of low-dose intravenous ketamine on continuous intercostal analgesia following thoracotomy. Ann. Card. Anaesth. 2012, 15, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Michelet, D.; Brasher, C.; Horlin, A.; Bellon, M.; Julien-Marsollier, F.; Vacher, T.; Pontone, S.; Dahmani, S. Ketamine for chronic non-cancer pain: A meta-analysis and trial sequential analysis of randomized controlled trials. Eur. J. Pain 2018, 22, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Wang, D. The Effect of Ketamine Infusion in the Treatment of Complex Regional Pain Syndrome: A Systemic Review and Meta-analysis. Curr. Pain Headache Rep. 2018, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Orhurhu, V.; Orhurhu, M.S.; Bhatia, A.; Cohen, S.P. Ketamine Infusions for Chronic Pain: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Anesth. Analg. 2019, 129, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, A.; Cubała, W.J. Safety and Tolerability of Ketamine Use in Treatment-Resistant Bipolar Depression Patients with Regard to Central Nervous System Symptomatology: Literature Review and Analysis. Medicina 2020, 56, 67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murck, H. Ketamine, magnesium and major depression—From pharmacology to pathophysiology and back. J. Psychiatr. Res. 2013, 47, 955–965. [Google Scholar] [CrossRef]
- Brinck, E.C.; Tiippana, E.; Heesen, M.; Bell, R.F.; Straube, S.; Moore, R.A.; Kontinen, V. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2018, 12, CD012033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balzer, N.; McLeod, S.L.; Walsh, C.; Grewal, K. Low-dose Ketamine for Acute Pain Control in the Emergency Department: A Systematic Review and Meta-analysis. Acad. Emerg. Med. 2021, 28, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, H.; Hai, Y.; Cheng, Y. Perioperative Low-Dose Ketamine for Postoperative Pain Management in Spine Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Res. Manag. 2022, 2022, 1507097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abouarab, A.H.; Brülle, R.; Aboukilila, M.Y.; Weibel, S.; Schnabel, A. Efficacy and safety of perioperative ketamine for the prevention of chronic postsurgical pain: A meta-analysis. Pain Pract. 2024, 24, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Niciu, M.J.; Luckenbaugh, D.A.; Ionescu, D.F.; Richards, E.M.; Voort, J.L.V.; Ballard, E.D.; Brutsche, N.E.; Furey, M.L.; Zarate, C.A. Ketamine’s antidepressant efficacy is extended for at least four weeks in subjects with a family history of an alcohol use disorder. Int. J. Neuropsychopharmacol. 2014, 18, pyu039, Erratum in Int. J. Neuropsychopharmacol. 2016, 19, pyw031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Reference | Country | Ketamine Group | Non-Ketamine Group | Results between Groups | ||
|---|---|---|---|---|---|---|
| Patients | Intervention | Patients | Intervention | |||
| Carver, et al., 2018 [24] | The United States of America | N = 45 Age = 49 Patients with multiples ribs fracture | Infusion of LDK (2.5 μg·kg−1·min−1) within 12 h of a patient’s arrival at the institution and were continued for a total of 48 h unless safety concerns prompted otherwise | N = 46 Age = 46 Patients with multiple rib fractures | A similar dose of placebo was administered | While no difference was noted in NPS or OME within the entire cohort at 12 h, 24 h, or 48 h, LDK significantly reduced OME utilization in severely injured patients (ISS, >15). NPS at 12–24 h: mean 5.9, SD 2.0, p = 0.36 NPS at 24–48 h: mean 5.7, SD 2.0, p = 0.77 OME at 12–24 h: mean 57.3, SD 57.1, p = 0.79 OME at 24–48 h, mean 99.6, SD 157.2, p = 0.63 |
