Antioxidant Activity, Antiproliferative Activity, Antiviral Activity, NO Production Inhibition, and Chemical Composition of Essential Oils and Crude Extracts of Leaves, Flower Buds, and Stems of Tetradenia riparia
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Antioxidant Activity
3.2. NO Production Inhibition
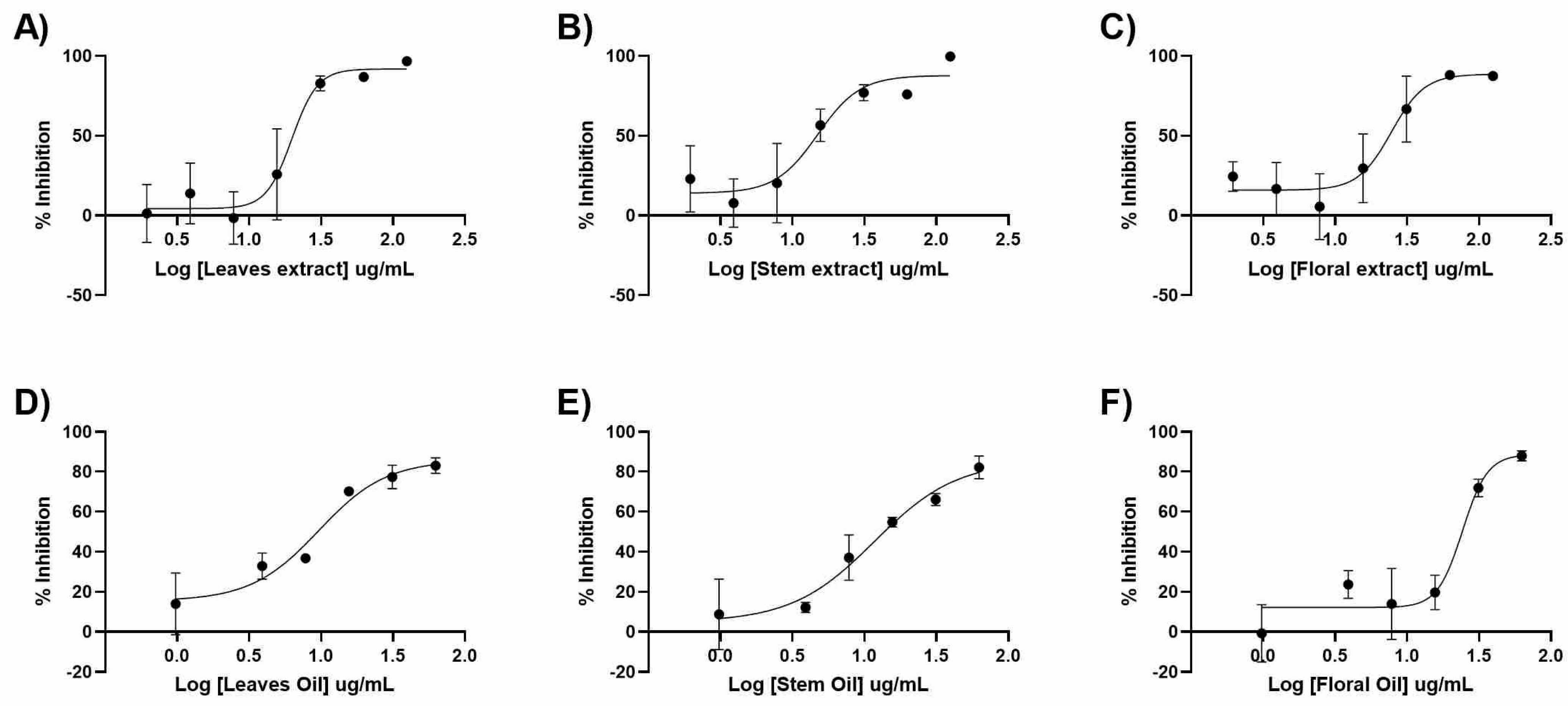
3.3. Antiviral Activity
4. Materials and Methods
4.1. Plant Material and Botanical Identification
4.2. Collection and Extraction of Essential Oils and Preparation of Crude Extracts from Tetradenia riparia Leaves, Flower Buds, and Stems
4.3. Chemical Identification of Essential Oils
4.4. Chemical Identification of Crude Extracts
4.5. Antioxidant Activity
4.5.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH•) Method
4.5.2. Ferric Reducing Antioxidant Power (FRAP)
4.5.3. Quantification of Total Phenols
4.5.4. β-Carotene–Linoleic Acid (BCLA) Method
4.6. Cellular Antioxidant Activity
4.7. NO Production Inhibition Assay
4.8. Antiproliferative Activity
4.9. Antiviral Activity
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramírez, J.; Gilardoni, G.; Radice, M.; Morocho, V. Phytochemistry, Bioactivity, and Ethnopharmacology of the Genus Lepechinia Willd. (Lamiaceae): A Review. Plants 2024, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Shimira, F. Tetradenia riparia, an ethnobotanical plant with diverse applications, from antimicrobial to anti-proliferative activity against cancerous cell lines: A systematic review. J. Herb. Med. 2022, 32, 100537. [Google Scholar] [CrossRef]
- Panda, S.K.; Gazim, Z.C.; Swain, S.S.; Bento, M.C.V.d.A.; Sena, J.d.S.; Mukazayire, M.J.; van Puyvelde, L.; Luyten, W. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Tetradenia riparia (Hochst.) Codd (Lamiaceae). Front. Pharmacol. 2022, 13, 896078. [Google Scholar] [CrossRef] [PubMed]
- Zelnik, R.; Rabenhorst, E.; Matida, A.K.; Gottlieb, H.E.; Lavie, D.; Panizza, S. Ibozol, a new diterpenoid from Iboza riparia. Phytochemistry 1978, 17, 1795–1797. [Google Scholar] [CrossRef]
- Weaver, D.K.; Dunkel, F.V.; van Puyvelde, L.; Richards, D.C.; Fitzgerald, G.W. Toxicity and protectant potential of the essential oil of Tetradenia riparia (Lamiales, Lamiaceae) against Zabrotes subfasciatus (Col., Bruchidae) infesting dried pinto beans (Fabales, Leguminosae). J. Appl. Entomol. 1994, 118, 179–196. [Google Scholar] [CrossRef]
- Gazim, Z.C.; Rodrigues, F.; Amorin, A.C.L.; de Rezende, C.M.; Soković, M.; Tešević, V.; Vučković, I.; Krstić, G.; Cortez, L.E.R.; Colauto, N.B.; et al. New Natural Diterpene-Type Abietane from Tetradenia riparia Essential Oil with Cytotoxic and Antioxidant Activities. Molecules 2014, 19, 514–524. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; de Kimpe, N.; Dubé, S.; Chagnon-Dubé, M.; Boily, Y.; Borremans, F.; Schamp, N.; Anteunis, M.J. 1′,2′-Dideacetylboronolide, an α-pyrone from Iboza riparia. Phytochemistry 1981, 20, 2753–2755. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; de Kimpe, N.; Borremans, F.; Zhang, W.; Schamp, N. 8(14),15-sandaracopimaradiene-2α,18-diol, a minor constituent of the rwandese medicinal plant Tetradenia riparia. Phytochemistry 1987, 26, 493–495. [Google Scholar] [CrossRef]
- Davies-Coleman, M.T.; Rivett, D.E. Structure of the 5,6-dihydro-α-pyrone, umuravumbolide. Phytochemistry 1995, 38, 791–792. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; de Kimpe, N. Tetradenolide, an α-PYRONE from Tetradenia riparia. Phytochemistry 1998, 49, 1157–1158. [Google Scholar] [CrossRef]
- Sabec, G.Z.; de Jesus, R.A.; de Oliveira, H.L.M.; Campo, C.F.d.A.A.; Jacomassi, E.; Gonçalves, J.E.; Gazim, Z.C. Tetradenia riparia (Lamiaceae) essential oil: An alternative to Rhipicephalus sanguineus. Aust. J. Crop Sci. 2020, 14, 1608–1615. [Google Scholar] [CrossRef]
