Comparing the Differences in Adverse Events among Chimeric Antigen Receptor T-Cell Therapies: A Real-World Pharmacovigilance Study
Abstract
1. Introduction
2. Results
2.1. Characteristics of CAR T-Cell Reports in the FAERS Database
2.2. CAR T-Cell Therapy-Associated Adverse Events
2.2.1. Common Adverse Events in Key Organs
2.2.2. Adverse Events of Special Interest
2.3. Clinical Characteristics of the Adverse Events
3. Discussion
4. Materials and Methods
4.1. Study Design and Data Source
4.2. Data Extraction and Mining
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tan, S.Y.E.; Zhao, H.; Ni, F.; Hu, Y.; Huang, H. CAR-T cells: The Chinese experience. Expert Opin. Biol. Ther. 2020, 20, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Guo, Y.; Wang, Y.; Wu, Z.; Bo, J.; Zhang, B.; Zhu, J.; Han, W. Clinical development of CAR T cell therapy in China: 2020 update. Cell. Mol. Immunol. 2021, 18, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Riviere, I.; Gonen, M.; Wang, X.; Senechal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, Z.; Luo, Y.; Shi, J.; Yu, J.; Pu, C.; Liang, Z.; Wei, G.; Cui, Q.; Sun, J.; et al. Potent Anti-leukemia Activities of Chimeric Antigen Receptor-Modified T Cells against CD19 in Chinese Patients with Relapsed/Refractory Acute Lymphocytic Leukemia. Clin. Cancer Res. 2017, 23, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, G.; Cheng, H.; Wei, C.; Qi, K.; Sang, W.; Zhenyu, L.; Shi, M.; Li, H.; Qiao, J.; et al. Potent anti-leukemia activities of humanized CD19-targeted Chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am. J. Hematol. 2018, 93, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, C.; Yin, P.; Guo, T.; Liu, L.; Xia, L.; Wu, Y.; Zhou, F.; Ai, L.; Shi, W.; et al. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: An open-label pragmatic clinical trial. Am. J. Hematol. 2019, 94, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhao, M.; Hu, Y.; Wang, Y.; Li, P.; Cao, J.; Shi, M.; Tan, J.; Zhang, M.; Xiao, X.; et al. Efficacy and safety of CD19-specific CAR T cell-based therapy in B-cell acute lymphoblastic leukemia patients with CNSL. Blood 2022, 139, 3376–3386. [Google Scholar] [CrossRef]
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: Phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 2021, 398, 491–502. [Google Scholar] [CrossRef]
- Wang, T.; Tang, Y.; Cai, J.; Wan, X.; Hu, S.; Lu, X.; Xie, Z.; Qiao, X.; Jiang, H.; Shao, J.; et al. Coadministration of CD19- and CD22-Directed Chimeric Antigen Receptor T-Cell Therapy in Childhood B-Cell Acute Lymphoblastic Leukemia: A Single-Arm, Multicenter, Phase II Trial. J. Clin. Oncol. 2023, 41, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Leahy, A.B.; Newman, H.; Li, Y.; Liu, H.; Myers, R.; DiNofia, A.; Dolan, J.G.; Callahan, C.; Baniewicz, D.; Devine, K.; et al. CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: A post-hoc analysis of pooled data from five clinical trials. Lancet Haematol. 2021, 8, e711–e722. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, H.; Adachi, S.; Umeda, K.; Kato, I.; Eldjerou, L.; Agostinho, A.C.; Natsume, K.; Tokushige, K.; Watanabe, Y.; Grupp, S.A. Efficacy and safety of tisagenlecleucel in Japanese pediatric and young adult patients with relapsed/refractory B cell acute lymphoblastic leukemia. Int. J. Hematol. 2020, 111, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Liu, Y.; Tong, C.; Wang, C.; Guo, Y.; Ti, D.; Yang, Q.; Qiao, S.; Wu, Z.; et al. Long-term activity of tandem CD19/CD20 CAR therapy in refractory/relapsed B-cell lymphoma: A single-arm, phase 1-2 trial. Leukemia 2022, 36, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.; Shi, M.; Yang, J.; Cao, J.; Xu, L.; Yan, D.; Yao, M.; Liu, H.; Li, W.; Zhang, B.; et al. Phase II trial of co-administration of CD19- and CD20-targeted chimeric antigen receptor T cells for relapsed and refractory diffuse large B cell lymphoma. Cancer Med. 2020, 9, 5827–5838. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Zhang, Y.; Liu, Y.; Ji, X.; Zhang, W.; Guo, Y.; Han, X.; Ti, D.; Dai, H.; Wang, C.; et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood 2020, 136, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Liu, J.; Wang, B.Y.; Chen, Y.X.; Cao, X.M.; Yang, Y.; Zhang, Y.L.; Wang, F.X.; Zhang, P.Y.; Lei, B.; et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Cao, J.; Cheng, H.; Qiao, J.; Zhang, H.; Wang, Y.; Shi, M.; Lan, J.; Fei, X.; Jin, L.; et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase 2 trial. Lancet Haematol. 2019, 6, e521–e529. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Li, C.; Jiang, H.; Zhao, X.; Huang, Z.; Jin, D.; Guo, T.; Kou, H.; Liu, L.; Tang, L.; et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2021, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.J.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves first CAR T therapy. Nat. Rev. Drug Discov. 2017, 16, 669. [Google Scholar] [CrossRef]
- FDA. FDA Approves Second CAR T-cell Therapy. Cancer Discov. 2018, 8, 5–6. [Google Scholar] [CrossRef]
- Hou, J.Z.; Ye, J.C.; Pu, J.J.; Liu, H.; Ding, W.; Zheng, H.; Liu, D. Novel agents and regimens for hematological malignancies: Recent updates from 2020 ASH annual meeting. J. Hematol. Oncol. 2021, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves first BCMA-targeted CAR-T cell therapy. Nat. Rev. Drug Discov. 2021, 20, 332. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves fourth CAR-T cell therapy. Nat. Rev. Drug Discov. 2021, 20, 166. [Google Scholar] [CrossRef]
- Cai, C.; Tang, D.; Han, Y.; Shen, E.; Abdihamid, O.; Guo, C.; Shen, H.; Zeng, S. A comprehensive analysis of the fatal toxic effects associated with CD19 CAR-T cell therapy. Aging (Albany Ny) 2020, 12, 18741–18753. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jager, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.M.; May, S.; Lei, M.; Qualls, S.; Bushey, K.; Rubin, D.B.; Barra, M.E. CAR T-Cell-Associated Neurotoxicity: Current Management and Emerging Treatment Strategies. Crit. Care Nurs. Q. 2020, 43, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Freyer, C.W.; Porter, D.L. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies. J. Allergy. Clin. Immunol. 2020, 146, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Van Oekelen, O.; Aleman, A.; Upadhyaya, B.; Schnakenberg, S.; Madduri, D.; Gavane, S.; Teruya-Feldstein, J.; Crary, J.F.; Fowkes, M.E.; Stacy, C.B.; et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat. Med. 2021, 27, 2099–2103. [Google Scholar] [CrossRef] [PubMed]
- Karschnia, P.; Miller, K.C.; Yee, A.J.; Rejeski, K.; Johnson, P.C.; Raje, N.; Frigault, M.J.; Dietrich, J. Neurologic toxicities following adoptive immunotherapy with BCMA-directed CAR T cells. Blood 2023, 142, 1243–1248. [Google Scholar] [CrossRef]
- Torre, M.; Solomon, I.H.; Sutherland, C.L.; Nikiforow, S.; DeAngelo, D.J.; Stone, R.M.; Vaitkevicius, H.; Galinsky, I.A.; Padera, R.F.; Trede, N.; et al. Neuropathology of a Case With Fatal CAR T-Cell-Associated Cerebral Edema. J. Neuropathol. Exp. Neurol. 2018, 77, 877–882. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, O.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- FDA. KYMRIAH® (Tisagenlecleucel) Suspension for Intravenous Infusion Initial U.S. Approval: 2017. Available online: https://www.fda.gov/media/107296/download?attachment (accessed on 23 April 2024).
