Acetylcholinesterase Inhibitor Ameliorates Early Cardiometabolic Disorders in Fructose-Overloaded Rat Offspring
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Arterial Catheterization and Hemodynamic Assessments
2.3. Autonomic Control of Heart Rate
2.4. Evaluation of Glycemia and Insulin Tolerance Test
2.5. Statistical Analysis
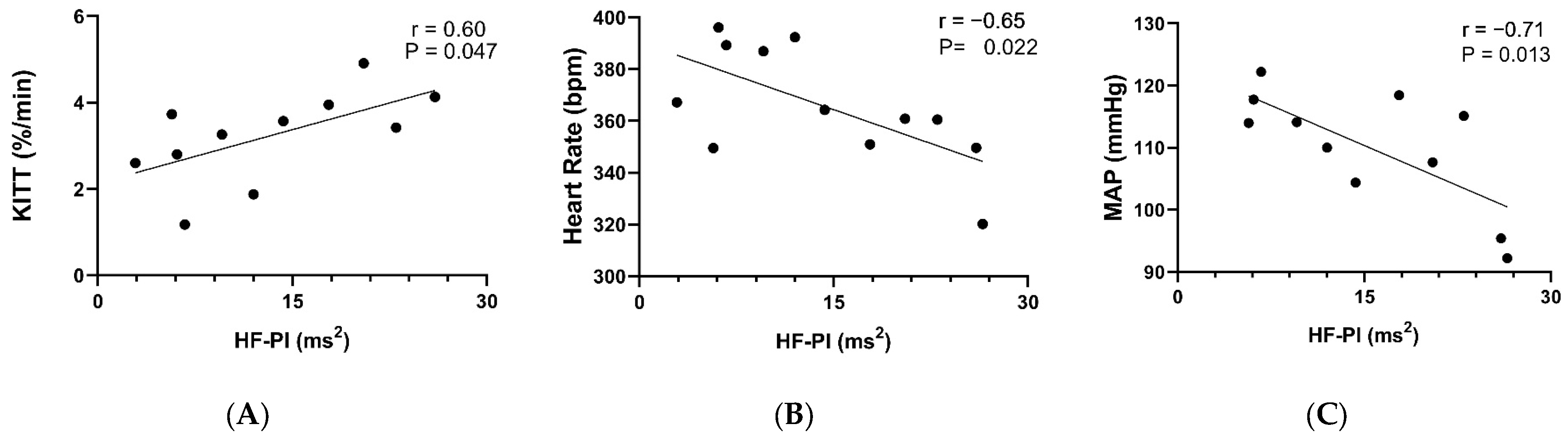
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tobar-Bernal, F.A.; Zamudio, S.R.; Quevedo-Corona, L. The high-fructose intake of dams during pregnancy and lactation exerts sex-specific effects on adult rat offspring metabolism. J. Dev. Orig. Health Dis. 2020, 12, 411–419. [Google Scholar] [CrossRef]
- Kumar, E.C.; Gaur, G.S.; Yerrabelli, D.; Sahoo, J.; Vairappan, B.; Goud, A.C. Association between metabolic syndrome components and cardiac autonomic modulation in southern Indian adults with pre-metabolic syndrome: Hyperglycemia is the major contributing factor. Korean J. Physiol. Pharmacol. 2023, 27, 49–59. [Google Scholar] [CrossRef]
- Farah, D.; Nunes, J.; Sartori, M.; Dias, D.S.; Sirvente, R.; Silva, M.B.; Fiorino, P.; Morris, M.; Llesuy, S.; Farah, V.; et al. Exercise Training Prevents Cardiovascular Derangements Induced by Fructose Overload in Developing Rats. PLoS ONE 2016, 11, e0167291. [Google Scholar] [CrossRef]
- Bernardes, N.; Dias, D.S.; Conti, F.F.S.; Monzani, J.O.B.; Malfatino, C.; Caldini, E.G.; Ulloa, L.; Llesuy, S.F.; Irigoyen, M.C.; De Angelis, K. Baroreflex Impairment Precedes Cardiometabolic Dysfunction in an Experimental Model of Metabolic Syndrome: Role of Inflammation and Oxidative Stress. Sci. Rep. 2018, 8, 8578. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; DeBosch, B.J. Maternal Fructose Diet-Induced Developmental Programming. Nutrients 2021, 13, 3278. [Google Scholar] [CrossRef]
- Mez, C.N.; Pavlov, V.A. Treating disorders across the lifespan by modulating cholinergic signaling with galantamine. J. Neurochem. 2021, 158, 1359–1380. [Google Scholar] [CrossRef]
- Ali, M.A.; El-Abhar, H.S.; Kamel, M.A.; Attia, A.S. Antidiabetic Effect of Galantamine: Novel Effect for a Known Centrally Acting Drug. PLoS ONE 2015, 10, e0134648. [Google Scholar] [CrossRef] [PubMed]
- Sangaleti, C.T.; Katayama, K.Y.; De Angelis, K.; Moraes, T.L.; Araújo, A.A.; Lopes, H.F.; Camacho, C.; Bortolotto, L.A.; Michelini, L.C.; Irigoyen, M.C.; et al. The Cholinergic Drug Galantamine Alleviates Oxidative Stress Alongside Anti-inflammatory and Cardio-Metabolic Effects in Subjects with the Metabolic. Front. Immunol. 2021, 12, 613979. [Google Scholar] [CrossRef]