| Czarnetzki, et al., 2019 [25] | Switzerland | N = 80 Age = 67.0 Patients subjected to major lower back surgery | Intravenous ketamine 0.25 mg/kg preoperatively, followed by 0.25 mg/kg/h intraoperatively and 0.1 mg/kg/h from 1 h before the end of surgery until medical discharge | N = 80 Age = 66.0 Patients subjected to major lower back surgery | Similar volume of placebo (physiological saline), the dosing of which was based on ideal body weight. | DN4 score ≥ 4 at baseline p = 1, DN4 score ≥ 4 at 6 months p = 0.607, DN4 ≥ 4 at 12 months p = 0.319 Total score COMI at baseline p = 0.957; at 6 months p = 0.946; at 12 months p = 0.841 * MEAN AND SD * |
| Jafarinia, et al., 2016 [26] | Iran | N = 20 Age = 40.7 Patients with depression and chronic pain | 50 mg ketamine (50 mg capsules) thrice daily for 6 weeks. | N = 20 Age = 38.95 Patients with depression and chronic pain | 50 mg diclofenac (50 mg capsules) thrice daily for 6 weeks. | There was no significant difference between the mean VAS scores for ketamine and diclofenac arms at baseline, 3 weeks post-treatment, and at the study end point (72 ± 17.95 vs. 69.50 ± 18.77, p-value = 0.669, 55.70 ± 29.91 vs. 55.35 ± 30.07, p-value = 0.960, 55.25 ± 26.08 vs. 49.95 ± 30.58, p-value = 0.577; Analysis of GLM repeated- measure ANOVA confirmed the effect size of time × treatment was not significant throughout the trial period (F1.71, 64.84 = 0.289, p-value = 0.715). Mean (95%CI) difference in changes in the VAS score between ketamine and diclofenac intervention groups were not statistically different at week 3 or the study endpoint at week 6 (16.30 ± 17.86 vs. 14.25 ± 14.17; mean difference: ketamine−diclofenac (95% CI): 2.05 (−8.27 to 12.37); Cohen’s d: 0.13; p-value = 0.690 and 16.65 ± 22.67 vs. 19.55 ± 24.69; mean difference: ketamine−diclofenac (95% CI): −2.90 (−18.07 to 12.27); Cohen’s d: −0.12; p-value = 0.701 |
| Jain, et al., 2022 [27] | India | N = 25 Age = 33.44 Patients undergoing elective laparoscopic cholecystectomy under general anesthesia of GRADE I or II with chronic pain | Ketamine (0.5 mg/kg) intravenous injection after LC | N = 25 Age = 37.64 Patients undergoing elective laparoscopic cholecystectomy under general anesthesia of GRADE I or II with chronic pain | Normal saline (2 mL) intravenous injection after LC | NRS at 1 h: mean 1.44; SD 0.77; p = 0.056 NRS at 2 h: mean 1.40; SD 0.76; p = 0.13 NRS at 4 h: mean 1.44; SD 0.82; p = 0.29 NRS at 6 h: mean 1.76; SD 1.09; p = 0.623 NRS at 8 h: mean 2.28; SD 1.34; p = 0.18 NRS at 12 h: mean 2.04; SD 1.17; p = 0.207 NRS at 24 h: mean 1.44; SD 0.82; p = 0.137 |
| Lauretti, et al., 1999 [28] | Brazil | N = 12 Age = 56 Patients with terminal cancer suffering from chronic pain | Received 0.2 mg/kg epidural ketamine (2 mL) | N1 = 12 Age = 54 N2 = 12 Age = 50 N3 = 12 Age = 55 Patients with terminal cancer suffering from chronic pain | Group 1: received 2 mg of epidural morphine (2 mL). Group 2: received 2 mg of epidural morphine (2 mL). Group 3: received 500 mg epidural midazolam (2 mL). | VAS score: mean: 9; DS: 1: p = 0.222 morp |
