The Stable Gastric Pentadecapeptide BPC 157 Pleiotropic Beneficial Activity and Its Possible Relations with Neurotransmitter Activity
Abstract
1. Introduction
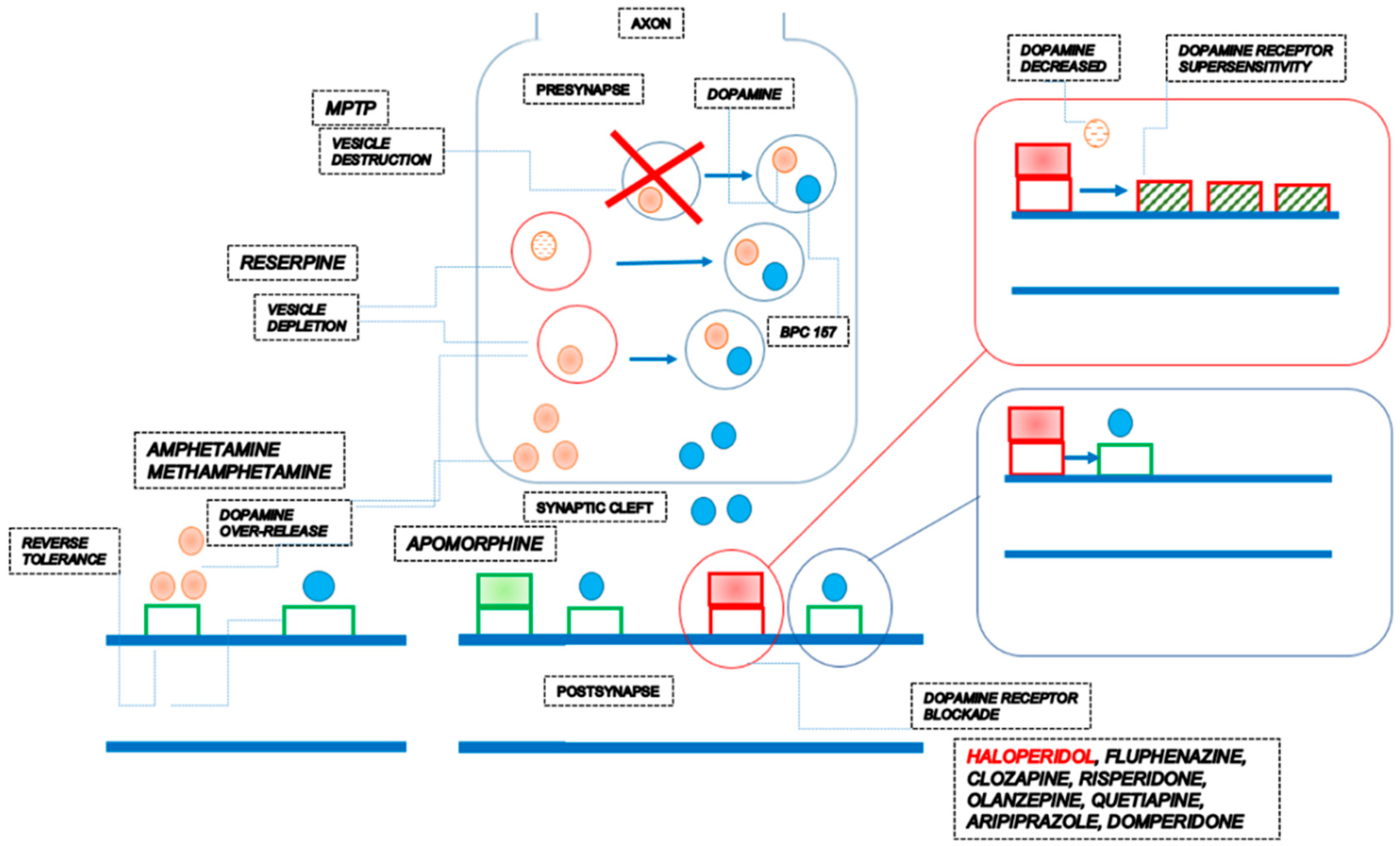
1.1. Dopamine
| Effect | Specification | Ref. |
|---|---|---|
| BPC 157 therapy can counteract the consequences of dopamine neurons destruction in the substantia nigra (neurotoxin MPTP) | BPC 157 counteracted tremor, rigor, akinesia, and MPTP mortality. BPC 157 counteracted MPTP-induced gastric lesions. | [69] |
| BPC 157 therapy can counteract the consequences of dopamine vesicle depletion (reserpine) | BPC 157 counteracted tremors, rigor, akinesia, and hypothermia. BPC 157 counteracted reserpine-induced gastric lesions. | [69] |
| BPC 157 therapy can counteract the consequences of dopamine receptor blockade (neuroleptics) | BPC 157 therapy counteracted catalepsy and somatosensory disorientation induced by dopamine antagonists [68,70]. BPC 157 therapy counteracted prolonged QTc intervals [89], gastric lesions, and lower esophageal sphincter and pyloric sphincter dysfunction induced by dopamine receptor antagonists [68,92,165], as well as gastric lesions induced by combined application of dopamine receptor antagonist and reserpine [166]. A common effect is a counteraction of occlusion/occlusion-like syndromes, peripherally and centrally, induced by haloperidol, fluphenazine, clozapine, risperidone, olanzapine, quetiapine, aripiprazole and domperidone [54]. BPC 157 therapy (activation of the collateral pathways, i.e., azygos vein direct flow delivery) instantly occurred. | [68,70] |
| BPC 157 can prevent and reverse amphetamine disturbances (and methamphetamine), acutely and chronically, schizophrenia positive symptoms-like models. | BPC 157 can prevent amphetamine disturbances, reverse already advanced disturbances, and counteract amphetamine “reverse tolerance” even after a very long period (i.e., 46 days) [70,153]. A common effect is a counteraction of occlusion/occlusion-like syndromes, peripherally and centrally, induced by amphetamine [54]. BPC 157 therapy (activation of the collateral pathways, i.e., azygos vein direct flow delivery) instantly occurred. | [70,153] |
| BPC 157 can reverse apomorphine motor disturbances, schizophrenia-positive symptoms-like models | BPC 157 can reverse continuous oral stereotypy (licking, gnawing) in apomorphine rats. | [70] |
| The counteraction of the dopamine receptor supersensitivity (and thereby counteraction of amphetamine over-activity in haloperidol pretreated mice, simultaneous counteraction of both haloperidol and amphetamine effects. | There is a prominent effect of BPC 157 on increased amphetamine-climbing behavior in mice pretreated with dopamine antagonists haloperidol (5 mg/kg ip) and subsequently treated with amphetamine (20 mg/kg ip challenge at 1, 2, 4, and 10 days after haloperidol pretreatment. An almost complete reversal occurred when BPC 157 was coadministered with haloperidol. | [152] |
| In conclusion, there is a restorative dimension of the BPC 157 therapy, given that it can react with the dopamine system depending on the condition [68,69,70,153]. | The restorative dimension of the BPC 157 therapy, and thereby BPC 157 activity over the dopamine system, as a likely neurotransmitter of its own, can be based on the following consistent evidence providing a wide range of influence on various, even opposite, activities [1]. BPC 157 therapy can counteract the consequences of dopamine neurons destruction in the substantia nigra (neurotoxin MPTP) [69], dopamine vesicle depletion (reserpine) [69], dopamine receptors blockade (neuroleptics) [68,70], dopamine over-release and re-uptake inhibition (amphetamine; methamphetamine) [70,152,153], dopamine receptor agonization (apomorphine) [70], dopamine receptor supersensitivity (haloperidol) [152] and reverse tolerance (amphetamine) [153]. | |
1.2. Serotonin
1.3. Glutamate
1.4. GABA
1.5. Acetylcholine
1.6. Adrenaline/Noradrenaline
1.7. NO
2. Nerve-Muscle
3. Nerve-Nerve
4. Channels
5. Receptors
6. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sikiric, P.; Gojkovic, S.; Krezic, I.; Smoday, I.M.; Kalogjera, L.; Zizek, H.; Oroz, K.; Vranes, H.; Vukovic, V.; Labidi, M.; et al. Stable gastric pentadecapeptide BPC 157 may recover brain-gut axis and gut-brain axis function. Pharmaceuticals 2023, 16, 676. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Kokot, A.; Kralj, T.; Zlatar, M.; Masnec, S.; Lazic, R.; Loncaric, K.; Oroz, K.; Sablic, M.; Boljesic, M.; et al. Stable gastric pentadecapeptide BPC 157-Possible novel therapy of glaucoma and other ocular conditions. Pharmaceuticals 2023, 16, 1052. [Google Scholar] [CrossRef] [PubMed]
- Staresinic, M.; Japjec, M.; Vranes, H.; Prtoric, A.; Zizek, H.; Krezic, I.; Gojkovic, S.; Smoday, I.M.; Oroz, K.; Staresinic, E.; et al. Stable gastric pentadecapeptide BPC 157 and striated, smooth, and heart muscle. Biomedicines 2022, 10, 3221. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Udovicic, M.; Barisic, I.; Balenovic, D.; Zivanovic Posilovic, G.; Strinic, D.; Uzun, S.; Sikiric, S.; Krezic, I.; Zizek, H.; et al. Stable gastric pentadecapeptide BPC 157 as useful cytoprotective peptide therapy in the heart disturbances, myocardial infarction, heart failure, pulmonary hypertension, arrhythmias, and thrombosis presentation. Biomedicines 2022, 10, 2696. [Google Scholar] [CrossRef]
- Vukojevic, J.; Milavić, M.; Perović, D.; Ilić, S.; Čilić, A.Z.; Đuran, N.; Štrbe, S.; Zoričić, Z.; Filipčić, I.; Brečić, P.; et al. Pentadecapeptide BPC 157 and the central nervous system. Neural. Regen. Res. 2022, 17, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, S.; Milavic, M.; Vukojevic, J.; Gojkovic, S.; Krezic, I.; Vuletic, L.B.; Pavlov, K.H.; Petrovic, A.; Sikiric, S.; Vranes, H.; et al. Stable gastric pentadecapeptide BPC 157 and wound healing. Front. Pharmacol. 2021, 12, 627533. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Hahm, K.B.; Blagaic, A.B.; Tvrdeic, A.; Pavlov, K.H.; Petrovic, A.; Kokot, A.; Gojkovic, S.; Krezic, I.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157, Robert’s stomach cytoprotection/adaptive cytoprotection/organoprotection, and Selye’s stress coping response: Progress, achievements, and the future. Gut Liver 2020, 14, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 rescued NSAID-cytotoxicity via stabilizing intestinal permeability and enhancing cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef]
- Sikiric, P.; Drmic, D.; Sever, M.; Klicek, R.; Blagaic, A.B.; Tvrdeic, A.; Kralj, T.; Kovac, K.K.; Vukojevic, J.; Siroglavic, M.; et al. Fistulas healing, stable gastric pentadecapeptide BPC 157 therapy. Curr. Pharm. Des. 2020, 26, 2991–3000. [Google Scholar] [CrossRef]
- Seiwerth, S.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. BPC 157 and standard angiogenic growth factors. Gastrointesinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr. Pharm. Des. 2018, 24, 1972–1989. [Google Scholar] [CrossRef]
