Anticancer Activity of Imidazolyl Gold(I/III) Compounds in Non-Small Cell Lung Cancer Cell Lines
Abstract
1. Introduction
2. Results and Discussion
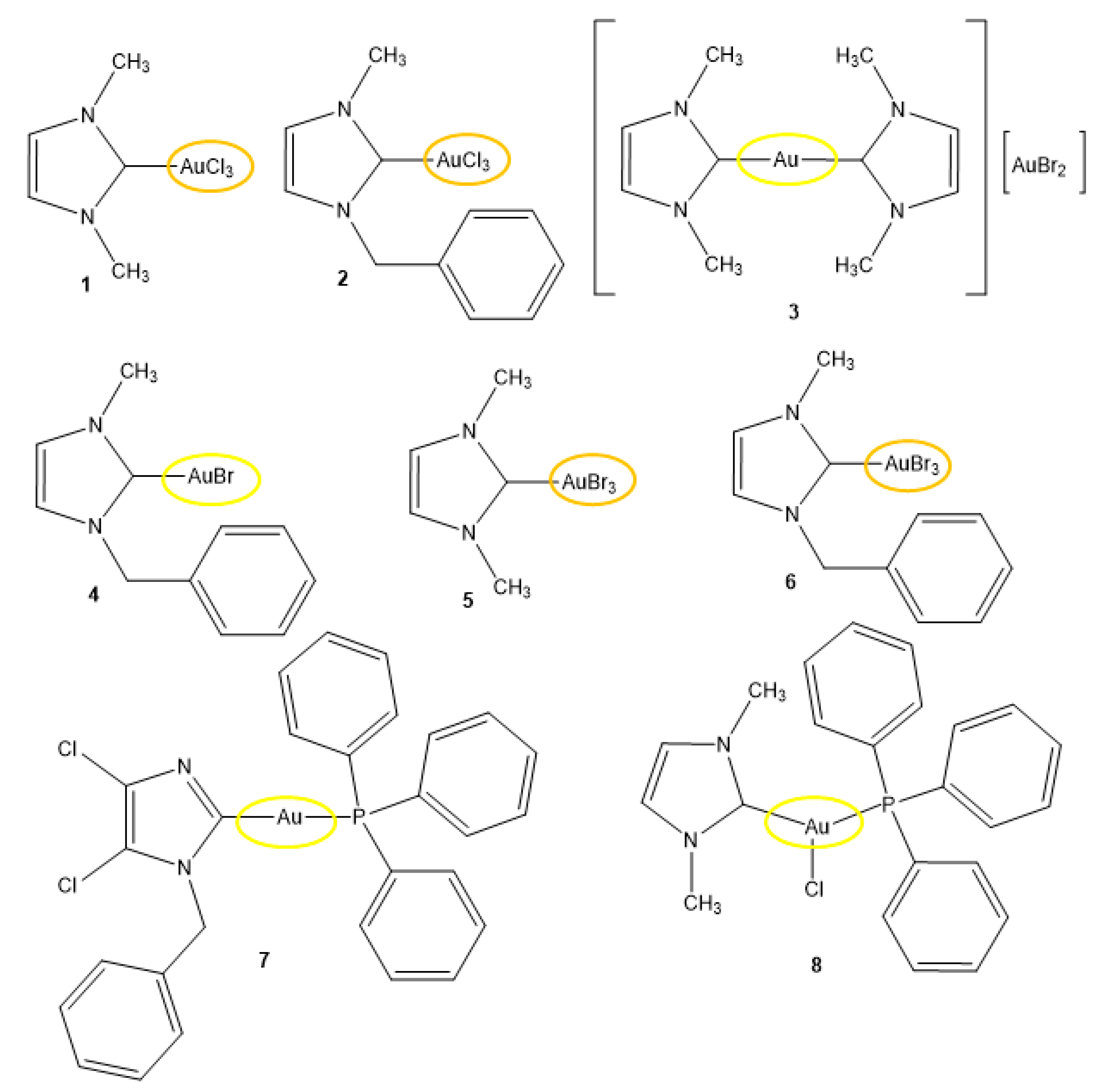
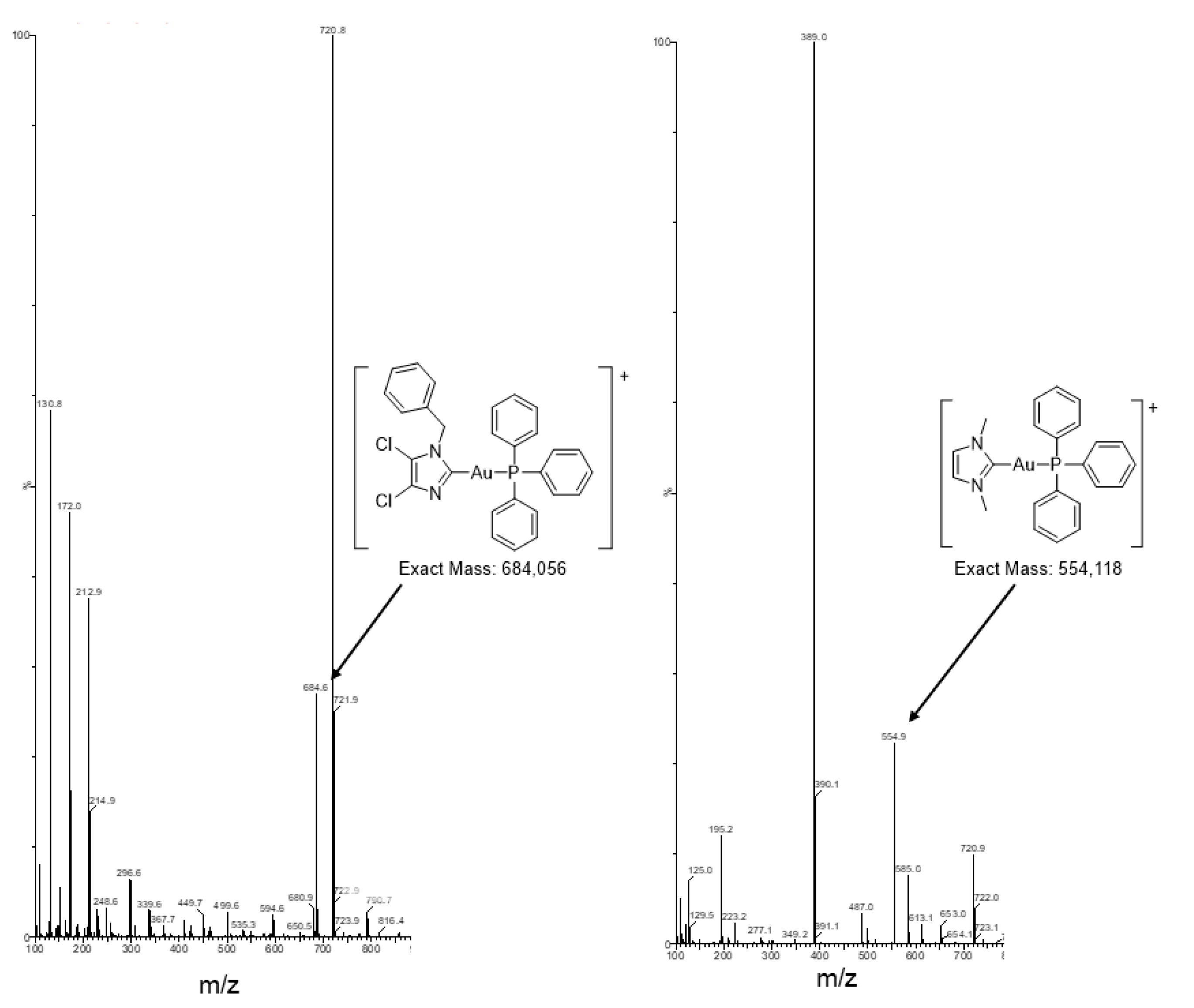
2.1. Synthesis of the Gold(I/III) Compounds
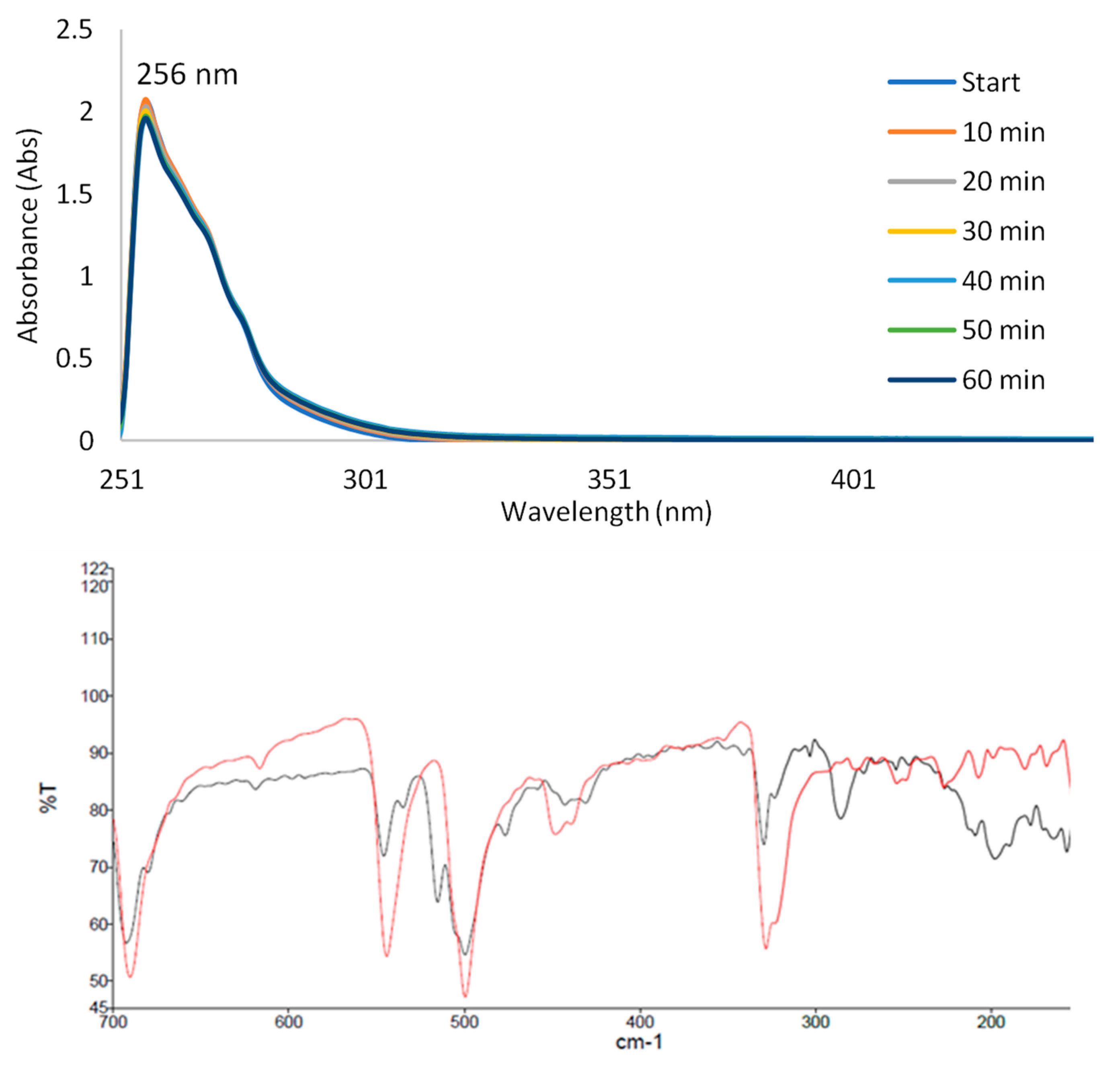
Solution Stability Studies
2.2. The Au(I) Compounds with the C-Au-P Environment Are the Most Cytotoxic
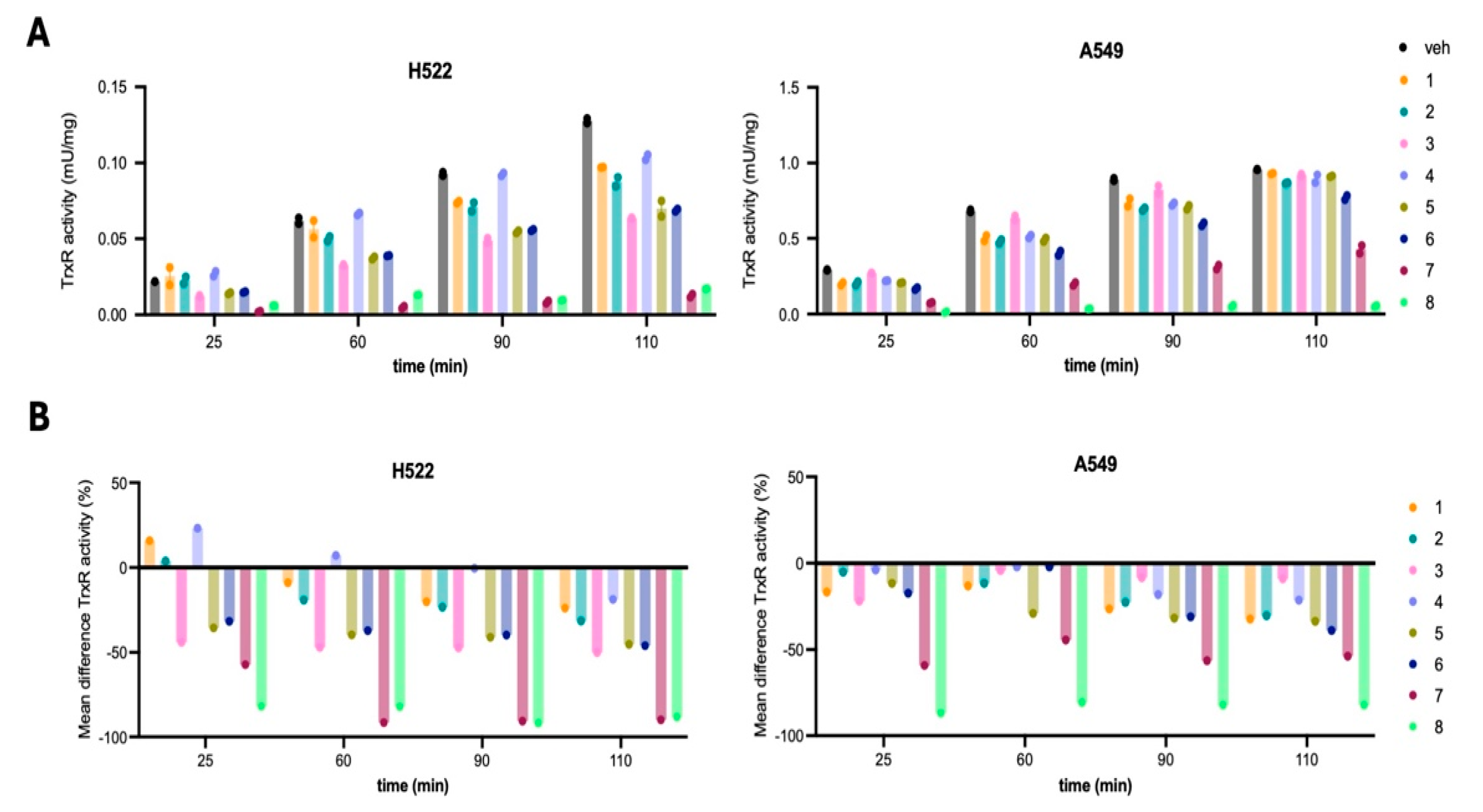
2.3. TrxR Inhibition Activity of the Au Compounds Correlates with Their Cytotoxic Capability
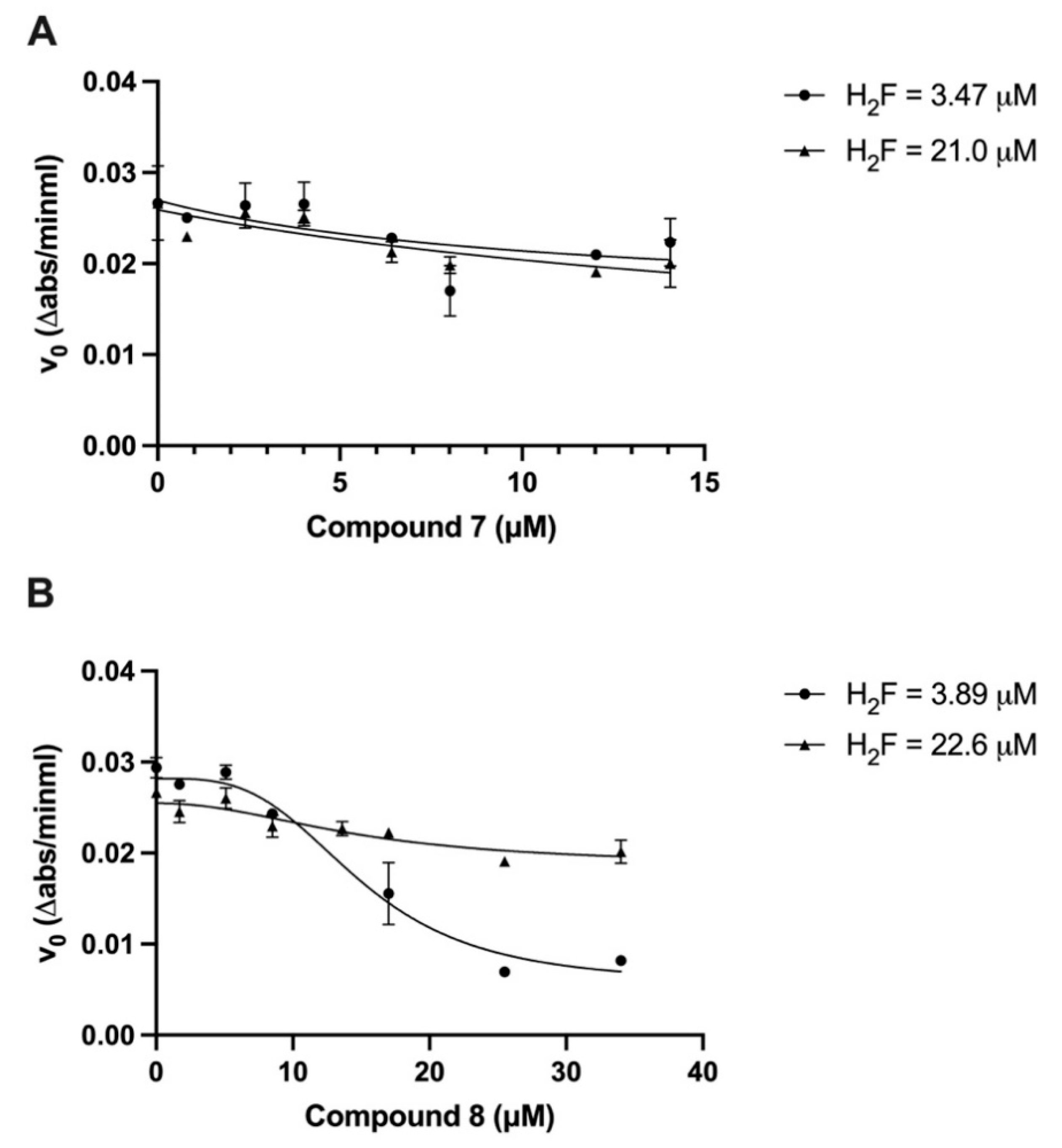
2.4. The Trigonal Au(I) Compound 8 with the C-Au-P Environment Inhibits Human DHFR
3. Materials and Methods
3.1. Materials
3.2. Characterizations
3.3. Preparations
3.3.1. Synthesis of Compound 1
3.3.2. Synthesis of Compound 2
3.3.3. Synthesis of Compound 3
3.3.4. Synthesis of Compound 4
3.3.5. Synthesis of Compound 5
3.3.6. Synthesis of Compound 6
3.3.7. Synthesis of Compound 7
3.3.8. Synthesis of Compound 8
3.4. Cell Lines
3.5. MTT Assays
3.6. Thioredoxin Reductase (TrxR) Activity
3.7. Cell-Free Human Dihydrofolate Reductase (hDHFR) Enzymatic Activity
3.8. Statistics, Data Representation, and Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Shao, W.; Mishina, Y.M.; Feng, Y.; Caponigro, G.; Cooke, V.G.; Rivera, S.; Wang, Y.; Shen, F.; Korn, J.M.; Griner, L.M.A.; et al. Antitumor Properties of RAF709, a Highly Selective and Potent Inhibitor of RAF Kinase Dimers, in Tumors Driven by Mutant RAS or BRAF. Cancer Res. 2018, 78, 1537–1548. [Google Scholar] [CrossRef]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Corcoran, R.B. A single inhibitor for all KRAS mutations. Nat. Cancer 2023, 4, 1060–1062. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ostrem, J.; Pellini, B.; Imbody, D.; Stern, Y.; Solanki, H.S.; Haura, E.B.; Villaruz, L.C. Overcoming KRAS-Mutant Lung Cancer; American Society of Clinical Oncology Educational Book: Alexandria, VA, USA, 2022; Volume 42, pp. 700–710. [Google Scholar]
- Mármol, I.; Quero, J.; Rodríguez-Yoldi, M.J.; Cerrada, E. Gold as a Possible Alternative to Platinum-Based Chemotherapy for Colon Cancer Treatment. Cancers 2019, 11, 780. [Google Scholar] [CrossRef]
