Phytochemical Profiling, Antioxidant and Antimicrobial Potentials of Ethanol and Ethyl Acetate Extracts of Chamaenerion latifolium L.
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield Analysis
2.2. Phytochemical Study
2.2.1. Total Phenolic and Flavonoid Content
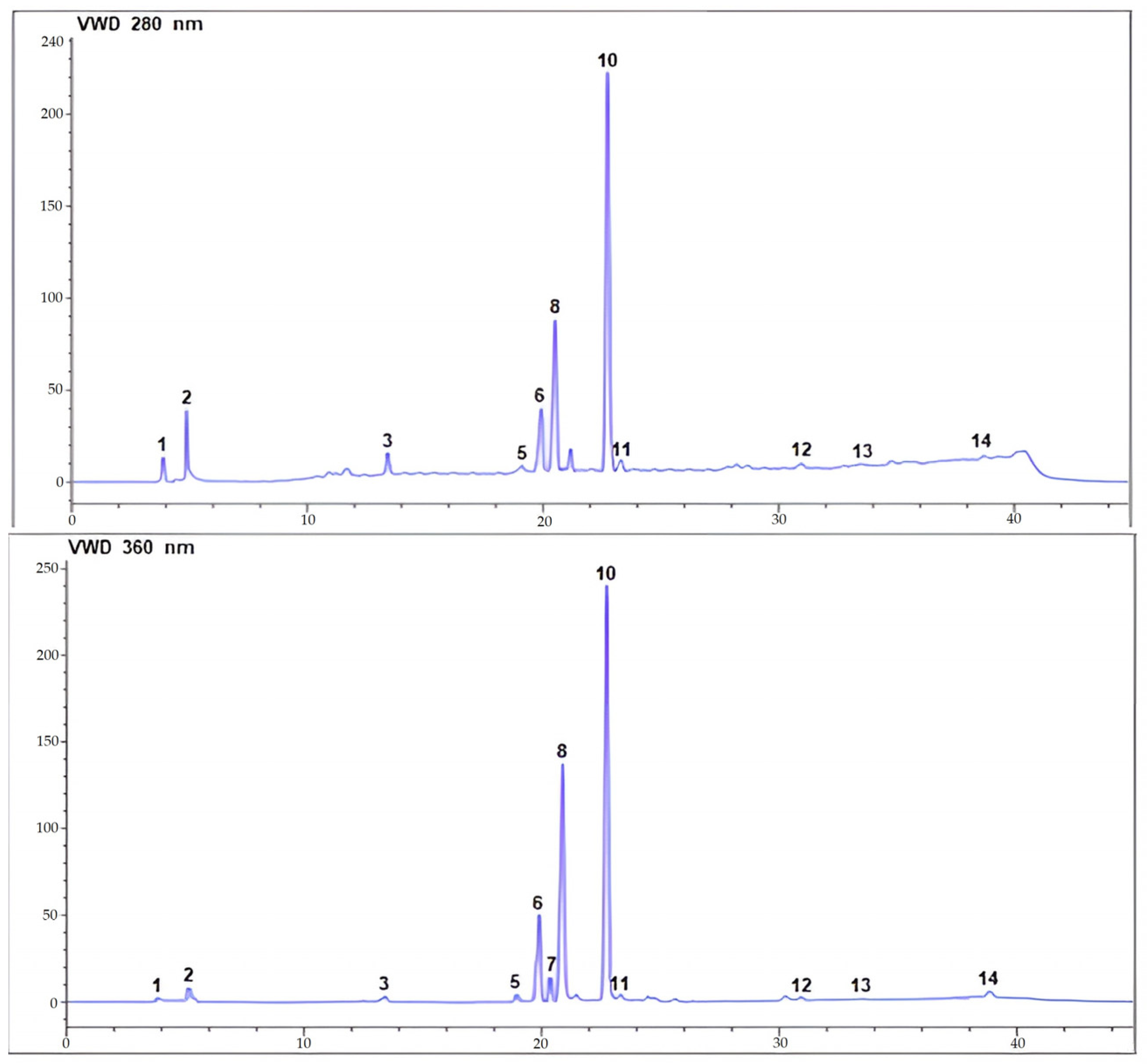
2.2.2. HPLC-UV-ESI/MS Analysis
2.2.3. FT-IR Analysis
2.3. Antioxidant Capacity
2.4. Antibacterial and Antifungal Activities
3. Materials and Methods
3.1. Reagents, Chemicals, and Standards
3.2. Plant Material
3.3. Extraction
3.4. Phytochemical Analysis
3.4.1. Total Phenolic and Flavonoid Content (TPC and TFC)
3.4.2. Analysis by High-Performance Liquid Chromatography with Ultraviolet Detection and Electrospray Ionization Mass Spectrometry (HPLC-UV-ESI/MS)
3.4.3. FTIR Analysis
3.5. Antioxidant Assays
3.5.1. DPPH Radical Scavenging Activity
3.5.2. FRAP Antioxidant Assay
3.6. Antibacterial and Antifungal Activity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H. Back to nature: Medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Rathor, L. Medicinal plants: A rich source of bioactive molecules used in drug development. In Evidence Based Validation of Traditional Medicines: A Comprehensive Approach; Mandal, S.C., Chakraborty, R., Sen, S., Eds.; Springer: Singapore, 2021; pp. 195–209. [Google Scholar]
- Yudaev, P.A.; Chistyakov, E.M. Progress in dental materials: Application of natural ingredients. Russ. Chem. Rev. 2024, 93, RCR5108. [Google Scholar] [CrossRef]
- Brinker, S.R. Discovery of Chamaenerion latifolium (L.) Holub (Onagraceae) in the Great Lakes Region. Great Lakes Bot. 2017, 55, 3–9. [Google Scholar]
- Wagner, W.L.; Hoch, P.C.; Raven, P.H. Revised classification of the Onagraceae. In Systematic Botany Monographs; Anderson, C., Ed.; American Society of Plant Taxonomists: Ann Arbor, MI, USA, 2007; Volume 83, pp. 1–240. [Google Scholar]
- Shawky, E.M.; Elgindi, M.R.; Ibrahim, H.A.; Baky, M.H. The potential and outgoing trends in traditional, phytochemical, economical, and ethnopharmacological importance of family Onagraceae: A comprehensive review. J. Ethnopharmacol. 2021, 281, 114450. [Google Scholar] [CrossRef]
- Iskakova, Z.; Kozhantayeva, A.; Tazhkenova, G.; Mashan, T.; Tosmaganbetova, K.; Tashenov, Y. Investigation of chemical constituents of Chamaenerion latifolium L. Antiinflamm. Antiallergy Agents Med. Chem. 2022, 21, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, V.; Kamarova, A.; Shalabayev, B.; Kussainov, A.; Anuarbekov, M.; Abeuov, S. Comparative analysis of the efficiency of medicinal plants for the treatment and prevention of COVID-19. Int. J. Biomater. 2022, 2022, 5943649. [Google Scholar] [CrossRef]
- Kozhantayeva, A.; Tashenov, Y.; Tosmaganbetova, K.; Tazhkenova, G.; Mashan, T.; Bazarkhankyzy, A.; Iskakova, Z.; Sapiyeva, A.; Gabbassova, A. Circaea lutetiana (L) plant and its chemical composition. Rasayan J. Chem. 2022, 15, 1653–1659. [Google Scholar] [CrossRef]
- Kozhantayeva, A.; Rakhmadiyeva, S.; Gulmira, O. Investigation of polyphenolic compounds of Chamaenerion latifolium (L.) plant. Rasayan J. Chem. 2020, 13, 2474–2482. [Google Scholar] [CrossRef]