- FDA. TECARTUS® (Brexucabtagene autoleucel) Suspension for Intravenous Infusion Initial U.S. Approval: 2020. Available online: https://www.fda.gov/media/140409/download?attachment (accessed on 23 April 2024).
- FDA. YESCARTA® (Axicabtagene ciloleucel) Suspension for Intravenous Infusion Initial U.S. Approval: 2017. Available online: https://www.fda.gov/media/108377/download?attachment (accessed on 23 April 2024).
- FDA. BREYANZI® (Lisocabtagene maraleucel) Suspension for Intravenous Infusion Initial U.S. Approval: 2021. Available online: https://www.fda.gov/media/145711/download?attachment (accessed on 23 April 2024).
- FDA. ABECMA® (Idecabtagene vicleucel), Suspension for Intravenous Infusion Initial U.S. Approval: 2021. Available online: https://www.fda.gov/media/147055/download?attachment (accessed on 23 April 2024).
- FDA. CARVYKTI® (Ciltacabtagene autoleucel) Suspension for Intravenous Infusion Initial U.S. Approval: 2022. Available online: https://www.fda.gov/media/156560/download?attachment (accessed on 23 April 2024).
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, W.; Wu, S.; Xu, K.; Qiu, L.; Xu, Y.; Yan, X.; Zhang, Q.; Zhang, M.; Wang, L.; et al. Derivation and validation of a novel score for early prediction of severe CRS after CAR-T therapy in haematological malignancy patients: A multi-centre study. Br. J. Haematol. 2023, 202, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Wudhikarn, K.; Palomba, M.L.; Pennisi, M.; Garcia-Recio, M.; Flynn, J.R.; Devlin, S.M.; Afuye, A.; Silverberg, M.L.; Maloy, M.A.; Shah, G.L.; et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Wesson, W.; Dima, D.; Suleman, N.; Saif, M.; Tabak, C.; Logan, E.; Davis, J.A.; McGann, M.; Furqan, F.; Mohan, M.; et al. Timing of Toxicities and Non-Relapse Mortality Following CAR T Therapy in Myeloma. Transpl. Cell. Ther. 2024, S2666–S6367. [Google Scholar] [CrossRef] [PubMed]
- Yakoub-Agha, I.; Chabannon, C.; Bader, P.; Basak, G.W.; Bonig, H.; Ciceri, F.; Corbacioglu, S.; Duarte, R.F.; Einsele, H.; Hudecek, M.; et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: Best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica 2020, 105, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Doan, A.; Pulsipher, M.A. Hypogammaglobulinemia due to CAR T-cell therapy. Pediatr. Blood Cancer 2018, 65, 1–2. [Google Scholar] [CrossRef]
- Schubert, M.L.; Schmitt, M.; Wang, L.; Ramos, C.A.; Jordan, K.; Muller-Tidow, C.; Dreger, P. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann. Oncol. 2021, 32, 34–48. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Li, D.; Hay, K.A.; Green, M.L.; Cherian, S.; Chen, X.; Riddell, S.R.; Maloney, D.G.; Boeckh, M.; Turtle, C.J. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018, 131, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, K.; Cheng, H.; Cao, J.; Shi, M.; Qiao, J.; Yan, Z.; Jing, G.; Pan, B.; Sang, W.; et al. Coagulation Disorders after Chimeric Antigen Receptor T Cell Therapy: Analysis of 100 Patients with Relapsed and Refractory Hematologic Malignancies. Biol. Blood Marrow Transplant. 2020, 26, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Alvi, R.M.; Frigault, M.J.; Fradley, M.G.; Jain, M.D.; Mahmood, S.S.; Awadalla, M.; Lee, D.H.; Zlotoff, D.A.; Zhang, L.; Drobni, Z.D.; et al. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J. Am. Coll. Cardiol. 2019, 74, 3099–3108. [Google Scholar] [CrossRef]