- Lum, C.T.; Sun, R.W.-Y.; Zoua, T.; Che, C.-M. Gold(III) complexes inhibit growth of cisplatin-resistant ovarian cancer in association with upregulation of proapoptotic PMS2 gene. Chem. Sci. 2014, 5, 1579–1584. [Google Scholar] [CrossRef]
- Juzheng, Z.; Yanping, L.; Ronghao, F.; Wei, W.; Yong, W.; Jiamin, J.; Feng, Y.; Jian, C. Organometallic gold(I) and gold(III) complexes for lung cancer treatment. Front. Pharmacol. 2022, 13, 979951. [Google Scholar] [CrossRef]
- Arambula, J.F.; McCall, R.; Sidoran, K.J.; Magda, D.; Mitchell, N.A.; Bielawski, C.W.; Lynch, V.M.; Sesslerg, J.L.; Arumug, K. Targeting antioxidant pathways with ferrocenylated N-heterocyclic carbene supported gold(i) complexes in A549 lung cancer cells. Chem. Sci. 2016, 7, 1245–1256. [Google Scholar] [CrossRef]
- Galassi, R.; Burini, A.; Ricci, S.; Pellei, M.; Rigobello, M.P.; Citta, A.; Dolmella, A.; Gandin, V.; Marzano, C. Synthesis and characterization of azolate gold(I) phosphane complexes as thioredoxin reductase inhibiting antitumor agents. Dalton Trans. 2012, 41, 5307–5318. [Google Scholar] [CrossRef]
- Gambini, V.; Tilio, M.; Maina, E.W.; Andreani, C.; Bartolacci, C.; Wang, J.; Iezzi, M.; Ferraro, S.; Ramadori, A.T.; Simon, O.C.; et al. In vitro and in vivo studies of gold(I) azolate/phosphane complexes for the treatment of basal like breast cancer. Eur. J. Med. Chem. 2018, 155, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Martynova, E.A.; Scattolin, T.; Cavarzerani, E.; Peng, M.; Van Hecke, K.; Rizzolio, F.; Nolan, S.P. A simple synthetic entryway into new families of NHC–gold-amido complexes and their in vitro antitumor activity. Dalton Trans. 2022, 51, 3462–3471. [Google Scholar] [CrossRef]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Galassi, R.; Luciani, L.; Gambini, V.; Vincenzetti, S.; Lupidi, G.; Amici, A.; Marchini, C.; Wang, J.; Pucciarelli, S. Multi-Targeted Anticancer Activity of Imidazolate Phosphane Gold(I) Compounds by Inhibition of DHFR and TrxR in Breast Cancer Cells. Front. Chem. 2020, 8, 602845. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, N.; Borghese, C.; Corona, G.; Aldinucci, D.; Adam, A.; Sulaiman, A.A.; Isab, A.A.; Ahmad, S.; Peedikakkal, A.M.P. Dinuclear gold(I) complexes based on carbene and diphosphane ligands: Bis [2-(dicyclohexylphosphano)ethyl]amine complex inhibits the proteasome activity, decreases stem cell markers and spheroid viability in lung cancer cells. JBIC J. Biol. Inorg. Chem. 2023, 28, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zheng, W.; Fu, X.; Li, X.; Wong, Y.