- Agnieszka, G.; Mariola, D.; Anna, P.; Piotr, K.; Natalia, W.; Aneta, S.; Marcin, O.; Bogna, O.; Zdzisław, Ł.; Aurelia, P.; et al. Qualitative and quantitative analyses of bioactive compounds from ex vitro Chamaenerion angustifolium (L.) (Epilobium Augustifolium) herb in different harvest times. Ind. Crop. Prod. 2018, 123, 208–220. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, R. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Tourabi, M.; Metouekel, A.; Ghouizi, A.E.L.; Jeddi, M.; Nouioura, G.; Laaroussi, H.; Hosen, M.E.; Benbrahim, K.F.; Bourhia, M.; Salamatullah, A.M.; et al. Efficacy of various extracting solvents on phytochemical composition, and biological properties of Mentha longifolia L. leaf extracts. Sci. Rep. 2023, 13, 18028. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Gopčević, K.; Grujić, S.; Arsenijević, J.; Džamić, A.; Veličković, I.; Izrael-Živković, L.; Medić, A.; Mudrić, J.; Soković, M.; Đurić, A. Bioactivity and phenolics profile of aqueous and ethyl acetate extracts of Satureja kitaibelii Wierzb. ex Heuff. obtained by ultrasound-assisted extraction. Sci. Rep. 2022, 12, 21221. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Tiwari, K.N.; Kumar, P.; Kumar, A.; Dixit, J.; Saini, R.; Mishra, S.K. Toxicity profiling and antioxidant activity of ethyl acetate extract of leaves of Premna integrifolia L. for its application as protective agent against xenobiotics. Toxicol. Rep. 2021, 8, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Olalere, O.A. Ethanolic extraction of flavonoids, phenolics and antioxidants from Vernonia amygdalina leaf using two-level factorial design. J. King Saud Univ.-Sci. 2020, 32, 7–16. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hossain, A.; Shamim, A.; Rahman, M.M. Phytochemical and pharmacological evaluation of ethanolic extract of Lepisanthes Rubiginosa L. leaves. BMC Complement. Altern. Med. 2017, 17, 496. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Kulaitiene, J.; Vaitkeviciene, N.; Jakiene, E.; Skiba, D.; Hallmann, E. Studies of the variability of biologically active compounds and antioxidant activity in organically, biodynamically, and naturally grown and fermented fireweed (Chamerion angustifolium (L.) Holub) leaves. Plants 2023, 12, 2345. [Google Scholar] [CrossRef] [PubMed]
- Kaškonienė, V.; Maruška, A.; Akuneca, I.; Stankevičius, M.; Ragažinskienė, O.; Bartkuvienė, V. Screening of antioxidant activity and volatile compounds composition of Chamerion angustifolium (L.) Holub ecotypes grown in Lithuania. Nat. Prod. Res. 2016, 30, 1373–1381. [Google Scholar] [CrossRef]
- Maruška, A.; Ugenskienė, R.; Raulinaitytė, D.; Juozaitytė, E.; Kaškonienė, V.; Drevinskas, T.; Stelmakienė, A.; Akuneca, I.; Makaravičius, T.; Tiso, N.; et al. Analysis of antiproliferative effect of Chamerion angustifolium water extract and its fractions on several breast cancer cell lines. Adv. Med. Sci. 2017, 62, 158–164. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Kulaitiene, J.; Adamaviciene, A.; Hallmann, E. The impact of solid-phase fermentation on flavonoids, phenolic acids, tannins and antioxidant activity in Chamerion angustifolium (L.) Holub (Fireweed) leaves. Plants 2023, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Hallmann, E.; Najman, K. Effect of different durations of solid-phase fermentation for Fireweed (Chamerion angustifolium (L.) Holub) leaves on the content of polyphenols and antioxidant activity in vitro. Molecules 2020, 25, 1011. [Google Scholar] [CrossRef] [PubMed]