- Kang, E.A.; Han, Y.M.; An, J.M.; Park, Y.J.; Sikiric, P.; Kim, D.H.; Kwon, K.A.; Kim, Y.J.; Yang, D.; Tchah, H.; et al. BPC157 as potential agent rescuing from cancer cachexia. Curr. Pharm. Des. 2018, 24, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Smelser, N.J.; Baltes, P.B. International Encyclopedia of the Social & Behavioral Sciences, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Cuevas, J. Neurotransmitters and Their Life Cycle. Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.S.; McNamara, J.O.; Williams, S.M. Peptide Neurotransmitters. Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Sikirić, P.; Petek, M.; Rucman, R.; Seiwerth, S.; Grabarević, Z.; Rotkvić, I.; Turković, B.; Jagić, V.; Mildner, B.; Duvnjak, M. A new gastric juice peptide, BPC. An overview of the stomach-stress-organoprotection hypothesis and beneficial effects of BPC. J. Physiol. 1993, 87, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Robert, A. Cytoprotection by prostaglandins. Gastroenterology 1979, 77 Pt 1, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Nezamis, J.E.; Lancaster, C.; Hanchar, A.J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology 1979, 77, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.; Trier, J.S.; Brown, A.; Schnoor, J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology 1985, 88 Pt 2, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Trier, J.S.; Szabo, S.; Allan, C.H. Ethanol-induced damage to mucosal capillaries of rat stomach. Ultrastructural features and effects of prostaglandin F2 beta and cysteamine. Gastroenterology 1987, 92, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Pihan, G.; Majzoubi, D.; Haudenschild, C.; Trier, J.S.; Szabo, S. Early microcirculatory stasis in acute gastric mucosal injury in the rat and prevention by 16,16-dimethyl prostaglandin E2 or sodium thiosulfate. Gastroenterology 1986, 91, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.; Pihan, G.; Trier, J.S. Alterations in blood vessels during gastric injury and protection. Scand. J. Gastroenterol. 1986, 125, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S. “Gastric cytoprotection” is still relevant. J. Gastroenterol. Hepatol. 2014, 29 (Suppl. 4), 124–132. [Google Scholar] [CrossRef]
- Szabo, S.; Tache, Y.; Tarnawski, A. The ‘gastric cytoprotection’ concept of Andre Robert and the origins of a new series of international symposia. In Cell/Tissue Injury and Cytoprotection/Organoprotection in the Gastrointestinal Tract: Mechanisms, Prevention and Treatment; Filaretova, L.P., Takeuchi, K., Eds.; Karger: Basel, Switzerland, 2012; Volume 30, pp. 1–23. [Google Scholar]
- Tarnawski, A.; Hollander, D.; Cergely, H. Cytoprotective drugs. Focus on essential fatty acids and sucralfate. Scand. J. Gastroenterol. 1987, 127, 39–43. [Google Scholar] [CrossRef]
- Szabó, S. Critical and timely review of the concept of gastric cytoprotection. Acta Physiol. Hung. 1989, 73, 115–127. [Google Scholar] [PubMed]
- Sikiric, P.; Gojkovic, S.; Knezevic, M.; Tepes, M.; Strbe, S.; Vukojevic, J.; Duzel, A.; Kralj, T.; Krezic, I.; Zizek, H.; et al. Stable gastric pentadecapeptide BPC 157: Prompt particular activation of collateral pathways. Curr. Med. Chem. 2023, 30, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Skrtic, A.; Gojkovic, S.; Krezic, I.; Zizek, H.; Lovric, E.; Sikiric, S.; Knezevic, M.; Strbe, S.; Milavic, M.; et al. Cytoprotective gastric pentadecapeptide BPC 157 resolves major vessel occlusion disturbances, ischemia-reperfusion injury following Pringle maneuver, and Budd-Chiari syndrome. World J. Gastroenterol. 2022, 28, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157, vascular recruitment and gastrointestinal tract healing. Curr. Pharm. Des. 2018, 24, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157: Novel therapy in gastrointestinal tract. Curr. Pharm. Des. 2011, 17, 1612–1632. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Ilic, S.; Kolenc, D. Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr. Pharm. Des. 2010, 16, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. A syndrome produced by diverse nocuous agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- Masson, G.; Selye, H. Réaction générale d’adaptation: Ses indications pratiques. Can. J. Comp. Med. 1938, 2, 282–285. [Google Scholar]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Drmic, D.; Stupnisek, M.; Kokot, A.; Sever, M.; Zoricic, I.; Zoricic, Z.; Batelja, L.; et al. Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157, Finally, do we have a solution? Curr. Pharm. Des. 2017, 23, 4012–4028. [Google Scholar] [CrossRef]
- Corbière, A.; Vaudry, H.; Chan, P.; Walet-Balieu, M.L.; Lecroq, T.; Lefebvre, A.; Pineau, C.; Vaudry, D. Strategies for the identification of bioactive neuropeptides in vertebrates. Front. Neurosci. 2019, 13, 948. [Google Scholar] [CrossRef]
- Fricker, L.D.; Devi, L.A. Orphan neuropeptides and receptors: Novel therapeutic targets. Pharmacol. Ther. 2018, 185, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Gadalla, M.M.; Snyder, S.H. Signaling by gasotransmitters. Sci. Signal 2009, 2, re2. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Grgic, T.; Strbe, S.; Zukanovic, G.; Crvenkovic, D.; et al. Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Curr. Neuropharmacol. 2016, 14, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Vukojević, J.; Vrdoljak, B.; Malekinušić, D.; Siroglavić, M.; Milavić, M.; Kolenc, D.; Boban Blagaić, A.; Batelja, L.; Drmić, D.; Seiwerth, S.; et al. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020, 10, e01726. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Horvat Pavlov, K.; Petrovic, A.; Batelja Vuletic, L.; Milavic, M.; Sikiric, S.; et al. BPC 157 therapy and permanent occlusion of the superior sagittal sinus in rat: Vascular recruitment. Biomedicines 2021, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Vranes, H.; Knezevic, T.; Barisic, I.; Horvat Pavlov, K.; et al. Occlusion of the superior mesenteric artery in rats reversed by collateral pathways activation: Gastric pentadecapeptide BPC 157 therapy counteracts multiple organ dysfunction syndrome; intracranial, portal, and caval hypertension; and aortal hypotension. Biomedicines 2021, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Vranes, H.; Drmic, D.; Staroveski, M.; et al. Occluded superior mesenteric artery and vein. Therapy with the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Horvat Pavlov, K.; Drmic, D.; et al. Complex syndrome of the complete occlusion of the end of the superior mesenteric vein, opposed with the stable gastric pentadecapeptide BPC 157 in rats. Biomedicines 2021, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Vukojević, J.; Siroglavić, M.; Kašnik, K.; Kralj, T.; Stanćić, D.; Kokot, A.; Kolarić, D.; Drmić, D.; Sever, A.Z.; Barišić, I.; et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul. Pharmacol. 2018, 106, 54–66. [Google Scholar] [CrossRef]
- Kolovrat, M.; Gojkovic, S.; Krezic, I.; Malekinusic, D.; Vrdoljak, B.; Kasnik Kovac, K.; Kralj, T.; Drmic, D.; Barisic, I.; Horvat Pavlov, K.; et al. Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion. World J. Hepatol. 2020, 12, 184–206. [Google Scholar] [CrossRef]
- Kralj, T.; Kokot, A.; Zlatar, M.; Masnec, S.; Kasnik Kovac, K.; Milkovic Perisa, M.; Batelja Vuletic, L.; Giljanovic, A.; Strbe, S.; Sikiric, S.; et al. Stable gastric pentadecapeptide BPC 157 therapy of rat glaucoma. Biomedicines 2021, 10, 89. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vrdoljak, B.; Malekinusic, D.; Barisic, I.; Petrovic, A.; Horvat Pavlov, K.; Kolovrat, M.; Duzel, A.; Knezevic, M.; et al. Pentadecapeptide BPC 157 resolves suprahepatic occlusion of the inferior caval vein, Budd-Chiari syndrome model in rats. World J. Gastrointest. Pathophysiol. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tepes, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Madzar, Z.; Santak, G.; Batelja, L.; Milavic, M.; Sikiric, S.; et al. Stable gastric pentadecapeptide BPC 157 therapy for primary abdominal compartment syndrome in rats. Front. Pharmacol. 2021, 12, 718147. [Google Scholar] [CrossRef]