- Ganatra, S.; Redd, R.; Hayek, S.S.; Parikh, R.; Azam, T.; Yanik, G.A.; Spendley, L.; Nikiforow, S.; Jacobson, C.; Nohria, A. Chimeric Antigen Receptor T-Cell Therapy-Associated Cardiomyopathy in Patients With Refractory or Relapsed Non-Hodgkin Lymphoma. Circulation 2020, 142, 1687–1690. [Google Scholar] [CrossRef]
- Lefebvre, B.; Kang, Y.; Smith, A.M.; Frey, N.V.; Carver, J.R.; Scherrer-Crosbie, M. Cardiovascular Effects of CAR T Cell Therapy: A Retrospective Study. Jacc-Cardiooncol. 2020, 2, 193–203. [Google Scholar] [CrossRef]
- Goldman, A.; Maor, E.; Bomze, D.; Liu, J.E.; Herrmann, J.; Fein, J.; Steingart, R.M.; Mahmood, S.S.; Schaffer, W.L.; Perales, M.A.; et al. Adverse Cardiovascular and Pulmonary Events Associated With Chimeric Antigen Receptor T-Cell Therapy. J. Am. Coll. Cardiol. 2021, 78, 1800–1813. [Google Scholar] [CrossRef]
- Steiner, R.E.; Banchs, J.; Koutroumpakis, E.; Becnel, M.; Gutierrez, C.; Strati, P.; Pinnix, C.C.; Feng, L.; Rondon, G.; Claussen, C.; et al. Cardiovascular events in patients treated with chimeric antigen receptor T-cell therapy for aggressive B-cell lymphoma. Haematologica 2022, 107, 1555–1566. [Google Scholar] [CrossRef]
- Shalabi, H.; Sachdev, V.; Kulshreshtha, A.; Cohen, J.W.; Yates, B.; Rosing, D.R.; Sidenko, S.; Delbrook, C.; Mackall, C.; Wiley, B.; et al. Impact of cytokine release syndrome on cardiac function following CD19 CAR-T cell therapy in children and young adults with hematological malignancies. J. Immunother. Cancer 2020, 8, e001159. [Google Scholar] [CrossRef]
- Totzeck, M.; Michel, L.; Lin, Y.; Herrmann, J.; Rassaf, T. Cardiotoxicity from chimeric antigen receptor-T cell therapy for advanced malignancies. Eur. Heart J. 2022, 43, 1928–1940. [Google Scholar] [CrossRef]
- Hazell, L.; Shakir, S.A. Under-reporting of adverse drug reactions: A systematic review. Drug. Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef]
- Locke, F.L.; Siddiqi, T.; Jacobson, C.A.; Ghobadi, A.; Ahmed, S.; Miklos, D.B.; Perales, M.A.; Munoz, J.; Fingrut, W.B.; Pennisi, M.; et al. Real-world and clinical trial outcomes in large B-cell lymphoma with axicabtagene ciloleucel across race and ethnicity. Blood. 2024, 143, 2722–2734. [Google Scholar] [CrossRef]
- Bate, A.; Lindquist, M.; Edwards, I.R.; Olsson, S.; Orre, R.; Lansner, A.; De Freitas, R.M. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 1998, 54, 315–321. [Google Scholar] [CrossRef]
- van Puijenbroek, E.; Diemont, W.; van Grootheest, K. Application of quantitative signal detection in the Dutch spontaneous reporting system for adverse drug reactions. Drug. Saf. 2003, 26, 293–301. [Google Scholar] [CrossRef]
- Evans, S.J.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef] [PubMed]