- Jariene, E.; Lasinskas, M.; Danilcenko, H.; Vaitkeviciene, N.; Slepetiene, A.; Najman, K. Polyphenols, antioxidant activity and volatile compounds in fermented leaves of medicinal plant rosebay willowherb (Chamerion angustifolium (L.) Holub). Plants 2020, 9, 1683. [Google Scholar] [CrossRef] [PubMed]
- Maruška, A.; Ragažinskienė, O.; Vyšniauskas, O.; Kaškonienė, V.; Bartkuvienė, V.; Kornysova, O.; Briedis, V.; Ramanauskienė, K. Flavonoids of willow herb (Chamerion angustifolium (L.) Holub) and their radical scavenging activity during vegetation. Adv. Med. Sci. 2014, 59, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Semenescu, A.D.; Moacă, E.A.; Iftode, A.; Dehelean, C.A.; Tchiakpe-Antal, D.S.; Vlase, L.; Vlase, A.M.; Muntean, D.; Chioibaş, R. Phytochemical and nutraceutical screening of ethanol and ethyl acetate phases of Romanian Galium verum Herba (Rubiaceae). Molecules 2023, 28, 7804. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Wang, S.-W.; Li, C.-W.; Lin, H.-R.; Yang, C.-S.; Chu, Y.-C.; Lee, T.-H.; Chen, J.-J. Comparison of various solvent extracts and major bioactive components from Portulaca oleracea for antioxidant, Anti-Tyrosinase, and Anti-α-Glucosidase Activities. Antioxidants 2022, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Thavamoney, N.; Sivanadian, L.; Tee, L.H.; Khoo, H.E.; Prasad, K.N.; Kong, K.W. Extraction and recovery of phytochemical components and antioxidative properties in fruit parts of Dacryodes rostrata influenced by different solvents. J. Food Sci. Technol. 2018, 55, 2523–2532. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Nowak, A.; Zagórska-Dziok, M.; Ossowicz-Rupniewska, P.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Adamiak-Giera, U.; Prowans, P.; Czapla, N.; Bargiel, P.; et al. Epilobium angustifolium L. extracts as valuable ingredients in cosmetic and dermatological products. Molecules 2021, 26, 3456. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Moomin, A.; Russell, W.R.; Knott, R.M.; Scobbie, L.; Mensah, K.B.; Adu-Gyamfi, P.K.T.; Duthie, S.J. Season, storage and extraction method impact on the phytochemical profile of Terminalia Ivorensis. BMC Plant Biol. 2023, 23, 162. [Google Scholar] [CrossRef]
- Kabtni, S.; Sdouga, D.; Bettaib Rebey, I.; Save, M.; Trifi-Farah, N.; Fauconnier, M.L.; Marghali, S. Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci. Rep. 2020, 10, 8293. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhang, H.S.; Yang, S.F. Phenolic compounds and its antioxidant activities in ethanolic extracts from seven cultivars of Chinese jujube. Food Sci. Hum. Wellness 2014, 3, 183–190. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and therapeutic potential of kaempferol and quercetin: New insights for plant and human health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial properties, sources, clinical, and traditional applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Hasnat, H.; Shompa, S.A.; Islam, M.M.; Alam, S.; Richi, F.T.; Emon, N.U.; Ashrafi, S.; Ahmed, N.U.; Chowdhury, M.N.R.; Fatema, N.; et al. Flavonoids: A treasure house of prospective pharmacological potentials. Heliyon 2024, 10, 27533. [Google Scholar]