- Zardeto, G.; de Jesus, R.A.; de Oliveira, H.L.M.; Gonçalves, J.E.; Junior, R.P.; Jacomassi, E.; Gazim, Z.C. Tetradenia riparia leaves, flower buds, and stem essential oils to control of Aedes aegypti larvae. Braz. J. Pharm. Sci. 2022, 58, 20556. [Google Scholar] [CrossRef]
- Scanavacca, J.; Faria, M.G.I.; Silva, G.C.C.; Inumaro, R.S.; Gonçalves, J.E.; Kupski, L.; Gazim, Z.C. Chemical analysis, antifungal and antimycotoxigenic activity of Tetradenia riparia essential oil and crude extract. Food Addit. Contam. Part A 2022, 39, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Cella, W.; Rahal, I.L.; Silva, G.C.C.; Jacomassi, E.; Junior, R.P.; Gonçalves, J.E.; Gonçalves, D.D.; Gazim, Z.C. Activity of essential oils from leaves, flower buds and stems of Tetradenia riparia on Rhipicephalus (Boophilus) microplus larvae. Rev. Bras. Parasitol. Vet. 2023, 32, e013522. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M. Essential Oils Art, Agriculture, Science, Industry and Entrepreneurship; Nova Science Publishers: New York, NY, USA, 2009; pp. 43–79. [Google Scholar]
- Barbieri, C.; Borsotto, P. Essential oils: Market and legislation. In Potential of Essential Oils; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2018; pp. 107–127. [Google Scholar] [CrossRef]
- Ahuja, I.S.; Singh, H. Evaluating the effectiveness of 5S implementation practices in Indian manufacturing industry. Int. J. Prod. Qual. Manag. 2018, 25, 506. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Abrini, J.; Talbaoui, A.; Fellah, H.; Bakri, Y.; Dakka, N. Lavandula stoechas essential oil from Morocco as novel source of antileishmanial, antibacterial and antioxidant activities. Biocatal. Agric. Biotechnol. 2017, 12, 179–184. [Google Scholar] [CrossRef]
- Dra, L.A.; Brahim, M.A.S.; Boualy, B.; Aghraz, A.; Barakate, M.; Oubaassine, S.; Markouk, M.; Larhsini, M. Chemical composition, antioxidant and evidence antimicrobial synergistic effects of Periploca laevigata essential oil with conventional antibiotics. Ind. Crops Prod. 2017, 109, 746–752. [Google Scholar] [CrossRef]
- Lou, Z.; Chen, J.; Yu, F.; Wang, H.; Kou, X.; Ma, C.; Zhu, S. The antioxidant, antibacterial, antibiofilm activity of essential oil from Citrus medica L. var. sarcodactylis and its nanoemulsion. LWT 2017, 80, 371–377. [Google Scholar] [CrossRef]
- Mezza, G.N.; Borgarello, A.V.; Grosso, N.R.; Fernandez, H.; Pramparo, M.C.; Gayol, M.F. Antioxidant activity of rosemary essential oil fractions obtained by molecular distillation and their effect on oxidative stability of sunflower oil. Food Chem. 2018, 242, 9–15. [Google Scholar] [CrossRef]
- Urbizu-González, A.L.; Castillo-Ruiz, O.; Martínez-Ávila, G.C.G.; Torres-Castillo, J.A. Natural variability of essential oil and antioxidants in the medicinal plant Turnera diffusa. Asian Pac. J. Trop. Med. 2017, 10, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Semerdjieva, I.B.; Dincheva, I.; Kacaniova, M.; Astatkie, T.; Radoukova, T.; Schlegel, V. Antimicrobial and antioxidant activity of Juniper galbuli essential oil constituents eluted at different times. Ind. Crops Prod. 2017, 109, 529–537. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, R.S.; Yamarik, T. Final Report on the Safety Assessment of BHT. Int. J. Toxicol. 2002, 21, 19–94. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.C.; Valentim, I.B.; Goulart, M.O.F.; Silva, C.A.; Bechara, E.J.H.; Trevisan, M.T.S. Fontes vegetais naturais de antioxidantes. Quim. Nova 2009, 32, 689–702. [Google Scholar] [CrossRef]
