Oral Nanoformulations in Cardiovascular Medicine: Advances in Atherosclerosis Treatment
Abstract
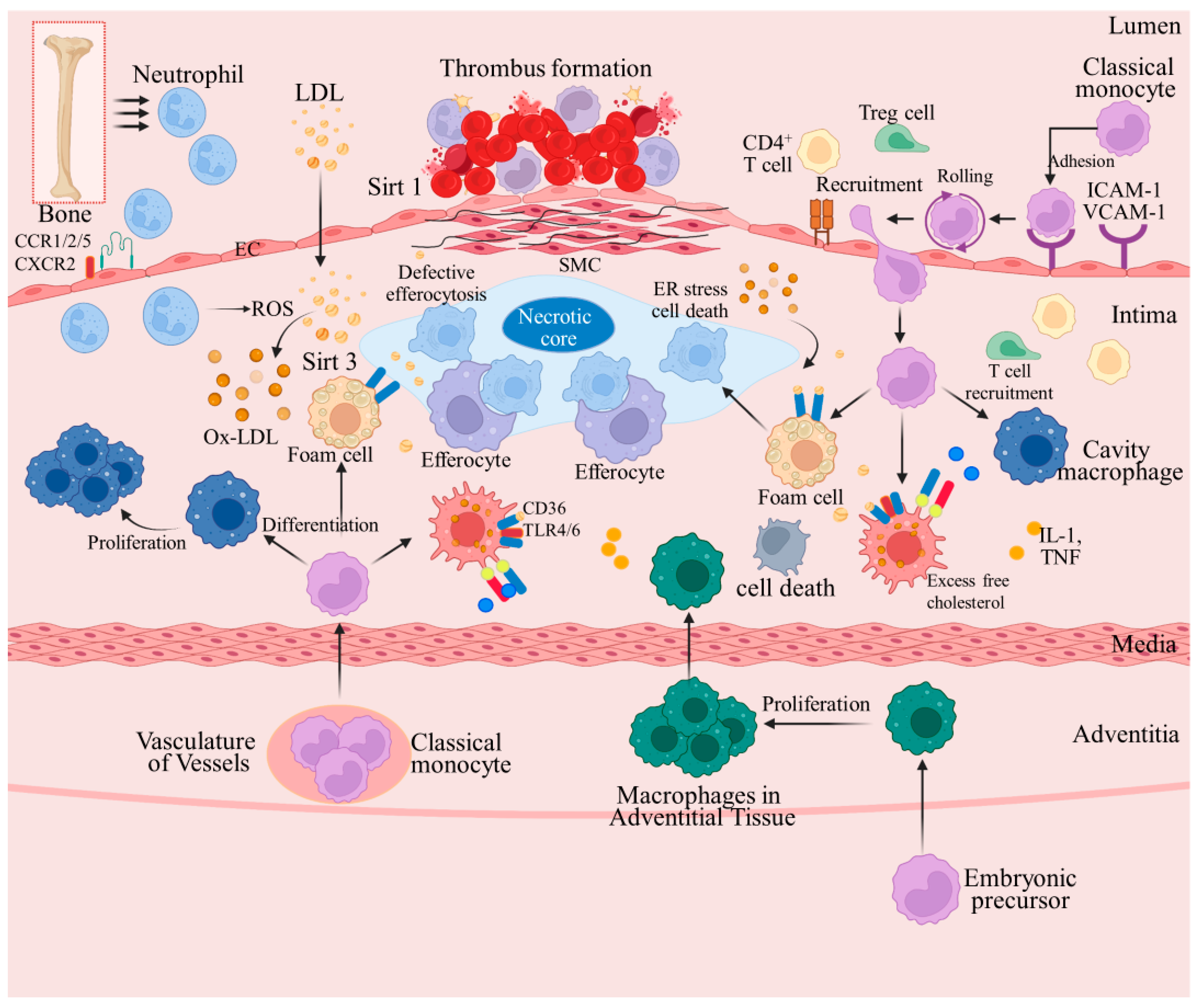
1. Introduction
2. AS Pathogenesis and Related Therapeutic Agents
2.1. Changes in Bioactive Substances Induced by Endothelial Cell Damage
2.2. Interaction between Lipid Deposition and Inflammatory Response
2.3. Intermediate AS Stages
2.4. Advanced AS Stages
3. Novel Oral Nanoformulations for AS Treatment
3.1. Oral NP-Loaded Drugs
3.2. Oral Drug-Loaded Nanoliposomes
3.3. Oral Drug-Loaded Solid Lipid NPs
3.4. Oral Drug-Loaded Nanoemulsions
3.5. Oral Drug-Loaded Nanocapsules
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, L.; Peng, C.; Tang, Y.; Yuan, Y.; Liu, J.; Xiang, T.; Liu, F.; Zhou, X.; Li, X. Biomimetic oral targeted delivery of bindarit for immunotherapy of atherosclerosis. Biomater. Sci. 2020, 8, 3640–3648. [Google Scholar] [CrossRef] [PubMed]
- Velpuri, P.; Rai, V.; Agrawal, D.K. Role of sirtuins in attenuating plaque vulnerability in atherosclerosis. Mol. Cell. Biochem. 2023, 479, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Tannaz, J.; Željko, R.; Maryam Matbou, R.; Prashant, K.; Ali, H.E.; Zahra, T.-N.; Amirhossein, S. The Effects of Statin Therapy on Circulating Levels of Trimethylamine N-oxide: A Systematic Review and Meta-analysis. Curr. Med. Chem. 2023, 31. [Google Scholar] [CrossRef]
- Rehman, Z.U. Rivaroxaban in Peripheral Arterial Disease (PAD) Management. J. Coll. Physicians Surg. Pak. 2023, 33, 832–833. [Google Scholar] [CrossRef] [PubMed]
- Nicolajsen, C.W.; Søgaard, M.; Jensen, M.; Eldrup, N.; Larsen, T.B.; Goldhaber, S.Z.; Behrendt, C.-A.; Nielsen, P.B. Antiplatelet Therapy in Patients with Abdominal Aortic Aneurysm without Symptomatic Atherosclerotic Disease. JAMA Netw. Open 2023, 6, e2339715. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.W.; Winkels, H.; Durant, C.P.; Zaitsev, K.; Ghosheh, Y.; Ley, K. Single Cell RNA Sequencing in Atherosclerosis Research. Circ. Res. 2020, 126, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.-S. Application of chitosan/alginate nanoparticle in oral drug delivery systems: Prospects and challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef]
- Hossaini Nasr, S.; Rashidijahanabad, Z.; Ramadan, S.; Kauffman, N.; Parameswaran, N.; Zinn, K.R.; Qian, C.; Arora, R.; Agnew, D.; Huang, X. Effective atherosclerotic plaque inflammation inhibition with targeted drug delivery by hyaluronan conjugated atorvastatin nanoparticles. Nanoscale 2020, 12, 9541–9556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Khan, A.R.; Fu, M.F.; Zhai, Y.J.; Ji, J.B.; Bobrovskaya, L.; Zhai, G.X. Advances in curcumin-loaded nanopreparations: Improving bioavailability and overcoming inherent drawbacks. J. Drug. Target. 2019, 27, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-J.; Chung, Y.S.; Lee, Y.J.; Yu, S.E.; Baek, S.; Kim, H.-S.; Kim, S.W.; Lee, J.-Y.; Kim, S.; Sung, H.-J. Cancer Patient Tissueoid with Self-Homing Nano-Targeting of Metabolic Inhibitor. Adv. Sci. 2021, 8, e2102640. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Liu, H.; Xie, Z.; Zheng, M. Biomimetic nanoprodrugs from fatty acid modified camptothecin and albumin for enhanced pharmacotherapy. J. Colloid Interface Sci. 2022, 630, 385–394. [Google Scholar] [CrossRef]
- Jiang, K.; Yu, Y.; Qiu, W.; Tian, K.; Guo, Z.; Qian, J.; Lu, H.; Zhan, C. Protein corona on brain targeted nanocarriers: Challenges and prospects. Adv. Drug Deliv. Rev. 2023, 202, 115114. [Google Scholar] [CrossRef]
- Mohammad, H.-U.-R.; Israt, J.; Tahmina, F.; Abu Bin, I. Bio-Inspired Nanomaterials for Micro/Nanodevices: A New Era in Biomedical Applications. Micromachines 2023, 14, 1786. [Google Scholar] [CrossRef]
- Sadhna, M.; Shalini, S.; Shikha, P.; Arvind, K.; Dipendra Kumar, M.; Pradeep, K.; Kaustubh Chandrakant, K.; Ashutosh, R. Enhancement in Biological Availability of Vitamins by Nano-engineering and its Applications: An Update. Curr. Pharm. Biotechnol. 2023, 25, 1523–1537. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, H.; Zhao, H.; Fu, S.; Li, R.; Wang, Z.; Wang, Y.; Lu, W.; Yang, X. Nanoparticle encapsulation using self-assembly abietic acid to improve oral bioavailability of curcumin. Food Chem. 2023, 436, 137676. [Google Scholar] [CrossRef] [PubMed]
- Raitakari, O.; Pahkala, K.; Magnussen, C.G. Prevention of atherosclerosis from childhood. Nat. Rev. Cardiol. 2022, 19, 543–554. [Google Scholar] [CrossRef]
- Mahtta, D.; Khalid, U.; Misra, A.; Samad, Z.; Nasir, K.; Virani, S.S. Premature Atherosclerotic Cardiovascular Disease: What Have We Learned Recently? Curr. Atheroscler. Rep. 2020, 22, 44. [Google Scholar] [CrossRef]
- Fernandez-Friera, L.; Penalvo, J.L.; Fernandez-Ortiz, A.; Ibanez, B.; Lopez-Melgar, B.; Laclaustra, M.; Oliva, B.; Mocoroa, A.; Mendiguren, J.; Martinez de Vega, V.; et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 2015, 131, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, I. Atherosclerosis: From Molecular Biology to Therapeutic Perspective 2.0. Int. J. Mol. Sci. 2022, 23, 15158. [Google Scholar] [CrossRef]
- Stone, N.J.; Smith, S.C., Jr.; Orringer, C.E.; Rigotti, N.A.; Navar, A.M.; Khan, S.S.; Jones, D.W.; Goldberg, R.; Mora, S.; Blaha, M.; et al. Managing Atherosclerotic Cardiovascular Risk in Young Adults: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.V.; Stancu, C.S.; Simionescu, M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009, 335, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Bloom, S.I.; Islam, M.T.; Lesniewski, L.A.; Donato, A.J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 2023, 20, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, V.; Balzan, S. Roles of LOX-1 in microvascular dysfunction. Microvasc. Res. 2016, 105, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Wanschel, A.; Guizoni, D.M.; Lorza-Gil, E.; Salerno, A.G.; Paiva, A.A.; Dorighello, G.G.; Davel, A.P.; Balkan, W.; Hare, J.M.; Oliveira, H.C.F. The Presence of Cholesteryl Ester Transfer Protein (CETP) in Endothelial Cells Generates Vascular Oxidative Stress and Endothelial Dysfunction. Biomolecules 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ding, M.-L.; Wu, F.; He, W.; Li, J.; Zhang, X.-Y.; Xie, W.-L.; Duan, S.-Z.; Xia, W.-H.; Tao, J. Impaired Endothelial Repair Capacity of Early Endothelial Progenitor Cells in Hypertensive Patients with Primary Hyperaldosteronemia. Hypertension 2016, 67, 430–439. [Google Scholar] [CrossRef]
- Diao, H.; Cheng, J.; Huang, X.; Huang, B.; Shao, X.; Zhao, J.; Lan, D.; Zhu, Q.; Yan, M.; Zhang, Y.; et al. The Chinese medicine Fufang Zhenzhu Tiaozhi capsule protects against atherosclerosis by suppressing EndMT via modulating Akt1/beta-catenin signaling pathway. J. Ethnopharmacol. 2022, 293, 115261. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Hu, C.F.; Wang, M.X.; Lin, J.; Li, J.M.; Wang, R.Z. Research on mechanism of PCS in damaging vascular endothelial cells and promoting formation of atherosclerosis via TLR4/TREM-1. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7533–7542. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Liu, H.; Tu, Z. Pro-inflammatory cytokines mediating senescence of vascular endothelial cells in atherosclerosis. Fundam. Clin. Pharmacol. 2023, 37, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Wang, Z.; Wang, C.; Ma, D. Endothelial-cell-mediated mechanism of coronary microvascular dysfunction leading to heart failure with preserved ejection fraction. Heart Fail. Rev. 2023, 28, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.L.; Wautier, M.P. Endothelial Cell Participation in Inflammatory Reaction. Int. J. Mol. Sci. 2021, 22, 6341. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.L.; Jia, Y.; Wan, Z.; An, Z.L.; Yang, S.; Han, F.F.; Gong, L.L.; Xuan, L.L.; Ren, L.L.; Zhang, W.; et al. Curcumin inhibits the formation of atherosclerosis in ApoE−/− mice by suppressing cytomegalovirus activity in endothelial cells. Life Sci. 2020, 257, 117658. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Hou, Y.; Zhou, H.; Li, Y.; Xue, Z.; Xue, X.; Huang, G.; Huang, K.; He, X.; Xu, W. Hypolipidemic, anti-inflammatory, and anti-atherosclerotic effects of tea before and after microbial fermentation. Food Sci. Nutr. 2021, 9, 1160–1170. [Google Scholar] [CrossRef]
