Comprehensive Chemical Profiling and Mechanistic Insight into Anticancer Activity of Annona muricata Leaves Extract
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterization
2.2. Molecular Findings
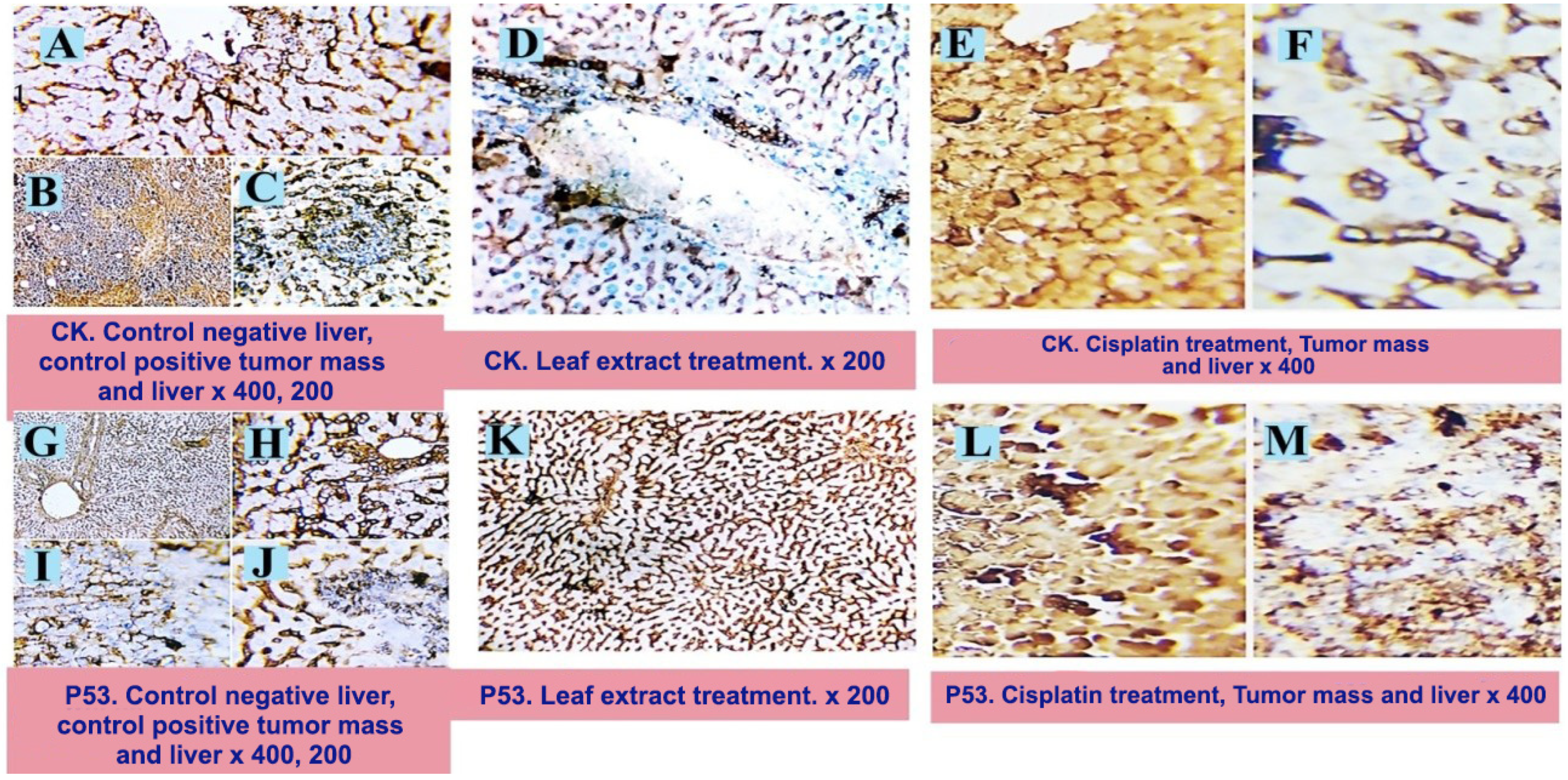
2.3. Histopathological Examination
3. Materials and Methods
3.1. Plant Materials and Extract Preparation
3.2. Analysis of A. muricata Extracts Using UPLC-ESI-MS/MS
3.2.1. Separation Technique and LC/MS Instrument Conditions and Parameters
3.2.2. UPLC-ESI-MS-MS Analysis
3.3. Cytotoxic Activity
3.3.1. Experimental Animals
3.3.2. Ehrlich Ascites Carcinoma (EAC)
3.3.3. Cisplatin
3.3.4. Cancer Induction by Ehrlich Ascites Carcinoma
3.3.5. Treatment Regimen
3.3.6. Tissue Samples
3.3.7. Molecular Determination
3.3.8. Histopathological Examination
3.3.9. Immunohistochemistry Investigation
3.3.10. Morphometric Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, I.; Rahman, H.; AbdEl-Salam, N.M.; Tawab, A.; Hussain, A.; Khan, T.A.; Khan, U.A.; Qasim, M.; Adnan, M.; Azizullah, A. Punica granatum peel extracts: HPLC fractionation and LCMS analysis to quest compound shaving activity against multidrug resistant bacteria. BMC Compl. Alter. Med. 2017, 17, 247. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; DelRio, D. Rapid and comprehensive evaluation of (poly) phenolic compounds in pomegranate (Punica granatum L.) juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef] [PubMed]
- Moraes, I.V.M.D.; Ribeiro, P.R.V.; Schmidt, F.L.; Canuto, K.M.; Zocolo, G.J.; Brito, E.S.D.; Luo, R.; Richards, K.M.; Tran, K.; Smith, R.E. UPLC–QTOF–MS and NMR analyses of graviola (Annona muricata) leaves. Rev. Bras. Farm. 2016, 26, 174–179. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): Areview of its traditional uses, isolated acetogenins and biological activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.; Ya’akob, H.; Rosdi, M.N.M. Acetogenins of Annona muricata leaves: Characterization and potential anticancer study. Integr. Cancer Sci. Ther. 2016, 3, 543–551. [Google Scholar]
- Abdallah, R.H.; Al-Saleem, M.S.; Abdel-Mageed, W.M.; Al-Attar, A.-S.R.; Shehata, Y.M.; Abdel-Fattah, D.M.; Atta, R.M. LCMS/MS Phytochemical Profiling, Molecular, Pathological, and Immune-Histochemical Studies on the Anticancer Properties of Annona muricata. Molecules 2023, 28, 5744. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, A.; Figadère, B.; Zafra-Polo, M.-C.; Barrachina, I.; Estornell, E.; Cortes, D. Acetogenins from Annonaceae: Recent progress in isolation, synthesis and mechanisms of action. Nat. Prod. Rep. 2005, 22, 269–303. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Bae, J.-Y.; Majrashi, T.; Wu, T.-Y.; Wang, Y.-H.; Wang, M.; Ali, Z.; Wu, Y.-C.; Khan, I.A. Targeted and non-targeted analysis of annonaceous alkaloids and acetogenins from Asimina and Annona species using UHPLC-QToF-MS. J. Pharm. Biom. Anal. 2018, 159, 548–566. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Rapak, A.; Ochmian, I. Profile and content of phenolic compounds in leaves, flowers, roots, and stalks of Sanguisorba officinalis L. determined with the LC-DAD-ESI-QTOF-MS/MS analysis and their in vitro antioxidant, antidiabetic, antiproliferative potency. Pharmaceuticals 2020, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L.(Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Nardo, L.; Gregori, M.; Ribeiro, I.; Mantegazza, F.; Delerue-Matos, C.; Masserini, M.; Grosso, C. Functionalized liposomes and phytosomes loading Annonamuricata L. aqueous extract: Potential nanoshuttles for brain-delivery of phenolic compounds. Phytomedicine 2018, 42, 233–244. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [PubMed]
- Marczak, Ł.; Znajdek-Awiżeń, P.; Bylka, W. The use of mass spectrometric techniques to differentiate isobaric and isomeric flavonoid conjugates from Axyris amaranthoides. Molecules 2016, 21, 1229. [Google Scholar] [CrossRef] [PubMed]
- Coria-Téllez, A.V.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; González-Ávila, M.; Martínez-Velázquez, M. Bioactivity, nutritional property, and rapid chemical characterization of aqueous extract of Annona muricata leaf from Mexico. Trop. J. Pharm. Res. 2019, 18, 611–617. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Wang, S.; Kuang, Y.; Hu, Z.-M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Justino, A.B.; Florentino, R.M.; França, A.; AntonioFilho, C.; Franco, R.R.; Saraiva, A.L.; Fonseca, M.C.; Leite, M.F.; Espindola, F.S. Alkaloid and acetogenin-rich fraction from Annona crassiflora fruit peel in hibits prolife ration and migration of human liver cancer HepG2 cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- Rady, I.; Bloch, M.B.; Chamcheu, R.-C.N.; Banang Mbeumi, S.; Anwar, M.R.; Mohamed, H.; Babatunde, A.S.; Kuiate, J.-R.; Noubissi, F.K.; ElSayed, K.A. Anticancer properties of graviola (Annona muricata): A comprehensive mechanistic review. Oxid. Med. Cell. Long. 2018, 2018, 1826170. [Google Scholar] [CrossRef] [PubMed]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; Chaves, D.S.D.A.; Romaniuk, A.; Rybczynska, M.; Gryszczynska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Rev. Bras. Farm. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Hou, Z.-F.; Xie, Z.-X.; Tu, Y.-Q.; Li, Y. Triterpenes and triterpene glycosides from Salvia tricupis. Indian J. Chem. B 2002, 41, 234–236. [Google Scholar]