-S.; Chen, T. Enhancement of auranofin-induced lung cancer cell apoptosis by selenocystine, a natural inhibitor of TrxR1 in vitro and in vivo. Cell Death Dis. 2014, 5, e1191. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobello, M.P.; Scutari, G.; Gabbiani, C.; Casini, A.; Messori, L. Thioredoxin reductase: A target for gold compounds acting as potential anticancer drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar] [CrossRef]
- Nobili, S.; Mini, E.; Landini, I.; Gabbiani, C.; Casini, A.; Messori, L. Gold compounds as anticancer agents: Chemistry, cellular pharmacology, and preclinical studies. Med. Res. Rev. 2010, 30, 550–1128. [Google Scholar] [CrossRef]
- Alhoshani, A.; Alrashdi, A.; Alhosaini, K.; Alanazi, F.E.; Alajez, N.M.; Altaf, M.; Isab, A.A.; Korashy, H.M. Gold-containing compound BDG-I inhibits the growth of A549 lung cancer cells through the deregulation of miRNA expression. Saudi Pharm. J. 2018, 26, 1035–1043. [Google Scholar] [CrossRef]
- Casini, A.; Hartinger, C.; Gabbiani, C.; Mini, E.; Dyson, P.J.; Keppler, B.K.; Messori, L. Gold(III) compounds as anticancer agents: Relevance of gold-protein interactions for their mechanism of action. J. Inorg. Biochem. 2008, 102, 564–575. [Google Scholar] [CrossRef]
- Zoppi, C.; Messori, L.; Pratesi, A. ESI MS studies highlight the selective interaction of Auranofin with protein free thiols. Dalton Trans. 2020, 49, 5906–5913. [Google Scholar] [CrossRef] [PubMed]
- Pucciarelli, S.; Vincenzetti, S.; Ricciutelli, M.; Simon, O.C.; Ramadori, A.T.; Luciani, L.; Galassi, R. Studies on the Interaction between Poly-Phosphane Gold(I) Complexes and Dihydrofolate Reductase: An Interplay with Nicotinamide Adenine Dinucleotide Cofactor. Int. J. Mol. Sci. 2019, 20, 1802. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bensdorf, K.; Proetto, M.; Abram, U.; Hagenbach, A.; Gust, G. NHC Gold Halide Complexes Derived from 4,5-Diarylimidazoles: Synthesis, Structural Analysis, and Pharmacological Investigations as Potential Antitumor Agents. J. Med. Chem. 2011, 54, 8605–8615. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Hanoian, P.; French, J.B.; Pringle, T.H.; Hammes-Schiffer, S.; Benkovic, S.J. Functional significance of evolving protein sequence in dihydrofolate reductase from bacteria to humans. Proc. Natl. Acad. Sci. USA 2013, 110, 10159–10164. [Google Scholar] [CrossRef]