- Yuan, P.; Hu, Q.; He, X.; Long, Y.; Song, X.; Wu, F.; He, Y.; Zhou, X. Laminar flow inhibits the Hippo/YAP pathway via autophagy and SIRT1-mediated deacetylation against atherosclerosis. Cell Death Dis. 2020, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 2013, 22, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.L.; Yuan, H.H.; Xie, L.L.; Guo, M.H.; Liao, D.F.; Zheng, X.L. New Dawn for Atherosclerosis: Vascular Endothelial Cell Senescence and Death. Int. J. Mol. Sci. 2023, 24, 15160. [Google Scholar] [CrossRef]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef]
- Yang, K.; Velagapudi, S.; Akhmedov, A.; Kraler, S.; Lapikova-Bryhinska, T.; Schmiady, M.O.; Wu, X.; Geng, L.; Camici, G.G.; Xu, A.; et al. Chronic SIRT1 supplementation in diabetic mice improves endothelial function by suppressing oxidative stres. Cardiovasc. Res. 2023, 119, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Pengnet, S.; Prommaouan, S.; Sumarithum, P.; Malakul, W. Naringin Reverses High-Cholesterol Diet-Induced Vascular Dysfunction and Oxidative Stress in Rats via Regulating LOX-1 and NADPH Oxidase Subunit Expression. BioMed Res. Int. 2019, 2019, 3708497. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, W.; Nie, P.; Lan, K.; Yang, X.; Yin, A.; Xiao, Q.; Shen, Y.; Xu, K.; Wang, X.; et al. Nur77 Deficiency Exacerbates Macrophage NLRP3 Inflammasome-Mediated Inflammation and Accelerates Atherosclerosis. Oxidative Med. Cell. Longev. 2022, 2022, 2017815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shao, C.; Zhou, H.; Yu, L.; Bao, Y.; Mao, Q.; Yang, J.; Wan, H. Salvianolic acid B inhibits atherosclerosis and TNF-alpha-induced inflammation by regulating NF-kappaB/NLRP3 signaling pathway. Phytomedicine 2023, 119, 155002. [Google Scholar] [CrossRef]
- Shen, Y.; Gao, Y.; Fu, J.; Wang, C.; Tang, Y.; Chen, S.; Zhao, Y. Lack of Rab27a attenuates foam cell formation and macrophage inflammation in uremic apolipoprotein E knockout mice. J. Mol. Histol. 2023, 54, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Lankin, V.Z.; Tikhaze, A.K.; Melkumyants, A.M. Dicarbonyl-Dependent Modification of LDL as a Key Factor of Endothelial Dysfunction and Atherosclerotic Vascular Wall Damage. Antioxidants 2022, 11, 1565. [Google Scholar] [CrossRef]
- Li, C.; Tan, Y.; Wu, J.; Ma, Q.; Bai, S.; Xia, Z.; Wan, X.; Liang, J. Resveratrol Improves Bnip3-Related Mitophagy and Attenuates High-Fat-Induced Endothelial Dysfunction. Front. Cell Dev. Biol. 2020, 8, 796. [Google Scholar] [CrossRef]
- Qiu, J.; Fu, Y.; Chen, Z.; Zhang, L.; Li, L.; Liang, D.; Wei, F.; Wen, Z.; Wang, Y.; Liang, S. BTK Promotes Atherosclerosis by Regulating Oxidative Stress, Mitochondrial Injury, and ER Stress of Macrophages. Oxidative Med. Cell. Longev. 2021, 2021, 9972413. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Wang, L.; Li, C.; Xu, M.; Duan, Y.; Ma, L.; Li, T.; Chen, Q.; Wang, Y.; et al. Targeting mitochondria-inflammation circle by renal denervation reduces atheroprone endothelial phenotypes and atherosclerosis. Redox Biol. 2021, 47, 102156. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jiang, L.Y.; Wang, Y.C.; Ma, D.F.; Li, X. Quercetin Attenuates Atherosclerosis via Modulating Oxidized LDL-Induced Endothelial Cellular Senescence. Front. Pharmacol. 2020, 11, 512. [Google Scholar] [CrossRef]
- Yu, S.; Kim, S.R.; Jiang, K.; Ogrodnik, M.; Zhu, X.Y.; Ferguson, C.M.; Tchkonia, T.; Lerman, A.; Kirkland, J.L.; Lerman, L.O. Quercetin Reverses Cardiac Systolic Dysfunction in Mice Fed with a High-Fat Diet: Role of Angiogenesis. Oxidative Med. Cell. Longev. 2021, 2021, 8875729. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, Y.; Li, J. Effects of Atorvastatin Combined with Nano-Selenium on Blood Lipids and Oxidative Stress in Atherosclerotic Rats. J. Nanosci. Nanotechnol. 2021, 21, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Lu, J.; Zhang, C.; Meng, L.; Zhu, Q. The proatherosclerotic function of BCAT1 in atherosclerosis development of aged-apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 2022, 631, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Miano, J.M.; Fisher, E.A.; Majesky, M.W. Fate and State of Vascular Smooth Muscle Cells in Atherosclerosis. Circulation 2021, 143, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, C.C. Lymphocytes in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Bornfeldt, K.E. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Guo, Z.; Liu, D.; Guo, Z.; Wu, Q.; Li, Q.; Lin, R.; Chen, P.; Ou, C.; Chen, M. Deficiency of PSRC1 accelerates atherosclerosis by increasing TMAO production via manipulating gut microbiota and flavin monooxygenase 3. Gut Microbes 2022, 14, 2077602. [Google Scholar] [CrossRef] [PubMed]
- Souilhol, C.; Harmsen, M.C.; Evans, P.C.; Krenning, G. Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc. Res. 2018, 114, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Jiang, X.; Sheng, W.; Zhang, Y.; Lin, Q.; Hong, S.; Zhao, J.; Wang, T.; Ye, X. Xinmaikang (XMK) tablets alleviate atherosclerosis by regulating the SREBP2-mediated NLRP3/ASC/Caspase-1 signaling pathway. J. Ethnopharmacol. 2023, 319, 117240. [Google Scholar] [CrossRef]
- Shen, S.; Sun, T.; Ding, X.; Gu, X.; Wang, Y.; Ma, X.; Li, Z.; Gao, H.; Ge, S.; Feng, Q. The exoprotein Gbp of Fusobacterium nucleatum promotes THP-1 cell lipid deposition by binding to CypA and activating PI3K-AKT/MAPK/NF-kappaB pathways. J. Adv. Res. 2023, 57, 93–105. [Google Scholar] [CrossRef]
- Cen, Y.; Xiong, Y.; Qin, R.; Tao, H.; Yang, Q.; Pan, X. Anti-malarial artesunate ameliorates atherosclerosis by modulating arterial inflammatory responses via inhibiting the NF-κB-NLRP3 inflammasome pathway. Front. Pharmacol. 2023, 14, 1123700. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Xu, Y.; Zhu, J.; Hu, X.; Wang, C.; Lu, D.; Gong, C.; Yang, J.; Zong, L. CLIC1 Inhibition Attenuates Vascular Inflammation, Oxidative Stress, and Endothelial Injury. PLoS ONE 2016, 11, e0166790. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, X.; Zhang, Y.; Ma, W.; Sun, L.; Wang, C.; Ren, B. Adrenomedullin, transcriptionally regulated by vitamin D receptors, alleviates atherosclerosis in mice through suppressing AMPK-mediated endothelial ferroptosis. Environ. Toxicol. 2023, 39, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Thanopoulou, K.; Salagianni, M.; Siasos, G.; Oikonomou, E.; Perrea, D.D.; Nirakis, N.; Filis, K.; Tsioufis, K.; Tousoulis, D.; et al. Rosuvastatin Attenuates Progression of Atherosclerosis and Reduces Serum IL6 and CCL2 Levels in Apolipoprotein-E-deficient Mice. In Vivo 2023, 37, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Palekar, R.U.; Jallouk, A.P.; Myerson, J.W.; Pan, H.; Wickline, S.A. Inhibition of Thrombin with PPACK-Nanoparticles Restores Disrupted Endothelial Barriers and Attenuates Thrombotic Risk in Experimental Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.; Savla, S.R.; Borkar, M.R.; Bhatt, L.K. Sulfamethizole attenuates poloxamer 407-induced atherosclerotic neointima formation via inhibition of mTOR in C57BL/6 mice. J. Biochem. Mol. Toxicol. 2023, 37, e23322. [Google Scholar] [CrossRef] [PubMed]
- Huwait, E.; Almassabi, R.; Almowallad, S.; Saddeek, S.; Karim, S.; Kalamegam, G.; Mirza, Z. Microarray Expression Profile of Myricetin-Treated THP-1 Macrophages Exhibits Alterations in Atherosclerosis-Related Regulator Molecules and LXR/RXR Pathway. Int. J. Mol. Sci. 2022, 24, 278. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Finigan, A.; Figg, N.L.; Uryga, A.K.; Bennett, M.R. SIRT6 Protects Smooth Muscle Cells From Senescence and Reduces Atherosclerosis. Circ. Res. 2021, 128, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Baardman, J.; Verberk, S.G.S.; van der Velden, S.; Gijbels, M.J.J.; van Roomen, C.; Sluimer, J.C.; Broos, J.Y.; Griffith, G.R.; Prange, K.H.M.; van Weeghel, M.; et al. Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques. Nat. Commun. 2020, 11, 6296. [Google Scholar] [CrossRef]
