Examining the Role of Oxytocinergic Signaling and Neuroinflammatory Markers in the Therapeutic Effects of MDMA in a Rat Model for PTSD
Abstract
1. Introduction
Top of Form
2. Results
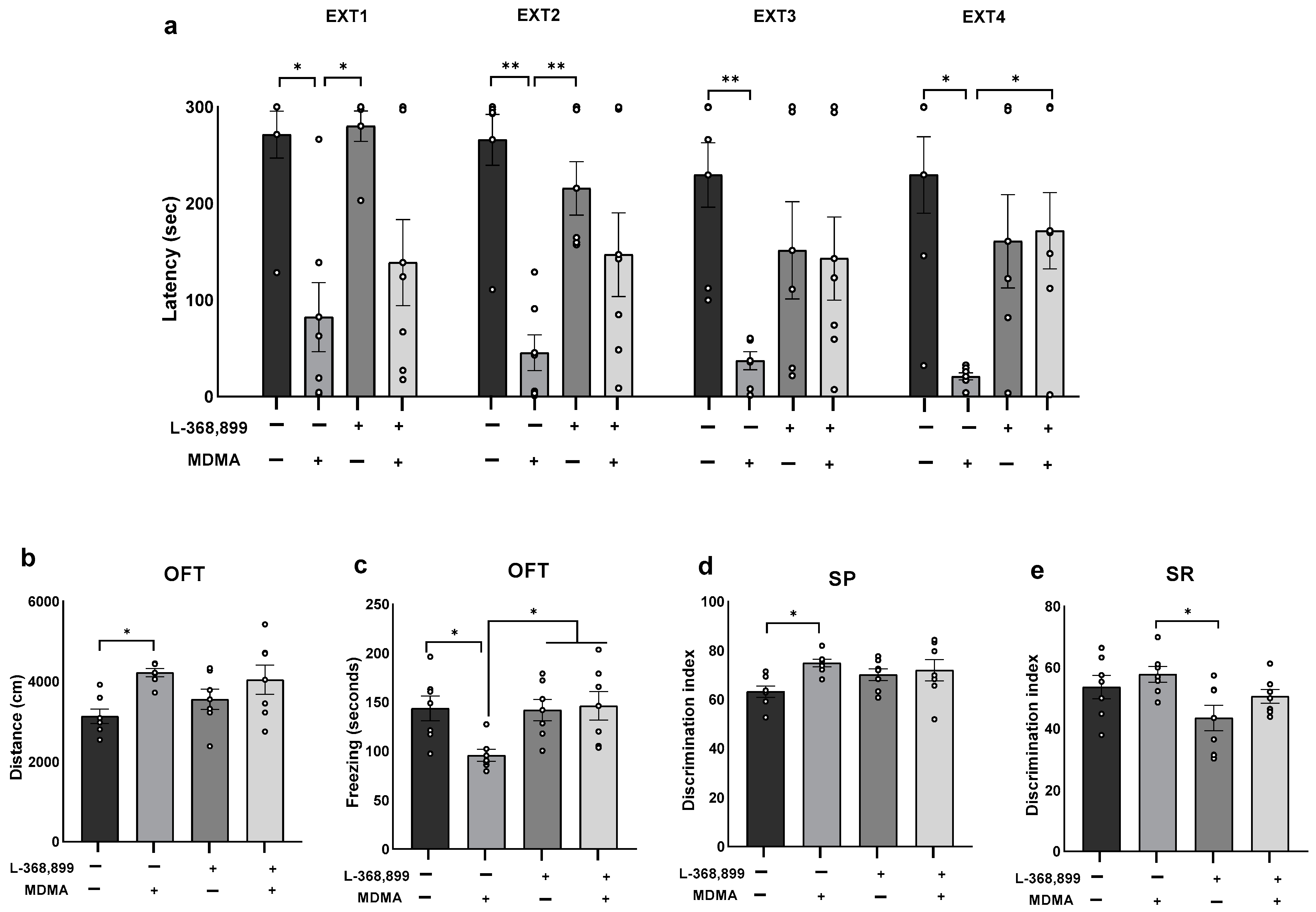
2.1. The Impact of MDMA on Behavior in Rats Subjected to Shock and Reminders
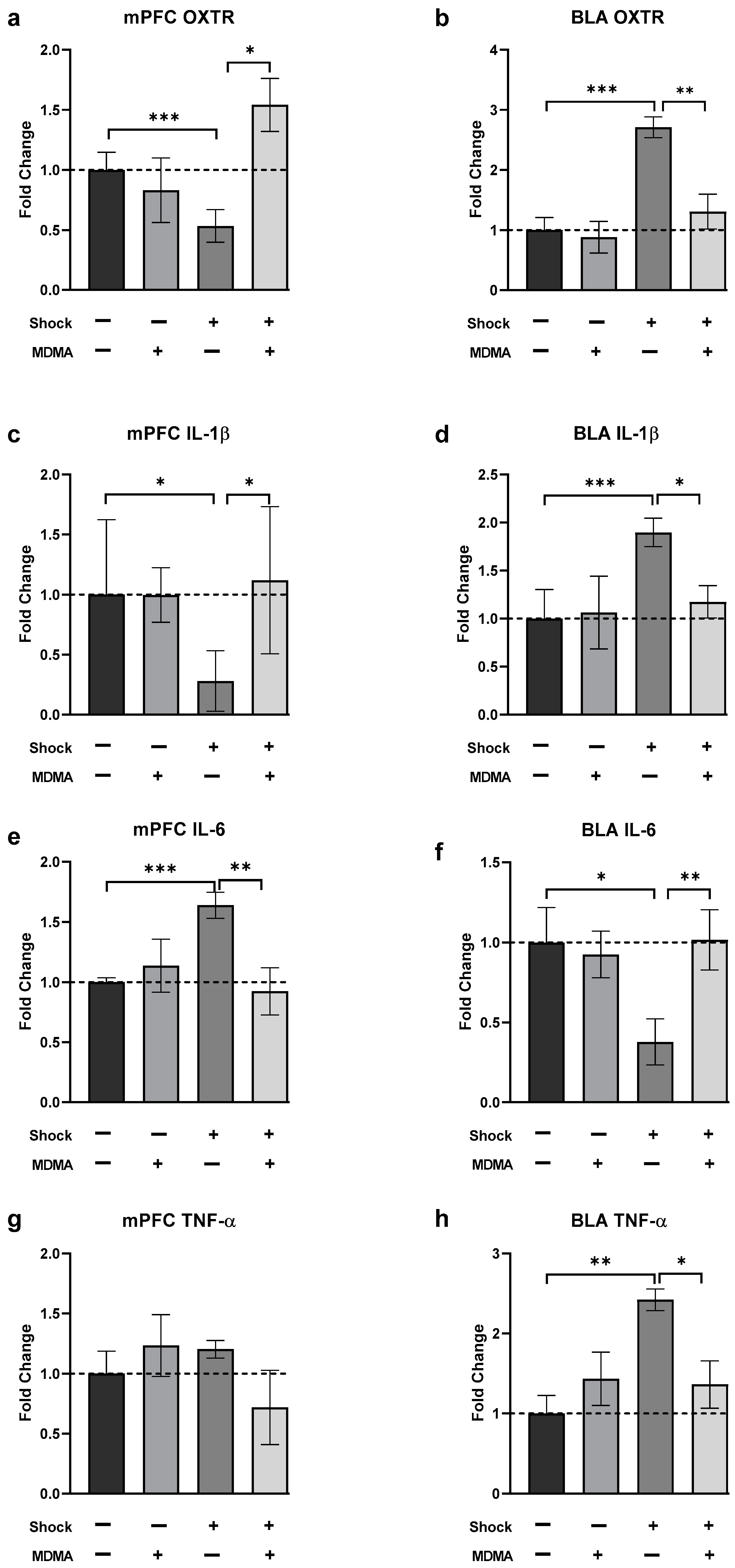
2.2. The Effects of MDMA on mRNA Levels of Oxytocin Receptor, IL-1β, IL-6, and TNF-α in Rats Exposed to Shock and Reminders
2.2.1. Oxytocin Receptor (OXT-R)
2.2.2. IL-1β
2.2.3. IL-6
2.2.4. TNF-α
2.3. Correlations between mRNA Expression and Behavior
2.4. The Effects of Co-Administration of MDMA and Oxytocin Receptor Antagonist on Behavior
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Drugs
4.3. Surgical Procedure and Microinjection
4.4. Shock and Situational Reminders
4.4.1. Shock
4.4.2. SRs
4.4.3. Extinction
4.5. Behavioral Tests
4.5.1. Open Field Test
4.5.2. Social Preference and Social Recognition
4.6. Real-Time (RT) PCR
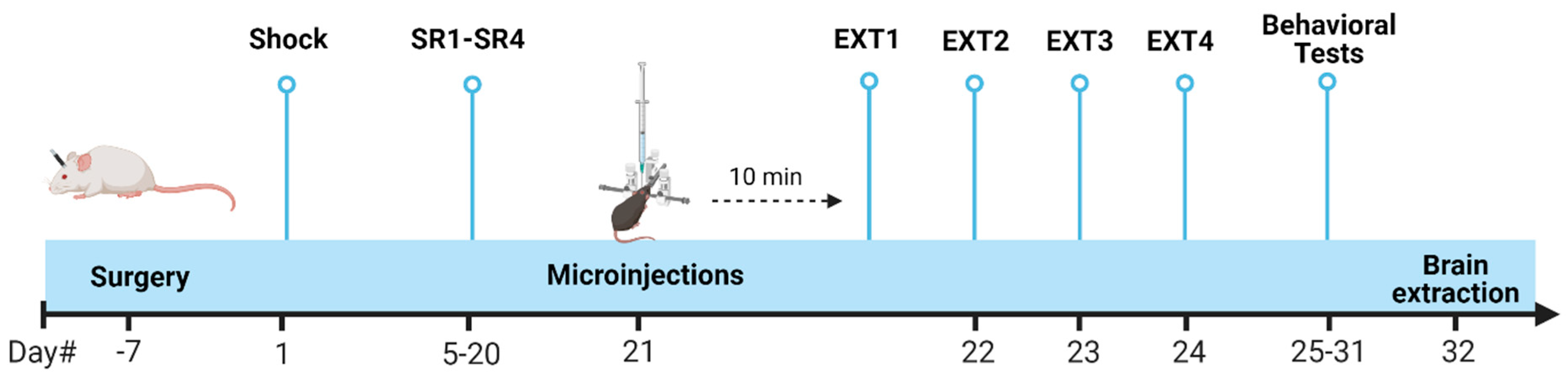
4.7. Experimental Design
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, M.O.; Garas, W.; Paleos, C.; Gorman, I.; et al. MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Mithoefer, A.T.; Feduccia, A.A.; Jerome, L.; Wagner, M.; Wymer, J.; Holland, J.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry 2018, 5, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, M.C.; Wagner, M.T.; Mithoefer, A.T.; Jerome, L.; Doblin, R. The safety and efficacy of ±3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. J. Psychopharmacol. 2011, 25, 439–452. [Google Scholar] [CrossRef]
- Oeri, H.E. Beyond ecstasy: Alternative entactogens to 3,4-methylenedioxymethamphetamine with potential applications in psychotherapy. J. Psychopharmacol. 2021, 35, 512–536. [Google Scholar] [CrossRef]
- Nichols, D.E. Entactogens: How the Name for a Novel Class of Psychoactive Agents Originated. Front. Psychiatry 2022, 13, 863088. [Google Scholar] [CrossRef] [PubMed]
- Heifets, B.D.; Malenka, R.C. MDMA as a Probe and Treatment for Social Behaviors. Cell 2016, 166, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Kamilar-Britt, P.; Bedi, G. The prosocial effects of 3,4-methylenedioxymethamphetamine (MDMA): Controlled studies in humans and laboratory animals. Neurosci. Biobehav. Rev. 2016, 57, 433–446. [Google Scholar] [CrossRef]
- Hake, H.S.; Davis, J.K.; Wood, R.R.; Tanner, M.K.; Loetz, E.C.; Sanchez, A.; Ostrovskyy, M.; Oleson, E.B.; Grigsby, J.; Doblin, R.; et al. 3,4-methylenedioxymethamphetamine (MDMA) impairs the extinction and reconsolidation of fear memory in rats. Physiol. Behav. 2019, 199, 343–350. [Google Scholar] [CrossRef]
- Nardou, R.; Lewis, E.M.; Rothhaas, R.; Xu, R.; Yang, A.; Boyden, E.; Dölen, G. Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature 2019, 569, 116–120. [Google Scholar] [CrossRef]
- Vizeli, P.; Straumann, I.; Duthaler, U.; Varghese, N.; Eckert, A.; Paulus, M.P.; Risbrough, V.; Liechti, M.E. Effects of 3,4-Methylenedioxymethamphetamine on Conditioned Fear Extinction and Retention in a Crossover Study in Healthy Subjects. Front. Pharmacol. 2022, 13, 906639. [Google Scholar] [CrossRef]
- Young, M.B.; Andero, R.; Ressler, K.J.; Howell, L.L. 3,4-Methylenedioxymethamphetamine facilitates fear extinction learning. Transl. Psychiatry 2015, 5, e634. [Google Scholar] [CrossRef] [PubMed]