| Lumanauw, et al., 2019 [29] | The United States of America | Group 1 N = 30 Age = 47.8 Group 2 N = 35 Age = 44.3 Patients with acute exacerbations of chronic pain | Group 1 = 0.5 mg/kg intravenous ketamine Group 2 = 0.25 mg/kg intravenous ketamine | n = 32 Age = 47.6 Patients with acute exacerbations of chronic pain | Similar volume of placebo (physiological saline), the dosing of which was based on ideal body weight | VAS at baseline: Pain group 1, mean 91.4, SD 8.5 Pain group 2, mean 93.2, SD 8.9 Pain group 3 (placebo), mean 91.2, SD 9.4 Both ketamine groups were superior to placebo, to successful improvement in their pain p = 0.001 |
| Nielsen, et al., 2017 [30] | The United States of America | N = 74 Age = 57 Patients subjected to spinal fusion surgery | Intraoperative S-ketamine bolus 0.5 mg/kg, followed by an infusion 0.25 mg/kg*h | N = 73 Age = 55 Patients subjected to spinal fusion surgery | Isotonic sodium chloride, bolus, and infusion, the dosing of which was based on ideal body weight | Pain (VAS) at rest in the ketamine group, mean 46, SD 19 Placebo group, mean 48, SD 20 p = 0.62 |
| Rakhman, et al., 2011 [31] | Israel | Group 1 (K1) N = 20 Age = 46 Group 2 (K2) N = 20 Age = 45 Group 3 (K3) N = 20 Age = 46 Patients undergoing tumor resection | Group 1 = Ketamine 25 mg at 4 h preoperatively Group 2 = ketamine 10 mg at 11 h preoperatively, and 25 mg at 4 h preoperatively Group 3 = ketamine 5 mg at 17 h preoperatively, 10 mg at 11 h preoperatively, and 25 mg at 4 h preoperatively | Group 1 (P1) N = 20 Age = 45 Group 2 (P2) N = 20 Age = 43 Group 3 (P3) N = 20 Age = 47 Patients undergoing tumor resection | Group 1 = 1 mL normal saline at 4 h preoperatively Group 2 = 1 mL normal saline at 11 and 4 h preoperatively Group 3 = 1 mL normal saline at 17, 11 and 4 h preoperatively | NRS (numerical rating scale), pain score K1 = NRS mean 5.4, SD = 2.06 K2 = NRS mean 6.41, SD = 0.95 K3 = NRS mean 6.28, SD = 1.1 P1 = NRS mean 5.0, SD = 1.71 P2 = NRS mean 4.69, SD = 1.69 P3 = NRS mean 5.16, SD = 1.75 Patients self-rated satisfaction scores were better in the K2 and K3 patients compared with their control counterparts (p < 0.005) |
| Rigo, et al., 2017 [32] | Brazil | Ketamine group N = 11 Age = 54 Patients with neuropathic chronic pain | Ketamine 30 mg oral, 3 times a day | Methadone group N = 13 Age = 52 Methadone + ketamine group N = 13 Age = 45 Patients with neuropathic chronic pain | Methadone 3 mg oral, 3 times a day Methadone + ketamine group = Methadone 3 mg, oral plus 30 mg of ketamine oral, 3 times a day | Visual analog scale (VAS) VAS after 90 days of treatment Ketamine group = mean 1.6, SD = 1.3 Methadone group = mean 1.3, SD = 1.0 Methadone + ketamine group= mean 2.2, SD = 1.1 p < 0.001 |
| Sigtermans, et al., 2009 [33] | The Netherlands | N = 30 Age = 43.7 Patients with complex regional pain syndrome type 1 | Ketamine 1.2 μg/kg per min, intravenous | N = 30 Age = 47.5 Patients with complex regional pain syndrome type 1 | Similar volume of placebo (physiological saline), the dosing of which was based on ideal body weight | NRS (numerical rating scale), pain score at end of week one, ketamine group = mean 2.68, SD = 0.51 Placebo group = mean 5.35, SD = 0.48 p < 0.001 |