- Wang, S.H.; Tsai, F.C.; Lin, H.H.; Yu, T.Y.; Kuo, C.H.; Li, H.Y.; Lin, M.S. Inhibition of monoamine oxidase B reduces atherosclerosis and fatty liver in mice. Clin. Sci. 2023, 137, 17–30. [Google Scholar] [CrossRef]
- Chen, J.; Lai, K.; Yong, X.; Yin, H.; Chen, Z.; Wang, H.; Chen, K.; Zheng, J. Silencing METTL3 Stabilizes Atherosclerotic Plaques by Regulating the Phenotypic Transformation of Vascular Smooth Muscle Cells via the miR-375-3p/PDK1 Axis. Cardiovasc. Drugs Ther. 2023, 37, 471–486. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, L.; Wu, P.; Zhao, L.; Wu, Y. Fusobacterium nucleatum Accelerates Atherosclerosis via Macrophage-Driven Aberrant Proinflammatory Response and Lipid Metabolism. Front. Microbiol. 2022, 13, 798685. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Hu, H.; Yin, M.; Lin, Y.; Yan, Y.; Han, P.; Liu, B.; Jing, B. HOXA1 participates in VSMC-to-macrophage-like cell transformation via regulation of NF-kappaB p65 and KLF4: A potential mechanism of atherosclerosis pathogenesis. Mol. Med. 2023, 29, 104. [Google Scholar] [CrossRef]
- Sakamoto, A.; Kawakami, R.; Mori, M.; Guo, L.; Paek, K.H.; Mosquera, J.V.; Cornelissen, A.; Ghosh, S.K.B.; Kawai, K.; Konishi, T.; et al. CD163+ macrophages restrain vascular calcification, promoting the development of high-risk plaque. JCI Insight 2023, 8, e154922. [Google Scholar] [CrossRef]
- Campia, U.; Gerhard-Herman, M.; Piazza, G.; Goldhaber, S.Z. Peripheral Artery Disease: Past, Present, and Future. Am. J. Med. 2019, 132, 1133–1141. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhu, P.; Liu, Y.; Zhu, H.; Geng, J.; Wang, B.; Yuan, G.; Peng, Y.; Xu, B. PM2.5 induces endothelial dysfunction via activating NLRP3 inflammasome. Environ. Toxicol. 2021, 36, 1886–1893. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef]
- Guo, X.; Guo, Y.; Wang, Z.; Cao, B.; Zheng, C.; Zeng, Z.; Wei, Y. Reducing the Damage of Ox-LDL/LOX-1 Pathway to Vascular Endothelial Barrier Can Inhibit Atherosclerosis. Oxidative Med. Cell. Longev. 2022, 2022, 7541411. [Google Scholar] [CrossRef]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, Q. Foam cell formation: A new target for fighting atherosclerosis and cardiovascular disease. Vasc. Pharmacol. 2019, 112, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Pinderski, L.J.; Fischbein, M.P.; Subbanagounder, G.; Fishbein, M.C.; Kubo, N.; Cheroutre, H.; Curtiss, L.K.; Berliner, J.A.; Boisvert, W.A. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circ. Res. 2002, 90, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Sawma, T.; Shaito, A.; Najm, N.; Sidani, M.; Orekhov, A.; El-Yazbi, A.F.; Iratni, R.; Eid, A.H. Role of RhoA and Rho-associated kinase in phenotypic switching of vascular smooth muscle cells: Implications for vascular function. Atherosclerosis 2022, 358, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Wadey, K.; Lopes, J.; Bendeck, M.; George, S. Role of smooth muscle cells in coronary artery bypass grafting failure. Cardiovasc. Res. 2018, 114, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Vacek, T.P.; Rehman, S.; Neamtu, D.; Yu, S.; Givimani, S.; Tyagi, S.C. Matrix metalloproteinases in atherosclerosis: Role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc. Health Risk Manag. 2015, 11, 173–183. [Google Scholar] [CrossRef]
- Beaudeux, J.L.; Giral, P.; Bruckert, E.; Foglietti, M.J.; Chapman, M.J. Matrix metalloproteinases, inflammation and atherosclerosis: Therapeutic perspectives. Clin. Chem. Lab. Med. 2004, 42, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Schlegel, M.P.; Afonso, M.S.; Brown, E.J.; Rahman, K.; Weinstock, A.; Sansbury, B.E.; Corr, E.M.; van Solingen, C.; Koelwyn, G.J.; et al. Regulatory T Cells License Macrophage Pro-Resolving Functions During Atherosclerosis Regression. Circ. Res. 2020, 127, 335–353. [Google Scholar] [CrossRef]
- Ammirati, E.; Moroni, F.; Magnoni, M.; Camici, P.G. The role of T and B cells in human atherosclerosis and atherothrombosis. Clin. Exp. Immunol. 2015, 179, 173–187. [Google Scholar] [CrossRef]
- van Diepen, S.; Fuster, V.; Verma, S.; Hamza, T.H.; Siami, F.S.; Goodman, S.G.; Farkouh, M.E. Dual Antiplatelet Therapy Versus Aspirin Monotherapy in Diabetics with Multivessel Disease Undergoing CABG: FREEDOM Insights. J. Am. Coll. Cardiol. 2017, 69, 119–127. [Google Scholar] [CrossRef]
- Batool, S.; Sohail, S.; ud Din, F.; Alamri, A.H.; Alqahtani, A.S.; Alshahrani, M.A.; Alshehri, M.A.; Choi, H.G. A detailed insight of the tumor targeting using nanocarrier drug delivery system. Drug Deliv. 2023, 30, 2183815. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, Q.; Wang, Y.; Zhong, X.; Liu, S.; Xie, R.; Ren, L. Functional nano drug delivery system with dual lubrication and immune escape for treating osteoarthritis. J. Colloid Interface Sci. 2023, 652, 2167–2179. [Google Scholar] [CrossRef]
- Movahedpour, A.; Taghvaeefar, R.; Asadi-Pooya, A.A.; Karami, Y.; Tavasolian, R.; Khatami, S.H.; Soltani Fard, E.; Taghvimi, S.; Karami, N.; Rahimi Jaberi, K.; et al. Nano-delivery systems as a promising therapeutic potential for epilepsy: Current status and future perspectives. CNS Neurosci. Ther. 2023, 29, 3150–3159. [Google Scholar] [CrossRef]
- Li, L.; Gao, Y.; Zhang, Y.; Yang, R.; Ouyang, Z.; Guo, R.; Yu, H.; Shi, X.; Cao, X. A Biomimetic Nanogel System Restores Macrophage Phagocytosis for Magnetic Resonance Imaging-Guided Synergistic Chemoimmunotherapy of Breast Cancer. Adv. Healthc. Mater. 2023, 12, e2300967. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Wang, X.; Su, M.; Xu, F.; Yang, L.; Jia, L.; Zhang, Z. Advances in Antitumor Nano-Drug Delivery Systems of 10-Hydroxycamptothecin. Int. J. Nanomed. 2022, 17, 4227–4259. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Chen, Z. Protein-Based Nano-Vessels Facilitates the Victoria Blue B Mediated Inhibition of Amyloid Fibrillation. Macromol. Rapid Commun. 2020, 41, e2000368. [Google Scholar] [CrossRef]
- Ze, L.; Zhixin, L.; Yilun, C.; Dong-Bing, C.; Taolei, S. MicroRNA therapeutics and nucleic acid nano-delivery systems in bacterial infection: A review. J. Mater. Chem. B 2023, 11, 7804–7833. [Google Scholar] [CrossRef]
- Wu, C.; Mao, J.; Wang, X.; Yang, R.; Wang, C.; Li, C.; Zhou, X. Advances in treatment strategies based on scavenging reactive oxygen species of nanoparticles for atherosclerosis. J. Nanobiotechnol. 2023, 21, 271. [Google Scholar] [CrossRef]
- Gao, F.; Cui, B.; Wang, C.; Li, X.; Li, B.; Zhan, S.; Shen, Y.; Zhao, X.; Sun, C.; Wang, C.; et al. Nano-EMB-SP improves the solubility, foliar affinity, photostability and bioactivity of emamectin benzoate. Pest Manag. Sci. 2022, 78, 3717–3724. [Google Scholar] [CrossRef]
- Fojtu, M.; Gumulec, J.; Stracina, T.; Raudenska, M.; Skotakova, A.; Vaculovicova, M.; Adam, V.; Babula, P.; Novakova, M.; Masarik, M. Reduction of Doxorubicin-Induced Cardiotoxicity Using Nanocarriers: A Review. Curr. Drug Metab. 2017, 18, 237–263. [Google Scholar] [CrossRef] [PubMed]
- Nenna, A.; Nappi, F.; Larobina, D.; Verghi, E.; Chello, M.; Ambrosio, L. Polymers and Nanoparticles for Statin Delivery: Current Use and Future Perspectives in Cardiovascular Disease. Polymers 2021, 13, 711. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.Y.; Lee, J.-S.; Lee, H.G. Chitosan/poly-γ-glutamic acid nanoparticles improve the solubility of lutein. Int. J. Biol. Macromol. 2016, 85, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Kalin, G.T.; Shi, D.; Kalinichenko, V.V. Nanoparticle Delivery Systems with Cell-Specific Targeting for Pulmonary Diseases. Am. J. Respir. Cell Mol. Biol. 2021, 64, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Laniado, N. The fragmentation of childrens’ oral health: Access to care in pediatric dentistry and orthodontics. Semin. Orthod. 2016, 22, 161–166. [Google Scholar] [CrossRef]
- Lina, Y.; Guanxiong, Z.; Zeyu, Z.; Zidan, X.; Weijie, P.; Liting, Z.; Yang, Y.; Siran, W.; Zhongxiao, L.; Xin, Z.; et al. Nano-Photosensitizer Directed Targeted Phototherapy Effective Against Oral Cancer in Animal Model. Int. J. Nanomed. 2023, 18, 6185–6198. [Google Scholar] [CrossRef]