- Young, M.B.; Norrholm, S.D.; Khoury, L.M.; Jovanovic, T.; Rauch, S.A.; Reiff, C.M.; Dunlop, B.W.; Rothbaum, B.O.; Howell, L.L. Inhibition of serotonin transporters disrupts the enhancement of fear memory extinction by 3,4-methylenedioxymethamphetamine (MDMA). Psychopharmacology 2017, 234, 2883–2895. [Google Scholar] [CrossRef] [PubMed]
- Han, D.D.; Gu, H.H. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Verrico, C.D.; Miller, G.M.; Madras, B.K. MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: Implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology 2005, 189, 489–503. [Google Scholar] [CrossRef]
- Aguilar, M.A.; García-Pardo, M.P.; Parrott, A.C. Of mice and men on MDMA: A translational comparison of the neuropsychobiological effects of 3,4-methylenedioxymethamphetamine (‘Ecstasy’). Brain Res. 2020, 1727, 146556. [Google Scholar] [CrossRef]
- Dumont, G.J.H.; Sweep, F.C.G.J.; van der Steen, R.; Hermsen, R.; Donders, A.R.T.; Touw, D.J.; van Gerven, J.M.A.; Buitelaar, J.K.; Verkes, R.J. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc. Neurosci. 2009, 4, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Callaghan, P.; Hunt, G.; Cornish, J.; McGregor, I. A role for oxytocin and 5-HT1A receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience 2007, 146, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Lahoud, N.; Maroun, M. Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquisition and extinction. Psychoneuroendocrinology 2013, 38, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Lin, C.-C.; Chen, C.-C.; Tzeng, N.-S.; Liu, Y.-P. Effects of Oxytocin on Fear Memory and Neuroinflammation in a Rodent Model of Posttraumatic Stress Disorder. Int. J. Mol. Sci. 2018, 19, 3848. [Google Scholar] [CrossRef]
- Olff, M.; Langeland, W.; Witteveen, A.; Denys, D. A Psychobiological Rationale for Oxytocin in the Treatment of Posttraumatic Stress Disorder. CNS Spectrums 2010, 15, 522–530. [Google Scholar] [CrossRef]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked Axonal Oxytocin Release in the Central Amygdala Attenuates Fear Response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef]
- Parekh, S.V.; Adams, L.O.; Barkell, G.A.; Lysle, D.T. MDMA administration attenuates hippocampal IL-β immunoreactivity and subsequent stress-enhanced fear learning: An animal model of PTSD. Brain Behav. Immun.-Health 2022, 26, 100542. [Google Scholar] [CrossRef]
- Hori, H.; Kim, Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 2019, 73, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.-Y.; Tong, L.; Li, M.-D.; Fu, C.-H.; Peng, J.-B.; Ji, L.-L. miR-142 downregulation alleviates rat PTSD-like behaviors, reduces the level of inflammatory cytokine expression and apoptosis in hippocampus, and upregulates the expression of fragile X mental retardation protein. J. Neuroinflamm. 2021, 18, 17. [Google Scholar] [CrossRef]
- Passos, I.C.; Vasconcelos-Moreno, M.P.; Costa, L.G.; Kunz, M.; Brietzke, E.; Quevedo, J.; Salum, G.; Magalhães, P.V.; Kapczinski, F.; Kauer-Sant’Anna, M. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015, 2, 1002–1012. [Google Scholar] [CrossRef]
- Peruzzolo, T.L.; Pinto, J.V.; Roza, T.H.; Shintani, A.O.; Anzolin, A.P.; Gnielka, V.; Kohmann, A.M.; Marin, A.S.; Lorenzon, V.R.; Brunoni, A.R.; et al. Inflammatory and oxidative stress markers in post-traumatic stress disorder: A systematic review and meta-analysis. Mol. Psychiatry 2022, 27, 3150–3163. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.B.; McLaughlin, L.D.; Nair, A.; Ebenezer, P.J.; Dange, R.; Francis, J. Inflammation and Oxidative Stress Are Elevated in the Brain, Blood, and Adrenal Glands during the Progression of Post-Traumatic Stress Disorder in a Predator Exposure Animal Model. PLoS ONE 2013, 8, e76146. [Google Scholar] [CrossRef]