- Da Silva, J.G.; Guimarães, V.P.; Lima, C.G.; da Silva, H.H.G.; Elias, C.N.; Mady, C.M.; Silva, V.V.d.M.e.; Nery, A.D.P.; da Rocha, K.R.; Rocha, C.; et al. Larvicidal and toxicological effect of the crude ethanolic extract of the bark of the stem of Magonia pubescens on Aedes aegypti (Diptera, Culicidae), in artificial breeding sites. J. Trop. Pathol. 2003, 32, 73–86. [Google Scholar] [CrossRef][Green Version]
- Idriss, H.; Siddig, B.; González-Maldonado, P.; Elkhair, H.M.; Alakhras, A.I.; Abdallah, E.M.; Elzupir, A.O.; Sotelo, P.H. Inhibitory Activity of Saussurea costus Extract against Bacteria, Candida, Herpes, and SARS-CoV-2. Plants 2023, 12, 460. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid. Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Procyanidins as Antioxidants and Tumor Cell Growth Modulators. J. Agric. Food Chem. 2006, 54, 2392–2397. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Chen, D.; Chen, S. Antioxidant Activity and Mechanism of Protocatechuic Acid In Vitro. Funct. Foods Health Dis. 2011, 1, 232–244. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Does antioxidant properties of the main component of essential oil reflect its antioxidant properties? The comparison of antioxidant properties of essential oils and their main components. Nat. Prod. Res. 2014, 28, 1952–1963. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.C.A.M.; Rosa, M.F.; Fernandez, C.M.M.; Bortolucci, W.C.; Melo, U.Z.; Siqueira, V.L.D.; Cortez, D.A.G.; Gonçalves, J.E.; Linde, G.A.; Gazim, Z.C. Antimicrobial and Antioxidant Activities of the Extract and Fractions of Tetradenia riparia (Hochst.) Codd (Lamiaceae) Leaves from Brazil. Curr. Microbiol. 2017, 74, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Richheimer, S.L.; Bernart, M.W.; King, G.A.; Kent, M.C.; Beiley, D.T. Antioxidant activity of lipid-soluble phenolic diterpenes from rosemary. J. Am. Oil Chem. Soc. 1996, 73, 507–514. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Structure–Activity Relationships of Flavonoids in the Cellular Antioxidant Activity Assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Finley, J. Potential Cell Culture Models for Antioxidant Research. J. Agric. Food Chem. 2005, 53, 4311–4314. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Gaire, B.; Parveen, A.; Kim, S.-Y. Nitric Oxide as a Target for Phytochemicals in Anti-Neuroinflammatory Prevention Therapy. Int. J. Mol. Sci. 2021, 22, 4771. [Google Scholar] [CrossRef] [PubMed]
- De, J.; Lu, Y.; Ling, L.; Peng, N.; Zhong, Y. Essential Oil Composition and Bioactivities of Waldheimia glabra (Asteraceae) from Qinghai-Tibet Plateau. Molecules 2017, 22, 460. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Ahamed, M.B.K.; Majid, A.M.A. In vivo anti-inflammatory activity of β-caryophyllene, evaluated by molecular imaging. Mol. Med. Chem. 2015, 1, 6. [Google Scholar] [CrossRef]
- Hernandez-Leon, A.; González-Trujano, M.E.; Narváez-González, F.; Pérez-Ortega, G.; Rivero-Cruz, F.; Aguilar, M.I. Role of β-Caryophyllene in the Antinociceptive and Anti-Inflammatory Effects of Tagetes lucida Cav. Essential Oil. Molecules 2020, 25, 675. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; la Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Cardoso, G.; Dantas, E.; Sousa, F.; Peron, A. Cytotoxicity of aqueous extracts of Rosmarinus officinalis L. (Labiatae) in plant test system. Braz. J. Biol. 2014, 74, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Demets, O.V.; Takibayeva, A.T.; Kassenov, R.Z.; Aliyeva, M.R. Methods of Betulin Extraction from Birch Bark. Molecules 2022, 27, 3621. [Google Scholar] [CrossRef] [PubMed]
- Jonnalagadda, S.C.; Suman, P.; Morgan, D.C.; Seay, J.N. Recent developments on the synthesis and applications of betulin and betulinic acid derivatives as therapeutic agents. Stud. Nat. Prod. Chem. 2017, 53, 45–84. [Google Scholar] [CrossRef]