- Maxius, G.; Veerakiet, B. Current applications of solid lipid nanoparticles and nanostructured lipid carriers as vehicles in oral delivery systems for antioxidant nutraceuticals: A review. Colloids Surf. B Biointerfaces 2023, 233, 113608. [Google Scholar] [CrossRef]
- Zi, Y.; Yang, K.; He, J.; Wu, Z.; Liu, J.; Zhang, W. Strategies to enhance drug delivery to solid tumors by harnessing the EPR effects and alternative targeting mechanisms. Adv. Drug Deliv. Rev. 2022, 188, 114449. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Valero-Muñoz, M.; Martín-Fernández, B.; Ballesteros, S.; Cachofeiro, V.; Lahera, V.; de Las Heras, N. Rosuvastatin improves insulin sensitivity in overweight rats induced by high fat diet. Role of SIRT1 in adipose tissue. Clín. Investig. Arterioscler. 2014, 26, 161–167. [Google Scholar] [CrossRef]
- Gualtero, D.F.; Viafara-Garcia, S.M.; Morantes, S.J.; Buitrago, D.M.; Gonzalez, O.A.; Lafaurie, G.I. Rosuvastatin Inhibits Interleukin (IL)-8 and IL-6 Production in Human Coronary Artery Endothelial Cells Stimulated with Aggregatibacter actinomycetemcomitans Serotype b. J. Periodontol. 2016, 88, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.; Wu, Y. Cholesterol (Blood lipid) lowering potential of Rosuvastatin chitosan nanoparticles for atherosclerosis: Preclinical study in rabbit model. Acta Biochim. Pol. 2020, 67, 495–499. [Google Scholar] [PubMed]
- Dash, R.; Yadav, M.; Biswal, J.; Chandra, A.; Goel, V.K.; Sharma, T.; Prusty, S.K.; Mohapatra, S. Modeling of chitosan modified PLGA atorvastatin-curcumin conjugate (AT-CU) nanoparticles, overcoming the barriers associated with PLGA: An approach for better management of atherosclerosis. Int. J. Pharm. 2023, 640, 123009. [Google Scholar] [CrossRef] [PubMed]
- Kilic, U.; Gok, O.; Elibol-Can, B.; Uysal, O.; Bacaksiz, A. Efficacy of statins on sirtuin 1 and endothelial nitric oxide synthase expression: The role of sirtuin 1 gene variants in human coronary atherosclerosis. Clin. Exp. Pharmacol. Physiol. 2015, 42, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Sun, Z.; Cao, Z.; Zhou, X.; Wang, D.; Wang, K.; Li, X.; Zuo, G. Atorvastatin regulates vascular smooth muscle cell phenotypic transformation by epigenetically modulating contractile proteins and mediating Akt/FOXO4 axis. Mol. Med. Rep. 2022, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, T.; Jiang, L.; Li, S.; Shi, B.; Li, F. Enhancing the biopharmaceutical attributes of atorvastatin calcium using polymeric and lipid-polymer hybrid nanoparticles: An approach for atherosclerosis treatment. Biomed. Pharmacother. 2023, 159, 114261. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yin, L.; Teng, Z.; Zhou, X.; Li, W.; Lai, Q.; Peng, C.; Zhang, C.; Lou, J.; Zhou, X. Prevention of Obesity Related Diseases through Laminarin-induced targeted delivery of Bindarit. Theranostics 2020, 10, 9544–9560. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Mao, L.; Xiao, J.; Wu, Y.; Liu, H. Selenium nanoparticles inhibit the formation of atherosclerosis in apolipoprotein E deficient mice by alleviating hyperlipidemia and oxidative stress. Eur. J. Pharmacol. 2021, 902, 174120. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xiao, J.; Liu, H. Enhanced oxidase-like activity of selenium nanoparticles stabilized by chitosan and application in a facile colorimetric assay for mercury (II). Biochem. Eng. J. 2019, 152, 107384. [Google Scholar] [CrossRef]
- Xiao, J.; Li, N.; Xiao, S.; Wu, Y.; Liu, H. Comparison of Selenium Nanoparticles and Sodium Selenite on the Alleviation of Early Atherosclerosis by Inhibiting Endothelial Dysfunction and Inflammation in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2021, 22, 11612. [Google Scholar] [CrossRef]
- Parveen, S.; Kumar, S.; Pal, S.; Yadav, N.P.; Rajawat, J.; Banerjee, M. Enhanced therapeutic efficacy of Piperlongumine for cancer treatment using nano-liposomes mediated delivery. Int. J. Pharm. 2023, 643, 123212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mintzer, E.; Uhrich, K.E. Synthesis and characterization of PEGylated bolaamphiphiles with enhanced retention in liposomes. J. Colloid Interface Sci. 2016, 482, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, K.; Fatemeh, A.; Rouzbeh, M.; Dina, M.; Farzad, K.; Mohsen, A.; Ebrahim, H.; Hassan, B. RGD-decorated nanoliposomes for combined delivery of arsenic trioxide and curcumin to prostate cancer cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 397, 2347–2357. [Google Scholar] [CrossRef]
- Xia, S.; Xu, S.; Zhang, X. Optimization in the preparation of coenzyme Q10 nanoliposomes. J. Agric. Food Chem. 2006, 54, 6358–6366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, H.; Liu, J.; Wang, J.; Zhang, S.; Zhang, S.; Wu, Z. Pharmacokinetics and atherosclerotic lesions targeting effects of tanshinone IIA discoidal and spherical biomimetic high density lipoproteins. Biomaterials 2013, 34, 306–319. [Google Scholar] [CrossRef]
- Liu, L.; Xing, R.; Xue, J.; Fan, J.; Zou, J.; Song, X.; Jia, R.; Zou, Y.; Li, L.; Zhou, X.; et al. Low molecular weight fucoidan modified nanoliposomes for the targeted delivery of the anti-inflammation natural product berberine. Int. J. Pharm. 2023, 642, 123102. [Google Scholar] [CrossRef] [PubMed]
- Darwitan, A.; Wong, Y.S.; Nguyen, L.T.H.; Czarny, B.; Vincent, A.; Nedumaran, A.M.; Tan, Y.F.; Muktabar, A.; Tang, J.K.; Ng, K.W.; et al. Liposomal Nanotherapy for Treatment of Atherosclerosis. Adv. Healthc. Mater. 2020, 9, e2000465. [Google Scholar] [CrossRef] [PubMed]
- Darwitan, A.; Tan, Y.F.; Wong, Y.S.; Nedumaran, A.M.; Czarny, B.; Venkatraman, S. Targeting efficiency of nanoliposomes on atherosclerotic foam cells: Polyethylene glycol-to-ligand ratio effects. Expert Opin. Drug Deliv. 2020, 17, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Moxon, J.V.; Jose, R.J.; Li, J.; Sahebkar, A.; Jaafari, M.R.; Hatamipour, M.; Liu, D.; Golledge, J. Anionic nanoliposomes reduced atherosclerosis progression in Low Density Lipoprotein Receptor (LDLR) deficient mice fed a high fat diet. J. Cell. Physiol. 2018, 233, 6951–6964. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, M.; Darwitan, A.; Muktabar, A.; Das, P.; Nguyen, L.T.H.; Cao, Y.; Vizetto-Duarte, C.; Tang, J.; Wong, Y.S.; Venkatraman, S.; et al. Anti-inflammatory potential of simvastatin loaded nanoliposomes in 2D and 3D foam cell models. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102434. [Google Scholar] [CrossRef]
- Zheng, Y.; Kou, J.; Wang, P.; Ye, T.; Wang, Z.; Gao, Z.; Cong, L.; Li, M.; Dong, B.; Yang, W.; et al. Berberine-induced TFEB deacetylation by SIRT1 promotes autophagy in peritoneal macrophages. Aging 2021, 13, 7096–7119. [Google Scholar] [CrossRef] [PubMed]
- Man, B.; Hu, C.; Yang, G.; Xiang, J.; Yang, S.; Ma, C. Berberine attenuates diabetic atherosclerosis via enhancing the interplay between KLF16 and PPARα in ApoE−/− mice. Biochem. Biophys. Res. Commun. 2022, 624, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.T.; Yen, T.T.H.; Nguyen, L.T.; Nguyen, T.-D.; Nguyen, T.-Q.-T.; Nghiem, T.-H.-L.; Pham, H.T.; Raal, A.; Heinämäki, J.; Pham, T.-M.-H. Berberine-loaded liposomes for oral delivery: Preparation, physicochemical characterization and in-vivo evaluation in an endogenous hyperlipidemic animal model. Int. J. Pharm. 2022, 616, 121525. [Google Scholar] [CrossRef] [PubMed]
- Lu, X. Structure and Function of Proprotein Convertase Subtilisin/kexin Type 9 (PCSK9) in Hyperlipidemia and Atherosclerosis. Curr. Drug Targets 2019, 20, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Prattichizzo, F.; Marfella, R.; Sardu, C.; Martino, E.; Scisciola, L.; Marfella, L.; La Grotta, R.; Frigé, C.; Paolisso, G.; et al. SIRT3 mediates the effects of PCSK9 inhibitors on inflammation, autophagy, and oxidative stress in endothelial cells. Theranostics 2023, 13, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, H.; Liu, H.; Liu, D.; Liu, J.; Jiang, J.; Zhang, Y.; Qin, Z.; Xu, Y.; Peng, Y.; et al. Evolocumab loaded Bio-Liposomes for efficient atherosclerosis therapy. J. Nanobiotechnol. 2023, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, K. Solid Lipid Nanoparticles: A Promising Nanomaterial in Drug Delivery. Curr. Pharm. Des. 2019, 25, 3943–3959. [Google Scholar] [CrossRef] [PubMed]
