Nalbuphine Potentiates Reversal of Fentanyl Overdose by Naloxone
Abstract
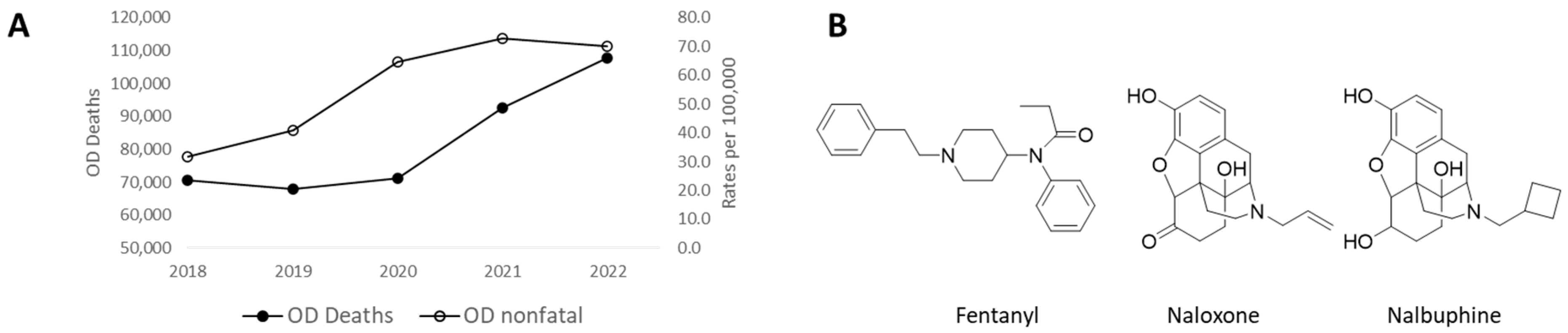
1. Introduction
2. Results and Discussion
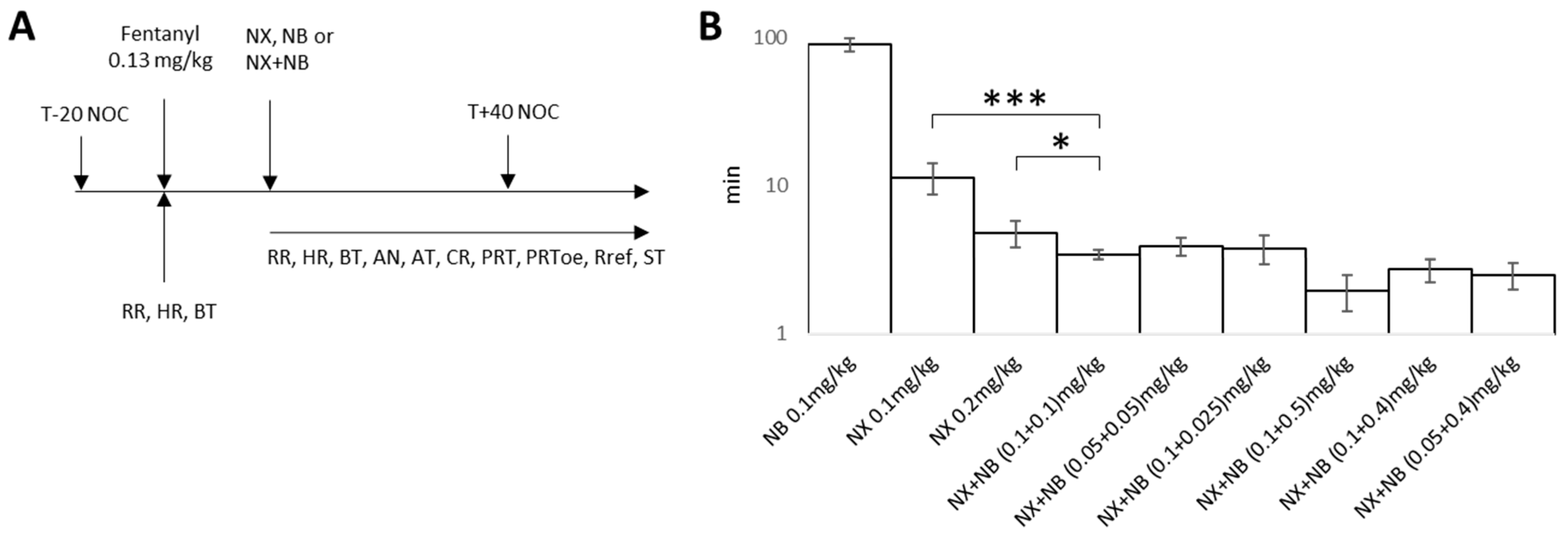
2.1. Evaluation of the Efficacy of NX, NB, and the Combination in a Fentanyl-Induced Overdose Model
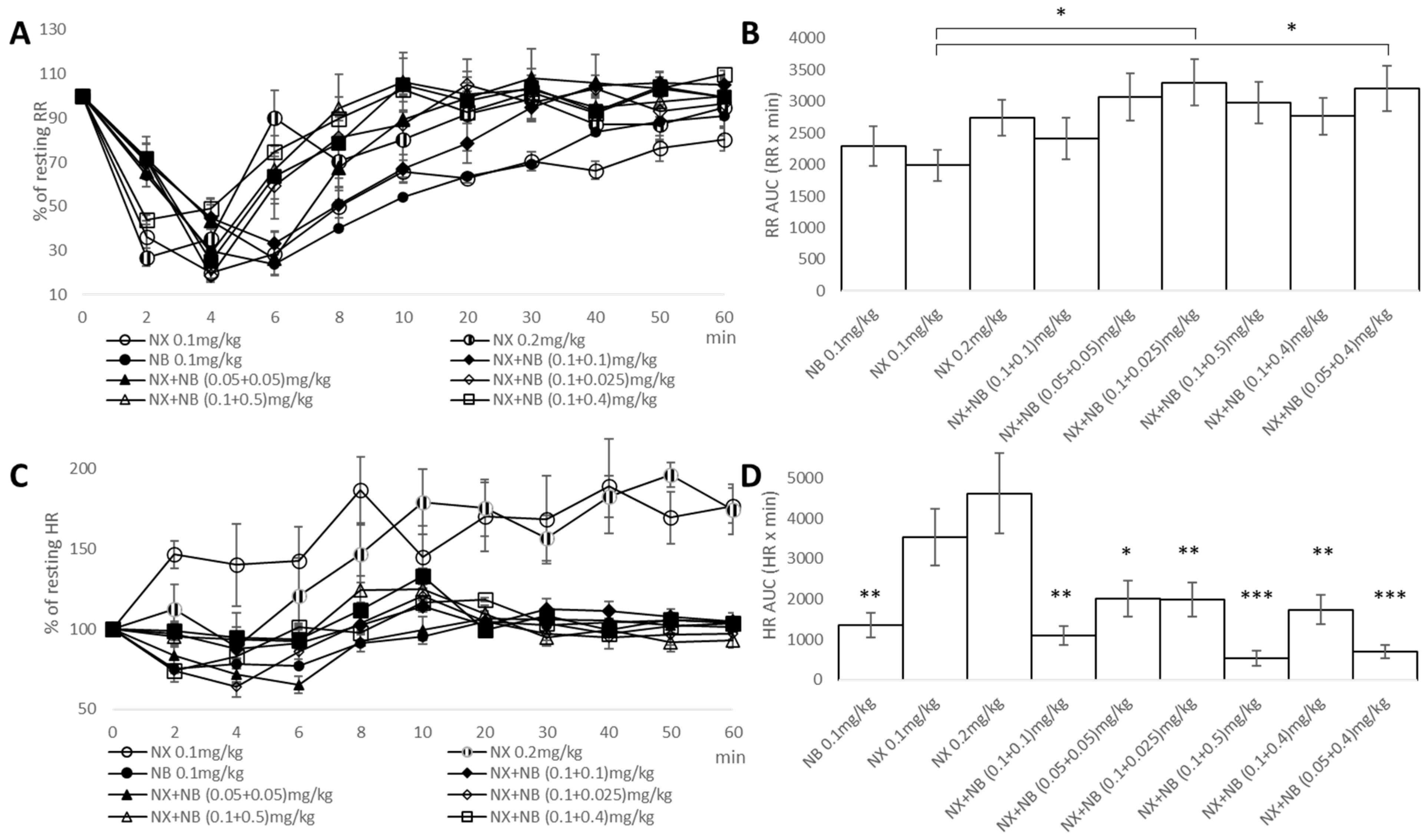
2.2. Evaluation of Respiratory and Cardiovascular Liability of NX, NB, and the Combination in a Fentanyl-Induced Overdose Model
2.3. Evaluation of Net Analgesia of NX, NB, and the Combination in Fentanyl-Induced Overdose Model
3. Materials and Methods
3.1. Chemicals
3.2. Fentanyl-Induced Overdose Model
3.3. Antinociception Evaluation
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, E.; Sutcliffe, K.; Cavallo, D.; Ramos-Gonzalez, N.; Alhosan, N.; Henderson, G. The Anomalous Pharmacology of Fentanyl. Br. J. Pharmacol. 2023, 180, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.B.; Cisewski, J.A.; Rossen, L.M.; Sutton, P. Provisional Drug Overdose Death Counts; Department of Health and Human Services, National Center for Health Statistics: Atlanta, GA USA, 2023. [Google Scholar]
- Somerville, N.J.; O’Donnell, J.; Gladden, R.M.; Zibbell, J.E.; Green, T.C.; Younkin, M.; Ruiz, S.; Babakhanlou-Chase, H.; Chan, M.; Callis, B.P.; et al. Characteristics of Fentanyl Overdose-Massachusetts, 2014–2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.; DeRienz, R.T.; Baker, D.D.; Casavant, M.; Spiller, H.A. Could Chest Wall Rigidity Be a Factor in Rapid Death from Illicit Fentanyl Abuse? Clin. Toxicol. 2016, 54, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P.; Behm, B. Reasons to Avoid Fentanyl. Ann. Palliat. Med. 2020, 9, 611–624. [Google Scholar] [CrossRef] [PubMed]
- DOSE Dashboard: Nonfatal Overdose Syndromic Surveillance Data. 2023. Available online: https://www.cdc.gov/overdose-prevention/data-research/facts-stats/dose-dashboard-nonfatal-surveillance-data.html (accessed on 24 May 2024).