- De Almeida, T.L.; Monteiro, J.A.; Lopes, G.K.P.; Chiavelli, L.U.R.; Santin, S.M.d.O.; da Silva, C.C.; Kaplum, V.; Scariot, D.B.; Nakamura, C.V.; Ruiz, A.L.T.G.; et al. Chemical study and antiproliferative, trypanocidal and leishmanicidal activities of Maxillaria picta. Quim. Nova 2014, 37, 1151–1157. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2009, 24, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Schnitzler, P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran. J. Microbiol. 2014, 6, 149–155. [Google Scholar] [PubMed]
- Gazim, Z.C.; Amorim, A.C.L.; Hovell, A.M.C.; Rezende, C.M.; Nascimento, I.A.; Ferreira, G.A.; Cortez, D.A.G. Seasonal Variation, Chemical Composition, and Analgesic and Antimicrobial Activities of the Essential Oil from Leaves of Tetradenia riparia (Hochst.) Codd in Southern Brazil. Molecules 2010, 15, 5509–5524. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectroscopy, 3rd ed.; Allured Publishing Corporation: Pennsylvania, PA, USA, 2001; p. 804. [Google Scholar]
- Rufino, M.S.M.; Alves., R.E.; de Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Jiménez, J.P.; Calixto, F.D.S. Metodologia científica: Determinação da atividade Antioxidante total em frutas pela Captura do Radical Livre DPPH. Comun. Técnico Embrapa 2007, 127, 1–4. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Jiménez, J.P.; Calixto, F.D.S. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pelo Método de Redução do Ferro (FRAP). Comun. Técnico Embrapa 2015, 125, 1–4. [Google Scholar]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 19, 63–68. [Google Scholar] [CrossRef]
- De Sousa Sá, P.G.; Guimarães, A.L.; Oliveira, A.P.; Filho, J.A.S.; Fontana, A.P.; Damasceno, P.K.F.; Branco, C.R.C.; Branco, A.; Almeida, J.R.G.S. Total phenols. total flavonoids and antioxidant activity of Selaginella convoluta (Arn.) Spring (Selaginellaceae). Rev. Ciências Farm. Básica Apl. 2012, 33, 561–566. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; Filho, J.M.; Moreira, A.V.B. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas no Sistema β-caroteno/Ácido Linoleico. Comun. Técnico Embrapa 2006, 126, 1–4. [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- De la Fuente, B.; Pinela, J.; Mandim, F.; Heleno, S.A.; Ferreira, I.C.; Barba, F.J.; Berrada, H.; Caleja, C.; Barros, L. Nutritional and bioactive oils from salmon (Salmo salar) side streams obtained by Soxhlet and optimized microwave-assisted extraction. Food Chem. 2022, 386, 132778. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; de Souza, A.H.P.; Calhelha, R.C.; Barros, L.; Glamoclija, J.; Sokovic, M.; Peralta, R.M.; Bracht, A.; Ferreira, I.C.F.R. Bioactive formulations prepared from fruiting bodies and submerged culture mycelia of the Brazilian edible mushroom Pleurotus ostreatoroseus Singer. Food Funct. 2015, 6, 2155–2164. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Gabaglio, S.; Alvarenga, N.; Cantero-González, G.; Degen, R.; Ferro, E.A.; Langjahr, P.; Chnaiderman, J.; Sotelo, P.H. A quantitative PCR assay for antiviral activity screening of medicinal plants against Herpes simplex 1. Nat. Prod. Res. 2019, 35, 2926–2930. [Google Scholar] [CrossRef]
| Compound | Molecular Formula | Theoretical m/z [M − H]− | Experimental m/z [M − H]− | Error (ppm) | Rt (min) | Sample |
|---|---|---|---|---|---|---|
| Anthocyanins | ||||||
| Pelargonidin | C15H11O5+ | 271.0601 [M]+ | 271.0594 | 2.58 | 4.41 | Leaves |
| 271.0593 | 2.95 | 4.43 | Stems | |||
| 271.0592 | 3.32 | 4.42 | Flower buds | |||
| Cyanidin | C15H11O6+ | 287.0550 [M]+ | 287.0542 | 2.79 | 4.27 | Leaves |
| 287.0543 | 2.44 | 4.25 | Stems | |||
| 287.0541 | 3.13 | 4.25 | Flower buds | |||
| Malvidin | C17H15O7+ | 331.0812 [M]+ | 331.0804 | 2.42 | 4.48 | Leaves |