- Yang, J.-J.; Jiang, W. Immune biomarkers alterations in post-traumatic stress disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 268, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Amini-Khoei, H.; Mohammadi-Asl, A.; Amiri, S.; Hosseini, M.-J.; Momeny, M.; Hassanipour, M.; Rastegar, M.; Haj-Mirzaian, A.; Mirzaian, A.H.; Sanjarimoghaddam, H.; et al. Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 76, 169–178. [Google Scholar] [CrossRef]
- Chen, W.; Man, X.; Zhang, Y.; Yao, G.; Chen, J. Medial prefrontal cortex oxytocin mitigates epilepsy and cognitive impairments induced by traumatic brain injury through reducing neuroinflammation in mice. Sci. Rep. 2023, 13, 5214. [Google Scholar] [CrossRef]
- Feduccia, A.A.; Mithoefer, M.C. MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84 Pt A, 221–228. [Google Scholar] [CrossRef]
- Abad, S.; Fole, A.; del Olmo, N.; Pubill, D.; Pallàs, M.; Junyent, F.; Camarasa, J.; Camins, A.; Escubedo, E. MDMA enhances hippocampal-dependent learning and memory under restrictive conditions, and modifies hippocampal spine density. Psychopharmacology 2014, 231, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Mechan, A.O.; Moran, P.M.; Elliott, M.J.; Young, A.M.; Joseph, M.H.; Green, R.A. A study of the effect of a single neurotoxic dose of 3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”) on the subsequent long-term behaviour of rats in the plus maze and open field. Psychopharmacology 2002, 159, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Maldonado, E. Acute and subchronic effects of MDMA (“ecstasy”) on anxiety in male mice tested in the elevated plus-maze. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Farooq, R.K.; Nasir, S.; Hanif, R.; Javed, A. Therapeutic effect of Thymoquinone on behavioural response to UCMS and neuroinflammation in hippocampus and amygdala in BALB/c mice model. Psychopharmacology 2022, 239, 47–58. [Google Scholar] [CrossRef]
- Ho, Y.-J.; Pawlak, C.R.; Guo, L.; Schwarting, R.K. Acute and long-term consequences of single MDMA administration in relation to individual anxiety levels in the rat. Behav. Brain Res. 2004, 149, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, V.; Mihov, Y.; Schwarting, R. Behavioral and neurochemical consequences of multiple MDMA administrations in the rat: Role of individual differences in anxiety-related behavior. Behav. Brain Res. 2007, 189, 52–64. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.G.; Kelly, J.P.; Leonard, B.E. Some behavioural and neurochemical aspects of subacute (±)3,4-methylenedioxymethamphetamine administration in rats. Pharmacol. Biochem. Behav. 1995, 52, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Fox, M.A.; Gallagher, P.S.; Murphy, D.L. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007, 6, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Shokry, I.M.; Callanan, J.J.; Sousa, J.; Tao, R. New Insights on Different Response of MDMA-Elicited Serotonin Syndrome to Systemic and Intracranial Administrations in the Rat Brain. PLoS ONE 2016, 11, e0155551. [Google Scholar] [CrossRef]