- Tepes, M.; Krezic, I.; Vranes, H.; Smoday, I.M.; Kalogjera, L.; Zizek, H.; Vukovic, V.; Oroz, K.; Kovac, K.K.; Madzar, Z.; et al. Stable gastric pentadecapeptide BPC 157 therapy: Effect of reperfusion following maintained intra-abdominal hypertension (grade III and IV) in rats. Pharmaceuticals 2023, 16, 1554. [Google Scholar] [CrossRef] [PubMed]
- Kalogjera, L.; Krezic, I.; Smoday, I.M.; Vranes, H.; Zizek, H.; Yago, H.; Oroz, K.; Vukovic, V.; Kavelj, I.; Novosel, L.; et al. Stomach perforation-induced general occlusion/occlusion-like syndrome and stable gastric pentadecapeptide BPC 157 therapy effect. World. J. Gastroenterol. 2023, 29, 4289–4316. [Google Scholar] [CrossRef]
- Smoday, I.M.; Petrovic, I.; Kalogjera, L.; Vranes, H.; Zizek, H.; Krezic, I.; Gojkovic, S.; Skorak, I.; Hriberski, K.; Brizic, I.; et al. Therapy effect of the stable gastric pentadecapeptide BPC 157 on acute pancreatitis as vascular failure-induced severe peripheral and central syndrome in rats. Biomedicines 2022, 10, 1299. [Google Scholar] [CrossRef]
- Smoday, I.M.; Krezic, I.; Kalogjera, L.; Vukovic, V.; Zizek, H.; Skoro, M.; Kovac, K.K.; Vranes, H.; Barisic, I.; Sikiric, S.; et al. Pentadecapeptide BPC 157 as therapy for inferior caval vein embolization: Recovery of sodium laurate-post-embolization syndrome in rats. Pharmaceuticals 2023, 16, 1507. [Google Scholar] [CrossRef]
- Premuzic Mestrovic, I.; Smoday, I.M.; Kalogjera, L.; Krezic, I.; Zizek, H.; Vranes, H.; Vukovic, V.; Oroz, K.; Skorak, I.; Brizic, I.; et al. Antiarrhythmic sotalol, occlusion/occlusion-like syndrome in rats, and stable gastric pentadecapeptide BPC 157 therapy. Pharmaceuticals 2023, 16, 977. [Google Scholar] [CrossRef] [PubMed]
- Barisic, I.; Balenovic, D.; Udovicic, M.; Bardak, D.; Strinic, D.; Vlainić, J.; Vranes, H.; Smoday, I.M.; Krezic, I.; Milavic, M.; et al. Stable gastric pentadecapeptide BPC 157 may counteract myocardial infarction induced by isoprenaline in rats. Biomedicines 2022, 10, 265. [Google Scholar] [CrossRef]
- Strbe, S.; Smoday, I.M.; Krezic, I.; Kalogjera, L.; Vukovic, V.; Zizek, H.; Gojkovic, S.; Vranes, H.; Barisic, I.; Sikiric, S.; et al. Innate vascular failure by application of neuroleptics, amphetamine, and domperedone rapidly induced severe occlusion/occlusion-like syndrome in rats and stable gastric pentadecapeptide BPC 157 as therapy. Pharmaceuticals 2023, 16, 788. [Google Scholar] [CrossRef]
- Strbe, S.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Barisic, I.; Strinic, D.; Orct, T.; Vukojevic, J.; Ilic, S.; et al. Over-dose lithium toxicity as an occlusive-like syndrome in rats and gastrci pentadecapeptide BPC 157. Biomedicines 2021, 9, 1506. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Batelja Vuletic, L.; Milavic, M.; Sikiric, S.; Stilinovic, I.; Simeon, P.; et al. Robert’s intragastric alcohol-induced gastric lesion model as an escalated general peripheral and central syndrome, counteracted by the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1300. [Google Scholar] [CrossRef]
- Sikiric, P.; Jelovac, N.; Jelovac-Gjeldum, A.; Dodig, G.; Staresinic, M.; Anic, T.; Zoricic, I.; Ferovic, D.; Buljat, G.; Prkacin, I.; et al. Anxiolytic effect of BPC-157, a gastric pentadecapeptide: Shock probe/burying test and light/dark test. Acta Pharmacol. Sin. 2001, 22, 225–230. [Google Scholar] [PubMed]
- Jelovac, N.; Sikiric, P.; Rucman, R.; Petek, M.; Perovic, D.; Marovic, A.; Anic, T.; Seiwerth, S.; Mise, S.; Pigac, B.; et al. The effect of a novel pentadecapeptide BPC 157 on development of tolerance and physical dependence following repeated administration of diazepam. Chin. J. Physiol. 1999, 42, 171–179. [Google Scholar] [PubMed]
- Zemba Cilic, A.; Zemba, M.; Cilic, M.; Strbe, S.; Ilic, S.; Vukojevic, J.; Zoricic, Z.; Filipcic, I.; Kokot, A.; Smoday, I.M.; et al. BPC 157, L-NAME, L-arginine, NO-relation, in the suited rat ketamine models resembling “negative-like” sympotms of schizophrenia. Biomedicines 2022, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Lozic, M.; Stambolija, V.; Krezic, I.; Dugandzic, A.; Zivanovic-Posilovic, G.; Gojkovic, S.; Kovacevic, J.; Vrdoljak, L.; Mirkovic, I.; Kokot, A.; et al. In relation to NO-system, stable pentadecapeptide BPC 157 counteracts lidocaine-induced adverse effects in rats and depolarisation in vitro. Emerg. Med. Int. 2020, 2020, 6805354. [Google Scholar] [CrossRef] [PubMed]
- Ilic, S.; Drmic, D.; Zarkovic, K.; Kolenc, D.; Coric, M.; Brcic, L.; Klicek, R.; Radic, B.; Sever, M.; Djuzel, V.; et al. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736). J. Physiol. Pharmacol. 2010, 61, 241–250. [Google Scholar]
- Ilic, S.; Brcic, I.; Mester, M.; Filipovic, M.; Sever, M.; Klicek, R.; Barisic, I.; Radic, B.; Zoricic, Z.; Bilic, V.; et al. Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. J. Physiol. Pharmacol. 2009, 60 (Suppl. 7), 107–114. [Google Scholar] [PubMed]
- Boban-Blagaic, A.; Blagaic, V.; Romic, Z.; Jelovac, N.; Dodig, G.; Rucman, R.; Petek, M.; Turkovic, B.; Seiwerth, S.; Sikiric, P. The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice. The effect of N(G)-nitro-L-arginine methyl ester and L-arginine. Med. Sci. 2006, 12, BR36–BR45. [Google Scholar] [PubMed]
- Blagaic, A.B.; Blagaic, V.; Romic, Z.; Sikiric, P. The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice. Eur. J. Pharmacol. 2004, 499, 285–290. [Google Scholar] [CrossRef]
- Sikiric, P.; Separovic, J.; Buljat, G.; Anic, T.; Stancic-Rokotov, D.; Mikus, D.; Marovic, A.; Prkacin, I.; Duplancic, B.; Zoricic, I.; et al. The antidepressant effect of an antiulcer pentadecapeptide BPC 157 in Porsolt’s test and chronic unpredictable stress in rats. A comparison with antidepressants. J. Physiol. Paris 2000, 94, 99–104. [Google Scholar] [CrossRef]
- Boban Blagaic, A.; Blagaic, V.; Mirt, M.; Jelovac, N.; Dodig, G.; Rucman, R.; Petek, M.; Turkovic, B.; Anic, T.; Dubovecak, M.; et al. Gastric pentadecapeptide BPC 157 effective against serotonin syndrome in rats. Eur. J. Pharmacol. 2005, 512, 173–179. [Google Scholar] [CrossRef]
- Tohyama, Y.; Sikirić, P.; Diksic, M. Effects of pentadecapeptide BPC157 on regional serotonin synthesis in the rat brain: α-Methyl-L-tryptophan autoradiographic measurements. Life. Sci. 2004, 76, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, N.; Sikiric, P.; Rucman, R.; Petek, M.; Marovic, A.; Perovic, D.; Seiwerth, S.; Mise, S.; Turkovic, B.; Dodig, G.; et al. Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: The effect on catalepsy and gastric ulcers in mice and rats. Eur. J. Pharmacol. 1999, 379, 19–31. [Google Scholar] [CrossRef]
- Sikiric, P.; Marovic, A.; Matoz, W.; Anic, T.; Buljat, G.; Mikus, D.; Stancic-Rokotov, D.; Separovic, J.; Seiwerth, S.; Grabarevic, Z.; et al. A behavioural study of the effect of pentadecapeptide BPC 157 in Parkinson’s disease models in mice and gastric lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydrophyridine. J. Physiol. Paris 1999, 93, 505–512. [Google Scholar] [CrossRef]