| Yazigi, et al., 2012 [34] | Lebanon | N = 30 Age = 57.3 Patients subjected to a thoracotomy | Ketamine (0.1 mg/kg as a preincisional bolus followed by a continuous infusion of 0.05 mg/kg/h) | N = 30 Age = 56.9 Patients subjected to a thoracotomy | Similar volume of placebo (physiological saline), the dosing of which was based on ideal body weight | They were not significantly different between the two groups at any time point of the study, at rest (p = 0.75) or during coughing (p = 0.70) |
| Aveline, et al., 2014 [10] | France | N = 24 Age = 71 Patients with osteoarthritis scheduled for elective tricompartmental TKA performed | Ketamine 0.2 mL/kg bolus over 20 min started before surgical incision, followed by a continuous infusion of 120 mg/kg/h until the end of surgery and then 60 mg/kg/h until the second post- operative day | N nefopam = 22 Age = 71 N placebo = 23 Age = 71 Patients with osteoarthritis scheduled for elective tricompartmental TKA | Nefopam 0.2 mL/kg bolus over 20 min started before surgical incision, followed by a continuous infusion of 120 mg/kg/h until the end of surgery and then 60 mg/kg/h until the second postoperative day Infusion of isotonic saline | The RR of having CP during movement was not significantly decreased by ketamine and nefopam (ketamine vs. placebo: RR 0.48 [95% CI, 0.14- 1.69, p = 0.25]; nefopam vs. placebo: RR 0.52 [95% CI, 0.15–1.84, p = 0.31]). Ketamine and nefopam did not decrease the RR of having a DN4 score Z4 at M12 compared with placebo (RR 0.48 [95% CI, 0.1–2.37], p = 0.36 and RR 0.27 [95% CI, 0.03–2.16], p = 0.22, respectively). No difference was documented between ketamine and nefopam (RR 1.91; 95% CI, 0.19–19.52; p = 0.59). |
| Hardy, et al., 2012 [3] | Australia | N = 93 Age = 63.0 Patients with cancer pain | Subcutaneous infusion of ketamine at three doses levels (100, 300, or 500 mg) | N = 92 Age = 64.3 Patients with cancer pain | Similar volume of placebo (physiological saline), the dosing of which was based on ideal body weight | BPI pain score Ketamine group average mean 5.43, SD = 1.3 Placebo group average mean 5.21, SD = 1.4 The difference in absolute terms is small (0.71) and was not clinically significant because the difference was not ≥BPI units. |
| Hassan, et al., 2021 [6] | Egypt | N = 44 Age = 50.14 Patients undergoing cancer breast surgeries | Ketamine group (K) 0.5 mg/kg bolus, then 0.12 mg/kg/h infusion for the first 24 h postoperatively | N = 43 Age = 50.91 Patients undergoing cancer breast surgeries | Group KM: ketamine 0.5 mg/kg and mg sulfate 50 mg/kg, then ketamine 0.12 mg/kg/h and Mg sulfate 8 mg/kg/h infusions for the first 24 h postoperatively | NRS (numerical rating scale), pain score Group K in the first 24 h at Rest = mean 1, SD = X Movement = mean 3, SD = X Group KM in the first 24 h Rest = mean 1, SD = X Movement = mean 3, SD = X Rest p = 0.193 Movement p = 0.255 |
| Author | Country | Total N in Experimental Group | Characteristics and Doses in Experimental Group | Total N in Control Group | Characteristics and Doses in Control Group | Outcomes |
|---|---|---|---|---|---|---|