- Herndon, J.M.; Cholanians, A.B.; Lau, S.S.; Monks, T.J. Glial Cell Response to 3,4-(±)-Methylenedioxymethamphetamine and Its Metabolites. Toxicol. Sci. 2014, 138, 130–138. [Google Scholar] [CrossRef]
- Heifets, B.D.; Salgado, J.S.; Taylor, M.D.; Hoerbelt, P.; Pinto, D.F.C.; Steinberg, E.E.; Walsh, J.J.; Sze, J.Y.; Malenka, R.C. Distinct neural mechanisms for the prosocial and rewarding properties of MDMA. Sci. Transl. Med. 2019, 11, eaaw6435. [Google Scholar] [CrossRef]
- Ramos, L.; Hicks, C.; Caminer, A.; Couto, K.; Narlawar, R.; Kassiou, M.; McGregor, I.S. MDMA (‘Ecstasy’), oxytocin and vasopressin modulate social preference in rats: A role for handling and oxytocin receptors. Pharmacol. Biochem. Behav. 2016, 150–151, 115–123. [Google Scholar] [CrossRef]
- Kuteykin-Teplyakov, K.; Maldonado, R. Looking for prosocial genes: ITRAQ analysis of proteins involved in MDMA-induced sociability in mice. Eur. Neuropsychopharmacol. 2014, 24, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Lin, C.-C.; Tzeng, N.-S.; Tung, C.-S.; Liu, Y.-P. Effects of oxytocin on prosocial behavior and the associated profiles of oxytocinergic and corticotropin-releasing hormone receptors in a rodent model of posttraumatic stress disorder. J. Biomed. Sci. 2019, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ma, L.; Ju, P.; Yang, B.; Wang, Y.-X.; Chen, J. Involvement of Oxytocin Receptor/Erk/MAPK Signaling in the mPFC in Early Life Stress-Induced Autistic-Like Behaviors. Front. Cell Dev. Biol. 2020, 8, 564485. [Google Scholar] [CrossRef]
- Mairesse, J.; Gatta, E.; Reynaert, M.-L.; Marrocco, J.; Morley-Fletcher, S.; Soichot, M.; Deruyter, L.; Van Camp, G.; Bouwalerh, H.; Fagioli, F.; et al. Activation of presynaptic oxytocin receptors enhances glutamate release in the ventral hippocampus of prenatally restraint stressed rats. Psychoneuroendocrinology 2015, 62, 36–46. [Google Scholar] [CrossRef]
- Conti, F.; Sertic, S.; Reversi, A.; Chini, B. Intracellular trafficking of the human oxytocin receptor: Evidence of receptor recycling via a Rab4/Rab5 “short cycle”. Am. J. Physiol. Metab. 2009, 296, E532–E542. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Slattery, D.A. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol. Psychiatry 2016, 79, 213–221. [Google Scholar] [CrossRef]
- Peters, S.; Slattery, D.A.; Uschold-Schmidt, N.; Reber, S.O.; Neumann, I.D. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 2014, 42, 225–236. [Google Scholar] [CrossRef]
- Shi, D.-D.; Zhang, Y.-D.; Ren, Y.-Y.; Peng, S.-Y.; Yuan, T.-F.; Wang, Z. Predictable maternal separation confers adult stress resilience via the medial prefrontal cortex oxytocin signaling pathway in rats. Mol. Psychiatry 2021, 26, 7296–7307. [Google Scholar] [CrossRef]
- Milad, M.R.; Vidal-Gonzalez, I.; Quirk, G.J. Electrical Stimulation of Medial Prefrontal Cortex Reduces Conditioned Fear in a Temporally Specific Manner. Behav. Neurosci. 2004, 118, 389–394. [Google Scholar] [CrossRef]
- Milad, M.R.; Quirk, G.J. Fear Extinction as a Model for Translational Neuroscience: Ten Years of Progress. Annu. Rev. Psychol. 2012, 63, 129–151. [Google Scholar] [CrossRef]
- Sabihi, S.; Dong, S.M.; Maurer, S.D.; Post, C.; Leuner, B. Oxytocin in the medial prefrontal cortex attenuates anxiety: Anatomical and receptor specificity and mechanism of action. Neuropharmacology 2017, 125, 1–12. [Google Scholar] [CrossRef]