- Hamed, K.M.; Dighriri, I.M.; Baomar, A.F.; Alharthy, B.T.; Alenazi, F.E.; Alali, G.H.; Alenazy, R.H.; Alhumaidi, N.T.; Alhulayfi, D.H.; Alotaibi, Y.B.; et al. Overview of Methotrexate Toxicity: A Comprehensive Literature Review. Cureus 2022, 14, e29518. [Google Scholar] [CrossRef]
- Gridelli, C.; Maione, P.; Rossi, A.; Bareschino, M.A.; Schettino, C.; Sacco, P.C.; Zeppa, R. Pemetrexed in advanced non-small cell lung cancer. Expert Opin. Drug Saf. 2011, 10, 311–317. [Google Scholar] [CrossRef]
- Chan, M.; Gravel, M.; Bramoullé, A.; Bridon, G.; Avizonis, D.; Shore, G.C.; Roulston, A. Synergy between the NAMPT inhibitor GMX1777(8) and pemetrexed in non-small cell lung cancer cells is mediated by PARP activation and enhanced NAD consumption. Cancer Res. 2014, 74, 5948–5954. [Google Scholar] [CrossRef]
- Hussein, E.M.; Alsantali, R.I.; Abd El-Galil, S.M.; Obaid, R.J.; Alharbi, A.; Abourehab, M.A.S.; Ahmed, S.A. Bioactive fluorenes. part I. Synthesis, pharmacological study and molecular docking of novel dihydrofolate reductase inhibitors based-2,7-dichlorofluorene. Heliyon 2019, 5, e01982. [Google Scholar] [CrossRef]
- Vásquez, A.F.; Gómez, L.A.; González Barrios, A.; Riaño-Pachón, D.M. Identification of Active Compounds against Melanoma Growth by Virtual Screening for Non--Classical Human DHFR Inhibitors. Int. J. Mol. Sci. 2022, 23, 13946. [Google Scholar] [CrossRef]
- Sargentoni, N.; Galassi, R.; Luciani, L.; Rominger, F.; Rudolph, M.; Hashmi, A.S.K. Oxidation state and halogen influence on the NHC-gold-halide catalyzed cyclization of propargyl amides. Adv. Synth. Catal. 2024, accepted. [Google Scholar]
- Galassi, R.; Sargentoni, N.; Luciani, L.; Manca, G.; Ienco, A. Halogen addition to NHC-gold(I) chloride complexes in the framework of the Inverted Ligand Field. Inorg. Chim. Acta 2024, 560, 121810. [Google Scholar] [CrossRef]
- Luciani, L.; Sargentoni, N.; Graiff, C.; Monge, M.; Rodríguez-Castillo, M.; López-de-Luzuriaga, J.M.; Galassi, R. Mechanochemical preparation of strongly emissive monosubstituted triarylphosphane gold(I) compounds activated by hydrogen bonding driven aggregations. RSC Adv. 2023, 13, 25425–25436. [Google Scholar] [CrossRef] [PubMed]