- Zemba Cilic, A.; Zemba, M.; Cilic, M.; Balenovic, I.; Strbe, S.; Ilic, S.; Vukojevic, J.; Zoricic, Z.; Filipcic, I.; Kokot, A.; et al. Pentadecapeptide BPC 157 counteracts L-NAME-induced catalepsy. BPC 157, L-NAME, L-arginine, NO-relation, in the suited rat acute and chronic models resembling ‘positive-like’ symptoms of schizophrenia. Behav. Brain. Res. 2021, 396, 112919. [Google Scholar] [CrossRef]
- Staresinic, M.; Petrovic, I.; Novinscak, T.; Jukic, I.; Pevec, D.; Suknaic, S.; Kokic, N.; Batelja, L.; Brcic, L.; Boban-Blagaic, A.; et al. Effective therapy of transected quadriceps muscle in rat: Gastric pentadecapeptide BPC 157. J. Orthop. Res. 2006, 24, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Novinscak, T.; Brcic, L.; Staresinic, M.; Jukic, I.; Radic, B.; Pevec, D.; Mise, S.; Tomasovic, S.; Brcic, I.; Banic, T.; et al. Gastric pentadecapeptide BPC 157 as an effective therapy for muscle crush injury in the rat. Surg. Today 2008, 38, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Pevec, D.; Novinscak, T.; Brcic, L.; Sipos, K.; Jukic, I.; Staresinic, M.; Mise, S.; Brcic, I.; Kolenc, D.; Klicek, R.; et al. Impact of pentadecapeptide BPC 157 on muscle healing impaired by systemic corticosteroid application. Med. Sci. 2010, 16, BR81–BR88. [Google Scholar]
- Mihovil, I.; Radic, B.; Brcic, L.; Brcic, I.; Vukoja, I.; Ilic, S.; Boban Blagaic, A.; Seiwerth, S.; Sikiric, P. Beneficial effect of pentadecapeptide BPC 157 on denervated muscle in rats. J. Physiol. Pharmacol. 2009, 60, 69. [Google Scholar]
- Japjec, M.; Horvat Pavlov, K.; Petrovic, A.; Staresinic, M.; Sebecic, B.; Buljan, M.; Vranes, H.; Giljanovic, A.; Drmic, D.; Japjec, M.; et al. Stable gastric pentadecapeptide BPC 157 as a therapy for the disable myotendinous junction in rats. Biomedicines 2021, 9, 1547. [Google Scholar] [CrossRef]
- Perovic, D.; Kolenc, D.; Bilic, V.; Somun, N.; Drmic, D.; Elabjer, E.; Buljat, G.; Seiwerth, S.; Sikiric, P. Stable gastric pentadecapeptide BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats. J. Orthop. Surg. Res. 2019, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Perovic, D.; Milavic, M.; Dokuzovic, S.; Krezic, I.; Gojkovic, S.; Vranes, H.; Bebek, I.; Bilic, V.; Somun, N.; Brizic, I.; et al. Novel therapeutic effects in rat spinal cord injuries: Recovery of the definitive and early spinal cord injury by the administration of pentadecapeptide BPC 157 therapy. Curr. Issues. Mol. Biol. 2022, 44, 1901–1927. [Google Scholar] [CrossRef] [PubMed]
- Tudor, M.; Jandric, I.; Marovic, A.; Gjurasin, M.; Perovic, D.; Radic, B.; Blagaic, A.B.; Kolenc, D.; Brcic, L.; Zarkovic, K.; et al. Traumatic brain injury in mice and pentadecapeptide BPC 157 effect. Regul. Pept. 2010, 160, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Gjurasin, M.; Miklic, P.; Zupancic, B.; Perovic, D.; Zarkovic, K.; Brcic, L.; Kolenc, D.; Radic, B.; Seiwerth, S.; Sikiric, P. Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Regul. Pept. 2010, 160, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Klicek, R.; Kolenc, D.; Suran, J.; Drmic, D.; Brcic, L.; Aralica, G.; Sever, M.; Holjevac, J.; Radic, B.; Turudic, T.; et al. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J. Physiol. Pharmacol. 2013, 64, 597–612. [Google Scholar] [PubMed]
- Stambolija, V.; Stambolija, T.P.; Holjevac, J.K.; Murselovic, T.; Radonic, J.; Duzel, V.; Duplancic, B.; Uzun, S.; Zivanovic-Posilovic, G.; Kolenc, D.; et al. BPC 157: The counteraction of succinylcholine, hyperkalemia, and arrhythmias. Eur. J. Pharmacol. 2016, 781, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Hrelec, M.; Klicek, R.; Brcic, L.; Brcic, I.; Cvjetko, I.; Seiwerth, S.; Sikiric, P. Abdominal aorta anastomosis in rats and stable gastric pentadecapeptide BPC 157, prophylaxis and therapy. J. Physiol. Pharmacol. 2009, 60 (Suppl. 7), 161–165. [Google Scholar] [PubMed]
- Barisic, I.; Balenovic, D.; Klicek, R.; Radic, B.; Nikitovic, B.; Drmic, D.; Udovicic, M.; Strinic, D.; Bardak, D.; Berkopic, L.; et al. Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul. Pept. 2013, 181, 50–66. [Google Scholar] [CrossRef]
- Balenovic, D.; Barisic, I.; Prkacin, I.; Horvat, I.; Udovicic, M.; Uzun, S.; Strinic, D.; Pevec, D.; Drmic, D.; Radic, B.; et al. Mortal furosemide-hypokalemia-disturbances in rats NO-system related. Shorten survival by L-NAME. Therapy benefit with BPC 157 peptide more than with L-arginine. J. Clin. Exp. Cardiolog. 2012, 3, 201. [Google Scholar] [CrossRef]
- Medvidovic-Grubisic, M.; Stambolija, V.; Kolenc, D.; Katancic, J.; Murselovic, T.; Plestina-Borjan, I.; Strbe, S.; Drmic, D.; Barisic, I.; Sindic, A.; et al. Hypermagnesemia disturbances in rats, NO-related: Pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen. Inflammopharmacology 2017, 25, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Kokot, A.; Zlatar, M.; Stupnisek, M.; Drmic, D.; Radic, R.; Vcev, A.; Seiwerth, S.; Sikiric, P. NO system dependence of atropine-induced mydriasis and L-NAME and L-arginine-induced miosis: Reversal by the pentadecapeptide BPC 157 in rats and guinea pigs. Eur. J. Pharmacol. 2016, 771, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Lovric-Bencic, M.; Sikiric, P.; Hanzevacki, J.S.; Seiwerth, S.; Rogic, D.; Kusec, V.; Aralica, G.; Konjevoda, P.; Batelja, L.; Blagaic, A.B. Doxorubicine-congestive heart failure-increased big endothelin-1 plasma concentration: Reversal by amlodipine, losartan, and gastric pentadecapeptide BPC157 in rat and mouse. J. Pharmacol. Sci. 2004, 95, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Udovicic, M.; Sever, M.; Kavur, L.; Loncaric, K.; Barisic, I.; Balenovic, D.; Zivanovic Posilovic, G.; Strinic, D.; Uzun, S.; Batelja Vuletic, L.; et al. Stable gastric pentadecapeptide BPC 157 therapy for monocrotaline-induced pulmonary hypertension in rats leads to prevention and reversal. Biomedicines 2021, 9, 822. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic-Posilovic, G.; Balenovic, D.; Barisic, I.; Strinic, D.; Stambolija, V.; Udovicic, M.; Uzun, S.; Drmic, D.; Vlainic, J.; Bencic, M.L.; et al. Stable gastric pentadecapeptide BPC 157 and bupivacaine. Eur. J. Pharmacol. 2016, 793, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Strinic, D.; Belosic Halle, Z.; Luetic, K.; Nedic, A.; Petrovic, I.; Sucic, M.; Zivanovic Posilovic, G.; Balenovic, D.; Strbe, S.; Udovicic, M.; et al. BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life. Sci. 2017, 186, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Balenovic, D.; Bencic, M.L.; Udovicic, M.; Simonji, K.; Hanzevacki, J.S.; Barisic, I.; Kranjcevic, S.; Prkacin, I.; Coric, V.; Brcic, L.; et al. Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: A relation with NO-system. Regul. Pept. 2009, 156, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Belosic Halle, Z.; Vlainic, J.; Drmic, D.; Strinic, D.; Luetic, K.; Sucic, M.; Medvidovic-Grubisic, M.; Pavelic Turudic, T.; Petrovic, I.; Seiwerth, S.; et al. Class side effects: Decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology 2017, 25, 511–522. [Google Scholar] [CrossRef]
- Vitaic, S.; Stupnisek, M.; Drmic, D.; Bauk, L.; Kokot, A.; Klicek, R.; Vcev, A.; Luetic, K.; Seiwerth, S.; Sikiric, P. Nonsteroidal anti-inflammatory drugs-induced failure of lower esophageal and pyloric sphincter and counteraction of sphincters failure with stable gatric pentadecapeptide BPC 157 in rats. J. Physiol. Pharmacol. 2017, 68, 265–272. [Google Scholar]