- Bloodgood, D.W.; Sugam, J.A.; Holmes, A.; Kash, T.L. Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Transl. Psychiatry 2018, 8, 60. [Google Scholar] [CrossRef]
- Tye, K.M.; Prakash, R.; Kim, S.-Y.; Fenno, L.E.; Grosenick, L.; Zarabi, H.; Thompson, K.R.; Gradinaru, V.; Ramakrishnan, C.; Deisseroth, K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 2011, 471, 358–362. [Google Scholar] [CrossRef]
- Frau, L.; Simola, N.; Porceddu, P.F.; Morelli, M. Effect of crowding, temperature and age on glia activation and dopaminergic neurotoxicity induced by MDMA in the mouse brain. NeuroToxicology 2016, 56, 127–138. [Google Scholar] [CrossRef]
- Orio, L.; O’Shea, E.; Sanchez, V.; Pradillo, J.M.; Escobedo, I.; Camarero, J.; Moro, M.A.; Green, A.R.; Colado, M.I. 3,4-Methylenedioxymethamphetamine increases interleukin-1β levels and activates microglia in rat brain: Studies on the relationship with acute hyperthermia and 5-HT depletion. J. Neurochem. 2004, 89, 1445–1453. [Google Scholar] [CrossRef]
- O’Shea, E.; Urrutia, A.; Green, A.R.; Colado, M.I. Current preclinical studies on neuroinflammation and changes in blood–brain barrier integrity by MDMA and methamphetamine. Neuropharmacology 2014, 87, 125–134. [Google Scholar] [CrossRef]
- Esteban, B.; O’Shea, E.; Camarero, J.; Sanchez, V.; Green, A.R.; Colado, M.I. 3,4-Methylenedioxymethamphetamine induces monoamine release, but not toxicity, when administered centrally at a concentration occurring following a peripherally injected neurotoxic dose. Psychopharmacology 2001, 154, 251–260. [Google Scholar] [CrossRef]
- O’Connor, K.A.; Johnson, J.D.; Hansen, M.K.; Frank, J.L.W.; Maksimova, E.; Watkins, L.R.; Maier, S.F. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003, 991, 123–132. [Google Scholar] [CrossRef]
- Vecchiarelli, H.A.; Gandhi, C.P.; Gray, J.M.; Morena, M.; Hassan, K.I.; Hill, M.N. Divergent responses of inflammatory mediators within the amygdala and medial prefrontal cortex to acute psychological stress. Brain Behav. Immun. 2016, 51, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Avital, A.; Goshen, I.; Kamsler, A.; Segal, M.; Iverfeldt, K.; Richter-Levin, G.; Yirmiya, R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 2003, 13, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Goshen, I.; Kreisel, T.; Ounallah-Saad, H.; Renbaum, P.; Zalzstein, Y.; Ben-Hur, T.; Levy-Lahad, E.; Yirmiya, R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 2007, 32, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Engler, H.; Doenlen, R.; Engler, A.; Riether, C.; Prager, G.; Niemi, M.-B.; Pacheco-López, G.; Krügel, U.; Schedlowski, M. Acute amygdaloid response to systemic inflammation. Brain Behav. Immun. 2011, 25, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Munshi, S.; Loh, M.K.; Ferrara, N.; DeJoseph, M.R.; Ritger, A.; Padival, M.; Record, M.J.; Urban, J.H.; Rosenkranz, J.A. Repeated stress induces a pro-inflammatory state, increases amygdala neuronal and microglial activation, and causes anxiety in adult male rats. Brain Behav. Immun. 2020, 84, 180–199. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Xue, J.; Yu, Y.; Wei, S.-G.; Beltz, T.G.; Felder, R.B.; Johnson, A.K. Predator Scent-Induced Sensitization of Hypertension and Anxiety-like Behaviors. Cell. Mol. Neurobiol. 2022, 42, 1141–1152. [Google Scholar] [CrossRef]