- Da Silva Dias, D.; Bernardes, N.; Conti, F.F.S.; Santos, C.P.; Araújo, A.A.; Llesuy, S.; Irigoyen, M.C.; De Angelis, K. Impact of combined exercise training on the development of cardiometabolic and neuroimmune complications induced by fructose consumption in hypertensive rats. PLoS ONE 2020, 15, e0233785. [Google Scholar] [CrossRef]
- Brito, J.O.; Ponciano, K.; Figueroa, D.; Bernardes, N.; Sanches, I.C.; Irigoyen, M.C.; De Angelis, K. Parasympathetic dysfunction is associated with insulin resistance in fructose-fed female rats. Braz. J. Med. Biol. Res. 2008, 41, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Wu, K.L.H.; Leu, S.; Tain, Y.L. Translational insights on developmental origins of metabolic syndrome: Focus on fructose consumption. Biomed. J. 2018, 41, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.V.L.; Dyson, R.M.; Weth, F.R.; Berry, M.J.; Gray, C. Maternal Fructose Intake, Programmed Mitochondrial Function and Predisposition to Adult Disease. Int. J. Mol. Sci. 2022, 23, 12215. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Plows, J.F.; Ventura, E.E. Effects of consuming sugars and alternative sweeteners during pregnancy on maternal and child health: Evidence for a secondhand sugar effect. Proc. Nutr. Soc. 2019, 78, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Alzamendi, A.; Zubiría, G.; Moreno, G.; Portales, A.; Spinedi, E.; Giovambattista, A. High Risk of Metabolic and Adipose Tissue Dysfunctions in Adult Male Progeny, Due to Prenatal and Adulthood Malnutrition Induced by Fructose Rich Diet. Nutrients 2016, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Astbury, S.; Song, A.; Zhou, M.; Nielsen, B.; Hoedl, A.; Willing, B.P.; Symonds, M.E.; Bell, R.C. High Fructose Intake during Pregnancy in Rats Influences the Maternal Microbiome and Gut Development in the Offspring. Front. Genet. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.P.; Nascimento-Filho, A.V.; Araujo, A.A.; Dias, D.S.; Silva, D.R.; Bernardes, N.; Shecaira, T.P.; Irigoyen, M.C.; De Angelis, K. Parental fructose consumption induces early baroreflex dysfunction in offspring: Impact on arterial pressure and on insulin resistance. Int. J. Obes. 2024, 48, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.; Fedorchak, S.; Boychuk, C.R. Interplay between Systemic Metabolic Cues and Autonomic Output: Connecting Cardiometabolic Function and Parasympathetic Circuits. Front. Physiol. 2021, 12, 624595. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, C.L.; Zou, H.D. Galantamine protects against lipopolysaccharide-induced acute lung injury in rats. Braz. J. Med. Biol. Res. 2016, 49, e5008. [Google Scholar] [CrossRef] [PubMed]