- Zeweil, M.M.; Sadek, K.M.; Taha, N.M.; El-Sayed, Y.; Menshawy, S. Graviola attenuates DMBA-induced breast cancer possibly through augmenting apoptosis and antioxidant pathway and down regulating estrogen receptors. Environ. Sci. Poll. Res. 2019, 26, 15209–15217. [Google Scholar] [CrossRef]
- Samin, B.; Fachrial, E.; Refilda; Chaidir, Z.; Almahdy. Protective Effect of Aqueous Extract of Annona muricata Leaves against Copper Induced Hepatotoxicity in Experimental Rats. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 880–885. [Google Scholar]
- Alzergy, A.; Haman, M.R.; Shushni, M.A.; Almagtouf, F.A. Phyto-pharmaceuticals and biological study on graviola (Annona muricata L.) fruit and dietary supplement of graviola sold on the Libyan market as a cancer cure against TCA induce hepatotoxicity in mice. Cancer Biol. Ther. 2018, 8, 1–23. [Google Scholar]
- AbdEl-Kaream, S.A. Biochemical and biophysical study of chemopreventive and chemotherapeutic anti-tumor potential of some Egyptian plant extracts. Bioch. Bioph. Rep. 2019, 18, 100637. [Google Scholar]
- Shukry, M.; El-Shehawi, A.M.; El-Kholy, W.M.; Elsisy, R.A.; Hamoda, H.S.; Tohamy, H.G.; Abumandour, M.M.; Farrag, F.A. Ameliorative effect of graviola (Annona muricata) on mono sodium glutamate-induced hepatic injury in rats: Antioxidant, apoptotic, anti-inflammatory, lipogenesis markers, and histopathological studies. Animals 2020, 10, 1996. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.K.; Varsha, V.; Devananda, D. Anti-cancer properties of Annona muricata (L.): A Review. Med. Plants-Int. J. Phytomedicines Relat. Ind. 2019, 11, 123–134. [Google Scholar] [CrossRef]
- AbdEldaim, M.A.; Tousson, E.; Soliman, M.M.; ElSayed, I.E.T.; AbdelAleem, A.A.H.; Elsharkawy, H.N. Grape seed extract ameliorated Ehrlich solid tumor-induced hepatic tissue and DNA damage with reduction of PCNA and P53 protein expression in mice. Environ. Sci. Poll. Res. 2021, 28, 44226–44238. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, S.A. Lack of the beneficial effects of mirazid (Commiphora molmol) when administered with chemotherapeutic agents on Ehrlich ascetic carcinoma bearing mice. Adv. Biol. Res. 2011, 5, 193–199. [Google Scholar]