- Muhie, S.; Gautam, A.; Chakraborty, N.; Hoke, A.; Meyerhoff, J.; Hammamieh, R.; Jett, M. Molecular indicators of stress-induced neuroinflammation in a mouse model simulating features of post-traumatic stress disorder. Transl. Psychiatry 2017, 7, e1135. [Google Scholar] [CrossRef] [PubMed]
- Patki, G.; Solanki, N.; Atrooz, F.; Allam, F.; Salim, S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013, 1539, 73–86. [Google Scholar] [CrossRef]
- Li, S.; Liao, Y.; Dong, Y.; Li, X.; Li, J.; Cheng, Y.; Cheng, J.; Yuan, Z. Microglial deletion and inhibition alleviate behavior of post-traumatic stress disorder in mice. J. Neuroinflamm. 2021, 18, 7. [Google Scholar] [CrossRef]
- Stepanichev, M.Y.; Peregud, D.I.; Manolova, A.O.; Lazareva, N.A.; Onufriev, M.V.; Gulyaeva, N.V. Chronic Mild Stress Increases the Expression of Genes Encoding Proinflammatory Cytokines in the Rat Brain. Biol. Bull. 2018, 45, 186–191. [Google Scholar] [CrossRef]
- Baier, P.C.; May, U.; Scheller, J.; Rose-John, S.; Schiffelholz, T. Impaired hippocampus-dependent and -independent learning in IL-6 deficient mice. Behav. Brain Res. 2009, 200, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.J.; Khom, S.; Bajo, M.; Vlkolinsky, R.; Polis, I.; Cates-Gatto, C.; Roberto, M.; Gruol, D.L. Increased IL-6 expression in astrocytes is associated with emotionality, alterations in central amygdala GABAergic transmission, and excitability during alcohol withdrawal. Brain Behav. Immun. 2019, 82, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Lin, W.; Tang, M. Comparison of stress-induced and LPS-induced depressive-like behaviors and the alterations of central proinflammatory cytokines mRNA in rats. PsyCh J. 2015, 4, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Hao, Y.; Bi, Q.; Zhang, J.; Yang, P. Intra-amygdala microinjection of TNF-α impairs the auditory fear conditioning of rats via glutamate toxicity. Neurosci. Res. 2015, 91, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, K.; Ito, H.; Ohgidani, M.; Yamawaki, Y.; Sahin, E.H.; Kitajima, T.; Katsumata, S.; Yamawaki, S.; Kato, T.A.; Aizawa, H. Antidepressant effect of the translocator protein antagonist ONO-2952 on mouse behaviors under chronic social defeat stress. Neuropharmacology 2020, 162, 107835. [Google Scholar] [CrossRef] [PubMed]
- Brill-Maoz, N.; Maroun, M. Extinction of fear is facilitated by social presence: Synergism with prefrontal oxytocin. Psychoneuroendocrinology 2016, 66, 75–81. [Google Scholar] [CrossRef]
- Tan, Y.; Singhal, S.M.; Harden, S.W.; Cahill, K.M.; Nguyen, D.-T.M.; Colon-Perez, L.M.; Sahagian, T.J.; Thinschmidt, J.S.; de Kloet, A.D.; Febo, M.; et al. Oxytocin Receptors Are Expressed by Glutamatergic Prefrontal Cortical Neurons That Selectively Modulate Social Recognition. J. Neurosci. 2019, 39, 3249–3263. [Google Scholar] [CrossRef]
- Burgos-Robles, A.; Vidal-Gonzalez, I.; Santini, E.; Quirk, G.J. Consolidation of Fear Extinction Requires NMDA Receptor-Dependent Bursting in the Ventromedial Prefrontal Cortex. Neuron 2007, 53, 871–880. [Google Scholar] [CrossRef]
- Maroun, M.; Kavushansky, A.; Holmes, A.; Wellman, C.; Motanis, H. Enhanced Extinction of Aversive Memories by High-Frequency Stimulation of the Rat Infralimbic Cortex. PLoS ONE 2012, 7, e35853. [Google Scholar] [CrossRef]
- Ninan, I. Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. J. Neurochem. 2011, 119, 324–331. [Google Scholar] [CrossRef]
- Dunlap, L.E.; Andrews, A.M.; Olson, D.E. Dark Classics in Chemical Neuroscience: 3,4-Methylenedioxymethamphetamine. ACS Chem. Neurosci. 2018, 9, 2408–2427. [Google Scholar] [CrossRef]