| Peyton, et al., 2017 [20] | The United States of America | N = 40 Age = 55.3 Patients with chronic pain after a thoracic or abdominal surgery | Ketamine 0.5 mg/kg preincision, 0.25 mg/kg/hour intraoperatively and 0.1 mg/kg/ hour for 24 h | N = 40 Age = 55.3 Patients with chronic pain after a thoracic or abdominal surgery | Similar volume of placebo (physiological saline), the dosing of which was based on ideal body weight. | NRS pain severity score (median [interquartile range, IQR]) for average pain in the previous 24 h among those patients reporting CPSP was 17.5/100 (IQR (0–40)) |
| Remérand, et al., 2009 [21] | France | N = 79 Age = 64 Patients with chronic pain after total hip arthroplasty | Ketamine 0.5 mg/kg IV before incision and a 24 h infusion of ketamine 2 μg/kg per min | N = 75 Age = 65 Patients with chronic pain after total hip arthroplasty | Similar volume of placebo (physiological saline), the dosing of which was based on ideal body weight. | NRS (numerical rating scale), worst pain score, day 0 to day 3 pain NRS Ketamine = mean 41, SD = 28 Placebo = mean 45, SD = 35 Worst, day 4 to day 7 pain NRS Ketamine = mean 31, SD = 25 Placebo = mean 37, SD = 23 |
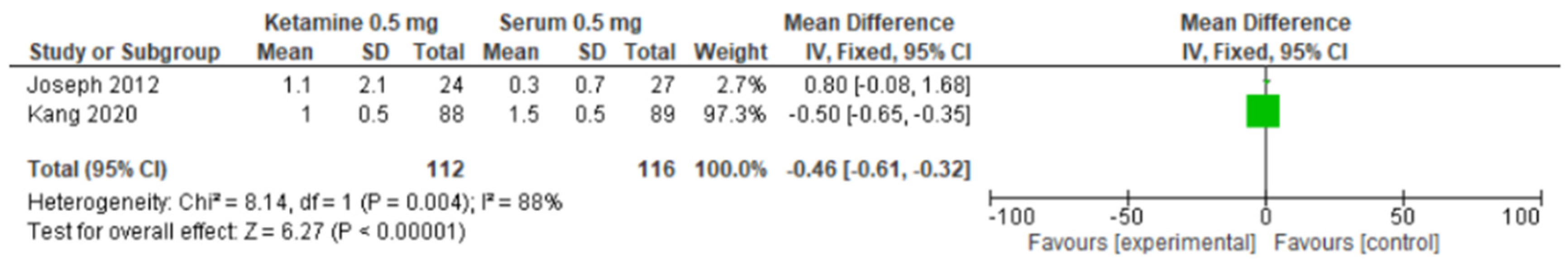
| Joseph, et al., 2012 [18] | France | N = 24 Age = 60 Patients planned for an elective partial pneumonectomy (partial or total lobectomy involving one or more lobes, except total pneumonectomy) by posterolateral or lateral thoracotomy | Received a combination of the continuous i.v. infusion of ketamine for 48 h and patient-controlled thoracic epidural analgesia (PCEA) with ropivacaine 1.5 mg/mL during the thoracotomy postoperative period. An i.v. ketamine infusion was standardized as follows: 0.5 mg/kg of ketamine during anesthesia induction and an intraoperative continuous i.v. infusion of ketamine 3 μg kg−1 min−1 following by a postoperative infusion of ketamine 1.5 μg kg−1 min−1 during the postoperative 48 h, starting at the end of the surgery | N = 27 Age = 60 Patients planned for an elective partial pneumonectomy (partial or total lobectomy involving one or more lobes, except total pneumonectomy) by posterolateral or lateral thoracotomy | Were given a combination of continuous i.v. infusion of saline solution and PCEA with ropivacaine 1.5 mg/mL during the thoracotomy postoperative period. The saline solution was administered using the same protocol and the same duration | NRS (rest) first month in ketamine group: mean: 0.9; SD: 1.2; p = 0.827 NRS (rest) third month in ketamine group: mean: 1.1; SD: 2.1; p = 0.385 NRS (abduction) first month in ketamine group: mean: 1.2; SD: 1.5; p = 0.909 NRS (abduction) third month in ketamine group: mean: 1.3; SD: 2.5; p = 0.589 |
| Kang, et al., 2020 [19] | South Korea | N = 88 Age = 49.7 Patients scheduled for elective unilateral breast cancer surgery | Infusion of 100 mg of ketamine (2 mg/mL) with 48 mL of 0.9% normal saline | N = 89 Age = 50.8 Patients scheduled for elective unilateral breast cancer surgery | Infusion of 50 mL of 0.9% normal saline | NRSr after 1 month: mean: 1.0; interquartile range: 0–3.0; p = 0.667 NRSr after 3 months: mean: 1.0; interquartile range: 0–2.0; p = 0.696 NRSr after 6 months: mean: 0; interquartile range: 0–2.0; p = 0.929 NRSd after 1 month: mean: 3.0; interquartile range: 1.0–4.0; p = 0.168 NRSd after 3 months: mean: 2.0; interquartile range: 0–3.0; p = 0.119 NRSd after 6 months: mean: 1.0; interquartile range: 0–3.0; p = 0.474 DN-4 after 1 month: mean: 5.0; p = 0115 DN-4 after 3 months: mean: 3.0; p = 0.720 DN4-4 after 6 months: mean: 1.0; p = 0.210 |