- Djakovic, Z.; Djakovic, I.; Cesarec, V.; Madzarac, G.; Becejac, T.; Zukanovic, G.; Drmic, D.; Batelja, L.; Zenko Sever, A.; Kolenc, D.; et al. Esophagogastric anastomosis in rats: Improved healing by BPC 157 and L-arginine, aggravated by L-NAME. World J. Gastroenterol. 2016, 22, 9127–9140. [Google Scholar] [CrossRef]
- Skorjanec, S.; Kokot, A.; Drmic, D.; Radic, B.; Sever, M.; Klicek, R.; Kolenc, D.; Zenko, A.; Lovric Bencic, M.; Belosic Halle, Z.; et al. Duodenocutaneous fistula in rats as a model for “wound healing-therapy” in ulcer healing: The effect of pentadecapeptide BPC 157, L-nitro-arginine methyl ester and L-arginine. J. Physiol. Pharmacol. 2015, 66, 581–590. [Google Scholar]
- Cesarec, V.; Becejac, T.; Misic, M.; Djakovic, Z.; Olujic, D.; Drmic, D.; Brcic, L.; Rokotov, D.S.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur. J. Pharmacol. 2013, 701, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, I.; Dobric, I.; Drmic, D.; Sever, M.; Klicek, R.; Radic, B.; Brcic, L.; Kolenc, D.; Zlatar, M.; Kunjko, K.; et al. BPC 157 therapy to detriment sphincters failure-esophagitis-pancreatitis in rat and acute pancreatitis patients low sphincters pressure. J. Physiol. Pharmacol. 2011, 62, 527–534. [Google Scholar] [PubMed]
- Dobric, I.; Drvis, P.; Petrovic, I.; Shejbal, D.; Brcic, L.; Blagaic, A.B.; Batelja, L.; Sever, M.; Kokic, N.; Tonkic, A.; et al. Prolonged esophagitis after primary dysfunction of the pyloric sphincter in the rat and therapeutic potential of the gastric pentadecapeptide BPC 157. J. Pharmacol. Sci. 2007, 104, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, I.; Dobric, I.; Drvis, P.; Shejbal, D.; Brcic, L.; Blagaic, A.B.; Batelja, L.; Kokic, N.; Tonkic, A.; Mise, S.; et al. An experimental model of prolonged esophagitis with sphincter failure in the rat and the therapeutic potential of gastric pentadecapeptide BPC 157. J. Pharmacol. Sci. 2006, 102, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Sucic, M.; Luetic, K.; Jandric, I.; Drmic, D.; Sever, A.Z.; Vuletic, L.B.; Halle, Z.B.; Strinic, D.; Kokot, A.; Seiwerth, R.S.; et al. Therapy of the rat hemorrhagic cystitis induced by cyclophosphamide. Stable gastric pentadecapeptide BPC 157, L-arginine, L-NAME. Eur. J. Pharmacol. 2019, 861, 172593. [Google Scholar] [CrossRef] [PubMed]
- Jandric, I.; Vrcic, H.; Jandric Balen, M.; Kolenc, D.; Brcic, L.; Radic, B.; Drmic, D.; Seiwerth, S.; Sikiric, P. Salutary effect of gastric pentadecapeptide BPC 157 in two different stress urinary incontinence models in female rats. Med. Sci. Monit. Basic. Res. 2013, 19, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Rasic, D.; Zenko Sever, A.; Rasic, F.; Strbe, S.; Rasic, Z.; Djuzel, A.; Duplancic, B.; Boban Blagaic, A.; Skrtic, A.; Seiwerth, S.; et al. Stable gastric pentadecapeptide BPC 157 heals established vesicovaginal fistula and counteracts stone formation in rats. Biomedicines 2021, 9, 1206. [Google Scholar] [CrossRef]
- Vuksic, T.; Zoricic, I.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Cesarec, V.; Berkopic, L.; Keller, N.; Blagaic, A.B.; et al. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL14736, Pliva, Croatia) heals ileoileal anastomosis in the rat. Surg. Today 2007, 37, 768–777. [Google Scholar] [CrossRef]
- Sever, M.; Klicek, R.; Radic, B.; Brcic, L.; Zoricic, I.; Drmic, D.; Ivica, M.; Barisic, I.; Ilic, S.; Berkopic, L.; et al. Gastric pentadecapeptide BPC 157 and short bowel syndrome in rats. Dig. Dis. Sci. 2009, 54, 2070–2083. [Google Scholar] [CrossRef]
- Drmic, D.; Kolenc, D.; Ilic, S.; Bauk, L.; Sever, M.; Zenko Sever, A.; Luetic, K.; Suran, J.; Seiwerth, S.; Sikiric, P. Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-NAME. World J. Gastroenterol. 2017, 23, 5304–5312. [Google Scholar] [CrossRef] [PubMed]
- Lojo, N.; Rasic, Z.; Zenko Sever, A.; Kolenc, D.; Vukusic, D.; Drmic, D.; Zoricic, I.; Sever, M.; Seiwerth, S.; Sikiric, P. Effects of diclofenac, L-NAME, L-arginine, and pentadecapeptide BPC 157 on gastrointestinal, liver, and brain lesions, failed anastomosis deterioration in 24 hour-short-bowel rats. PLoS ONE 2016, 11, e0162590. [Google Scholar] [CrossRef] [PubMed]
- Ilic, S.; Drmic, D.; Zarkovic, K.; Kolenc, D.; Brcic, L.; Radic, B.; Djuzel, V.; Blagaic, A.B.; Romic, Z.; Dzidic, S.; et al. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur. J. Pharmacol. 2011, 667, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Ilic, S.; Drmic, D.; Franjic, S.; Kolenc, D.; Coric, M.; Brcic, L.; Klicek, R.; Radic, B.; Sever, M.; Djuzel, V.; et al. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: Diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011, 88, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr. Pharm. Des. 2013, 19, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Duzel, A.; Vlainic, J.; Antunovic, M.; Malekinusic, D.; Vrdoljak, B.; Samara, M.; Gojkovic, S.; Krezic, I.; Vidovic, T.; Bilic, Z.; et al. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J. Gastroenterol. 2017, 23, 8465–8488. [Google Scholar] [CrossRef] [PubMed]
- Zlatar, M.; Kokot, A.; Vuletic, L.B.; Masnec, S.; Kralj, T.; Perisa, M.M.; Barisic, I.; Radic, B.; Milanovic, K.; Drmic, D.; et al. BPC 157, as a therapy for retinal ischemia induced by retrobulbar application of L-NAME in rats. Front. Pharmacol. 2021, 12, 632295. [Google Scholar] [CrossRef] [PubMed]
- Kralj, T.; Kokot, A.; Kasnik, K.; Drmic, D.; Zlatar, M.; Seiwerth, S.; Sikiric, P. Effects of pentadecapeptide BPC 157 on experimental rat model of dry eye. FASEB J. 2017, 31 (Suppl. 1), 993.3. [Google Scholar] [CrossRef]
- Masnec, S.; Kokot, A.; Zlatar, M.; Kalauz, M.; Kunjko, K.; Radic, B.; Klicek, R.; Drmic, D.; Lazic, R.; Brcic, L.; et al. Perforating corneal injury in rat and pentadecapeptide BPC 157. Exp. Eye. Res. 2015, 136, 9–15. [Google Scholar] [CrossRef]
- Lazić, R.; Gabrić, N.; Dekaris, I.; Bosnar, D.; Boban-Blagaić, A.; Sikirić, P. Gastric pentadecapeptide BPC 157 promotes corneal epithelial defects healing in rats. Antropol. 2005, 29, 321–325. [Google Scholar]
- Huang, B.S.; Huang, S.C.; Chen, F.H.; Chang, Y.; Mei, H.F.; Huang, H.Y.; Chen, W.Y.; Pang, J.S. Pentadecapeptide BPC 157 efficiently reduces radiation-induced liver injury and lipid accumulation through Kruppel-like factor 4 upregulation both in vivo and in vitro. Life Sci. 2022, 310, 121072. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Gu, J.; Zhang, W.; Wang, Y.; et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Dev. Ther. 2015, 9, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Tsai, W.C.; Hsu, Y.H.; Pang, J.H. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 2014, 19, 19066–19077. [Google Scholar] [CrossRef]
- Chang, C.H.; Tsai, W.C.; Lin, M.S.; Hsu, Y.H.; Pang, J.H. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 1985, 110, 774–780. [Google Scholar] [CrossRef]