- Hunt, G.E.; McGregor, I.S.; Cornish, J.L.; Callaghan, P.D. MDMA-induced c-Fos expression in oxytocin-containing neurons is blocked by pretreatment with the 5-HT-1A receptor antagonist WAY 100635. Brain Res. Bull. 2011, 86, 65–73. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The oxytocin receptor: From intracellular signaling to behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Sabihi, S.; Durosko, N.E.; Dong, S.M.; Leuner, B. Oxytocin in the prelimbic medial prefrontal cortex reduces anxiety-like behavior in female and male rats. Psychoneuroendocrinology 2014, 45, 31–42. [Google Scholar] [CrossRef]
- Scantamburlo, G.; Hansenne, M.; Geenen, V.; Legros, J.; Ansseau, M. Additional intranasal oxytocin to escitalopram improves depressive symptoms in resistant depression: An open trial. Eur. Psychiatry 2015, 30, 65–68. [Google Scholar] [CrossRef]
- Patin, A.; Scheele, D.; Hurlemann, R. Oxytocin and Interpersonal Relationships. In Behavioral Pharmacology of Neuropeptides: Oxytocin; Hurlemann, R., Grinevich, V., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 389–420. [Google Scholar] [CrossRef]
- Quinones, M.M.; Gallegos, A.M.; Lin, F.V.; Heffner, K. Dysregulation of inflammation, neurobiology, and cognitive function in PTSD: An integrative review. Cogn. Affect. Behav. Neurosci. 2020, 20, 455–480. [Google Scholar] [CrossRef]
- Wilson, C.B.; McLaughlin, L.D.; Ebenezer, P.J.; Nair, A.R.; Dange, R.; Harre, J.G.; Shaak, T.L.; Diamond, D.M.; Francis, J. Differential effects of sertraline in a predator exposure animal model of post-traumatic stress disorder. Front. Behav. Neurosci. 2014, 8, 256. [Google Scholar] [CrossRef]
- Lou, C.; Fang, M.; Ye, S.; Fang, Z.; Amin, N.; Chen, Y. Fluoxetine protects against inflammation and promotes autophagy in mice model of post-traumatic stress disorder. Behav. Brain Res. 2022, 433, 114004. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, S.; Bai, X.; Gao, Y.; Liu, G.; Wang, X.; Liu, D.; Li, T.; Hao, A.; Wang, Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J. Neuroinflamm. 2016, 13, 77. [Google Scholar] [CrossRef]
- Reguilón, M.D.; Ferrer-Pérez, C.; Miñarro, J.; Rodríguez-Arias, M. Oxytocin reverses ethanol consumption and neuroinflammation induced by social defeat in male mice. Horm. Behav. 2021, 127, 104875. [Google Scholar] [CrossRef]
- Costa, G.; Gołembiowska, K. Neurotoxicity of MDMA: Main effects and mechanisms. Exp. Neurol. 2022, 347, 113894. [Google Scholar] [CrossRef] [PubMed]
- Calcagnoli, F.; Kreutzmann, J.C.; de Boer, S.F.; Althaus, M.; Koolhaas, J.M. Acute and repeated intranasal oxytocin administration exerts anti-aggressive and pro-affiliative effects in male rats. Psychoneuroendocrinology 2015, 51, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Khazen, T.; Narattil, N.R.; Ferreira, G.; Maroun, M. Hippocampal oxytocin is involved in spatial memory and synaptic plasticity deficits following acute high-fat diet intake in juvenile rats. Cereb. Cortex 2023, 33, 3934–3943. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, N.; Segev, A.; Abush, H.; Zer-Aviv, T.M.; Akirav, I. Cannabinoids prevent the differential long-term effects of exposure to severe stress on hippocampal- and amygdala-dependent memory and plasticity. Hippocampus 2017, 27, 1093–1109. [Google Scholar] [CrossRef]