| Randy, et al., 2010 [22] | The United States of America | N = 52 Age = 51.7 Patients with chronic back pain undergoing back surgery | Ketamine 0.5 mg/kg intravenous on induction of anesthesia, and a continuous infusion at 10 μg/kg per min on induction and terminated at wound closure. | N = 50 Age = 51.4 Patients with chronic back pain undergoing back surgery | Similar volume of placebo (physiological saline), the dosing of which was based on ideal body weight. | Visual analog scale (VAS) VAS 24 h Ketamine group = mean 4.7, SD = 2.7 Placebo group = mean 4.8, SD = 2.4 p = 0.902 VAS 48 h Ketamine group = mean 5.4, SD = 2.1 Placebo group = mean 5.3, SD = 2.2 p = 0.838 VAS 6 weeks Ketamine group = mean 3.1, SD = 2.4 Placebo group = mean 4.2, SD = 2.4 p = 0.026 |
| Lee, et al., 2017 [23] | South Korea | N = 32 Age = 37 Patients scheduled for robotic thyroidectomy | Bolus dose of 0.15 mg/kg of racemic ketamine after anesthetic induction. Racemic ketamine was also infused continuously until the end of the surgery at a rate of 2 mg/kg/min | N = 32 Age = 38 Patients scheduled for robotic thyroidectomy | Similar volume of placebo (physiological saline), the dosing of which was based on ideal body weight. | There was a statistically significant difference in the VAS pain scores at rest and while coughing until 24 h postoperatively between the two groups (p = 0.028 and p = 0.039, respectively) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruna-Mejias, A.; Baeza, V.; Gamboa, J.; Baez Flores, B.; San Martin, J.; Astorga, C.; Leyton, J.; Nova-Baeza, P.; Orellana-Donoso, M.; Suazo-Santibañez, A.; et al. Use of Ketamine in Patients with Multifactorial Neuropathic Pain: A Systematic Review and Meta-Analysis. Pharmaceuticals 2024, 17, 1165. https://doi.org/10.3390/ph17091165
Bruna-Mejias A, Baeza V, Gamboa J, Baez Flores B, San Martin J, Astorga C, Leyton J, Nova-Baeza P, Orellana-Donoso M, Suazo-Santibañez A, et al. Use of Ketamine in Patients with Multifactorial Neuropathic Pain: A Systematic Review and Meta-Analysis. Pharmaceuticals. 2024; 17(9):1165. https://doi.org/10.3390/ph17091165
Chicago/Turabian StyleBruna-Mejias, Alejandro, Vicente Baeza, Javiera Gamboa, Belen Baez Flores, Jessica San Martin, Constanza Astorga, Javiera Leyton, Pablo Nova-Baeza, Mathias Orellana-Donoso, Alejandra Suazo-Santibañez, and et al. 2024. "Use of Ketamine in Patients with Multifactorial Neuropathic Pain: A Systematic Review and Meta-Analysis" Pharmaceuticals 17, no. 9: 1165. https://doi.org/10.3390/ph17091165
APA StyleBruna-Mejias, A., Baeza, V., Gamboa, J., Baez Flores, B., San Martin, J., Astorga, C., Leyton, J., Nova-Baeza, P., Orellana-Donoso, M., Suazo-Santibañez, A., Becerra-Farfán, A., Oyanedel-Amaro, G., & Valenzuela-Fuenzalida, J. J. (2024). Use of Ketamine in Patients with Multifactorial Neuropathic Pain: A Systematic Review and Meta-Analysis. Pharmaceuticals, 17(9), 1165. https://doi.org/10.3390/ph17091165