- Yu, Q.; Li, W.; Liang, M.; Li, G.; Wu, Z.; Long, J.; Yuan, C.; Mei, W.; Xia, X. Preparation, characterization, and antioxidant activities of extracts from Amygdalus persica L. Flowers. Molecules 2024, 29, 633. [Google Scholar] [CrossRef] [PubMed]
- Ella Nkogo, L.F.; Mikala Mouendou, M.S.; Dumarçay, S.; Edou Engonga, P.; Gérardin, P. Phytochemical study, FTIR and GC-MS characterization and evaluation of the antioxidant activity of Letestua durissima extracts. Forests 2024, 15, 429. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; de Oliveira, F.C.S.; Passos, T.M.; Quilty, B.; Thiré, R.M.d.S.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matér. Rio Jan. 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manoj, P.; Giridhar, P. Fourier transform infrared spectroscopy (FTIR) analysis, chlorophyll content and antioxidant properties of native and defatted foliage of green leafy vegetables. J. Food Sci. Technol. 2015, 52, 8131–8139. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Sathasivam, R.; Park, S.U.; Lee, H.; Kim, M.S.; Baek, I.; Cho, B.K. Application of Fourier transform infrared spectroscopy and multivariate analysis methods for the non-destructive evaluation of phenolics compounds in Moringa powder. Agriculture 2022, 12, 10. [Google Scholar] [CrossRef]
- Johnson, J.; Mani, J.; Ashwath, N.; Naiker, M. Potential for Fourier transform infrared (FTIR) spectroscopy toward predicting antioxidant and phenolic contents in powdered plant matrices. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 233, 118228. [Google Scholar] [CrossRef] [PubMed]
- Wongsa, P.; Phatikulrungsun, P.; Prathumthong, S. FT-IR characteristics, phenolic profiles and inhibitory potential against digestive enzymes of 25 herbal infusions. Sci. Rep. 2022, 12, 6631. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic potential of quercetin: New insights and perspectives for human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Wu, L. Effect of chlorogenic acid on antioxidant activity of flos lonicerae extracts. J. Zhejiang Univ.-Sci. B 2007, 8, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, S.A.; Farhoosh, R.; Sharif, A. Antioxidant activity of gallic acid as affected by an extra carboxyl group than pyrogallol in various oxidative environments. Eur. J. Lipid Sci. Technol. 2019, 120, 1800319. [Google Scholar]
- Okello, D.; Chung, Y.; Kim, H.; Lee, J.; Rahmat, E.; Komakech, R.; Kim, Y.-G.; Omujal, F.; Kang, Y. Antioxidant activity, polyphenolic content, and FT-NIR analysis of different Aspilia africana medicinal plant tissues. Evid. Based Complement. Alternat. Med. 2021, 2021, 9917810. [Google Scholar] [CrossRef]