- Hirtenlehner, C.; Krims, C.; Hölbling, J.; List, M.; Zabel, M.; Fleck, M.; Berger, R.J.F.; Schoefberger, W.; Monkowius, U. Syntheses, crystal structures, reactivity, and photochemistry of gold(III) bromides bearing N-heterocyclic carbenes. Dalton Trans. 2011, 40, 9899–9910. [Google Scholar] [CrossRef]
- Crabtree, R.H. NHC ligands versus cyclopentadienyls and phosphines as spectator ligands in organometallic catalysis. J. Organomet. Chem. 2005, 690, 5451–5457. [Google Scholar] [CrossRef]
- Huynh, H.V.; Guo, S.; Wu, W. Detailed Structural, Spectroscopic, and Electrochemical Trends of Halido Mono- and Bis(NHC) Complexes of Au(I) and Au(III). Organometallics 2013, 32, 4591–4600. [Google Scholar] [CrossRef]
- de Frémont, P.; Singh, R.; Stevens, E.D.; Petersen, J.L.; Nolan, S.P. Synthesis, Characterization and Reactivity of N-Heterocyclic Carbene Gold(III) Complexes. Organometallics 2007, 26, 1376. [Google Scholar] [CrossRef]
- Gazdar, A.F.; Girard, L.; Lockwood, W.W.; Lam, W.L.; Minna, J.D. Lung Cancer Cell Lines as Tools for Biomedical Discovery and Research. JNCI J. Natl. Cancer Inst. 2010, 102, 1310–1321. [Google Scholar] [CrossRef]
- Leiya, K.; Shuang, W.; Pei, K. Current Progress and Perspectives on Using Gold Compounds for the Modulation of Tumor Cell Metabolism. Front. Chem. 2021, 9, 733463. [Google Scholar] [CrossRef]
- Lim, J.K.M.; Leprivier, G. The impact of oncogenic RAS on redox balance and implications for cancer development. Cell Death Dis. 2019, 10, 955. [Google Scholar] [CrossRef]
- Wallace, L.A.; Matthews, C.R. Highly divergent dihydrofolate reductases conserve complex folding mechanism. J. Mol. Biol. 2002, 315, 193–211. [Google Scholar] [CrossRef]
- Moreno-Alcántar, G.; Picchetti, P.; Casini, A. Gold Complexes in Anticancer Therapy: From New Design Principles to Particle-Based Delivery Systems. Angew. Chem. Int. Ed. 2023, 62, e202218000. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, P.; Pisani, M.; Giorgini, E.; Rossi, B.; Damin, A.; Vita, F.; Francescangeli, O.; Luciani, L.; Galassi, R. Synchrotron Characterization of Hexagonal and Cubic Lipidic Phases Loaded with Azolate/Phosphane Gold(I) Compounds: A New Approach to the Uploading of Gold(I)-Based Drugs. Nanomaterials 2020, 10, 1851. [Google Scholar] [CrossRef]