- Daminelli, E.N.; Martinelli, A.E.M.; Bulgarelli, A.; Freitas, F.R.; Maranhão, R.C. Reduction of Atherosclerotic Lesions by the Chemotherapeutic Agent Carmustine Associated to Lipid Nanoparticles. Cardiovasc. Drugs Ther. 2016, 30, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Oumzil, K.; Ramin, M.A.; Lorenzato, C.; Hémadou, A.; Laroche, J.; Jacobin-Valat, M.J.; Mornet, S.; Roy, C.-E.; Kauss, T.; Gaudin, K.; et al. Solid Lipid Nanoparticles for Image-Guided Therapy of Atherosclerosis. Bioconjug. Chem. 2016, 27, 569–575. [Google Scholar] [CrossRef]
- Zhang, Z.; Bu, H.; Gao, Z.; Huang, Y.; Gao, F.; Li, Y. The characteristics and mechanism of simvastatin loaded lipid nanoparticles to increase oral bioavailability in rats. Int. J. Pharm. 2010, 394, 147–153. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.-X.; Ning, D.-S.; Chen, J.; Li, S.-X.; Mo, Z.-W.; Peng, Y.-M.; He, S.-H.; Chen, Y.-T.; Zheng, C.-J.; et al. Simvastatin inhibits POVPC-mediated induction of endothelial to mesenchymal cell transition. J. Lipid Res. 2021, 62, 100066. [Google Scholar] [CrossRef]
- Du, G.; Song, Y.; Zhang, T.; Ma, L.; Bian, N.; Chen, X.; Feng, J.; Chang, Q.; Li, Z. Simvastatin attenuates TNF-α-induced apoptosis in endothelial progenitor cells via the upregulation of SIRT1. Int. J. Mol. Med. 2014, 34, 177–182. [Google Scholar] [CrossRef]
- Rizvi, S.Z.H.; Shah, F.A.; Khan, N.; Muhammad, I.; Ali, K.H.; Ansari, M.M.; Din, F.U.; Qureshi, O.S.; Kim, K.-W.; Choe, Y.-H.; et al. Simvastatin-loaded solid lipid nanoparticles for enhanced anti-hyperlipidemic activity in hyperlipidemia animal model. Int. J. Pharm. 2019, 560, 136–143. [Google Scholar] [CrossRef]
- Elkhayat, D.; Abdelmalak, N.S.; Amer, R.; Awad, H.H. Ezetimibe-Loaded Nanostructured Lipid Carrier for Oral Delivery: Response Surface Methodology; In Vitro Characterization and Assessing the Antihyperlipidemic Effect in Rats. ACS Omega 2024, 9, 8103–8116. [Google Scholar] [CrossRef]
- Devel, L.; Almer, G.; Cabella, C.; Beau, F.; Bernes, M.; Oliva, P.; Navarro, F.; Prassl, R.; Mangge, H.; Texier, I. Biodistribution of Nanostructured Lipid Carriers in Mice Atherosclerotic Model. Molecules 2019, 24, 3499. [Google Scholar] [CrossRef] [PubMed]
- Vigne, J.; Cabella, C.; Dezsi, L.; Rustique, E.; Couffin, A.C.; Aid, R.; Anizan, N.; Chauvierre, C.; Letourneur, D.; Le Guludec, D.; et al. Nanostructured lipid carriers accumulate in atherosclerotic plaques of ApoE−/− mice. Nanomedicine 2020, 25, 102157. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Sabjan, K.B.; Munawar, S.M.; Rajendiran, D.; Vinoji, S.K.; Kasinathan, K. Nanoemulsion as Oral Drug Delivery—A Review. Curr. Drug Res. Rev. 2020, 12, 4–15. [Google Scholar] [CrossRef]
- Shukr, M.H.; Farid, O.A.A. Brain targeting of agomelatine egg lecithin based chitosan coated nanoemulsion. Pharm. Dev. Technol. 2021, 26, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Nishimon, Y.; Ueta, H.; Matsuno, K.; Takada, K. Utility of nano-sized, water-in-oil emulsion as a sustained release formulation of glycyrrhizin. Biol. Pharm. Bull. 2011, 34, 300–305. [Google Scholar] [CrossRef]
- Sun, W.; Ma, X.; Wei, X.; Xu, Y. Nano Composite Emulsion for Sustained Drug Release and Improved Bioavailability. Pharm. Res. 2014, 31, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Z.; Liu, H.; Hu, L. Nanoemulsion-based delivery approaches for nutraceuticals: Fabrication, application, characterization, biological fate, potential toxicity and future trends. Food Funct. 2021, 12, 1933–1953. [Google Scholar] [CrossRef]
- Bonacucina, G.; Cespi, M.; Misici-Falzi, M.; Palmieri, G.F. Colloidal soft matter as drug delivery system. J. Pharm. Sci. 2009, 98, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Kukadiya, H.; Mashru, R.; Surti, N.; Mandal, S. Development of microemulsion for solubility enhancement of clopidogrel. Iran. J. Pharm. Res. IJPR 2010, 9, 327–334. [Google Scholar] [PubMed]
- Santos Rodrigues, A.P.; Souza, B.S.F.E.; Alves Barros, A.S.; de Oliveira Carvalho, H.; Lobato Duarte, J.; Leticia Elizandra Boettger, M.; Barbosa, R.; Maciel Ferreira, A.; Maciel Ferreira, I.; Fernandes, C.P.; et al. The effects of Rosmarinus officinalis L. essential oil and its nanoemulsion on dyslipidemic Wistar rats. J. Appl. Biomed. 2020, 18, 126–135. [Google Scholar] [CrossRef]
- Prévot, G.; Kauss, T.; Lorenzato, C.; Gaubert, A.; Larivière, M.; Baillet, J.; Laroche-Traineau, J.; Jacobin-Valat, M.J.; Adumeau, L.; Mornet, S.; et al. Iron oxide core oil-in-water nanoemulsion as tracer for atherosclerosis MPI and MRI imaging. Int. J. Pharm. 2017, 532, 669–676. [Google Scholar] [CrossRef]
- Ahsan, M.N.; Prasad Verma, P.R. Solidified self nano-emulsifying drug delivery system of rosuvastatin calcium to treat diet-induced hyperlipidemia in rat: In vitro and in vivo evaluations. Ther. Deliv. 2017, 8, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wu, G.; Yang, H.; Zhang, Y.; Shen, X.; Tao, L. Diethylaminoethyl-dextran and monocyte cell membrane coated 1,8-cineole delivery system for intracellular delivery and synergistic treatment of atherosclerosis. Int. J. Biol. Macromol. 2023, 253, 127365. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Xu, J.; Zhang, J.; Xu, S.; Zhang, Q.; Huang, J.; Peng, J.; Xu, H.; Du, Q.; et al. Fabrication of a Polysaccharide-Protein/Protein Complex Stabilized Oral Nanoemulsion to Facilitate the Therapeutic Effects of 1,8-Cineole on Atherosclerosis. ACS Nano 2023, 17, 9090–9109. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef]
- Ikeuchi-Takahashi, Y.; Murata, S.; Murata, W.; Kobayashi, A.; Ishihara, C.; Onishi, H. Development of Morin-Loaded Nanoemulsions Containing Various Polymers; Role of Polymers in Formulation Properties and Bioavailability. AAPS PharmSciTech 2020, 21, 150. [Google Scholar] [CrossRef] [PubMed]
- Dnyandev, G.; Mural, Q.; Akanksha, R.U.; Mimansa, G.; Vivek, G. A Nanoemulgel for Nose-to-Brain Delivery of Quetiapine—QbD-Enabled Formulation Development & In-vitro Characterization. Int. J. Pharm. 2023, 648, 123566. [Google Scholar] [CrossRef]
- Riddhi, V.; Caitlin, C.; Lu, L.; Amit Chandra, D.; Rebecca, M.; Fatih, Z.; Yalcin, K.; Vijay, S.G.; Jelena, M.J. A Reversibly Thermoresponsive, Theranostic Nanoemulgel for Tacrolimus Delivery to Activated Macrophages: Formulation and In Vitro Validation. Pharmaceutics 2023, 15, 2372. [Google Scholar] [CrossRef] [PubMed]
- Purohit, D.; Jalwal, P.; Manchanda, D.; Saini, S.; Verma, R.; Kaushik, D.; Mittal, V.; Kumar, M.; Bhattacharya, T.; Rahman, M.H.; et al. Nanocapsules: An Emerging Drug Delivery System. Recent Pat. Nanotechnol. 2022, 17, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Chi, C.; Wang, T.; He, Y.; Chen, L.; Li, X. Multi-responsive starch-based nanocapsules for colon-targeting delivery of peptides: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2023, 242, 124953. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Ma, J.; Kang, K.; Gu, Z. Bioreducible nanocapsules for folic acid-assisted targeting and effective tumor-specific chemotherapy. Int. J. Nanomed. 2018, 13, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Khattab, W.M.; Zein El-Dein, E.E.; El-Gizawy, S.A. Formulation of lyophilized oily-core poly-Ɛ-caprolactone nanocapsules to improve oral bioavailability of Olmesartan Medoxomil. Drug Dev. Ind. Pharm. 2020, 46, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Campión, R.; Gonzalez-Navarro, C.J.; Luisa Martínez López, A.; Cristina Martínez-Oharriz, M.; Matías, C.; Sáiz-Abajo, M.-J.; Collantes, M.; Peñuelas, I.; Irache, J.M. Zein-based nanospheres and nanocapsules for the encapsulation and oral delivery of quercetin. Int. J. Pharm. 2023, 643, 123216. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.L.d.; do Espirito Santo Pereira, A.; Fernandes Fraceto, L.; Bueno dos Reis Martinez, C. Can atrazine loaded nanocapsules reduce the toxic effects of this herbicide on the fish Prochilodus lineatus? A multibiomarker approach. Sci. Total Environ. 2019, 663, 548–559. [Google Scholar] [CrossRef]