| 331.0803 | 2.72 | 4.46 | Stems | |||
| 331.0803 | 2.72 | 4.46 | Flower buds | |||
| Flavonoids | ||||||
| Astragalin | C21H20O11 | 447.0921 [M − H]− | 447.0903 | 4.03 | 4.21 | Leaves |
| 447.0906 | 3.36 | 4.44 | Stems | |||
| 447.0904 | 3.80 | 4.17 | Flower buds | |||
| Luteolin | C15H10O6 | 285.0393 [M − H]− | 285.0389 | 1.40 | 5.16 | Leaves |
| 285.0388 | 1.75 | 5.14 | Stems | |||
| 285.0387 | 2.10 | 5.15 | Flower buds | |||
| Apigenin | C15H10O5 | 269.0444 [M − H]− | 269.0439 | 1.86 | 5.40 | Leaves |
| 269.0439 | 1.86 | 5.44 | Stems | |||
| 269.0438 | 2.23 | 5.42 | Flower buds | |||
| Phenolic acids | ||||||
| Sagerinic acid | C36H32O16 | 721.1763 [M + H]+ | 721.1734 | 4.02 | 4.12 | Leaves |
| 721.1732 | 4.29 | 4.10 | Flower buds | |||
| 4-Hydroxybenzoic acid | C7H6O3 | 137.0233 [M − H]− | 137.0233 | 0 | 4.03 | Leaves |
| 137.0233 | 0 | 3.99 | Stems | |||
| 137.0234 | −0.73 | 4.01 | Flower buds | |||
| p-Coumaric acid | C9H8O3 | 163.0389 [M − H]− | 163.0389 | 0 | 4.58 | Leaves |
| 163.0389 | 0 | 4.59 | Stems | |||
| 163.0388 | 0.61 | 4.60 | Flower buds | |||
| Ferulic acid | C10H10O4 | 193.0495 [M − H]− | 193.0492 | 1.55 | 4.72 | Leaves |
| 193.0495 | 0 | 4.74 | Stems | |||
| 193.0489 | 3.11 | 4.75 | Flower buds | |||
| Protocatechuic acid | C7H6O4 | 153.0182 [M − H]− | 153.0182 | 0 | 4.78 | Leaves |
| Caffeic acid | C9H8O4 | 179.0350 [M − H]− | 179.0336 | 7.82 | 4.29 | Leaves |
| 179.0336 | 7.82 | 4.25 | Stems | |||
| 179.0335 | 8.38 | 4.19 | Flower buds | |||
| Rosmarinic acid | C18H16O8 | 361.0917 [M + H]+ | 361.0905 | 3.32 | 4.11 | Leaves |
| 361.0903 | 3.88 | 4.10 | Flower buds | |||
| Tannins | ||||||
| Procyanidin | C30H26O13 | 593.1289 [M − H]− | 593.1266 | 3.88 | 5.46 | Leaves |
| Terpenes | ||||||
| Sandaracopimaradienolal | C20H30O2 | 303.2318 [M + H]+ | 303.2307 | 3.63 | 4.52 | Leaves |
| 303.2310 | 2.64 | 4.50 | Stems | |||
| 303.2306 | 3.96 | 4.87 | Flower buds | |||
| 14-Hydroxyhumulene | C15H24O | 221.1899 [M + H]+ | 221.1894 | 2.26 | 5.31 | Leaves |
| 221.1892 | 3.16 | 5.27 | Stems | |||
| 221.1892 | 3.16 | 5.27 | Flower buds | |||
| Abieta-7,9 (11) -dien-13β-ol | C20H32O | 289.2525 [M + H]+ | 289.2517 | 2.77 | 8.82 | Leaves |
| 289.2511 | 4.84 | 8.82 | Flower buds | |||
| 8(14),15-Sandaracopimaradiene-2α, 18-diol | C20H32O2 | 305.2475 [M + H]+ | 305.2463 | 3.93 | 6.55 | Leaves |
| 305.2464 | 3.60 | 6.17 | Stems | |||
| 305.2463 | 3.93 | 6.18 | Flower buds | |||
| Campesterol | C28H48O | 423.3597 [M + H]+ | 423.3601 | −0.94 | 13.87 | Leaves |
| 423.3600 | −0.71 | 13.84 | Flower buds | |||
| Eugenol | C10H12O2 | 165.0910 [M + H]+ | 165.0906 | 2.42 | 6.14 | Stems |
| Carnosic acid | C20H28O4 | 333.2060 [M + H]+ | 333.2053 | 2.10 | 7.02 | Leaves |
| 333.2051 | 2.70 | 6.51 | Stems | |||
| 333.2044 | 4.80 | 6.99 | Flower buds | |||
| Carnosol | C20H26O4 | 331.1903 [M + H]+ | 331.1892 | 3.32 | 6.94 | Leaves |
| 331.1893 | 3.02 | 6.94 | Stems | |||
| 331.1892 | 3.32 | 6.94 | Flower buds | |||
| Rosmanol | C20H26O5 | 347.1853 [M + H]+ | 347.1843 | 2.88 | 6.62 | Leaves |
| 347.1840 | 3.74 | 6.63 | Flower buds | |||
| Rosmarinidifenol | C20H28O3 | 317.2111 [M + H]+ | 317.2100 | 3.47 | 6.13 | Leaves |
| 317.2105 | 1.89 | 6.34 | Stems | |||
| 317.2099 | 3.78 | 6.01 | Flower buds | |||
| Rosmariniquinone | C19H22O2 | 283.1692 [M + H]+ | 283.1685 | 2.47 | 8.30 | Stems |
| Betulin aldehyde | C30H48O2 | 441.3727 [M + H]+ | 441.3706 | 4.76 | 10.04 | Leaves |
| Betulin | C30H50O2 | 443.3883 [M + H]+ | 443.3869 | 3.16 | 11.45 | Leaves |
| 443.3862 | 4.74 | 11.50 | Stems | |||
| Ketobetulinic acid | C30H46O4 | 471.3468 [M + H]+ | 471.3451 | 3.61 | 5.21 | Leaves |
| 471.3455 | 2.76 | 5.25 | Stems | |||
| 471.3454 | 2.97 | 5.22 | Flower buds | |||