- van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.; Lindquist, M.; Orre, R.; Egberts, A.C. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
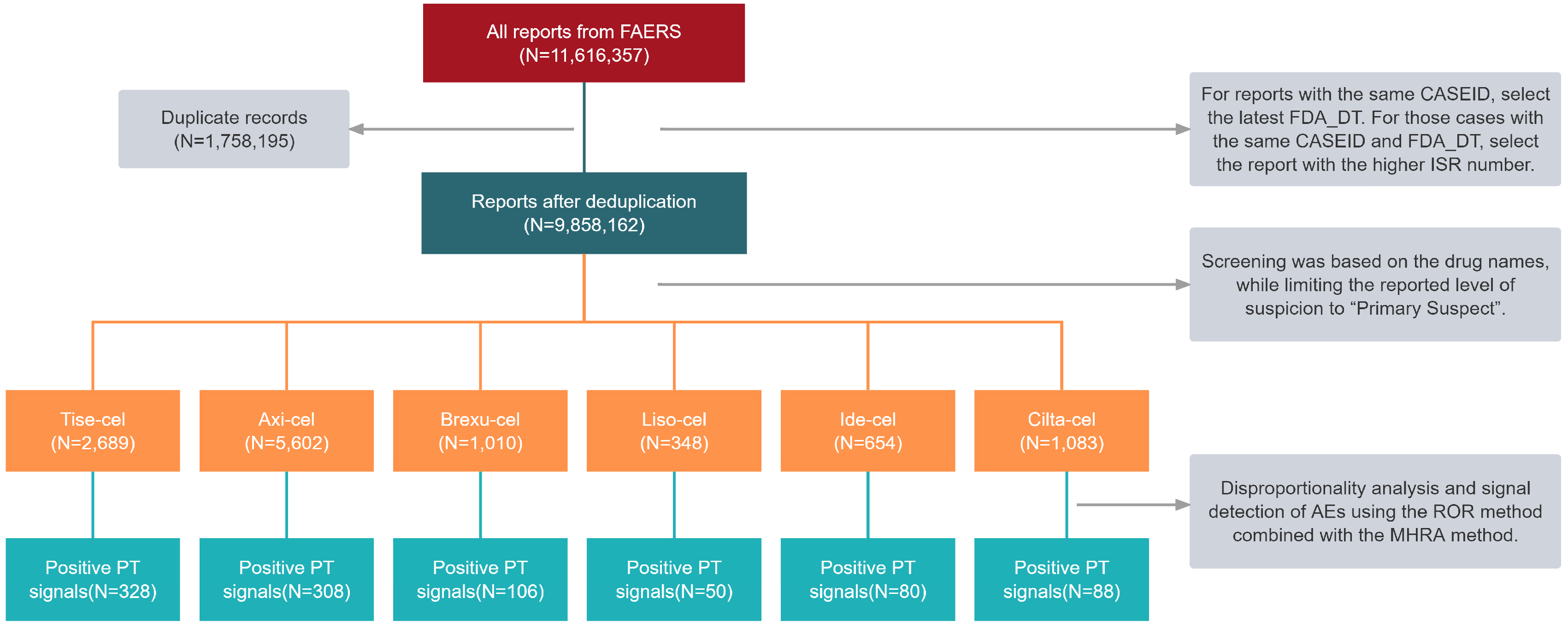
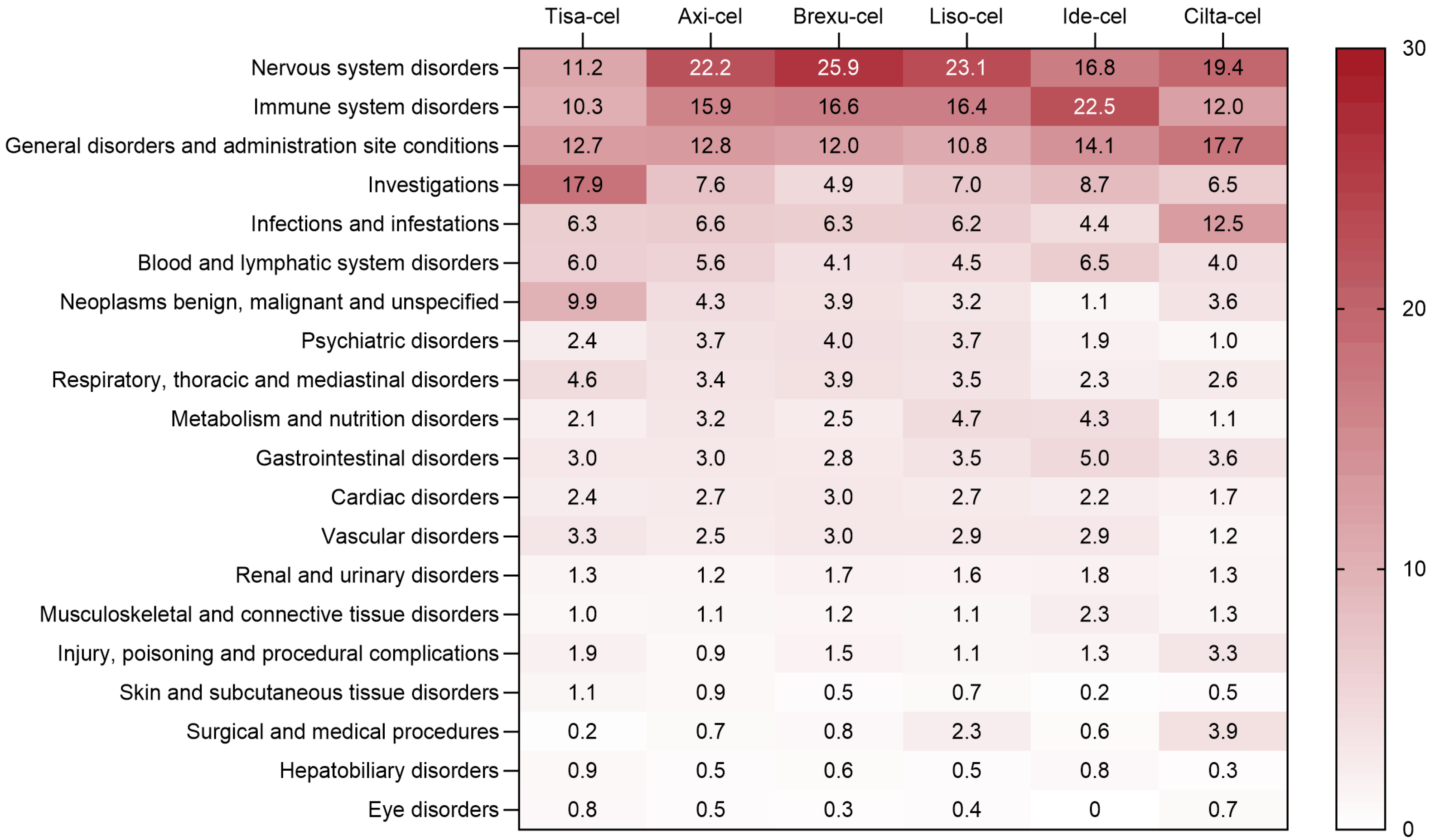
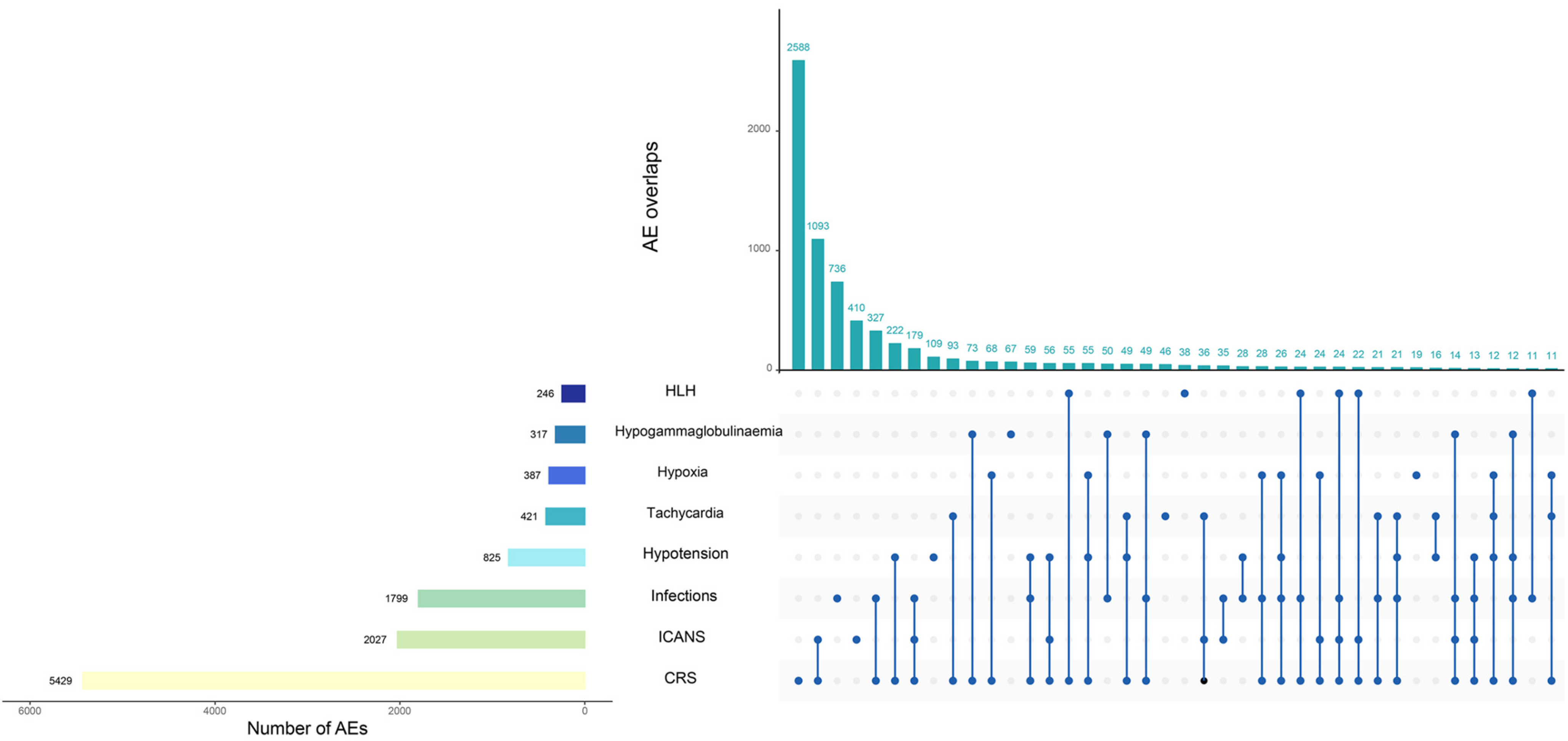
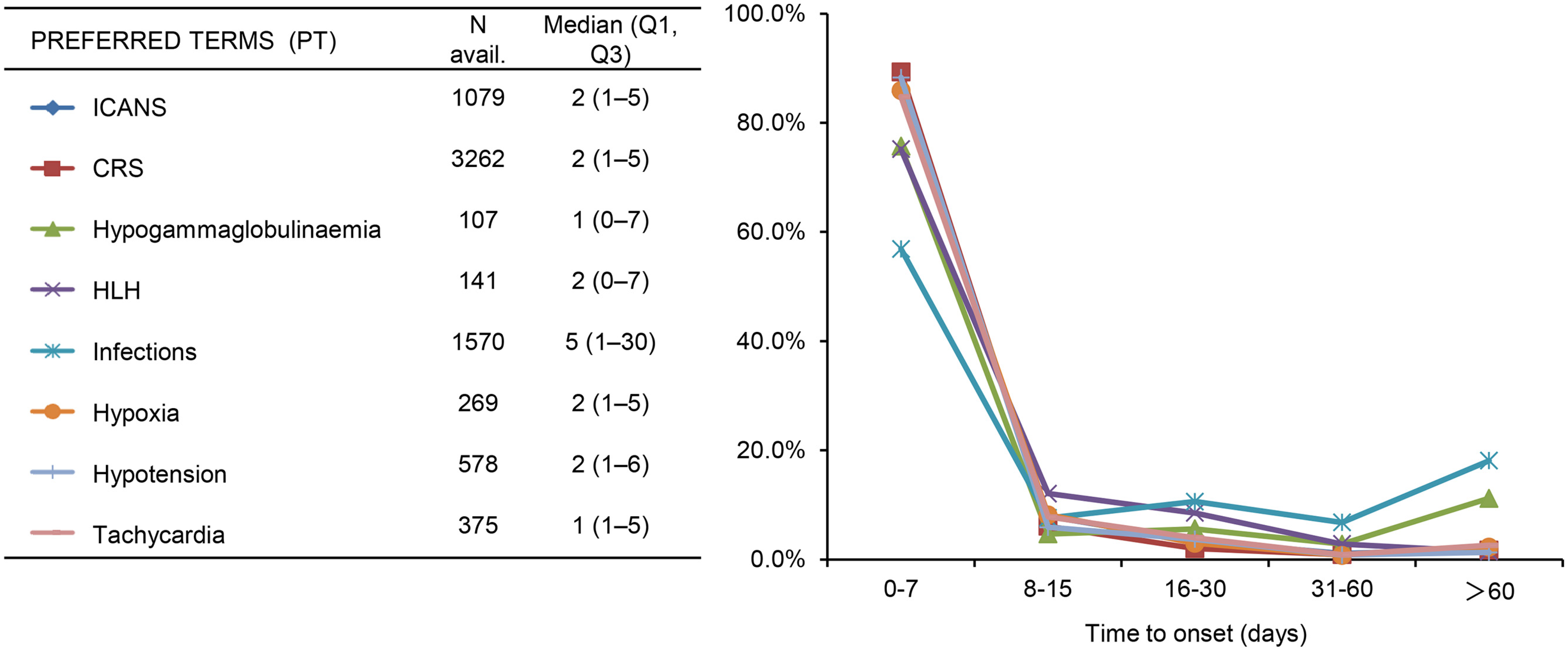
| Variable (n, %) | Tisa-cel | Axi-cel | Brexu-cel | Liso-cel | Ide-cel | Cilta-cel |
|---|---|---|---|---|---|---|
| No. of patients | 2689 | 5602 | 1010 | 348 | 654 | 1083 |
| Sex | ||||||
| Female | 835 (31.1) | 1734 (31.0) | 206 (20.4) | 127 (36.5) | 231 (35.3) | 273 (25.2) |
| Male | 1297 (48.2) | 2716 (48.5) | 630 (62.4) | 208 (59.8) | 326 (49.8) | 364 (33.6) |
| Not specified | 557 (20.7) | 1152 (20.6) | 174 (17.2) | 13 (3.7) | 97 (14.8) | 446 (41.2) |
| Age, years | ||||||
| <18 | 578 (21.5) | 16 (0.3) | 8 (0.8) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| 18–64 | 701 (26.1) | 2323 (41.5) | 341 (33.8) | 99 (28.4) | 198 (30.3) | 144 (13.3) |
| ≥65 | 458 (17.0) | 1522 (27.2) | 344 (34.1) | 218 (62.6) | 307 (46.9) | 205 (18.9) |
| Not specified | 952 (35.4) | 1741 (31.1) | 317 (31.4) | 30 (8.6) | 149 (22.8) | 734 (67.8) |
| Reporting year | ||||||
| 2017 | 9 (0.3) | 1 (0.0) | - | - | - | - |
| 2018 | 135 (5.0) | 479 (8.6) | - | - | - | - |
| 2019 | 302 (11.2) | 785 (14.0) | - | - | - | - |
| 2020 | 601 (22.4) | 861 (15.4) | 25 (2.5) | - | - | - |
| 2021 | 535 (19.9) | 678 (12.1) | 206 (20.4) | 86 (24.7) | 94 (14.4) | - |
| 2022 | 556 (20.7) | 898 (16.0) | 283 (28.0) | 124 (35.6) | 264 (40.4) | 161 (14.9) |
| 2023 | 424 (15.8) | 1313 (23.4) | 362 (35.8) | 104 (29.9) | 218 (33.3) | 607 (56.0) |
| 2024 | 127 (4.7) | 587 (10.5) | 134 (13.3) | 34 (9.8) | 78 (11.9) | 315 (29.1) |
| Reporter | ||||||
| Health professional | 2284 (84.9) | 4820 (86.0) | 831 (82.3) | 240 (69.0) | 459 (70.2) | 569 (52.5%) |
| Consumer | 331 (12.3) | 393 (7.0) | 58 (5.7) | 29 (8.3) | 84 (12.8) | 463 (42.8) |
| Not specified | 74 (2.8) | 389 (6.9) | 121 (12.0) | 79 (22.7) | 111 (17.0) | 51 (4.7) |
| Reporting region | ||||||
| North America | 1767 (65.7) | 3421 (61.1) | 574 (56.8) | 177 (50.9) | 405 (61.9) | 878 (81.1) |
| Europe | 476 (17.7) | 1427 (25.5) | 247 (24.5) | 15 (4.3) | 81 (12.4) | 72 (6.6) |
| Asia | 207 (7.7) | 219 (3.9) | 7 (0.7) | 32 (9.2) | 19 (2.9) | 16 (1.5) |
| Not specified/other | 239 (8.9) | 535 (9.6) | 182 (18.0) | 124 (35.6) | 149 (22.8) | 117 (10.8) |
| Indication | ||||||
| Acute lymphoblastic leukemia | 1054 (39.2) | 31 (0.6) | 161 (15.9) | 1 (0.3) | - | - |
| Large B-cell lymphoma | 1004 (37.3) | 3256 (58.1) | 16 (1.6) | 237 (68.1) | 3 (0.5) | 2 (0.2) |
| Follicular lymphoma | 49 (1.8) | 148 (2.6) | - | 17 (4.9) | - | - |
| Mantle cell lymphoma | 5 (0.2) | 39 (0.7) | 517 (51.2) | 7 (2.0) | - | - |
| Multiple myeloma | - | - | - | - | 578 (88.4) | 417 (38.5) |
| Not specified/other | 577 (21.5) | 2128 (38.0) | 316 (31.3) | 86 (24.7) | 73 (11.2) | 664 (61.3) |
| AE Severity | ||||||
| Serious | 2499 (92.9) | 5394 (96.3) | 955 (94.6) | 309 (88.8) | 572 (87.5) | 759 (70.1) |
| Non-serious | 190 (7.1) | 208 (3.7) | 55 (5.4) | 39 (11.2) | 82 (12.5) | 324 (29.9) |