- Wang, X.Y.; Qu, M.; Duan, R.; Shi, D.; Jin, L.; Gao, J.; Wood, J.D.; Li, J.; Wang, G.D. Cytoprotective mechanism of the novel gastric peptide BPC157 in gastrointestinal tract and cultured enteric neurons and glial cells. Neurosci. Bull. 2019, 35, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Tkalcević, V.I.; Cuzić, S.; Brajsa, K.; Mildner, B.; Bokulić, A.; Situm, K.; Perović, D.; Glojnarić, I.; Parnham, M.J. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Lee, C.H.; Chueh, H.Y.; Chang, G.J.; Huang, H.Y.; Lin, Y.; Pang, J.S. Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway. Sci. Rep. 2020, 10, 17078. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Liu, H.T.; Wang, C.N.; Huang, H.Y.; Lin, Y.; Ko, Y.S.; Wang, J.S.; Chang, V.H.; Pang, J.S. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J. Mol. Med. 2017, 95, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wei, M.; Li, N.; Lu, Q.; Shrestha, S.M.; Tan, J.; Zhang, Z.; Wu, G.; Shi, R. Clopidogrel-induced gastric injury in rats is attenuated by stable gastric pentadecapeptide BPC 157. Drug Des. Dev. Ther. 2020, 14, 5599–5619. [Google Scholar] [CrossRef]
- Sikiric, P.; Drmic, D.; Boban Blagaic, A.; Tvrdeic, A.; Krezic, I.; Gojkovic, S.; Zizek, H.; Sikiric, S.; Strbe, S.; Smoday, I.M.; et al. Stable gastric pentadecapeptide BPC 157 and No-system. In Nitric Oxide: From Research to Therapeutics, Advances in Biochemistry in Health and Disease 22; Ray, A., Gulati, K., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 349–375. [Google Scholar]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr. Pharm. Des. 2014, 20, 1126–1135. [Google Scholar] [CrossRef]
- Sikirić, P.; Seiwerth, S.; Grabarević, Z.; Rucman, R.; Petek, M.; Jagić, V.; Turković, B.; Rotkvić, I.; Mise, S.; Zoricić, I.; et al. The influence of a novel pentadecapeptide, BPC 157, on NG-nitro-l-arginine methylester and l-arginine effects on stomach mucosa integrity and blood pressure. Eur. J. Pharmacol. 1997, 332, 23–33. [Google Scholar] [CrossRef]
- Turkovic, B.; Sikiric, P.; Seiwerth, S.; Mise, S.; Anic, T.; Petek, M. Stable gastric pentadecapeptide BPC 157 studied for inflammatory bowel disease (PLD-116, PL14736, Pliva) induces nitric oxide synthesis. Gastroenterology 2004, 126, 287. [Google Scholar]
- Stupnisek, M.; Kokot, A.; Drmic, D.; Hrelec Patrlj, M.; Zenko Sever, A.; Kolenc, D.; Radic, B.; Suran, J.; Bojic, D.; Vcev, A.; et al. Pentadecapeptide BPC 157 reduces bleeding and thrombocytopenia after amputation in rats treated with heparin, warfarin, L-NAMe and L-arginine. PLoS ONE 2015, 10, e0123454. [Google Scholar] [CrossRef]
- Konosic, S.; Petricevic, M.; Ivancan, V.; Konosic, L.; Goluza, E.; Krtalic, B.; Drmic, D.; Stupnisek, M.; Seiwerth, S.; Sikiric, P. Intragastric application of aspirin, clopidogrel, cilostazol, and BPC 157 in rats: Platelet aggregation and blood clot. Oxid. Med. Cell. Longev. 2019, 2019, 9084643. [Google Scholar] [CrossRef]
- Stupnisek, M.; Franjic, S.; Drmic, D.; Hrelec, M.; Kolenc, D.; Radic, B.; Bojic, D.; Vcev, A.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb. Res. 2012, 129, 652–659. [Google Scholar] [CrossRef]
- Cesar, L.B.; Gojkovic, S.; Krezic, I.; Malekinusic, D.; Zizek, H.; Vuletic, L.B.; Petrovic, A.; Pavlov, K.H.; Drmic, D.; Kokot, A.; et al. Bowel adhesion and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine in rats. World J. Gastrointest. Pharmacol. Ther. 2020, 11, 93–109. [Google Scholar] [CrossRef]
- Duplancic, B.; Stambolija, V.; Holjevac, J.; Zemba, M.; Balenovic, I.; Drmic, D.; Suran, J.; Radic, B.; Filipovic, M.; Blagaic, A.B.; et al. Pentadecapeptide BPC 157 and anaphylactoid reaction in rats and mice after intravenous dextran and white egg administration. Eur. J. Pharmacol. 2014, 727, 75–79. [Google Scholar] [CrossRef]
- Luetic, K.; Sucic, M.; Vlainic, J.; Halle, Z.B.; Strinic, D.; Vidovic, T.; Luetic, F.; Marusic, M.; Gulic, S.; Pavelic, T.T.; et al. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology 2017, 25, 255–264. [Google Scholar] [CrossRef]
- Sever, A.Z.; Sever, M.; Vidovic, T.; Lojo, N.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Kokot, A.; Zoricic, I.; Coric, M.; et al. Stable gastric pentadecapeptide BPC 157 in the therapy of the rats with bile duct ligation. Eur. J. Pharmacol. 2019, 847, 130–142. [Google Scholar] [CrossRef]
- Staresinic, M.; Sebecic, B.; Patrlj, L.; Jadrijevic, S.; Suknaic, S.; Perovic, D.; Aralica, G.; Zarkovic, N.; Borovic, S.; Srdjak, M.; et al. Gastric pentadecapeptide BPC 157 accelerates healing of transected rat Achilles tendon and in vitro stimulates tendocytes growth. J. Orthop. Res. 2003, 21, 976–983. [Google Scholar] [CrossRef]
- Gamulin, O.; Oroz, K.; Coric, L.; Krajacic, M.; Skrabic, M.; Dretar, V.; Strbe, S.; Talapko, J.; Juzbasic, M.; Krezic, I.; et al. Fourier transform infrared spectroscopy reveals molecular changes in blood vessels of rats treated with pentadecapeptide BPC 157. Biomedicines 2022, 10, 3130. [Google Scholar] [CrossRef]
- Grabarevic, Z.; Tisljar, M.; Artukovic, B.; Bratulic, M.; Dzaja, P.; Seiwerth, S.; Sikiric, P.; Peric, J.; Geres, D.; Kos, J. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicks. J. Physiol. Paris 1997, 91, 139–149. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Smodiš Škerl, M.I.; Šoštarić, P.; Šuran, J.; Sikirić, P.; Vlainić, J. Physiological and immunological status of adult honeybees (Apis mellifera) fed sugar syrup supplemented with pentadecapeptide BPC 157. Biology 2021, 10, 891. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Ribarić, J.; Smodiš Škerl, M.; Vlainić, J.; Sikirić, P. Stable gastric pentadecapeptide BPC 157 in honeybee (Apis mellifera) therapy, to control Nosema ceranae invasions in apiary conditions. J. Vet. Pharmacol. Ther. 2018, 41, 614–621. [Google Scholar] [CrossRef]
- Filosevic, A.; Andretic Waldowski, R.; Sikiric, P.; Drmic, D. Stable gatric pentadecapeptide BPC 157 antagonizes hydrogen peroxide induced oxidative stress in Drosophila melanogaster. FASEB J. 2018, 31 (Suppl. 1), 667.14. [Google Scholar] [CrossRef]
- Konturek, S.J.; Brzozowski, T.; Piastucki, I.; Radecki, T.; Dupuy, D.; Szabo, S. Gastric mucosal protection by agents altering gastric mucosal sulfhydryls. Role of endogenous prostaglandins. Digestion 1987, 37, 67–71. [Google Scholar] [CrossRef]
- Szabo, S.; Usadel, K.H. Cytoprotection—Organoprotection by somatostatin: Gastric and hepatic lesions. Experientia 1982, 38, 254–256. [Google Scholar] [CrossRef]
- Hernandez, D.E.; Drago, F.; Mason, G.A.; Stanley, D.A.; Prange, A.J., Jr. Effect of dopamine agonists and antagonists on neurotensin-induced antinociception. Pharmacol. Biochem. Behav. 1986, 24, 425–428. [Google Scholar] [CrossRef]
- Petkov, V.; Radomirov, R. Adrenergic transmission and prostaglandins. Acta Physiol. Pharmacol. Bulg. 1982, 8, 115–124. [Google Scholar]
- Petkov, V.; Radomirov, R. On the origin of prostaglandin and its role in the sympathetic nerve transmission in vas deferens. Gen. Pharmacol. 1980, 11, 275–282. [Google Scholar] [CrossRef]
- Brody, M.J.; Kadowitz, P.J. Prostaglandins as modulators of the autonomic nervous system. Fed. Proc. 1974, 33, 48–60. [Google Scholar] [PubMed]
- Timimi, K.S.; Bedwani, J.R.; Stanton, T.W. Effects of prostaglandin E2 and a prostaglandin endoperoxide analogue on neuroeffector transmission in the rat anococcygeus muscle. Br. J. Pharmacol. 1978, 63, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Yoon, J.; Lee, S.H. The role of neuropeptide somatostatin in the brain and its application in treating neurological disorders. Exp. Mol. Med. 2021, 53, 328338. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Arancibia, L.; Pares-Herbuté, N.; Astier, H. Calcium dependence of somatostatin (SRIF) release and cyclic AMP levels in cultured diencephalic neurons. Neuroendocrinology 1989, 49, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Gamse, R.; Vaccaro, D.E.; Gamse, G.; DiPace, M.; Fox, T.O.; Leeman, S.E. Release of immunoreactive somatostatin from hypothalamic cells in culture: Inhibition by gamma-aminobutyric acid. Proc. Natl. Acad. Sci. USA 1980, 77, 5552–5556. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.E.; Stanley, D.A.; Melvin, J.A.; Prange, A.J., Jr. Role of brain neurotransmitters on neurotensin-induced gastric cytoprotection. Pharmacol. Biochem. Behav. 1985, 22, 509–513. [Google Scholar] [CrossRef]