- Voronkov, M.; Ataiants, J.; Cocchiaro, B.; Stock, J.B.; Lankenau, S.E. A Vicious Cycle of Neuropathological, Cognitive and Behavioural Sequelae of Repeated Opioid Overdose. Int. J. Drug Policy 2021, 97, 103362. [Google Scholar] [CrossRef] [PubMed]
- France, C.P.; Ahern, G.P.; Averick, S.; Disney, A.; Enright, H.A.; Esmaeli-Azad, B.; Federico, A.; Gerak, L.R.; Husbands, S.M.; Kolber, B.; et al. Countermeasures for Preventing and Treating Opioid Overdose. Clin. Pharmacol. Ther. 2021, 109, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Zuurmond, W.W.; Meert, T.F.; Noorduin, H. Partial versus Full Agonists for Opioid-Mediated Analgesia--Focus on Fentanyl and Buprenorphine. Acta Anaesthesiol. Belg. 2002, 53, 193–201. [Google Scholar] [PubMed]
- Yassen, A.; Olofsen, E.; van Dorp, E.; Sarton, E.; Teppema, L.; Danhof, M.; Dahan, A. Mechanism-Based Pharmacokinetic-Pharmacodynamic Modelling of the Reversal of Buprenorphine-Induced Respiratory Depression by Naloxone. Clin. Pharmacokinet. 2007, 46, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Bisaga, A. What Should Clinicians Do as Fentanyl Replaces Heroin? Addiction 2019, 114, 782–783. [Google Scholar] [CrossRef]
- Bennett, A.S.; Freeman, R.; Des Jarlais, D.C.; Aronson, I.D. Reasons People Who Use Opioids Do Not Accept or Carry No-Cost Naloxone: Qualitative Interview Study. JMIR Form. Res. 2020, 4, e22411. [Google Scholar] [CrossRef]
- Lai, J.T.; Goldfine, C.E.; Chapman, B.P.; Taylor, M.M.; Rosen, R.K.; Carreiro, S.P.; Babu, K.M. Nobody Wants to Be Narcan’d: A Pilot Qualitative Analysis of Drug Users’ Perspectives on Naloxone. West. J. Emerg. Med. 2021, 22, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, M.; Alshami, A.; Douedi, S.; Al-Taei, M.; Alsaoudi, G.; Costanzo, E. Naloxone-Induced Acute Pulmonary Edema Is Dose-Dependent: A Case Series. Am. J. Case Rep. 2021, 22, e929412. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Huecker, M.R. Opioid Withdrawal; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Li, T.; Hou, Y.; Cao, W.; Yan, C.-X.; Chen, T.; Li, S.-B. Naloxone-Precipitated Withdrawal Enhances ERK Phosphorylation in Prefrontal Association Cortex and Accumbens Nucleus of Morphine-Dependent Mice. Neurosci. Lett. 2010, 468, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Neale, J.; Strang, J. Naloxone—Does over-Antagonism Matter? Evidence of Iatrogenic Harm after Emergency Treatment of Heroin/Opioid Overdose. Addiction 2015, 110, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.G.; Baker, O.; Bernson, D.; Schuur, J.D. One-Year Mortality of Patients After Emergency Department Treatment for Nonfatal Opioid Overdose. Ann. Emerg. Med. 2020, 75, 13–17. [Google Scholar] [CrossRef]
- Uddin, O.; Jenne, C.; Fox, M.E.; Arakawa, K.; Keller, A.; Cramer, N. Divergent Profiles of Fentanyl Withdrawal and Associated Pain in Mice and Rats. Pharmacol. Biochem. Behav. 2021, 200, 173077. [Google Scholar] [CrossRef] [PubMed]
- van Dorp, E.L.A.; Yassen, A.; Dahan, A. Naloxone Treatment in Opioid Addiction: The Risks and Benefits. Expert Opin. Drug Saf. 2007, 6, 125–132. [Google Scholar] [CrossRef]
- Rabadán, J.V.; Milanés, M.V.; Laorden, M.L. Changes in Right Atrial Catecholamine Content in Naïve Rats and after Naloxone-Induced Withdrawal. Br. J. Anaesth. 1998, 80, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Kienbaum, P.; Scherbaum, N.; Thürauf, N.; Michel, M.C.; Gastpar, M.; Peters, J. Acute Detoxification of Opioid-Addicted Patients with Naloxone during Propofol or Methohexital Anesthesia: A Comparison of Withdrawal Symptoms, Neuroendocrine, Metabolic, and Cardiovascular Patterns. Crit. Care Med. 2000, 28, 969–976. [Google Scholar] [CrossRef]
- Torralva, R.; Janowsky, A. Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J. Pharmacol. Exp. Ther. 2019, 371, 453–475. [Google Scholar] [CrossRef]