- Shanks, J.; Ramchandra, R. Angiotensin II and the Cardiac Parasympathetic Nervous System in Hypertension. Int. J. Mol. Sci. 2021, 22, 12305. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.K.; Ochani, M.; Dancho, M.; Hudson, L.K.; Rosas-Ballina, M.; Valdes-Ferrer, S.I.; Olofsson, P.S.; Harris, Y.T.; Roth, J.; Chavan, S.; et al. Galantamine Alleviates Inflammation and Other Obesity-Associated Complications in High-Fat Diet–Fed Mice. Mol. Med. 2011, 17, 599–606. [Google Scholar] [CrossRef] [PubMed]

| C | F | GAL | |
|---|---|---|---|
| DAP (mmHg) | 84 ± 3.4 | 95 ± 2.47 | 88 ± 3.8 |
| SAP (mmHg) | 117 ± 2.7 | 130 ± 3.5 | 121 ± 4.9 |
| RMSSD (ms) | 4.84 ± 0.73 | 4.67 ± 0.46 | 8.54 ± 0.52 *& |
| HF–PI (ms2) | 7.65 ± 1.92 | 7.15 ± 1.30 | 21.41 ± 1.99 *& |
| LF–PI (ms2) | 2.39 ± 0.67 | 3.15 ± 0.37 | 3.27 ± 0.27 |
| LF/HF | 0.33 ± 0.01 | 0.52 ± 0.10 * | 0.17 ± 0.02 & |
| VAR–SAP (mmHg2) | 13.80 ± 1.68 | 20.47 ± 2.38 * | 11.51 ± 2.07 & |
| LF–SAP (mmHg2) | 2.03 ± 0.48 | 4.13 ± 0.50 * | 1.85 ± 0.39 & |
| Alpha LF index (ms/mmHg) | 1.21 ± 0.13 | 0.85 ± 0.10 | 1.47 ± 0.14 & |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Miranda, V.H.M.; Dos Santos, C.P.; Neves, P.P.; Nascimento-Filho, A.V.; Dutra, M.R.H.; Bernardes, N.; Irigoyen, M.C.; De Angelis, K. Acetylcholinesterase Inhibitor Ameliorates Early Cardiometabolic Disorders in Fructose-Overloaded Rat Offspring. Pharmaceuticals 2024, 17, 1055. https://doi.org/10.3390/ph17081055
de Miranda VHM, Dos Santos CP, Neves PP, Nascimento-Filho AV, Dutra MRH, Bernardes N, Irigoyen MC, De Angelis K. Acetylcholinesterase Inhibitor Ameliorates Early Cardiometabolic Disorders in Fructose-Overloaded Rat Offspring. Pharmaceuticals. 2024; 17(8):1055. https://doi.org/10.3390/ph17081055
Chicago/Turabian Stylede Miranda, Victor Hugo Martins, Camila Paixão Dos Santos, Pietra Petrica Neves, Antonio Viana Nascimento-Filho, Marina Rascio Henriques Dutra, Nathalia Bernardes, Maria Claúdia Irigoyen, and Kátia De Angelis. 2024. "Acetylcholinesterase Inhibitor Ameliorates Early Cardiometabolic Disorders in Fructose-Overloaded Rat Offspring" Pharmaceuticals 17, no. 8: 1055. https://doi.org/10.3390/ph17081055
APA Stylede Miranda, V. H. M., Dos Santos, C. P., Neves, P. P., Nascimento-Filho, A. V., Dutra, M. R. H., Bernardes, N., Irigoyen, M. C., & De Angelis, K. (2024). Acetylcholinesterase Inhibitor Ameliorates Early Cardiometabolic Disorders in Fructose-Overloaded Rat Offspring. Pharmaceuticals, 17(8), 1055. https://doi.org/10.3390/ph17081055