- Benvegnú, D.M.; Barcelos, R.C.S.; Boufleur, N.; Pase, C.S.; Reckziegel, P.; Flores, F.C.; Ourique, A.F.; Nora, M.D.; da Silva, C.d.B.; Beck, R.C.R.; et al. Haloperidol-loaded polysorbate-coated polymeric nanocapsules decrease its adverse motor side effects and oxidative stress markers in rats. Neurochem. Int. 2012, 61, 623–631. [Google Scholar] [CrossRef]
- Prego, C.; Torres, D.; Fernandez-Megia, E.; Novoa-Carballal, R.; Quiñoá, E.; Alonso, M.J. Chitosan-PEG nanocapsules as new carriers for oral peptide delivery. Effect of chitosan pegylation degree. J. Control. Release 2006, 111, 299–308. [Google Scholar] [CrossRef] [PubMed]
- de Castro Leao, M.; Raffin Pohlmann, A.; de Cristo Soares Alves, A.; Helena Poliselli Farsky, S.; Klimuk Uchiyama, M.; Araki, K.; Sandri, S.; Staniscuaski Guterres, S.; Alves Castro, I. Docosahexaenoic acid nanoencapsulated with anti-PECAM-1 as co-therapy for atherosclerosis regression. Eur. J. Pharm. Biopharm. 2021, 159, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, M.F.; Kazuma, S.M.; Bender, E.A.; Adorne, M.D.; Ullian, M.; Veras, M.M.; Saldiva, P.H.N.; Maranhão, A.Q.; Guterres, S.S.; Pohlmann, A.R.; et al. A nanoformulation containing a scFv reactive to electronegative LDL inhibits atherosclerosis in LDL receptor knockout mice. Eur. J. Pharm. Biopharm. 2016, 107, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.M.; Roy, J.; Pitta, I.R.; Abdalla, D.S.P.; Grabe-Guimarães, A.; Mosqueira, V.C.F.; Richard, S. Polylactide Nanocapsules Attenuate Adverse Cardiac Cellular Effects of Lyso-7, a Pan-PPAR Agonist/Anti-Inflammatory New Thiazolidinedione. Pharmaceutics 2021, 13, 1521. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; de Carvalho, G.M.; de Oliveira Zanuso, B.; Figueira, M.E.; Direito, R.; de Alvares Goulart, R.; Buglio, D.S.; Barbalho, S.M. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics 2023, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- de Castro Leão, M.; Di Piazza, I.; Caria, S.J.; Broering, M.F.; Farsky, S.H.P.; Uchiyama, M.K.; Araki, K.; Bonjour, K.; Cogliati, B.; Pohlmann, A.R.; et al. Effect of nanocapsules containing docosahexaenoic acid in mice with chronic inflammation. Biomed. Pharmacother. 2023, 167, 115474. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, L.; Liang, R.; Wei, J. Ultrasound and magnetic resonance molecular imaging of atherosclerotic neovasculature with perfluorocarbon magnetic nanocapsules targeted against vascular endothelial growth factor receptor 2 in rats. Mol. Med. Rep. 2017, 16, 5986–5996. [Google Scholar] [CrossRef] [PubMed]
- John, R.B.; Amanda, J.H. MK-0616: An oral PCSK9 inhibitor for hypercholesterolemia treatment. Expert Opin. Investig. Drugs 2023, 32, 873–878. [Google Scholar] [CrossRef]
- Czyzynska-Cichon, I.; Janik-Hazuka, M.; Szafraniec-Szczesny, J.; Jasinski, K.; Weglarz, W.P.; Zapotoczny, S.; Chlopicki, S. Low Dose Curcumin Administered in Hyaluronic Acid-Based Nanocapsules Induces Hypotensive Effect in Hypertensive Rats. Int. J. Nanomed. 2021, 16, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Salaheldin, T.A.; Godugu, K.; Bharali, D.J.; Fujioka, K.; Elshourbagy, N.; Mousa, S.A. Novel oral nano-hepatic targeted anti-PCSK9 in hypercholesterolemia. Nanomed. Nanotechnol. Biol. Med. 2021, 40, 102480. [Google Scholar] [CrossRef]
- Maranhão, R.C.; Leite, A.C.A. Development of anti-atherosclerosis therapy based on the inflammatory and proliferative aspects of the disease. Curr. Pharm. Des. 2014, 21, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-Y.; Shi, X.-Y.; Wu, Y.-R.; Zhang, Y.; Yao, Y.-H.; Qu, H.-L.; Zhang, W.; Guo, Y.-L.; Xu, R.-X.; Li, J.-J. Berberine attenuates atherosclerotic lesions and hepatic steatosis in ApoE−/− mice by down-regulating PCSK9 via ERK1/2 pathway. Ann. Transl. Med. 2021, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
- Ochin, C.C.; Garelnabi, M. Berberine Encapsulated PLGA-PEG Nanoparticles Modulate PCSK-9 in HepG2 Cells. Cardiovasc. Hematol. Disord.-Drug Targets 2018, 18, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-R.; Tong, Q.; Lin, Y.; Pan, L.-B.; Fu, J.; Peng, R.; Zhang, X.-F.; Zhao, Z.-X.; Li, Y.; Yu, J.-B.; et al. Berberine treats atherosclerosis via a vitamine-like effect down-regulating Choline-TMA-TMAO production pathway in gut microbiota. Signal Transduct. Target. Ther. 2022, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Zhang, O.; Xiang, H.; Yao, Y.-H.; Fang, Z.-Y. Curcumin improves atherosclerosis by inhibiting the epigenetic repression of lncRNA MIAT to miR-124. Vascular 2022, 30, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Gong, Y.; Zhang, J.; Mu, X.; Song, Z.; Feng, R.; Zhang, H. A novel curcumin-loaded nanoparticle restricts atherosclerosis development and promotes plaques stability in apolipoprotein E deficient mice. J. Biomater. Appl. 2018, 33, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Xu, Y.; Yin, J.-F.; Jin, J.; Jiang, Y.; Du, Q. Improving the Effectiveness of (−)-Epigallocatechin Gallate (EGCG) against Rabbit Atherosclerosis by EGCG-Loaded Nanoparticles Prepared from Chitosan and Polyaspartic Acid. J. Agric. Food Chem. 2014, 62, 12603–12609. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Protective Effect of Epigallocatechin Gallate on Endothelial Disorders in Atherosclerosis. J. Cardiovasc. Pharmacol. 2020, 75, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.-J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Giugliano, R.P.; Wiviott, S.D.; Atar, D.; Keech, A.; Kuder, J.F.; Im, K.; Murphy, S.A.; Flores-Arredondo, J.H.; López, J.A.G.; et al. Long-Term Evolocumab in Patients with Established Atherosclerotic Cardiovascular Disease. Circulation 2022, 146, 1109–1119. [Google Scholar] [CrossRef]
- Tan, C.; Zhou, L.; Wen, W.; Xiao, N. Curcumin promotes cholesterol efflux by regulating ABCA1 expression through miR-125a-5p/SIRT6 axis in THP-1 macrophage to prevent atherosclerosis. J. Toxicol. Sci. 2021, 46, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Pai, P.-Y.; Chou, W.-C.; Chan, S.-H.; Wu, S.-Y.; Chen, H.-I.; Li, C.-W.; Hsieh, P.-L.; Chu, P.-M.; Chen, Y.-A.; Ou, H.-C.; et al. Epigallocatechin Gallate Reduces Homocysteine-Caused Oxidative Damages through Modulation SIRT1/AMPK Pathway in Endothelial Cells. Am. J. Chin. Med. 2020, 49, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Om, H.; El-Naggar, M.E.; El-Banna, M.; Fouda, M.M.G.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Combating atherosclerosis with targeted Diosmin nanoparticles-treated experimental diabetes. Investig. New Drugs 2020, 38, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Nersesyan, A.; Pentland, K.; Melin, M.M.; Levy, R.M.; Ebong, E.E. Diosmin and its glycocalyx restorative and anti-inflammatory effects on injured blood vessels. FASEB J. 2022, 36, e22630. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.K.; Busui, R.P.; Deanfield, J.; Inzucchi, S.E.; Mann, J.F.E.; Marx, N.; Mulvagh, S.L.; Poulter, N.; Engelmann, M.D.M.; Hovingh, G.K.; et al. Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: Design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes. Metab. 2023, 25, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, M.; Wang, C.; Liu, Y.; Naruse, K.; Takahashi, K. The Mechanisms of the Development of Atherosclerosis in Prediabetes. Int. J. Mol. Sci. 2021, 22, 4108. [Google Scholar] [CrossRef] [PubMed]
- Ian, O.C.; Jayer, C. Contemporary Medical Management of Peripheral Arterial Disease. Cardiovasc. Drugs Ther. 2023. [Google Scholar] [CrossRef]
- Yen, C.-C.; Lii, C.-K.; Chen, C.-C.; Li, C.-C.; Tseng, M.-H.; Lo, C.-W.; Liu, K.-L.; Yang, Y.-C.; Chen, H.-W. Andrographolide Inhibits Lipotoxicity-Induced Activation of the NLRP3 Inflammasome in Bone Marrow-Derived Macrophages. Am. J. Chin. Med. 2022, 51, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lu, H. Ganoderic acid A inhibits ox-LDL-induced THP-1-derived macrophage inflammation and lipid deposition via Notch1/PPARgamma/CD36 signaling. Adv. Clin. Exp. Med. 2021, 30, 1031–1041. [Google Scholar] [CrossRef]