- DeSousa, O.V.; Vieira, G.D.-V.; de Jesus RG de Pinho, J.; Yamamoto, C.H.; Alves, M.S. Antinociceptive and anti-inflammatory activities of the ethanol extract of muricata L. leaves in animal models. Inter. J. Mol. Sci. 2010, 11, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, S.; Layton, C. The Hematoxylins and Eosin. Bancroft’s Theory Pract. Histol. Tech. 2012, 7, 173–186. [Google Scholar]
- Hsu, S.-M.; Raine, L.; Fanger, H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am. J. Clin. Path. 1981, 75, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Carson, H.J.; Reddy, V.; Taxy, J.B. Proliferation markers and prognosis in Merkel cell carcinoma. J. Cutan. Path. 1998, 25, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Harlow, E.; Lane, D.A. laboratory manual. N. Y. Cold Spring Harb. Lab. 1988, 579, 44. [Google Scholar]
- Hashish, H.; Kamal, R. Effect of curcumin on the expression of Caspase-3 and Bcl-2 in the spleen of diabetic rats. J. Exp. Clin. Anat. 2015, 14, 18–23. [Google Scholar] [CrossRef]



| No | Cpd-Name | Type | Rt | Mwt | M± | Ms/MsFragment | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 🏶🏶 Sanguisorbic acid dilactone | phenolic | 0.74 | 470 | 471 | 469,314,301,286 | [9] |
| 2 | ✓ galloyl-quinic acid-rutinoside | phenolic | 0.91 | 652 | 653 | 563,345,308 | [10] |
| 3 | 🏶🏶 mucic acid-kaempferol-malic acid-rhamnose | phenolic | 3.13 | 740 | 739 | 739,547,192 | [10] |
| 4 | 🏶🏶 squamolone | alkaliod | 4.41 | 128 | 129 | 129,112 | [8] |
| 5 | 🏶🏶 galloyl pyrogallol drv. | phenolic | 4.43 | 358 | 357 | 358,277 | [10] |
| 6 | 🏶🏶 cyanidin-acetylglucoside pyruvic acid | flavonoid | 6.61 | 559 | 558 | 558,359 | [12] |
| 7 | 🏶🏶 chrysoeriol-7-O-glucouronyl-glucouronic acid | flavonoid | 7.07 | 652 | 653 | 653,602,351,301 | [13] |
| 8 | 🏶🏶 digallic acid drv. | phenolic | 7.17 | 378 | 377 | 377,322(100%) | [10] |
| 9 | 🏶 catechin drv.+caffeic acid drv. | phenolic | 7.30 | 470 | 471 | 471,289,179,135 | [11] |
| 10 | 🏶🏶 isopiline | alkaliod | 8.78 | 297 | 298 | 297,265 | [16] |
| 11 | 🏶 vinblastine | alkaliod | 10.29 | 811 | 810 | 810 | [17] |
| 12 | 🏶 quercetin-rhamnose-sophoroside | flavonoid | 11.70 | 756 | 757 | 757,308,302,146 | [4] |
| 13 | 🏶 kaempferol-3-O-glucose-rhamnose-glucoside | flavonoid | 11.81 | 756 | 757 | 755,448,470 | [4] |
| 14 | 🏶🏶 phillygenin-O-hexose-O-pentose | lignan | 17.12 | 666 | 667 | 667,373,534 | [18] |
| 15 | 🏶🏶 Tangeretin-drv. | flavonoid | 17.53 | 740 | 739 | 739,371 | [14] |
| 16 | 🏶🏶 visIdulin III-drv. | flavonoid | 17.60 | 740 | 739 | 739,345 | [15] |
| 17 | 🏶 Cohibins A/B | acetogenin | 17.71 | 548 | 547 | 548 | [4,7] |