| Outcome | ||||||
| Life-threatening | 273 (10.2) | 361 (6.4) | 102 (10.1) | 31 (8.9) | 36 (5.5) | 46 (4.2) |
| Hospitalization | 831 (30.9) | 2011 (35.9) | 405 (40.1) | 205 (58.9) | 241 (36.9) | 402 (37.1) |
| Disability | 42 (1.6) | 82 (1.5) | 18 (1.8) | 2 (0.6) | 7 (1.1) | 4 (0.4) |
| Death | 707 (26.3) | 1322 (23.6) | 247 (24.5) | 61 (17.5) | 60 (9.2) | 127 (11.7) |
| Other | 1821 (67.7) | 4536 (81.0) | 744 (73.7) | 140 (40.2) | 396 (60.6) | 348 (32.1) |
| Preferred Terms (PT) | Tisa-cel | Axi-cel | Brexu-cel | Liso-cel | Ide-cel | Cilta-cel | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | ROR (95% CI) | N | ROR (95% CI) | N | ROR (95% CI) | N | ROR (95% CI) | N | ROR (95% CI) | N | ROR (95% CI) | |
| Nervous system disorders | ||||||||||||
| ICANS | 268 | 223.33 (196.65–253.63) | 1202 | 1198.80 (1109.64–1295.11) | 324 | 801.69 (709.79–905.49) | 64 | 426.30 (329.95–550.79) | 109 | 313.35 (257.36–381.52) | 60 | 178.74 (137.74–231.96) |
| Neurotoxicity | 288 | 63.36 (56.29–71.33) | 1156 | 209.88 (197.02–223.57) | 151 | 145.20 (123.07–171.29) | 72 | 236.75 (186.02–301.32) | 55 | 79.03 (60.38–103.44) | 43 | 71.22 (52.55–96.52) |
| Encephalopathy | 67 | 12.40 (9.75–15.77) | 247 | 33.92 (29.88–38.51) | 45 | 36.13 (26.90–48.54) | 6 | 16.10 (7.21–35.94) | 7 | 8.52 (4.06–17.90) | 6 | 8.43 (3.78–18.79) |
| Aphasia | 48 | 7.64 (5.75–10.14) | 204 | 24.13 (21.00–27.73) | 33 | 24.35 (17.27–34.33) | 14 | 35.22 (20.77–59.72) | 5 | 5.54 (2.30–13.33) | 3 | 3.89 (1.25–12.07) |
| Tremor a | - | - | 198 | 4.28 (3.72–4.92) | 45 | 6.26 (4.67–8.40) | 11 | 5.16 (2.85–9.34) | 27 | 5.74 (3.93–8.39) | - | - |
| Depressed level of consciousness | 43 | 5.06 (3.75–6.82) | 56 | 4.77 (3.67–6.20) | 17 | 9.05 (5.61–14.57) | 5 | 8.90 (3.74–21.67) | 18 | 23.97 (15.06–38.16) | - | - |
| Dysgraphia | 16 | 11.30 (6.91–18.48) | 56 | 32.33 (24.80–42.14) | 8 | 29.24 (14.58–58.64) | - | - | - | - | - | - |
| Apraxia | 3 | 9.43 (3.03–29.32) * | 3 | 6.80 (2.19–21.14) | - | - | - | - | - | - | - | - |
| Brain oedema | 18 | 7.13 (4.49–11.33) | 43 | 12.51 (9.26–16.90) | 14 | 24.55 (14.50–41.54) | - | - | - | - | 3 | 8.95 (2.88–27.80) |
| Cerebellar infarction | 4 | 13.75 (5.14–36.76) | 6 | 15.17 (6.79–33.92) | - | - | - | - | - | - | - | - |
| Brain stem infarction | - | - | 3 | 14.69 (4.71–45.83) | - | - | - | - | - | - | - | - |
| Embolic stroke | - | - | 5 | 5.05 (2.10–12.16) | - | - | - | - | - | - | - | - |
| Intracranial hemorrhage | - | - | - | - | - | - | - | - | - | - | 5 | 18.47 (7.67–44.48) * |
| Cerebral hemorrhage | - | - | - | - | - | - | - | - | - | - | 3 | 3.81 (1.23–11.82) * |
| Cranial nerve disorder | 3 | 19.93 (6.39–62.16) * | - | - | - | - | - | - | - | - | 8 | 991.24 (467.81–2100.39) |
| Facial nerve disorder | - | - | - | - | - | - | - | - | - | - | 6 | 727.22 (310.87–1701.16) |
| Bell’s palsy | - | - | - | - | - | - | - | - | - | - | 33 | 253.07 (178.12–359.57) |
| Facial paralysis b | 22 | 6.24 (4.11–9.49) * | - | - | 4 | 5.56 (2.08–14.83) * | - | - | - | - | 35 | 95.69 (68.32–134.02) |