- Ng, H.L.; Ma, X.; Chew, E.H.; Chui, W.K. Design, Synthesis, and Biological Evaluation of Coupled Bioactive Scaffolds as Potential Anticancer Agents for Dual Targeting of Dihydrofolate Reductase and Thioredoxin Reductase. J. Med. Chem. 2017, 60, 1734–1745. [Google Scholar] [CrossRef]
- Liang, J.; Lu, T.; Chen, Z.; Zhan, C.; Wang, Q. Mechanisms of resistance to pemetrexed in non-small cell lung cancer. Transl. Lung Cancer Res. 2019, 8, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Ozasa, H.; Oguri, T.; Uemura, T.; Miyazaki, M.; Maeno, K.; Sato, S.; Ueda, R. Significance of thymidylate synthase for resistance to pemetrexed in lung cancer. Cancer Sci. 2010, 101, 161–166. [Google Scholar] [CrossRef]
- Bär, S.I.; Gold, M.; Schleser, S.W.; Rehm, T.; Bär, A.; Köhler, L.; Carnell, L.R.; Biersack, B.; Schobert, R. Guided Antitumoural Drugs: (Imidazol-2-ylidene)(L)gold(I) Complexes Seeking Cellular Targets Controlled by the Nature of Ligand, L. Chemistry 2021, 27, 5003–5010. [Google Scholar] [CrossRef]
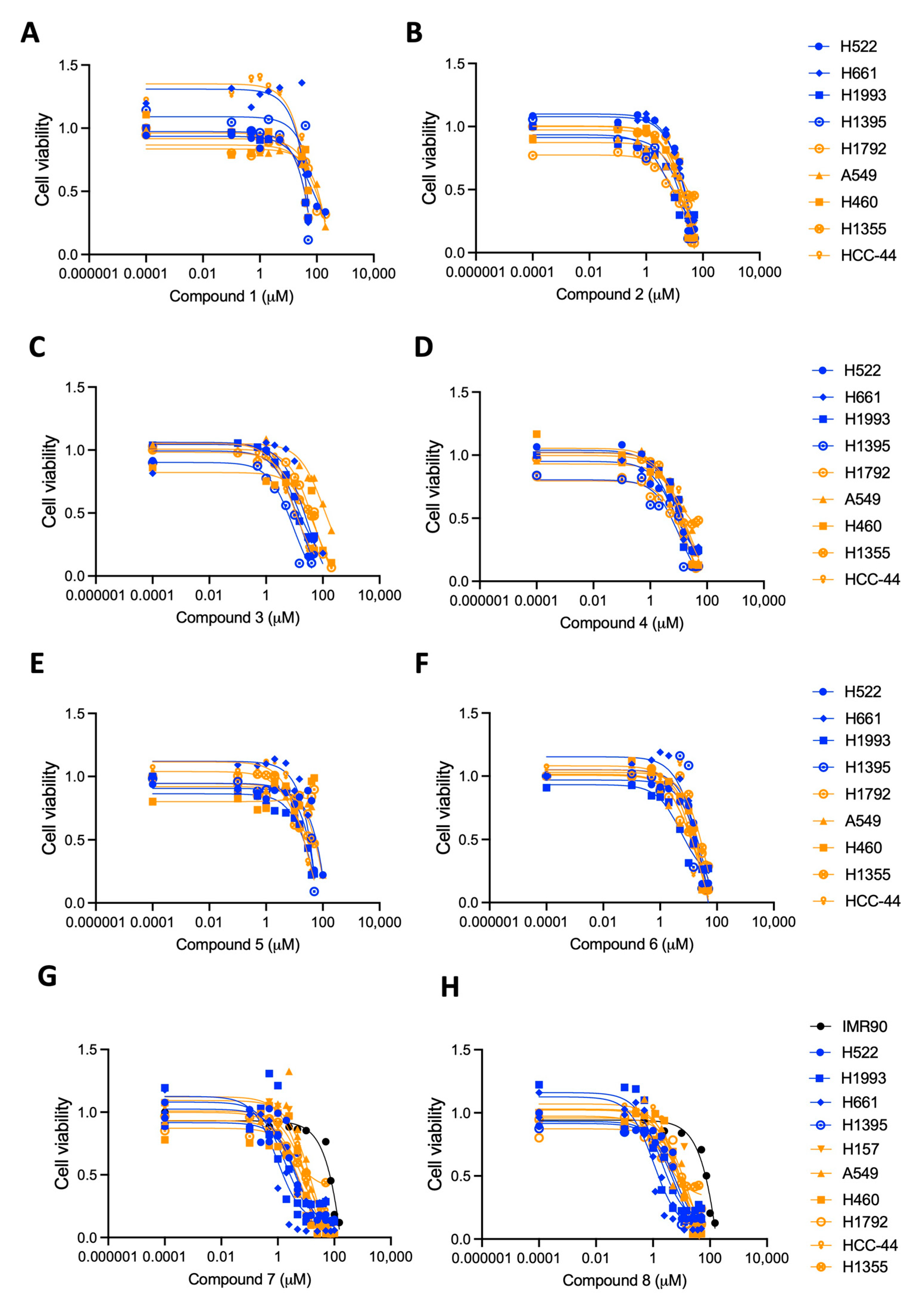

| # | H522 | H661 | H1395 | H1993 | IC50 * | H157 | A549 | H460 | H1792 | HCC-44 | H1355 | IC50 * | IMR90 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 ± 0.89 | 47 ± 1.1 | 47 ± 0.4 | 37 ± 1.3 | 52 | N.D. | >100 | 52 ± 1.2 | 60 ± 1.4 | 45 ± 0.25 | 41 ± 0.33 | 62 | N.D. |
| 2 | 53 ± 2.1 | 45 ± 1.7 | 17 ± 0.8 | 6.1 ± 0.76 | 30 | N.D. | 56 ± 1.6 | 65 ± 2.2 | 24 ± 0.98 | 26 ± 1.3 | 34 ± 1.4 | 41 | N.D. |
| 3 | 15 ± 1.1 | 27 ± 1.2 | 11 ± 0.63 | 11 ± 0.96 | 16 | N.D. | 91 ± 1.03 | 68 ± 0.10 | 50 ± 1.2 | 10 ± 1.1 | 9.1 ± 1.03 | 46 | N.D. |
| 4 | 5.3 ± 0.88 | 7 ± 0.81 | 11 ± 0.88 | 12 ± 1.03 | 9 | N.D. | 19 ± 1.0 | 11 ± 1.2 | 16 ± 0.94 | 14 ± 1.1 | 8.1 ± 0.66 | 14 | N.D. |
| 5 | 52 ± 0.37 | 42 ± 1.7 | 49 ± 0.11 | 70 ± 1.6 | 53 | N.D. | 49 ± 0.33 | >100 | >100 | 32 ± 1.6 | 12 ± 0.72 | 59 | N.D. |
| 6 | 55 ± 1.9 | 26 ± 1.5 | 62 ± 2.4 | 5.6 ± 0.76 | 37 | N.D. | 13 ± 1.03 | 37 ± 1.8 | 15 ± 1.1 | 19 ± 1.4 | 59 ± 1.7 | 29 | N.D. |
| 7 | 6.5 ± 0.97 | 0.95 ± 1.0 | 6.3 ± 0.98 | 1.8 ± 0.96 | 4 | 8.5 ± 1.2 | 16 ± 1.3 | 6.6 ± 1.02 | 12 ± 1.04 | 9.2 ± 1.02 | 7.8 ± 0.61 | 10 | 63 ± 2.5 |
| 8 | 3.3 ± 0.93 | 1.1 ± 1.1 | 5.9 ± 0.97 | 1.8 ± 1.05 | 3 | 17 ± 1.2 | 9.1 ± 1.2 | 13 ± 1.4 | 15 ± 1.1 | 7.3 ± 1.05 | 6.4 ± 0.65 | 11 | 87 ± 4.9 |