| Maslinic acid | C30H48O4 | 473.3625 [M + H]+ | 473.3609 | 3.38 | 6.57 | Leaves |
| 473.3615 | 2.11 | 6.59 | Stems | |||
| 473.3611 | 2.96 | 5.57 | Flower buds | |||
| Sandaracopimaradienediol | C20H32O2 | 305.2475 [M + H]+ | 305.2464 | 3.60 | 5.46 | Leaves |
| 305.2464 | 3.60 | 6.17 | Stems | |||
| 305.2462 | 4.26 | 6.17 | Flower buds | |||
| Sandaracopimaric acid | C20H30O2 | 303.2318 [M + H]+ | 303.2312 | 1.98 | 5.68 | Leaves |
| 303.2309 | 2.97 | 5.70 | Stems | |||
| 303.2306 | 3.96 | 6.41 | Flower buds | |||
| 6,7-Dehydroroyleanone | C20H26O3 | 315.1954 [M + H]+ | 315.1930 | 7.61 | 5.67 | Leaves |
| 315.1943 | 3.49 | 5.77 | Stems | |||
| 315.1943 | 3.49 | 6.12 | Flower buds | |||
| Peak | RT | Compounds | RI Lit. | RI Calc. | Relative Area (%) | Identification Methods | ||
|---|---|---|---|---|---|---|---|---|
| Leaves | Flower Buds | Stems | ||||||
| 1 | 3.178 | α-Pinene | 932 | 930 | 1.6 | 0.5 | 1.8 | a. b. c |
| 2 | 3.369 | Camphene | 946 | 935 | 1.2 | 0.4 | 1.6 | a. b. c |
| 3 | 3.645 | Sabinene | 969 | 975 | 1.4 | 0.5 | a. b. c | |
| 4 | 3.722 | β-Pinene | 974 | 986 | 0.7 | 0.3 | 1.0 | a. b. c |
| 5 | 4.463 | Limonene | 1024 | 1030 | 1.2 | 0.5 | 0.3 | a. b. c |
| 6 | 4.533 | Trans-β-ocimene | 1044 | 1041 | 0.5 | 0.3 | - | a. b. c |
| 7 | 5.624 | Fenchone | 1083 | 1094 | 11.6 | 4.7 | 0.4 | a. b. c |
| 8 | 6.106 | Endo fenchol | 1114 | 1119 | 0.8 | 0.4 | - | a. b. c |
| 9 | 6.834 | Camphor | 1141 | 1156 | 2.4 | 0.9 | 0.1 | a. b. c |
| 10 | 7.317 | Endo-carvacrol | 1165 | 1178 | 0.8 | 0.4 | 1.5 | a. b. c |
| 11 | 7.593 | Terpinen-4-ol | 1174 | 1190 | 0.5 | - | - | a. b. c |
| 12 | 7.912 | α-Terpineol | 1186 | 1196 | 0.4 | 0.3 | a. b. c | |
| 13 | 11.930 | δ-Elemene | 1335 | 1332 | 0.3 | 0.3 | 0.4 | a. b. c |
| 14 | 13.067 | α-copaene | 1374 | 1357 | 0.9 | 0.8 | 0.3 | a. b. c |
| 15 | 13.523 | β-Elemene | 1389 | 1377 | 0.7 | 0.5 | - | a. b. c |
| 16 | 14.059 | α-Gurjunene | 1409 | 1403 | 1.7 | 1.5 | 1.2 | a. b. c |
| 17 | 14.371 | β-Caryophyllene | 1417 | 1416 | 5.7 | 3.9 | 5.0 | a. b. c |
| 18 | 14.774 | α-Bergamotene | 1432 | 1430 | 0.8 | 0.6 | 0.4 | a. b. c |
| 19 | 14.922 | Humulene | 1436 | 1432 | 0.6 | 0.5 | 2.5 | a. b. c |
| 20 | 15.317 | Aromadendrene | 1439 | 1438 | 0.4 | 0.3 | 1.2 | a. b. c |
| 21 | 15.476 | Allo-aromadendrene | 1458 | 1459 | - | - | 1.9 | a. b. c |
| 22 | 15.560 | Aromadendrene dehydro- | 1460 | 1461 | - | - | 2.4 | a. b. c |
| 23 | 15.971 | γ-Muurolene | 1478 | 1475 | 0.3 | 0.2 | 0.3 | a. b. c |
| 24 | 16.205 | Germacrene D | 1484 | 1489 | - | - | 1.0 | a. b. c |
| 25 | 16.206 | β-Guaiene | 1492 | 1490 | 0.2 | 0.5 | a. b. c | |
| 26 | 16.342 | Valenceno | 1496 | 1493 | 0.3 | - | - | a. b. c |
| 27 | 16.433 | Viridiflorene | 1496 | 1495 | 0.6 | 0.6 | - | a. b. c |
| 28 | 16.658 | α-Muurolene | 1500 | 1500 | 0.8 | 0.6 | - | a. b. c |
| 29 | 16.612 | β-Himachalene | 1500 | 1502 | 0.5 | 1.6 | 0.3 | a. b. c |
| 30 | 16.658 | Byciclogermacrene | 1500 | 1505 | 3.7 | 3.2 | 6.0 | a. b. c |
| 31 | 16.856 | α-Farnesene | 1505 | 1512 | 1.2 | 0.3 | - | a. b. c |
| 32 | 17.061 | γ-Cadinene | 1513 | 1514 | 1.2 | 0.9 | 1.4 | a. b. c |
| 33 | 17.230 | 7-Epi-α-selinene | 1520 | 1519 | 1.3 | 2.0 | 1.1 | a. b. c |
| 34 | 17.233 | δ-Cadinene | 1523 | 1520 | 4.6 | 3.3 | 2.7 | a. b. c |
| 35 | 17.239 | γ-Dehydro-arhimachalene | 1532 | 1524 | 0.8 | - | 0.5 | a. b. c |
| 36 | 17.345 | 10-Epi-cubebol | 1533 | 1530 | 0.2 | 1.3 | 0.4 | a. b. c |
| 37 | 17.409 | Palustrol | 1567 | 1564 | - | 0.2 | 0.4 | a. b. c |
| 38 | 17.720 | Germacrene D-4-ol | 1574 | 1573 | - | - | 0.9 | a. b. c |
| 39 | 17.745 | Spathulenol | 1577 | 1579 | 2.9 | - | 9.1 | a. b. c |
| 40 | 17.896 | Caryophyllene oxide | 1582 | 1587 | 1.3 | 4 | 4.6 | a. b. c |