| Parkinsonism | - | - | - | - | - | - | - | - | 4 | 13.00 (4.87–34.72) | 43 | 168.90 (124.33–229.44) |
| Motor dysfunction | 12 | 5.40 (3.06–9.52) | - | - | - | - | - | - | ||||
| Optic neuritis | 6 | 3.57 (1.60–7.94) | - | - | - | - | - | - | - | - | - | - |
| Guillain–Barre syndrome | 4 | 3.81 (1.43–10.15) * | - | - | - | - | - | - | - | - | 3 | 19.92 (6.41–61.92) |
| Cerebellar syndrome | 3 | 7.82 (2.52–24.31) * | 3 | 5.92 (1.90–18.39) * | - | - | - | - | - | - | - | - |
| Dysmetria | - | - | 3 | 10.30 (3.31–32.07) * | - | - | - | - | - | - | - | - |
| Balance disorder | - | - | - | - | - | - | - | - | 18 | 6.62 (4.16–10.53) | - | - |
| Cerebral mass effect | - | - | 3 | 10.58 (3.40–32.96) * | - | - | - | - | - | - | - | - |
| Cerebral venous sinus thrombosis | - | - | 4 | 8.70 (3.25–23.25) | - | - | - | - | - | - | - | - |
| Paraplegia | - | - | 6 | 7.17 (3.21–15.98) * | - | - | - | - | - | - | - | - |
| Myelopathy | - | - | 4 | 6.00 (2.25–16.03) * | - | - | - | - | - | - | - | - |
| Ageusia | - | - | - | - | - | - | - | - | - | - | 4 | 5.22 (1.96–13.94) * |
| Immune system disorders | ||||||||||||
| CRS c | 1139 | 269.77 (253.08–287.57) | 2965 | 676.42 (646.44–707.79) | 539 | 421.97 (383.85–463.88) | 153 | 369.83 (311.01–439.77) | 448 | 515.97 (464.10–573.64) | 198 | 213.05 (183.57–247.27) |
| Hypogammaglobulinemia | 213 | 151.64 (131.80–174.47) | 65 | 31.74 (24.81–40.60) | - | - | 4 | 33.71 (12.61–90.10) * | 30 | 113.34 (78.80–163.02) | 4 | 16.28 (6.09–43.47) |
| HLH d | 78 | 30.82 (24.63–38.57) | 87 | 24.99 (20.21–30.91) | 23 | 34.84 (23.08–52.57) | 11 | 54.44 (30.02–98.72) | 17 | 37.52 (23.25–60.54) | 30 | 75.22 (52.33–108.13) |
| Graft versus host disease e | 21 | 6.51 (4.24–10.00) | 7 | 5.67 (2.70–11.91) * | - | - | - | - | - | - | - | - |
| Immunodeficiency | - | - | 6 | 133.41 (57.74–308.26) * | - | - | - | - | 22 | 32.43 (21.29–49.41) | 3 | 4.96 (1.60–15.41) |
| Infections and infestations | ||||||||||||
| Bacterial infections f | 244 | 4.59 (4.04–5.20) | 273 | 3.47 (3.08–3.91) | 36 | 3.69 (2.66–5.12) | 9 | 8.30 (4.30–16.00) | 32 | 7.82 (5.51–11.08) | 40 | 7.16 (5.23–9.79) |
| Fungal infections f | 85 | 3.14 (2.53–3.88) | 133 | 3.61 (3.04–4.28) | 26 | 4.94 (3.36–7.26) | 4 | 5.52 (2.07–14.74) | 10 | 4.92 (2.64–9.15) | 17 | 8.17 (5.07–13.18) |
| Viral infections f | - | - | - | - | - | - | - | - | - | - | 79 | 2.63 (2.10–3.30) |
| Investigations | ||||||||||||
| White blood cell count decreased | 229 | 8.63 (7.57–9.84) | 118 | 3.19 (2.66–3.82) | - | - | 6 | 2.96 (1.33–6.60) | 21 | 4.61 (3.00–7.09) | 8 | 2.02 (1.01–4.05) |
| Platelet count decreased | 232 | 9.33 (8.19–10.62) | 111 | 3.21 (2.66–3.86) | - | - | 10 | 5.26 (2.82–9.80) | - | - | 13 | 3.49 (2.02–6.03) |
| Hemoglobin decreased | 222 | 10.44 (9.14–11.92) | - | - | - | - | - | - | - | - | - | - |
| Neutrophil count decreased | 198 | 20.43 (17.75–23.52) | 124 | 9.15 (7.66–10.92) | - | - | - | - | 3 | 26.21 (8.43–81.53) | 5 | 3.09 (1.28–7.43) |
| Aspartate aminotransferase increased | 30 | 3.30 (2.30–4.72) | 95 | 7.63 (6.24–9.34) * | 22 | 10.31 (6.78–15.69) | 3 | 4.91 (1.58–15.26) * | 47 | 34.99 (26.19–46.75) | 9 | 7.50 (3.90–14.45) |