- Levine, R.; Veliz, S.; Singer, D. Wooden Chest Syndrome: Beware of Opioid Antagonists, Not Just Agonists. Am. J. Emerg. Med. 2020, 38, 411.e5–411.e6. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, M.; Nikonov, G.; Ataiants, J.; Isakulyan, L.; Stefanut, C.; Cernea, M.; Abernethy, J. Modifying Naloxone to Reverse Fentanyl-Induced Overdose. Int. J. Pharm. 2021, 611, 121326. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, M.; Ocheret, D.; Bondarenko, S.; Yu, Y.; Koren, S. Administration of Nalbuphine to Heroin Addicts. Feasibility and Short-Term Effects. Heroin Addict. Relat. Clin. Probl. 2008, 10, 19–24. [Google Scholar]
- Wang, Y.; Sun, J.; Tao, Y.; Chi, Z.; Liu, J. The Role of κ-Opioid Receptor Activation in Mediating Antinociception and Addiction. Acta Pharmacol. Sin. 2010, 31, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Spanagel, R.; Almeida, O.F.; Bartl, C.; Shippenberg, T.S. Endogenous Kappa-Opioid Systems in Opiate Withdrawal: Role in Aversion and Accompanying Changes in Mesolimbic Dopamine Release. Psychopharmacology 1994, 115, 121–127. [Google Scholar] [CrossRef]
- Dosaka-Akita, K.; Tortella, F.C.; Holaday, J.W.; Long, J.B. The Kappa Opioid Agonist U-50,488H Antagonizes Respiratory Effects of Mu Opioid Receptor Agonists in Conscious Rats. J. Pharmacol. Exp. Ther. 1993, 264, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Knapp, B.I.; Bidlack, J.M.; Neumeyer, J.L. Pharmacological Properties of Bivalent Ligands Containing Butorphan Linked to Nalbuphine, Naltrexone, and Naloxone at Mu, Delta, and Kappa Opioid Receptors. J. Med. Chem. 2007, 50, 2254–2258. [Google Scholar] [CrossRef]
- Gear, R.W.; Gordon, N.C.; Miaskowski, C.; Paul, S.M.; Heller, P.H.; Levine, J.D. Dose Ratio Is Important in Maximizing Naloxone Enhancement of Nalbuphine Analgesia in Humans. Neurosci. Lett. 2003, 351, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.L.; Gear, R.W.; Levine, J.D. Response of Neuropathic Trigeminal Pain to the Combination of Low-Dose Nalbuphine plus Naloxone in Humans. Neurosci. Lett. 2003, 343, 144–146. [Google Scholar] [CrossRef]