| Dunnett’s Comparison | H522 | A549 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean Diff. | 95.00% CI of Diff. | Summary | Adj p Value | Mean Diff. | 95.00% CI of Diff. | Summary | Adj p Value | |
| 25 min | ||||||||
| 1 vs. veh | 15.84 | −6.504 to 38.19 | ns | 0.2655 | −16.73 | −32.31 to −1.152 | * | 0.0306 |
| 2 vs. veh | 3.864 | −18.48 to 26.21 | ns | 0.9981 | −5.148 | −20.73 to 10.43 | ns | 0.918 |
| 3 vs. veh | −44.05 | −66.40 to −21.70 | **** | <0.0001 | −21.88 | −37.46 to −6.300 | ** | 0.0027 |
| 4 vs. veh | 23.18 | 0.8373 to 45.53 | * | 0.0391 | −3.861 | −19.44 to 11.72 | ns | 0.9819 |
| 5 vs. veh | −35.55 | −57.90 to −13.20 | *** | 0.0006 | −11.58 | −27.16 to 3.996 | ns | 0.2233 |
| 6 vs. veh | −31.68 | −54.03 to −9.338 | ** | 0.0024 | −17.37 | −32.95 to −1.795 | * | 0.023 |
| 7 vs. veh | −57.19 | −79.53 to −34.84 | **** | <0.0001 | −59.2 | −74.78 to −43.62 | **** | <0.0001 |
| 8 vs. veh | −81.92 | −104.3 to −59.57 | **** | <0.0001 | −86.87 | −102.5 to −71.29 | **** | <0.0001 |
| 60 min | ||||||||
| 1 vs. veh | −8.894 | −31.24 to 13.45 | ns | 0.8206 | −13.1 | −28.68 to 2.479 | ns | 0.1319 |
| 2 vs. veh | −19.16 | −41.50 to 3.190 | ns | 0.1201 | −11.64 | −27.22 to 3.934 | ns | 0.2189 |
| 3 vs. veh | −47.07 | −69.42 to −24.72 | **** | <0.0001 | −4.221 | −19.80 to 11.36 | ns | 0.97 |
| 4 vs. veh | 7.116 | −15.23 to 29.46 | ns | 0.9312 | −2.183 | −17.76 to 13.40 | ns | 0.9996 |
| 5 vs. veh | −39.68 | −62.03 to −17.34 | *** | 0.0001 | −29.11 | −44.69 to −13.53 | **** | <0.0001 |
| 6 vs. veh | −37.22 | −59.57 to −14.87 | *** | 0.0003 | −2.183 | −17.76 to 13.40 | ns | 0.9996 |
| 7 vs. veh | −91.41 | −113.8 to −69.06 | **** | <0.0001 | −44.4 | −59.97 to −28.82 | **** | <0.0001 |
| 8 vs. veh | −82.1 | −104.4 to −59.75 | **** | <0.0001 | −80.79 | −96.36 to −65.21 | **** | <0.0001 |
| 90 min | ||||||||
| 1 vs. veh | −20.1 | −42.44 to 2.252 | ns | 0.0938 | −26.55 | −42.13 to −10.98 | *** | 0.0002 |
| 2 vs. veh | −23.38 | −45.73 to −1.037 | * | 0.0368 | −22.67 | −38.25 to −7.090 | ** | 0.0018 |
| 3 vs. veh | −47.31 | −69.66 to −24.97 | **** | <0.0001 | −8.42 | −24.00 to 7.159 | ns | 0.5454 |
| 4 vs. veh | −0.3654 | −22.71 to 21.98 | ns | >0.9999 | −18.13 | −33.71 to −2.556 | * | 0.0163 |
| 5 vs. veh | −41.1 | −63.45 to −18.76 | **** | <0.0001 | −31.74 | −47.31 to −16.16 | **** | <0.0001 |
| 6 vs. veh | −39.82 | −62.17 to −17.48 | *** | 0.0001 | −31.09 | −46.67 to −15.51 | **** | <0.0001 |