| 18 | 🏶 stepharine | alkaloid | 18.15 | 297 | 298 | 297,146 | [1,4] |
| 19 | 🏶 Desacetyl uvaricin | acetogenin | 19.77 | 606 | 607 | 607,571,553 | [8] |
| 20 | 🏶 Muricin I | acetogenin | 19.95 | 606 | 607 | 607,571,553 | [4,7] |
| 21 | 🏶 5-Cis-reticulatacin-10-one | acetogenin | 19.97 | 606 | 607 | 607,571,553 | [4,7] |
| 22 | 🏶🏶🏶 2,3,19,23-tetra-OH-urs-12en-28oic-acid-glucose | triterpene | 20.17 | 666 | 667 | 667,503,162 | [19] |
| 23 | 🏶 Muricin D | acetogenin | 22.44 | 568 | 569 | 569,533 | [4] |
| 24 | 🏶 Muricin E | acetogenin | 23.02 | 568 | 569 | 569,533 | [4] |
| 25 | 🏶 Solamin | acetogenin | 25.77 | 564 | 563 | 563 | [4] |
| 26 | 🏶 Panatellin | acetogenin | 28.58 | 564 | 563 | 563 | [4] |
| Parameters | GII (EAC) | GIII (EAC + Cisplatin) | GIV (EAC + Capsule) |
|---|---|---|---|
| BAX | 1.00 ± 0.43 | 6.08 ± 1.33 b | 1.95 ± 0.16 |
| Bcl-2 | 1.00 ± 0.17 | 0.41 ± 0.11 b | 0.48 ± 0.14 b |
| Casp-3 | 1.00 ± 0.32 | 5.91 ± 1.00 b | 1.55 ± 0.52 |
| Parameters | P53 | CK | |
|---|---|---|---|
| Groups | |||
| Control −ve (GI) | 1.68 ± 0.27 | 1.93 ± 0.36 | |
| Control +ve(GII) | 4.50 ± 0.49 * | 41.69 ± 4.95 * | |
| Cisplatin | 19.35 ± 0.44 $b | 17.66 ± 0.59 $a | |
| Capsule | 3.30 ± 0.12 *# | 2.65 ± 0.16 a# | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, R.H.; Al-Attar, A.-s.R.; Shehata, Y.M.; Abdel-Fattah, D.M.; Atta, R.M.; Fantoukh, O.I.; Mustafa, A.M. Comprehensive Chemical Profiling and Mechanistic Insight into Anticancer Activity of Annona muricata Leaves Extract. Pharmaceuticals 2024, 17, 614. https://doi.org/10.3390/ph17050614
Abdallah RH, Al-Attar A-sR, Shehata YM, Abdel-Fattah DM, Atta RM, Fantoukh OI, Mustafa AM. Comprehensive Chemical Profiling and Mechanistic Insight into Anticancer Activity of Annona muricata Leaves Extract. Pharmaceuticals. 2024; 17(5):614. https://doi.org/10.3390/ph17050614
Chicago/Turabian StyleAbdallah, Rehab H., Al-sayed R. Al-Attar, Youssef M. Shehata, Doaa M. Abdel-Fattah, Rahnaa M. Atta, Omer I. Fantoukh, and Ahmed M. Mustafa. 2024. "Comprehensive Chemical Profiling and Mechanistic Insight into Anticancer Activity of Annona muricata Leaves Extract" Pharmaceuticals 17, no. 5: 614. https://doi.org/10.3390/ph17050614
APA StyleAbdallah, R. H., Al-Attar, A.-s. R., Shehata, Y. M., Abdel-Fattah, D. M., Atta, R. M., Fantoukh, O. I., & Mustafa, A. M. (2024). Comprehensive Chemical Profiling and Mechanistic Insight into Anticancer Activity of Annona muricata Leaves Extract. Pharmaceuticals, 17(5), 614. https://doi.org/10.3390/ph17050614