- Portugalov, A.; Zaidan, H.; Gaisler-Salomon, I.; Hillard, C.J.; Akirav, I. FAAH Inhibition Restores Early Life Stress-Induced Alterations in PFC microRNAs Associated with Depressive-Like Behavior in Male and Female Rats. Int. J. Mol. Sci. 2022, 23, 16101. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Dunnett, C.W. Pairwise Multiple Comparisons in the Unequal Variance Case. J. Am. Stat. Assoc. 1980, 75, 796–800. [Google Scholar] [CrossRef]

| mPFC | BLA | |
|---|---|---|
| Ext 1 | r = −0.612 *** | r = 0.501 ** |
| p < 0.001 | p < 0.01 | |
| Ext 2 | r = −0.599 *** | r = 0.508 ** |
| p < 0.001 | p < 0.01 | |
| Ext 3 | r = −0.265 | r = 0.511 ** |
| p = 0.157 | p < 0.01 | |
| Ext 4 | r = −0.612 ** | r = 0.421 * |
| p < 0.01 | p < 0.05 | |
| OFT–Distance | r = −0.0.024 | r = 0.135 |
| p = 0.901 | p = 0.477 | |
| OFT–Freezing | r = −0.161 | r = 0.2 |
| p = 0.394 | p = 0.288 | |
| SP-DI | r = 0.193 | r = 0.269 |
| p = 0.306 | p = 0.151 | |
| SR-DI | r = −0.188 | r = 0.083 |
| p = 0.320 | p = 0.664 |
| mPFC | BLA | |||||
|---|---|---|---|---|---|---|
| IL-1β | IL-6 | TNF-α | IL-1β | IL-6 | TNF-α | |
| Ext 1 | r = −0.407 * | r = 0.121 | r = 0.066 | r = 0.536 * | r = −0.384 | r = 0.645 ** |
| p < 0.05 | p = 0.573 | p = 0.758 | p < 0.05 | p = 0.105 | p < 0.01 | |
| Ext 2 | r = −0.364 | r = 0.28 | r = 0.148 | r = 0.416 | r = −0.012 | r = 0.457 |
| p = 0.062 | p = 0.184 | p = 0.491 | p = 0.068 | p = 0.961 | p = 0.056 | |
| Ext 3 | r = −0.371 | r = 0.280 | r = 0.082 | r = 0.523 * | r = −0.369 | r = 0.001 |
| p = 0.057 | p = 0.184 | p = 0.703 | p < 0.05 | p = 0.120 | p = 0.999 | |
| Ext 4 | r = −0.364 | r = 0.303 | r = 0.037 | r = 0.412 | r = −0.432 | r = 0.269 |
| p = 0.062 | p = 0.149 | p = 0.865 | p = 0.071 | p = 0.065 | p = 0.280 | |
| OFT–Distance | r = −0.248 | p = 0.075 | r = 0.2 | r = 0.245 | r = −0.126 | r = −0.225 |
| p = 0.213 | p = 0.728 | p = 0.349 | p = 0.299 | p = 0.477 | p = 0.369 | |
| OFT–Freezing | r = −0.2 | r = 0.105 | r = 0.093 | r = 0.270 | r = −0.069 | r = 0.181 |
| p = 0.316 | p = 0.626 | p = 0.666 | p = 0.250 | p = 0.780 | p = 0.472 | |
| SP- DI | r = −0.058 | r = 0.078 | r = 0.083 | r = 0.234 | r = 0.129 | r = −0.541 * |
| p = 0.780 | p = 0.718 | p = 0.701 | p = 0.320 | p = 0.578 | p < 0.05 | |
| SR-DI | r = −0.317 | r = 0.198 | r = −0.048 | r = −0.090 | r = −0.070 | r = −0.168 |
| p = 0.114 | p = 0.353 | p = 0.822 | p = 0.707 | p = 0.764 | p = 0.479 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avgana, H.; Toledano, R.S.; Akirav, I. Examining the Role of Oxytocinergic Signaling and Neuroinflammatory Markers in the Therapeutic Effects of MDMA in a Rat Model for PTSD. Pharmaceuticals 2024, 17, 846. https://doi.org/10.3390/ph17070846
Avgana H, Toledano RS, Akirav I. Examining the Role of Oxytocinergic Signaling and Neuroinflammatory Markers in the Therapeutic Effects of MDMA in a Rat Model for PTSD. Pharmaceuticals. 2024; 17(7):846. https://doi.org/10.3390/ph17070846
Chicago/Turabian StyleAvgana, Haron, Roni Shira Toledano, and Irit Akirav. 2024. "Examining the Role of Oxytocinergic Signaling and Neuroinflammatory Markers in the Therapeutic Effects of MDMA in a Rat Model for PTSD" Pharmaceuticals 17, no. 7: 846. https://doi.org/10.3390/ph17070846
APA StyleAvgana, H., Toledano, R. S., & Akirav, I. (2024). Examining the Role of Oxytocinergic Signaling and Neuroinflammatory Markers in the Therapeutic Effects of MDMA in a Rat Model for PTSD. Pharmaceuticals, 17(7), 846. https://doi.org/10.3390/ph17070846