| 7 vs. veh | −90.61 | −113.0 to −68.26 | **** | <0.0001 | −56.35 | −71.93 to −40.77 | **** | <0.0001 |
| 8 vs. veh | −91.89 | −114.2 to −69.54 | **** | <0.0001 | −82.25 | −97.83 to −66.68 | **** | <0.0001 |
| 110 min | ||||||||
| 1 vs. veh | −23.89 | −46.24 to −1.545 | * | 0.0316 | −32.38 | −47.96 to −16.80 | **** | <0.0001 |
| 2 vs. veh | −31.46 | −53.80 to −9.111 | ** | 0.0026 | −30.44 | −46.02 to −14.86 | **** | <0.0001 |
| 3 vs. veh | −50.17 | −72.52 to −27.83 | **** | <0.0001 | −9.068 | −24.65 to 6.512 | ns | 0.4662 |
| 4 vs. veh | −18.72 | −41.06 to 3.631 | ns | 0.1345 | −21.37 | −36.95 to −5.794 | ** | 0.0034 |
| 5 vs. veh | −45.26 | −67.61 to −22.91 | **** | <0.0001 | −33.68 | −49.26 to −18.10 | **** | <0.0001 |
| 6 vs. veh | −46.06 | −68.40 to −23.71 | **** | <0.0001 | −38.86 | −54.44 to −23.28 | **** | <0.0001 |
| 7 vs. veh | −89.86 | −112.2 to −67.51 | **** | <0.0001 | −53.76 | −69.34 to −38.18 | **** | <0.0001 |
| 8 vs. veh | −88.27 | −110.6 to −65.92 | **** | <0.0001 | −82.25 | −97.83 to −66.68 | **** | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galassi, R.; Sargentoni, N.; Renzi, S.; Luciani, L.; Bartolacci, C.; Pattabhi, P.; Andreani, C.; Pucciarelli, S. Anticancer Activity of Imidazolyl Gold(I/III) Compounds in Non-Small Cell Lung Cancer Cell Lines. Pharmaceuticals 2024, 17, 1133. https://doi.org/10.3390/ph17091133
Galassi R, Sargentoni N, Renzi S, Luciani L, Bartolacci C, Pattabhi P, Andreani C, Pucciarelli S. Anticancer Activity of Imidazolyl Gold(I/III) Compounds in Non-Small Cell Lung Cancer Cell Lines. Pharmaceuticals. 2024; 17(9):1133. https://doi.org/10.3390/ph17091133
Chicago/Turabian StyleGalassi, Rossana, Nicola Sargentoni, Sofia Renzi, Lorenzo Luciani, Caterina Bartolacci, Prasad Pattabhi, Cristina Andreani, and Stefania Pucciarelli. 2024. "Anticancer Activity of Imidazolyl Gold(I/III) Compounds in Non-Small Cell Lung Cancer Cell Lines" Pharmaceuticals 17, no. 9: 1133. https://doi.org/10.3390/ph17091133
APA StyleGalassi, R., Sargentoni, N., Renzi, S., Luciani, L., Bartolacci, C., Pattabhi, P., Andreani, C., & Pucciarelli, S. (2024). Anticancer Activity of Imidazolyl Gold(I/III) Compounds in Non-Small Cell Lung Cancer Cell Lines. Pharmaceuticals, 17(9), 1133. https://doi.org/10.3390/ph17091133