- Jelovac, N.; Sikirić, P.; Rucman, R.; Petek, M.; Perović, D.; Konjevoda, P.; Marović, A.; Seiwerth, S.; Grabarević, Z.; Sumajstorcić, J.; et al. A novel pentadecapeptide, BPC 157, blocks the stereotypy produced acutely by amphetamine and the development of haloperidol-induced supersensitivity to amphetamine. Biol. Psychiatry 1998, 43, 511–519. [Google Scholar] [CrossRef]
- Sikiric, P.; Jelovac, N.; Jelovac-Gjeldum, A.; Dodig, G.; Staresinic, M.; Anic, T.; Zoricic, I.; Rak, D.; Perovic, D.; Aralica, G.; et al. Pentadecapeptide BPC 157 attenuates chronic amphetamine-induced behavior disturbances. Acta Pharmacol. Sin. 2002, 23, 412–422. [Google Scholar] [PubMed]
- Yaffe, D.; Forrest, L.R.; Schuldiner, S. The ins and outs of vesicular monoamine transporters. J. Gen. Physiol. 2018, 150, 671–682. [Google Scholar] [CrossRef]
- Eiden, L.E.; Weihe, E. VMAT2: A dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. N. Y. Acad. Sci. 2011, 1216, 86–98. [Google Scholar] [CrossRef]
- Frim, D.M.; Uhler, T.A.; Galpern, W.R.; Beal, M.F.; Breakefield, X.O.; Isacson, O. Implanted fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc. Natl. Acad. Sci. USA 1994, 91, 5104–5108. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Quan, Y.; Sherer, T.B.; Greenamyre, J.T.; Miller, G.W. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol. Sci. 2005, 88, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Stacy, M.; Silver, D. Apomorphine for the acute treatment of “off” episodes in Parkinson’s disease. Park. Relat. Disord. 2008, 14, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Brams, M.; Mao, A.R.; Doyle, R.L. Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder. Postgrad. Med. 2008, 120, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.M. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. 2011, 116, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Lewin, A.H.; Miller, G.M.; Gilmour, B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg. Med. Chem. 2011, 19, 7044–7048. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J.; Maiofiss, L.; Cussac, D.; Audinot, V.; Boutin, J.A.; Newman-Tancredi, A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor subtypes. J. Pharmacol. Exp. Ther. 2002, 303, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A.; Uliana, D.L. Insights into the, mechanims of action of antipsychotic drugs derived from animal models: Standard of care versus novel targets. Int. J. Mol. Sci. 2023, 24, 12374. [Google Scholar] [CrossRef]
- Christensen, A.V.; Fjalland, B.; Nielsen, I.M. On the supersensitivity of dopamine receptors, induced by neuroleptics. Psychopharmacology 1976, 48, 1–6. [Google Scholar] [CrossRef]
- Sikiric, P.; Separovic, J.; Buljat, G.; Anic, T.; Stancic-Rokotov, D.; Mikus, D.; Duplancic, B.; Marovic, A.; Zoricic, I.; Prkacin, I.; et al. Gastric mucosal lesions induced by complete dopamine system failure in rats. The effects of dopamine agents, ranitidine, atropine, omeprazole and pentadecapeptide BPC 157. J. Physiol. Paris 2000, 94, 105–110. [Google Scholar] [CrossRef]
- Young, S.N. How to increase serotonin in the human brain without drugs. Psychiatry Neurosci. 2007, 32, 394–399. [Google Scholar]
- Robinson, T.E.; Kolb, B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 1997, 17, 8491–8497. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.E. LTP may trigger addiction. Mol. Interv. 2003, 3, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Parikh, V.; Sarter, M. Sensitized attentional performance and Fos-immunoreactive cholinergic neurons in the basal forebrain of amphetamine-pretreated rats. Biol. Psychiatry. 2005, 57, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, R.E.; Kapur, S.; Fletcher, P.J. The amphetamine-induced sensitized state as a model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1556–1571. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed El-Sisi, A.; Sokkar, S.S.; El-Sayed El-Sayad, M.; Sayed Ramadan, E.; Osman, E.Y. Celecoxib and omega-3 fatty acids alone and in combination with risperidone affect the behavior and brain biochemistry in amphetamine-induced model of schizophrenia. Biomed. Pharmacother. 2016, 82, 425–431. [Google Scholar] [CrossRef]
- Bilic, I.; Zoricic, I.; Anic, T.; Separovic, J.; Stancic-Rokotov, D.; Mikus, D.; Buljat, G.; Ivankovic, D.; Aralica, G.; Prkacin, I.; et al. Haloperidol-stomach lesions attenuation by pentadecapeptide BPC 157, omeprazole, bromocriptine, but not atropine, lansoprazole, pantoprazole, ranitidine, cimetidine and misoprostol in mice. Life Sci. 2001, 68, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Bilic, Z.; Gojkovic, S.; Kalogjera, L.; Krezic, I.; Malekinusic, D.; Knezevic, M.; Sever, M.; Lojo, N.; Kokot, A.; Kasnik, K.; et al. Novel insight into Robert’s cytoprotection: Complex therapeutic effect of cytoprotective pentadecapeptide pentadecapeptide BPC 157 in rats with perforated stomach throughout modulation of nitric oxide-system. Comparison with L-arginine, ranitidine and pantoprazole therapy and L-NG-nitro-L-arginine methyl ester worsening. J. Physiol. Pharmacol. Data 2021, 72, 939–955. [Google Scholar] [CrossRef]
- Drmic, D.; Samara, M.; Vidovic, T.; Malekinusic, D.; Antunovic, M.; Vrdoljak, B.; Ruzman, J.; Milkovic Perisa, M.; Horvat Pavlov, K.; Jeyakumar, J.; et al. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5462–5476. [Google Scholar] [CrossRef]
- Azmitia, E.C.; Segal, M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 1978, 179, 641–667. [Google Scholar] [CrossRef]
- Diksic, M. Labelled alpha-methyl-L-tryptophan as a tracer for the study of the brain serotonergic system. J. Psychiatry Neurosci. 2001, 26, 293–303. [Google Scholar]
- Steranka, L.R.; Sanders-Bush, E. Long-term effects of fenfluramine on central serotonergic mechanisms. Neuropharmacology 1979, 18, 895–903. [Google Scholar] [CrossRef]
- Tohyama, Y.; Yamane, F.; Merid, M.F.; Diksic, M. Effects of selective 5-HT1A receptor antagonists on regional serotonin synthesis in the rat brain: An autoradiographic study with alpha-14Cmethyl-L-tryptophan. Eur. Neuropsychopharmacol. 2001, 11, 193–202. [Google Scholar] [CrossRef]
- Tohyama, Y.; Yamane, F.; Fikre Merid, M.; Blier, P.; Diksic, M. Effects of serotonine receptors agonists, TFMPP and CGS12066B, on regional serotonin synthesis in the rat brain: An autoradiographic study. J. Neurochem. 2002, 80, 788–798. [Google Scholar] [CrossRef]
- Diksic, M.; Nagahiro, S.; Grdisa, M. The regional rate of serotonin synthesis estimated by the alpha-methyl-tryptophan method in rat brain from a single time point. J. Cereb. Blood Flow Metab. 1995, 15, 806–813. [Google Scholar] [CrossRef]
- Diksic, M.; Young, S.N. Study of the brain serotonergic system with labeled alpha-methyl-L-tryptophan. J. Neurochem. 2001, 78, 1185–1200. [Google Scholar] [CrossRef]