| 41 | 18.049 | Globulol | 1590 | 1592 | - | 1.0 | 0.5 | a. b. c |
| 42 | 18.054 | Viridiflorol | 1592 | 1592 | 0.3 | 0.3 | 0.2 | a. b. c |
| 43 | 18.351 | Carotol | 1594 | 1600 | - | - | 1.1 | a. b. c |
| 44 | 18.464 | Ledol | 1602 | 1605 | 0.5 | 0.5 | 1.3 | a. b. c |
| 45 | 18.581 | α-Acorenol | 1632 | 1629 | 1.6 | 0.2 | 0.1 | a. b. c |
| 46 | 18.817 | Epi-α-cadinol | 1638 | 1636 | 3.4 | 2.8 | 0.8 | a. b. c |
| 47 | 19.295 | Allo-aromadendrene epoxide | 1639 | 1637 | - | - | 0.6 | a. b. c |
| 48 | 19.921 | Epi-α-muurolol | 1640 | 1642 | 0.3 | 0.3 | 0.6 | a. b. c |
| 49 | 20.207 | Caryophyll-4(12),8(13)-dien | 1640 | 1645 | - | - | 0.4 | a. b. c |
| 50 | 20.274 | α-Muurolol | 1644 | 1647 | 3.4 | 3.4 | 0.6 | a. b. c |
| 51 | 20.413 | Cubenol | 1646 | 1649 | 0.2 | - | 0.3 | a. b. c |
| 52 | 20.528 | β-Eudesmol | 1649 | 1650 | - | 1.6 | 0.1 | a. b. c |
| 53 | 20.683 | α-Cadinol | 1652 | 1657 | 12.2 | 10.7 | 7.4 | a. b. c |
| 54 | 20.771 | 14-Hydroxy-9-epi-caryophyllene | 1668 | 1671 | 8.6 | 14.2 | 5.1 | a. b. c |
| 55 | 20.931 | 2,3-Dihydrofarnesol | 1688 | 1696 | - | - | 0.9 | a. b. c |
| 56 | 21.047 | Siobinol | 1688 | 1700 | - | - | 3.2 | a. b. c |
| 57 | 21.378 | Cedrenol | - | 1702 | - | - | 2.1 | a. b. c |
| 58 | 21.470 | Guaiol acetate | 1725 | 1731 | - | - | 1.5 | a. b. c |
| 59 | 22.056 | Valerenol | - | 1733 | - | - | 2.2 | a. b. c |
| 60 | 22.381 | γ-Costol | 1746 | 1745 | - | - | 4.1 | a. b. c |
| 61 | 22.459 | Cis-lanceol | 1760 | 1770 | - | - | 2.2 | a. b. c |
| 62 | 28.070 | Cembrene | 1937 | 1920 | - | 1.0 | - | a. b. c |
| 63 | 29.476 | 9β,13β-Epoxi-7-abietene | - | 1945 | 7.3 | 8.8 | 4.3 | d* |
| 64 | 29.537 | Manooil oxide | 1987 | 1957 | 0.3 | 0.5 | 0.1 | a. b. c |
| 65 | 31.389 | Abieta-8,11,13-triene | 2055 | 2051 | 1.4 | 1.5 | 0.2 | a. b. c |
| 66 | 31.595 | Abietadiene | 2087 | 2085 | 0.3 | 5.5 | 0.2 | a. b. c |
| 67 | 33.409 | n.i. | - | 2087 | - | 1.0 | - | a. b. c |
| 68 | 33.859 | 6,7-Dehydroroyleanone | - | 2094 | 5.8 | 7.5 | 7.5 | a. b. c |
| 69 | 37.330 | n. i | - | 2105 | - | 1.6 | - | a. b. c |
| Total Identified | 99.6 | 96.7 | 98.3 | |||||
| Monoterpene hydrocarbons | 6.6 | 2.3 | 4.3 | |||||
| Oxygenated monoterpenes | 16.4 | 6.8 | 2.0 | |||||
| Sesquiterpene hydrocarbons | 26.4 | 21.5 | 29.3 | |||||
| Oxygenated sesquiterpenes | 35.0 | 41.2 | 50.5 | |||||
| Diterpenes | 15.1 | 24.8 | 12.3 | |||||
| Unidentified | - | 2.6 | - | |||||
| Samples | Plant Parts | Concentrations (mg mL−1) | |||
|---|---|---|---|---|---|
| 1.00 | 0.75 | 0.50 | 0.25 | ||
| Essential oils | Leaves | 78.99 ± 0.27 Ab | 76.98 ± 0.08 Bc | 68.00 ± 0.08 Cd | 67.45 ± 0.16 Dd |
| Stems | 76.13 ± 5.20 Ab | 76.75 ± 0.25 Ac | 73.72 ± 0.20 Ac | 70.76 ± 0.07 Ac | |
| Flower buds | 80.28 ± 0.20 Ab | 78.08 ± 0.2 Bb | 75.14 ± 0.16 Cb | 74.41 ± 0.16 Db | |
| Crude extracts | Leaves | 102.25 ± 0.07 Aa | 99.54 ± 0.3 Ba | 98.55 ± 0.20 Ca | 95.98 ± 0.32 Da |
| Stems | 65.94 ± 0.07 Ac | 61.41 ± 0.20 Be | 56.27 ± 0.12 Cf | 55.07 ± 0.30 Df | |
| Flower buds | 69.46 ± 0.16 Ac | 64.58 ± 0.3 Bd | 61.50 ± 0.23 Ce | 57.86 ± 0.19 De | |
| Samples | Plant Parts | DPPH | FRAP | Total Phenols |
|---|---|---|---|---|
| IC50 (mg mL−1) | (µM Ferrous Sulfate mg−1) | (µg GAE mg−1) | ||
| Essential oils | Leaves | 5.62 ± 0.69 d | 0.44 ± 0.00 d | 10.70 ± 0.55 f |
| Stems | 8.47 ± 0.50 e | 0.44 ± 0.00 d | 15.37 ± 0.74 e | |
| Flower buds | 4.47 ± 0.60 c | 0.45 ± 0.00 d | 27.07 ± 0.72 d | |
| CrudeMILOSextracts | Leaves | 1.91 ± 0.01 b | 0.35 ± 0.03 e | 75.60 ± 0.36 b |
| Stems | 0.51 ± 0.03 a | 0.50 ± 0.01 c | 111.68 ± 0.66 a | |
| Flower buds | 0.91 ± 0.05 ab | 0.81 ± 0.01 b | 65.22 ± 0.60 c | |
| Quercetin | - | 0.01 ± 0.01 a | - | - |
| Trolox | - | - | 9.17 ± 0.01 a | - |