- Jin, P.; Gao, D.; Cong, G.; Yan, R.; Jia, S. Role of PCSK9 in Homocysteine-Accelerated Lipid Accumulation in Macrophages and Atherosclerosis in ApoE−/− Mice. Front. Cardiovasc. Med. 2021, 8, 746989. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Zhang, M.; Tian, J.; Liu, M.; Jin, T.; Pan, J.; Gao, M.; An, F. WISP1 alleviates lipid deposition in macrophages via the PPARgamma/CD36 pathway in the plaque formation of atherosclerosis. J. Cell. Mol. Med. 2020, 24, 11729–11741. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.C.; Truijman, M.T.; Hussain, B.; Zadi, T.; Saiedie, G.; de Rotte, A.A.; Liem, M.I.; van der Steen, A.F.; Daemen, M.J.; Koudstaal, P.J.; et al. Intraplaque Hemorrhage and the Plaque Surface in Carotid Atherosclerosis: The Plaque At RISK Study (PARISK). AJNR Am. J. Neuroradiol. 2015, 36, 2127–2133. [Google Scholar] [CrossRef]
- Babaniamansour, P.; Mohammadi, M.; Babaniamansour, S.; Aliniagerdroudbari, E. The Relation between Atherosclerosis Plaque Composition and Plaque Rupture. J. Med. Signals Sens. 2020, 10, 267–273. [Google Scholar] [CrossRef]
- Doring, Y.; Soehnlein, O.; Weber, C. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ. Res. 2017, 120, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Li, H.; Xia, N. Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis. Int. J. Mol. Sci. 2021, 22, 676. [Google Scholar] [CrossRef] [PubMed]
- Marx, C.; Novotny, J.; Salbeck, D.; Zellner, K.R.; Nicolai, L.; Pekayvaz, K.; Kilani, B.; Stockhausen, S.; Burgener, N.; Kupka, D.; et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood 2019, 134, 1859–1872. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.W.; Fang, L.J.; Cheng, S.Q.; Wang, X.; Liu, N.F. Programmed cell death in atherosclerosis and vascular calcification. Cell Death Dis. 2022, 13, 467. [Google Scholar] [CrossRef]
- Ouyang, L.; Yu, C.; Xie, Z.; Su, X.; Xu, Z.; Song, P.; Li, J.; Huang, H.; Ding, Y.; Zou, M.H. Indoleamine 2,3-Dioxygenase 1 Deletion-Mediated Kynurenine Insufficiency in Vascular Smooth Muscle Cells Exacerbates Arterial Calcification. Circulation 2022, 145, 1784–1798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Ning, X.; Wang, M.; Wang, H.; Xing, G.; Wang, L.; Lu, C.; Yu, A.; Wang, Y. A ROS-Responsive Simvastatin Nano-Prodrug and its Fibronectin-Targeted Co-Delivery System for Atherosclerosis Treatment. ACS Appl. Mater. Interfaces 2022, 14, 25080–25092. [Google Scholar] [CrossRef]
- Golledge, J. Update on the pathophysiology and medical treatment of peripheral artery disease. Nat. Rev. Cardiol. 2022, 19, 456–474. [Google Scholar] [CrossRef]
- Espinola-Klein, C. When and How to Combine Antiplatelet and Anticoagulant Drugs? Hämostaseologie 2022, 42, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Pothineni, N.V.K.; Goel, A.; Lüscher, T.F.; Mehta, J.L. PCSK9 and inflammation: Role of shear stress, pro-inflammatory cytokines and LOX-1 4. Cardiovasc. Res. 2019, 116, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Urbinati, S. Time has come to develop routine exercise training interventions in patients with chronic coronary syndrome. Eur. J. Prev. Cardiol. 2022, 29, 1072–1073. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.J.; Chen, D.C.; Westin, G.G.; Singh, S.; McCoach, C.E.; Bang, H.; Yeo, K.-K.; Anderson, D.; Amsterdam, E.A.; Laird, J.R. Adherence to guideline-recommended therapy is associated with decreased major adverse cardiovascular events and major adverse limb events among patients with peripheral arterial disease. J. Am. Heart Assoc. 2014, 3, e000697. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Centurion, F.; Chen, R.; Gu, Z. Intravascular Imaging of Atherosclerosis by Using Engineered Nanoparticles. Biosensors 2023, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Cao, Y.; Zhang, S.; Sun, J.; Wang, Y.; Song, S.; Zhang, H. Targeting the Microenvironment of Vulnerable Atherosclerotic Plaques: An Emerging Diagnosis and Therapy Strategy for Atherosclerosis. Adv. Mater. 2022, 34, 2110660. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zhang, Z.; Xu, J.; Liu, H.; Cai, L.; Huang, G.; Wang, C.; Chen, Y.; Xia, L.; Ding, X.; et al. Atherosclerosis treatment with nanoagent: Potential targets, stimulus signals and drug delivery mechanisms. Front. Bioeng. Biotechnol. 2023, 11, 1205751. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, Y.; Wijaya, A.; Liu, B.; Maruf, A.; Wang, J.; Xu, J.; Liao, X.; Wu, W.; Wang, G. ROS-responsive biomimetic nanoparticles for potential application in targeted anti-atherosclerosis. Regen. Biomater. 2021, 8, rbab033. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qi, Z.; Han, S.; Li, X.; Liu, B.; Liu, Y. Advances and Applications of Metal-Organic Framework Nanomaterials as Oral Delivery Carriers: A Review. Mini Rev. Med. Chem. 2022, 22, 2564–2580. [Google Scholar] [CrossRef] [PubMed]
- Yingyu, L.; Haiyan, L.; Susu, G.; Yifan, Z.; Jin, Q.; Ran, Z.; Jianing, R.; Huaiyi, C.; Mingrui, Z.; Xiuping, W.; et al. A review of carbon nanomaterials/bacterial cellulose composites for nanomedicine applications. Carbohydr. Polym. 2023, 323, 121445. [Google Scholar] [CrossRef]
- Zong, C.; Bronckaers, A.; Willems, G.; He, H.; Cadenas de Llano-Pérula, M. Nanomaterials for Periodontal Tissue Regeneration: Progress, Challenges and Future Perspectives. J. Funct. Biomater. 2023, 14, 290. [Google Scholar] [CrossRef] [PubMed]

| Pathogenesis | Cell | Relevant Targets | Inflammatory Factors/Therapeutic Pathways | Function | Location of Injury | Therapeutic Drug | References |
|---|---|---|---|---|---|---|---|
| Endothelial cell damage | Endothelial cell | CAMs (ICAM-1, VCAM-1) E-selectin | TNF-α; IL-1β; NF-κB; NLRP3; ROS | Endothelial cell activation and increased expression of adhesion molecules promote leukocyte adhesion to the vessel wall. | Endothelial layer of the arterial vascular wall | Sal B; Artesunate | [44,61,76,77,78] |
| Inflammatory response | Macrophages; T lymphocytes; Neutrophils; Monocytes; Dendritic cells | Cytokines; Adhesion molecules; Inflammatory mediators | TNF-α; IL-1β; IL-6; CRP; MCP-1; ICAM-1; VCAM-1; NO | Inflammatory mediators stimulate cell proliferation, migration of smooth muscle cells, and hasten damage to the arterial wall. | Arterial blood vessel wall | Curcumin; Naringin | [35,42,79] |
| Lipid deposition | Smooth muscle cells; Foam cells; Endothelial cells; Lipid plaques | LDL; ox-LDL-C; CD68; CD36 | IL-1; IL-6 IL-8; IL-10; IL-18; ApoE; TLR | Oxidized low-density lipoprotein (LDL) initiates inflammation, promotes phagocytosis by macrophages, and facilitates foam cell formation. | Middle layer of the arterial vascular wall | Quercetin; Atorvastatin combined with nano-selenium | [50,80,81,82] |
| Smooth muscle cell migration and proliferation | Vascular smooth muscle cells; Vascular endothelial cells; Macrophages | Growth factors (e.g., PDGF); Transforming growth factor-β (TGF-β) | PDGF; TGF-β; IL-8 | Activates the proliferation of smooth muscle cells, resulting in the deposition of collagen and the formation of fibrous plaques. | Middle layer of the arterial vascular wall | Rosuvastatin | [64,83,84] |
| Plaque stability formation | Smooth muscle cells; Inflammatory cells | Collagen in plaques; Elastin; Inflammatory cells | Matrix metalloproteinases (MMPs) | Activation of platelets leads to the rupture of plaque, ultimately resulting in thrombosis. | Patch area | Myricetin; ACE-inhibitor | [67,85,86,87] |
| Thrombosis | Blood platelet | Platelet activity; Coagulation factors | Thromboxane released by platelets | Forms blood clots that obstruct blood vessels. | Plaque rupture sites within the arterial luminal vessels. | Warfarin | [6,88] |
| Immune response | T-lymphocytes | T-Lymphocyte activation | CD4+ T helper cells (Th cells); Th17 cells; Regulatory T cells (Treg cells); Memory T cells; Cytotoxic T cells (CD8+ T cells); NK cells; γδ T cells; Interferon-γ (IFN-γ); Interleukin-17 (IL-17) | CD4+ T cells are activated and differentiate into different subpopulations (e.g., Th1 and Th17 subpopulations), releasing pro-inflammatory cytokines, such as interferon-gamma (IFN-γ) and interleukin-17 (IL-17), which are involved in the inflammatory process; Insufficient numbers or dysfunctional Treg cells can create an immune regulation imbalance, which exacerbates the inflammatory response. Memory T cells undergo repeated activation in response to sustained inflammation and immune response. Activation of CD8+ T cells may lead to cytotoxicity; The killing of endothelial cells and macrophages is linked to the activation of NK cells. | Arterial blood vessels | Aspires | [89,90,91] |