- Panat, N.A.; Amrute, B.K.; Bhattu, S.; Haram, S.K.; Sharma, G.K.; Ghaskadbi, S.S. Antioxidant profiling of C3 quercetin glycosides: Quercitrin, quercetin 3-β-d-glucoside and quercetin 3-O-(6″-O-malonyl)-β-D glucoside in cell free environment. Free Radic. Antiox. 2015, 5, 90–100. [Google Scholar]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic acid and its derivatives: Antimicrobial drugs toward microbial pathogens. J. Agric. Food. Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Tian, Q.M.; Wei, S.M.; Su, H.R.; Zheng, S.M.; Xu, S.Y.; Liu, M.J.; Bo, R.N.; Li, J.G. Bactericidal activity of gallic acid against multi-drug resistance Escherichia coli. Microb. Pathog. 2022, 173, 105824. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Safwat, N.A.; Kashef, M.T.; Aziz, R.K.; Amer, K.F.; Ramadan, M.A. Quercetin 3-O-glucoside recovered from the wild Egyptian Sahara plant, Euphorbia paralias L., inhibits glutamine synthetase and has antimycobacterial activity. Tuberculosis 2018, 108, 106–113. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Martínez, G.; Regente, M.; Jacobi, S.; Del Rio, M.; Pinedo, M.; de la Canal, L. Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pestic. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar] [CrossRef]
- Patra, A.K. An Overview of antimicrobial properties of different classes of phytochemicals. In Dietary Phytochemicals and Microbes; Springer: Amsterdam, The Netherlands, 2012; pp. 1–32. [Google Scholar]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Al Aboody, M.S.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Boon, S.A.; Ijaz, M.K.; McKinney, J.; Gerba, C.P. Antifungal activity and mechanism of action of natural product derivates as potential environmental disinfectants. J. Ind. Microbiol. Biotechnol. 2023, 50, kuad036. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, J.; Luyck, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.; Peres, A.; Silva, A.; Domingues, M.; Cardoso, S. Simultaneous characterization, and quantification of phenolic compounds in Thymus x citriodorus using a validated HPLC–UV and ESI-MS combined method. Food Res. Int. 2013, 54, 1773–1780. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Chem. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Skubel, S.A.; Dushenkov, V.; Graf, B.L.; Niu, Q.; Poulev, A.; Kalariya, H.M.; Foxcroft, L.C.; Raskin, I. Rapid, field-deployable method for collecting and preserving plant metabolome for biochemical and functional characterization. PLoS ONE 2018, 13, e0203569. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]




| Sample | TFC (mg QE/g DM) | TPC (mg GAE/g DM) |
|---|---|---|
| ChL-EtOAc | 21.72 ± 0.54 | 42.68 ± 2.48 |
| ChL-EtOH | 24.18 ± 1.06 | 267.48 ± 3.44 |
| Peak No. | Retention Time Rt (min) | [M+H]− (m/z) | Identified Metabolites | Subclass | Molecular Formula | Quantity * | |
|---|---|---|---|---|---|---|---|
| ChL-EtOH | ChL-EtOAc | ||||||
| 1 | 3.928 | 179 | Caffeic acid | Phenolic acid | C9H8O4 | 4.40 | 2.10 |
| 2 | 5.092 | 169 | Gallic acid | Phenolic acid | C7H6O5 | 35.00 | 0.80 |
| 3 | 13.466 | 353 | Chlorogenic acid | Phenolic acid (glycoside) | C16H18O9 | 26.80 | 3.00 |