| Alanine aminotransferase increased | 29 | 2.49 (1.73–3.58) | 78 | 4.86 (3.89–6.07) * | 23 | 4.71 (3.13–7.10) | - | - | 42 | 24.46 (18.01–33.20) | 8 | 5.31 (2.65–10.63) |
| Blood fibrinogen decreased | 24 | 112.61 (74.61–169.94) | 21 | 70.43 (45.42–109.19) | 3 | 55.78 (17.87–174.15) | - | - | - | - | ||
| Serum ferritin increased | 84 | 79.22 (63.66–98.59) | 71 | 48.25 (38.07–61.15) * | 13 | 56.30 (32.56–97.36) * | 6 | 90.51 (40.46–202.43) * | - | - | 3 | 21.70 (6.98–67.49) |
| Activated partial thromboplastin time prolonged | 19 | 30.32 (19.27–47.72) * | - | - | - | - | - | - | - | - | - | - |
| Prothrombin time prolonged | 16 | 24.03 (14.67–39.35) | - | - | - | - | - | - | - | - | - | - |
| Fibrin D dimer increased | 15 | 18.49 (11.12–30.75) | - | - | - | - | - | - | - | - | - | - |
| Preferred Terms (PT) | Tisa-cel | Axi-cel | Brexu-cel | Liso-cel | Ide-cel | Cilta-cel | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | ROR (95% CI) | N | ROR (95% CI) | N | ROR (95% CI) | N | ROR (95% CI) | N | ROR (95% CI) | N | ROR (95% CI) | |
| Respiratory, thoracic, and mediastinal disorders | ||||||||||||
| Hypoxia | 167 | 21.53 (18.47–25.09) | 171 | 15.95 (13.71–18.55) | 32 | 17.24 (12.16–24.43) | 4 | 7.32 (2.74–19.55) | 11 | 9.22 (5.10–16.68) | - | - |
| Respiratory failure a | 72 | 3.68 (2.92–4.64) | 74 | 2.76 (2.19–3.47) | 20 | 4.71 (3.03–7.31) | 4 | 12.27 (4.59–32.76) | - | - | 15 | 6.65 (4.00–11.06) |
| Tachypnoea | 38 | 12.19 (8.86–16.77) | 37 | 8.59 (6.21–11.87) | 12 | 15.80 (8.96–27.87) | - | - | 5 | 9.88 (4.10–23.77) | 3 | 6.87 (2.21–21.33) |
| Pleural effusion b | 36 | 2.79 (2.01–3.87) | 70 | 3.97 (3.14–5.02) | 15 | 5.40 (3.25–8.98) | 7 | 8.81 (4.19–18.52) | 6 | 3.36 (1.51–7.48) * | - | - |
| Atelectasis | 12 | 7.87 (4.46–13.88) * | 6 | 2.85 (1.28–6.35) * | 4 | 11.27 (4.22–30.08) * | - | - | - | - | - | - |
| Respiratory distress | 38 | 4.08 (2.96–5.61) | 17 | 3.29 (2.05–5.30) * | - | - | - | - | - | - | 3 | 6.14 (1.98–19.06) * |
| Pulmonary oedema | 23 | 2.46 (1.63–3.70) | - | - | - | - | - | - | - | - | - | - |
| Pulmonary hemorrhage | 8 | 5.29 (2.64–10.59) | 8 | 3.87 (1.93–7.75) | - | - | - | - | - | - | ||
| Pulmonary mass | 8 | 2.05 (1.02–4.10) * | - | - | - | - | - | - | - | - | - | - |
| Lung consolidation | 8 | 5.99 (2.99–11.98) * | 3 | 4.07 (1.31–12.65) * | - | - | - | - | - | - | - | - |
| Organizing pneumonia | 6 | 5.00 (2.24–11.14) * | - | - | - | - | - | - | - | - | - | - |
| Apnea | 4 | 2.84 (1.07–7.58) * | - | - | - | - | - | - | - | - | - | - |
| Laryngeal oedema | 4 | 3.40 (1.28–9.08) * | - | - | - | - | - | - | 5 | 30.63 (12.71–73.82) | - | - |
| Pharyngeal hemorrhage | 3 | 10.64 (3.42–33.10) | - | - | - | - | - | - | - | - | - | - |
| Aspiration | - | - | 12 | 4.19 (2.38–7.39) * | - | - | - | - | - | - | - | - |
| Lung infiltration | - | - | 6 | 3.56 (1.60–7.94) * | - | - | - | - | - | - | - | - |
| Pulmonary alveolar hemorrhage | - | - | 5 | 2.98 (1.24–7.16) | - | - | - | - | - | - | - | - |
| Cardiac disorders | ||||||||||||