| Medicines | Related SIRT Proteins | Clinical Usage | NCT Number | Reference |
|---|---|---|---|---|
| Atorvastatin | SIRT-1 | Assessment of the effect of atorvastatin on coronary atherosclerotic plaque morphology | NCT00576576 | [114] |
| Effects of atorvastatin in patients with atherosclerosis | NCT00115817 | |||
| Rosuvastatin | SIRT-1 | Evaluating the effect of rosuvastatin 10–20 mg on the progression of carotid atherosclerosis in Chinese patients | NCT00885872 | [110] |
| Evaluating the effect of rosuvastatin 20 mg for 76 weeks on coronary atherosclerotic plaque in Chinese patients with coronary heart disease (CHD) hyperlipidaemia compared with baseline | NCT01382277 | |||
| Simvastatin | SIRT-1 | Anti-inflammatory effects of simvastatin | NCT04638400 | [142] |
| PCSK9 antibody inhibitors | SIRT-3 | Effect of PCSK9 inhibitors on coronary microvascular dysfunction in patients with atherosclerotic cardiovascular disease requiring coronary angiographic confirmation of myocardial ischaemia | NCT04338165 | [135] |
| Berberine | SIRT1 | Hypolipidemic and vascular effects of nutritional combinations on HIV-infected patients on stable antiretroviral therapy | NCT03470376 | [41] |
| Epigallocatechin gallate (EGCG) | SIRT1 | EGCG improves endothelial function | NCT01662232 | [192] |
| Curcumin | SIRT6 | Effects of curcumin on diabetic patients with atherosclerotic cardiovascular risk | NCT05753436 | [191] |
| Effect of short-term supplementation with curcumin and polyphenols on the anti-inflammatory properties of high-density lipoprotein (PSI) | NCT02998918 |
| Types of Nanoparticles | Nanomedicine | Preparation Methods | Pathways/Targets | Vantage | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| Nanoparticle | Epigallocatechin gallate (EGCG) loaded nanoparticles | Polyelectrolyte composite nanoparticle preparation method | Nrf2/HO-1 pathway; ICAM-1; intercellular cell adhesion molecule-1 | Increase drug stability; enhanced efficacy | Causes adverse reactions | [187,188] |
| Berberine PLGA-PEG nanoparticles | Nanoprecipitation method | ERK1/2 pathway; Cholesterol efflux from HepG2 cells; ↑ (Upregulates) LDLR; ↓ (Downregulates) PCSK9 expression | Enhance pharmacokinetic properties and expected target outcomes of drugs | Absence of animal studies | [182,183] | |
| Curcumin nanoparticles | Filming–rehydration method | MIAT/miR-124 pathway; HMGB1-TLRS-NF-κB pathway; LDL-C, TC, TG level | Improving solubility, release performance, and stability of curcumin nanoparticles | Curcumin exhibits poor water solubility and instability during preparation | [35,185,186] | |
| Diosmin nanoparticles | Emulsion–solvent evaporation method, acid-base neutralization method | TGF-β1; Ang II; TC; TG; HDL-C; PON1 | Increased bioavailability, solubility, targeted action | Difficult control over release rate and release site | [193,194] | |
| pBIN; LApBIN | Dialysis method (pBIN); ultrasonic vibration method (LApBIN) | MCP-1; CCL2; TNF-α | Oral adsorption and transport to monocytes, effectively inhibiting inflammation. | There is a lack of sufficient long-term clinical data to assess its long-term efficacy and safety. | [117] | |
| Rosuvastatin-chitosan nanoparticles | Chitosan gel preparation; o/w emulsion preparation; TPP addition and stirring; nanoparticle separation | NF-κB-p65; IL-6; IL-8; ICAM-1; PECAM-1 | Targeted drug delivery, enhanced drug accumulation at the site of lesion, reduced impact on normal tissues. | Stability and efficacy of drug nanocarriers | [111,112] | |
| Polymer-lipid hybrid nanoparticles of atorvastatin | Single emulsion method, ultrasonication | Akt/FOXO4; α-SMA; SM-MHC; SM22α | Enhancing oral drug absorption, improving bioavailability, enhancing drug efficacy. | Lack of formulation toxicity studies | [112,115] | |
| Nanoemulsion | Rosuvastatin calcium solidified self-nanoemulsifying drug delivery system | Colloidal silica adsorption immobilization technology | NF-κB-p65 passway; IL-6; IL-8; ICAM-1; PECAM-1 | Physically stable, conducive to large-scale production; enhanced in vitro dissolution | Stability of self-emulsifying nanoemulsion systems | [111,157] |
| Oral nanodispersions stabilized by polysaccharide-protein/protein complexes | Micro jet and ultraviolet irradiation method | Inhibiting inflammatory factors | Improving stability both internally and externally, enhancing mucosal permeability | Individual differences may influence drug efficacy. | [158,159] | |
| Solid lipid nanoparticles | Simvastatin-loaded solid lipid nanoparticles (SLNs) | Ultrasonic emulsification and solidification of nanoparticles | TGF-β/Smad passway; Snail-1; Twist-1; EndMT | Improving oral bioavailability | Preparation and stability of lipid nanoparticles | [140,141] |
| Simvastatin solid lipid nanoparticles | High-temperature preparation of nanoemulsions; stabilizing and solidifying nanoemulsions through rapid cooling | TGF-β/Smad passway; Snail-1; Twist-1; EndMT | Reducing dosage administration | Needing more safety assessment and monitoring | [141,143] | |
| Nanosomes | Berberine precursor liposome | The air suspension coating method | KLF16; PPARα | Enhancing the bioavailability of water-insoluble drugs | Challenges in ensuring dose consistency | [132,133] |
| Nanocapsules | Oral nano liver-targeted anti-PCSK9 drug | Nanocapsule technology | ldl-c; non-HDL-C; apoB; Lp(α) | The first oral nano liver-targeted anti-PCSK9 drug | Complex process; high cost | [178,180] |
| Drug Type | Medications | Mechanisms | Current Status and Future Prospects | References |
|---|---|---|---|---|
| Statin Drugs | Atorvastatin | Akt/FOXO4 pathway; VSMCs phenotypic modulation; prevention and treatment of atherosclerosis |
| [11,113,115,116] |
| Rosuvastatin | ↓ NF-κB-p65; ↓ expression of IL-6, IL-8, ICAM-1, and PECAM-1; slowing the progression of atherosclerosis |
| [111,112,157] | |
| Simvastatin | ↓ Oxidative stress, TGF-β/Smad signalling ↓, inactivation of Snail-1 and Twist-1; ↓ EndMT induced by povpc; ↓ atherosclerosis |
| [140,141,143,209] | |
| Peptide Drugs | PCSK9 antibody inhibitors | ↓ LDL, apoB, Lp(a); ↓ risk of atherosclerosis |
| [136,178,180] |
| Natural Drugs | Berberine | ↑ Expression of KLF16; ↑ PPARα; ↑ interaction of KLF16 and PPARα; ↓ atherosclerosis |
| [132,133,182,183] |
| 1,8-Cineole (CIN) | ↓ Lipid parameters, ↓ expression of inflammatory factors and proteins |
| [158,159] | |
| Curcumin | MIAT/miR-124, HMGB1-TLRS-NF-κB signalling pathway; ↓ serum LDL-C, TC, TG levels; ↓ atherosclerosis |
| [35,185,186] | |
| Epigallocatechin gallate (EGCG) | Mediates Nrf2/HO-1 pathway; ↓ ICAM-1 and PECAM-1; ↓ monocyte adhesion → treatment of atherosclerosis |
| [187,188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Jia, X.; Tan, Z.; Fan, D.; Chen, M.; Cui, N.; Liu, A.; Liu, D. Oral Nanoformulations in Cardiovascular Medicine: Advances in Atherosclerosis Treatment. Pharmaceuticals 2024, 17, 919. https://doi.org/10.3390/ph17070919
Sun X, Jia X, Tan Z, Fan D, Chen M, Cui N, Liu A, Liu D. Oral Nanoformulations in Cardiovascular Medicine: Advances in Atherosclerosis Treatment. Pharmaceuticals. 2024; 17(7):919. https://doi.org/10.3390/ph17070919
Chicago/Turabian StyleSun, Xu, Xushuang Jia, Zhaolin Tan, Dongmei Fan, Meiqi Chen, Ning Cui, Aidong Liu, and Da Liu. 2024. "Oral Nanoformulations in Cardiovascular Medicine: Advances in Atherosclerosis Treatment" Pharmaceuticals 17, no. 7: 919. https://doi.org/10.3390/ph17070919
APA StyleSun, X., Jia, X., Tan, Z., Fan, D., Chen, M., Cui, N., Liu, A., & Liu, D. (2024). Oral Nanoformulations in Cardiovascular Medicine: Advances in Atherosclerosis Treatment. Pharmaceuticals, 17(7), 919. https://doi.org/10.3390/ph17070919