- Schmidt, W.K.; Tam, S.W.; Shotzberger, G.S.; Smith, D.H.J.; Clark, R.; Vernier, V.G. Nalbuphine. Drug Alcohol Depend. 1985, 14, 339–362. [Google Scholar] [CrossRef]
- Miller, R.R. Evaluation of Nalbuphine Hydrochloride. Am. J. Hosp. Pharm. 1980, 37, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.-S.; Wang, J.-J.; Yoa-Pu Hu, O.; Ho, S.-T.; Chen, Y.-W. The Antinociceptive Effect of Nalbuphine and Its Long-Acting Esters in Rats. Anesth. Analg. 2003, 97, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Ho, S.T.; Hu, O.Y.; Chu, K.M. An Innovative Cold Tail-Flick Test: The Cold Ethanol Tail-Flick Test. Anesth. Analg. 1995, 80, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Motsinger-Reif, A. Current Methods for Quantifying Drug Synergism. Proteom. Bioinform. Curr. Res. 2019, 1, 43–48. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cernea, M.; Nikonov, G.; Ataiants, J.; Ştefănuţ, C.; Abernethy, J.; Voronkov, M. Nalbuphine Potentiates Reversal of Fentanyl Overdose by Naloxone. Pharmaceuticals 2024, 17, 866. https://doi.org/10.3390/ph17070866
Cernea M, Nikonov G, Ataiants J, Ştefănuţ C, Abernethy J, Voronkov M. Nalbuphine Potentiates Reversal of Fentanyl Overdose by Naloxone. Pharmaceuticals. 2024; 17(7):866. https://doi.org/10.3390/ph17070866
Chicago/Turabian StyleCernea, Mihai, Georgiy Nikonov, Janna Ataiants, Cristina Ştefănuţ, John Abernethy, and Michael Voronkov. 2024. "Nalbuphine Potentiates Reversal of Fentanyl Overdose by Naloxone" Pharmaceuticals 17, no. 7: 866. https://doi.org/10.3390/ph17070866
APA StyleCernea, M., Nikonov, G., Ataiants, J., Ştefănuţ, C., Abernethy, J., & Voronkov, M. (2024). Nalbuphine Potentiates Reversal of Fentanyl Overdose by Naloxone. Pharmaceuticals, 17(7), 866. https://doi.org/10.3390/ph17070866