| 4 | 17.187 | 289 | Epicatechin | Flavonoid | C15H14O6 | - | - |
| 5 | 19.166 | 609 | Rutin | Flavonoid (glycoside) | C27H30O16 | 8.80 | 0.55 |
| 6 | 20.030 | 463 | Quercetin 3-glucoside | Flavonoid (glycoside) | C21H19O12 | 71.90 | 2.30 |
| 7 | 20.352 | 301 | Not identified | - | - | - | - |
| 8 | 20.685 | 447 | Not identified | - | - | - | - |
| 9 | 20.792 | 163 | p-Coumaric acid | Phenolic acid | C9H8O3 | 2.70 | - |
| 10 | 22.820 | 431 | Not identified | - | - | - | - |
| 11 | 23.327 | 317 | Myricetin | Flavonoid | C15H10O8 | 0.03 | 0.52 |
| 12 | 30.956 | 301 | Quercetin | Flavonoid | C15H10O7 | 0.90 | 0.08 |
| 13 | 33.723 | 271 | Naringenin | Flavonoid | C15H12O5 | 0.34 | 0.01 |
| 14 | 38.924 | 285 | Kaempferol | Flavonoid | C15H10O6 | 0.44 | 0.82 |
| ChL-EtOAc | ChL-EtOH | BHT | AA | |
|---|---|---|---|---|
| DPPH Test IC50 (μg/mL) | 22.60 ± 0.44 a | 21.31 ± 0.65 b | 20.02 ± 0.48 c | ND |
| FRAP Test IC50 (μg/mL) | 21.62 ± 0.22 a | 18.13 ± 0.15 b | ND | 18.64 ± 0.32 d |
| Microorganisms Tested | Gram Type | Extract | Positive Control | ||
|---|---|---|---|---|---|
| ChL-EtOH, IZD, mm | ChL-EtOAc, IZD, mm | Penicillin, IZD, mm | Nystatin, IZD, mm | ||
| Escherichia coli | Gram − | 8.53 ± 0.12 | NA | NA | - |
| Klebsiella pneumonia | Gram − | 9.26 ± 0.08 | NA | NA | - |
| Staphylococcus aureus | Gram + | 11.06 ± 0.30 | NA | 30.26 ± 0.88 | - |
| Bacillus cereus | Gram + | 9.60 ± 0.24 | NA | 18.63 ± 0.32 | - |
| Candida albicans | Fungus | 14.27 ± 0.65 | 8.58 ± 0.22 | - | 11.28 ± 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhantayeva, A.; Tursynova, N.; Kolpek, A.; Aibuldinov, Y.; Tursynova, A.; Mashan, T.; Mukazhanova, Z.; Ibrayeva, M.; Zeinuldina, A.; Nurlybayeva, A.; et al. Phytochemical Profiling, Antioxidant and Antimicrobial Potentials of Ethanol and Ethyl Acetate Extracts of Chamaenerion latifolium L. Pharmaceuticals 2024, 17, 996. https://doi.org/10.3390/ph17080996
Kozhantayeva A, Tursynova N, Kolpek A, Aibuldinov Y, Tursynova A, Mashan T, Mukazhanova Z, Ibrayeva M, Zeinuldina A, Nurlybayeva A, et al. Phytochemical Profiling, Antioxidant and Antimicrobial Potentials of Ethanol and Ethyl Acetate Extracts of Chamaenerion latifolium L. Pharmaceuticals. 2024; 17(8):996. https://doi.org/10.3390/ph17080996
Chicago/Turabian StyleKozhantayeva, Akmaral, Nurgul Tursynova, Ainagul Kolpek, Yelaman Aibuldinov, Arailym Tursynova, Togzhan Mashan, Zhazira Mukazhanova, Manshuk Ibrayeva, Aizhan Zeinuldina, Aisha Nurlybayeva, and et al. 2024. "Phytochemical Profiling, Antioxidant and Antimicrobial Potentials of Ethanol and Ethyl Acetate Extracts of Chamaenerion latifolium L." Pharmaceuticals 17, no. 8: 996. https://doi.org/10.3390/ph17080996
APA StyleKozhantayeva, A., Tursynova, N., Kolpek, A., Aibuldinov, Y., Tursynova, A., Mashan, T., Mukazhanova, Z., Ibrayeva, M., Zeinuldina, A., Nurlybayeva, A., Iskakova, Z., & Tashenov, Y. (2024). Phytochemical Profiling, Antioxidant and Antimicrobial Potentials of Ethanol and Ethyl Acetate Extracts of Chamaenerion latifolium L. Pharmaceuticals, 17(8), 996. https://doi.org/10.3390/ph17080996