| Tachycardia c | 143 | 5.30 (4.50–6.25) | 201 | 5.44 (4.74–6.26) | 47 | 7.84 (5.88–10.46) | 5 | 3.34 (1.39–8.04) | 29 | 7.82 (5.42–11.28) | 8 | 2.99 (1.49–5.99) |
| Arrhythmia | 94 | 1.23 (1.01–1.51) | 200 | 1.856 (1.61–2.126) | 37 | 2.31 (1.67–3.20) | 17 | 4.45 (2.75–7.18) | 18 | 1.75 (1.10–2.79) | - | - |
| Cardiorenal syndrome | 8 | 44.41 (22.03–89.50) * | 35 | 150.61 (106.25–213.50) * | - | - | - | - | - | - | - | - |
| Pericardial effusion | 12 | 2.41 (1.37–4.25) * | - | - | - | - | - | - | - | - | - | - |
| Left ventricular dysfunction | 8 | 5.27 (2.63–10.54) | - | - | - | - | - | - | - | - | - | - |
| Mitral valve disease | 8 | 3.78 (1.89–7.56) * | - | - | - | - | - | - | - | - | - | - |
| Aortic valve incompetence | 3 | 4.71 (1.52–14.64) * | - | - | - | - | - | - | - | - | - | - |
| Cardiomyopathy | - | - | 25 | 4.43 (3.00–6.57) * | - | - | - | - | - | - | - | - |
| Cardiopulmonary failure | - | - | 4 | 4.41 (1.65–11.77) * | - | - | - | - | - | - | - | - |
| Vascular disorders | ||||||||||||
| Hypotension d | 335 | 6.98 (6.27–7.78) | 346 | 5.22 (4.69–5.81) | 76 | 7.12 (5.67–8.94) | 26 | 8.15 (5.52–12.03) | 41 | 6.21 (4.56–8.46) | 16 | 2.76 (1.69–4.51) |
| Shock e | 22 | 3.20 (2.11–4.87) * | 32 | 2.87 (2.03–4.06) * | 7 | 4.31 (2.05–9.06) * | - | - | 3 | 4.41 (1.42–13.70) * | - | - |
| Hemodynamic instability | 9 | 5.23 (2.72–10.06) * | 9 | 3.75 (1.95–7.22) * | - | - | - | - | - | - | - | - |
| Circulatory collapse | 7 | 2.21 (1.05–4.64) * | - | - | - | - | - | - | - | - | - | - |
| Veno-occlusive disease | 5 | 7.03 (2.92–16.91) * | - | - | - | - | - | - | - | - | - | - |
| Hypoperfusion | 4 | 21.54 (8.04–57.69) * | - | - | - | - | - | - | - | - | - | - |
| Ischemia | 3 | 3.80 (1.22–11.79 * | - | - | - | - | - | - | - | - | - | - |
| Hypertensive emergency | - | - | 3 | 7.85 (2.52–24.43) * | - | - | - | - | - | - | - | - |
| Deep vein thrombosis | - | - | 25 | 1.85 (1.25–2.74) | - | - | - | - | - | - | - | - |
| Hypertension | - | - | - | - | - | - | - | - | 18 | 2.40 (1.51–3.82) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Ding, Y.; Wang, M.; Zhai, Q.; Liu, J.; Du, Q. Comparing the Differences in Adverse Events among Chimeric Antigen Receptor T-Cell Therapies: A Real-World Pharmacovigilance Study. Pharmaceuticals 2024, 17, 1025. https://doi.org/10.3390/ph17081025
Guo Z, Ding Y, Wang M, Zhai Q, Liu J, Du Q. Comparing the Differences in Adverse Events among Chimeric Antigen Receptor T-Cell Therapies: A Real-World Pharmacovigilance Study. Pharmaceuticals. 2024; 17(8):1025. https://doi.org/10.3390/ph17081025
Chicago/Turabian StyleGuo, Zihan, Yunlan Ding, Mengmeng Wang, Qing Zhai, Jiyong Liu, and Qiong Du. 2024. "Comparing the Differences in Adverse Events among Chimeric Antigen Receptor T-Cell Therapies: A Real-World Pharmacovigilance Study" Pharmaceuticals 17, no. 8: 1025. https://doi.org/10.3390/ph17081025
APA StyleGuo, Z., Ding, Y., Wang, M., Zhai, Q., Liu, J., & Du, Q. (2024). Comparing the Differences in Adverse Events among Chimeric Antigen Receptor T-Cell Therapies: A Real-World Pharmacovigilance Study. Pharmaceuticals, 17(8), 1025. https://doi.org/10.3390/ph17081025