| Samples | Plant Parts | Inhibition at Maximum Concentration Tested (%) |
|---|---|---|
| Essential oils | Leaves | 64% |
| Stems | 45% | |
| Flower buds | 46% | |
| Crude extracts | Leaves | 82% |
| Stems | 47% | |
| Flower buds | 54% |
| NO Production Inhibition | |||
|---|---|---|---|
| Samples | Plant Parts | EC50 (µg mL−1) | |
| Murine macrophage cells | Essential oils | Leaves | 95 ± 1 c |
| Stems | 76 ± 1 b | ||
| Flower buds | 247 ± 5 f | ||
| Crude extracts | Leaves | 192 ± 11 de | |
| Stems | 185 ± 5 d | ||
| Flower buds | 205 ± 2 dc | ||
| Dexamethasone | - | 16 ± 1 a | |
| Samples | Plant Parts | AGS | SI | CaCo-2 | SI | MCF-7 | SI | NCI-H460 | SI | VERO |
|---|---|---|---|---|---|---|---|---|---|---|
| Essential oils | Leaves | 34 ± 3 cA | 4.26 | 31 ± 2 bA | 4.69 | 204 ± 17 dC | 0.71 | 134 ± 7 dB | 1.08 | 145 ± 9 bB |
| Stems | 50.0 ± 0.4 dA | 2.86 | 41 ± 4 bcA | 3.48 | 163 ± 14 cB | 0.87 | 55 ± 4 bA | 2.6 | 143 ± 11 bB | |
| Flower buds | 42 ± 4 cdA | 4.30 | 63 ± 5 dA | 2.87 | 174 ± 17 cdB | 1.04 | 149 ± 14 dB | 1.21 | 181 ± 16 cB | |
| CrudeMILOSextracts | Leaves | 20 ± 1 bA | 8.8 | 52 ± 4 cdB | 3.38 | 59 ± 4 bB | 2.98 | 77 ± 2 cC | 2.28 | 176 ± 3 bcD |
| Stems | 40 ± 3 cA | 4.75 | 107 ± 7 fC | 1.77 | 71 ± 7 dB | 2.67 | 75 ± 1 cB | 2.53 | 190 ± 15 cD | |
| Flower buds | 67 ± 7 eA | 2.74 | 83 ± 3 eA | 2.21 | 145 ± 12 cB | 1.26 | 74 ± 6 cA | 2.48 | 184 ± 18 cC | |
| Ellipticine | 1.23 ± 0.03 aB | 1 | 1.21 ± 0.02 aB | 1 | 1.02 ± 0.02 aA | 1 | 1.01 ± 0.01 aA | 0.91 | 1.4 ± 0.1 aC |
| Samples | Plant Parts | IC50 (µg mL−1) | SI |
|---|---|---|---|
| Essential oils | Leaves | 9.64 | 15.01 |
| Stems | 11.75 | 12.17 | |
| Flower buds | 24.24 | 7.46 | |
| Crude extracts | Leaves | 18.84 | 9.34 |
| Stems | 15.01 | 12.65 | |
| Flower buds | 24.55 | 7.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sena, J.d.S.; Rodrigues, S.A.; Sakumoto, K.; Inumaro, R.S.; González-Maldonado, P.; Mendez-Scolari, E.; Piau, R., Jr.; Gonçalves, D.D.; Mandim, F.; Vaz, J.; et al. Antioxidant Activity, Antiproliferative Activity, Antiviral Activity, NO Production Inhibition, and Chemical Composition of Essential Oils and Crude Extracts of Leaves, Flower Buds, and Stems of Tetradenia riparia. Pharmaceuticals 2024, 17, 888. https://doi.org/10.3390/ph17070888
Sena JdS, Rodrigues SA, Sakumoto K, Inumaro RS, González-Maldonado P, Mendez-Scolari E, Piau R Jr., Gonçalves DD, Mandim F, Vaz J, et al. Antioxidant Activity, Antiproliferative Activity, Antiviral Activity, NO Production Inhibition, and Chemical Composition of Essential Oils and Crude Extracts of Leaves, Flower Buds, and Stems of Tetradenia riparia. Pharmaceuticals. 2024; 17(7):888. https://doi.org/10.3390/ph17070888
Chicago/Turabian StyleSena, Jéssica da Silva, Selma Alves Rodrigues, Karina Sakumoto, Rodrigo Sadao Inumaro, Pamela González-Maldonado, Emilio Mendez-Scolari, Ranulfo Piau, Jr., Daniela Dib Gonçalves, Filipa Mandim, Josiana Vaz, and et al. 2024. "Antioxidant Activity, Antiproliferative Activity, Antiviral Activity, NO Production Inhibition, and Chemical Composition of Essential Oils and Crude Extracts of Leaves, Flower Buds, and Stems of Tetradenia riparia" Pharmaceuticals 17, no. 7: 888. https://doi.org/10.3390/ph17070888
APA StyleSena, J. d. S., Rodrigues, S. A., Sakumoto, K., Inumaro, R. S., González-Maldonado, P., Mendez-Scolari, E., Piau, R., Jr., Gonçalves, D. D., Mandim, F., Vaz, J., Gonçalves, J. E., Sotelo, P. H., Valle, J. S. d., & Gazim, Z. C. (2024). Antioxidant Activity, Antiproliferative Activity, Antiviral Activity, NO Production Inhibition, and Chemical Composition of Essential Oils and Crude Extracts of Leaves, Flower Buds, and Stems of Tetradenia riparia. Pharmaceuticals, 17(7), 888. https://doi.org/10.3390/ph17070888