- Volpi-Abadie, J.; Kaye, A.M.; Kaye, A.D. Serotonin syndrome. Ochsner J. 2013, 13, 533–540. [Google Scholar]
- Ener, R.A.; Meglathery, S.B.; Van Decker, W.A.; Gallagher, R.M. Serotonin syndrome and other serotonergic disorders. Pain Med. 2003, 4, 63–74. [Google Scholar] [CrossRef]
- Gillman, P.K. A review of serotonin toxicity data: Implications for the mechanisms of antidepressant drug action. Biol. Psychiatry 2006, 59, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, L.C.; McClernon, F.J.; Kollins, S.H. Cognitive enhancers for the treatment of ADHD. Pharmacol. Biochem. Behav. 2011, 99, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, J. Enteric glial cells in immunological disorders of the gut. Front Cell. Neurosci. 2022, 16, 895871. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Thornberg, S.A.; Saklad, S.R. A review of NMDA receptors and the phencyclidine model of schizophrenia. Pharmacotherapy 1996, 16, 82–93. [Google Scholar] [CrossRef]
- Stone, J.M.; Erlandsson, K.; Arstad, E.; Squassante, L.; Teneggi, V.; Bressan, R.A.; Krystal, J.H.; Ell, P.J.; Pilowsky, L.S. Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy: A (123)ICNS-1261 SPET study. Psychopharmacology 2008, 197, 401–408. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Farber, N.B.; Jevtovic-Todorovic, V.; Selke, G.; Melson, A.K.; Hershey, T.; Craft, S.; Olney, J.W. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 1999, 20, 106–118. [Google Scholar] [CrossRef]
- Olney, J.W.; Newcomer, J.W.; Farber, N.B. NMDA receptor hypofunction model of schizophrenia. J. Psychiatr. Res. 1999, 33, 523–533. [Google Scholar] [CrossRef]
- Morris, B.J.; Cochran, S.M.; Pratt, J.A. PCP: From pharmacology to modelling schizophrenia. Curr. Opin. Pharmacol. 2005, 5, 101–106. [Google Scholar] [CrossRef]
- Frohlich, J.; Van Horn, J.D. Reviewing the ketamine model for schizophrenia. J. Psychopharmacol. 2014, 28, 287–302. [Google Scholar] [CrossRef]
- Olney, J.W.; Farber, N.B. NMDA antagonists as neurotherapeutic drugs, psychotogens, neurotoxins, and research tools for studying schizophrenia. Neuropsychopharmacology 1995, 13, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Schooler, N.R. Negative symptoms in schizophrenia: A review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.H.; Karper, L.P.; Seibyl, J.P.; Freeman, G.K.; Delaney, R.; Bremner, J.D.; Heninger, G.R.; Bowers, M.B., Jr.; Charney, D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 1994, 51, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Białoń, M.; Wąsik, A. Advantages and limitations of animal schizophrenia models. Int. J. Mol. Sci. 2022, 23, 5968. [Google Scholar] [CrossRef] [PubMed]
- Suarez Santiago, J.E.; Roldan Roldan, G.; Picazo, O. Ketamine as a pharmacological tool for the preclinical study of memory deficit in schizophrenia. Behav. Pharmacol. 2023, 34, 80–91. [Google Scholar] [CrossRef] [PubMed]
- McEntee, W.J.; Crook, T.H. Glutamate: Its role in learning, memory, and the aging brain. Psychopharmacology 1993, 111, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Shinohe, A.; Hashimoto, K.; Nakamura, K.; Tsujii, M.; Iwata, Y.; Tsuchiya, K.J.; Sekine, Y.; Suda, S.; Suzuki, K.; Sugihara, G.; et al. Increased serum levels of glutamate in adult patients with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Tully, J.L.; Dahlén, A.D.; Haggarty, C.J.; Schiöth, H.B.; Brooks, S. Ketamine treatment for refractory anxiety: A systematic review. Br. J. Clin. Pharmacol. 2022, 88, 4412–4426. [Google Scholar] [CrossRef] [PubMed]
- Hess, E.M.; Riggs, L.M.; Michaelides, M.; Gould, T.D. Mechanisms of ketamine and its metabolites as antidepressants. Biochem. Pharmacol. 2022, 197, 114892. [Google Scholar] [CrossRef] [PubMed]
- Trullas, R.; Skolnick, P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur. J. Pharmacol. 1990, 185, 1–10. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef]
- Kapur, S.; Seeman, P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D2 and serotonin 5-HT2 receptors-implications for models of schizophrenia. Mol. Psychiatry 2002, 7, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Boultadakis, A.; Georgiadou, G.; Pitsikas, N. Effects of the nitric oxide synthase inhibitor L-NAME on different memory components as assessed in the object recognition task in the rat. Behav. Brain Res. 2010, 20, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Pitsikas, N.; Boultadakis, A.; Sakellaridis, N. Effects of sub-anesthetic doses of ketamine on rats’ spatial and non-spatial recognition memory. Neuroscience 2008, 154, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Trevlopoulou, A.; Touzlatzi, N.; Pitsikas, N. The nitric oxide donor sodium nitroprusside attenuates recogniton memory deficits and social withdrawal produced by the NMDA receptor antagonist ketamine and induces anxiolytic-like behaviour in rats. Psychopharmacology 2016, 233, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Koros, E.; Rosenbrock, H.; Birk, G. The selective mGlu5 receptor antagonist MTEP, similar to NMDA receptor antagonists, induces social isolation in rats. Neuropsychopharmacology 2007, 32, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Zoupa, E.; Gravanis, A.; Pitsikas, N. The novel dehydroepiandrosterone (DHEA) derivative BNN27 counteracts behavioural deficits induced by the NMDA receptor antagonist ketamine in rats. Neuropharmacology 2019, 151, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005, 52, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Kandratavicius, L.; Balista, P.A.; Wolf, D.C.; Abrao, J.; Evora, P.R.; Rodrigues, A.J.; Chaves, C.; Maia-de-Oliveira, J.P.; Leite, J.P.; Dursun, S.M.; et al. Effects of nitric oxide-related compounds in the acute ketamine animal model of schizophrenia. BMC Neurosci. 2015, 16, 9. [Google Scholar] [CrossRef]
- Mazarati, A.; Shin, D.; Auvin, S. Kindling epileptogenesis in immature rats leads to persistent depressive behavior. Epilepsy Behav. 2007, 10, 377–383. [Google Scholar] [CrossRef]
- Grivas, V.; Markou, A.; Pitsikas, N. The metabotropic glutamate 2/3 receptor agonist LY379268 induces anxiety-like behavior at the highest dose tested in two rat models of anxiety. Eur. J. Pharmacol. 2013, 715, 105–110. [Google Scholar] [CrossRef]
- Wallach, J.; Kang, H.; Colestock, T.; Morris, H.; Bortolotto, Z.A.; Collingridge, G.L.; Lodge, D.; Halberstadt, A.L.; Brandt, S.D.; Adejare, A. Pharmacological investigations of the dissociative “legal heights” diphenide, methoxphenidine and analogues. PLoS ONE 2016, 11, e0157021. [Google Scholar] [CrossRef] [PubMed]
- Pitsikas, N. The role of nitric oxide donors in schizophrenia: Basic studies and clinical applications. Eur. J. Pharmacol. 2015, 766, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zemba, M.; Cilic, A.Z.; Balenovic, I.; Cilic, M.; Radic, B.; Suran, J.; Drmic, D.; Kokot, A.; Stambolija, V.; Murselovic, T.; et al. BPC 157 antagonized the general anaesthetic potency of thiopental and reduced prolongation of anaesthesia induced by L-NAME/thiopental combination. Inflammopharmacology 2015, 23, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Costa, E. From GABAA receptor diversity emerges a unified vision of GABAergic inhibition. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 321–350. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.H.; Katz, J.L.; Winger, G. Benzodiazepines: Use, abuse, and consequences. Pharmacol. Rev. 1992, 44, 151–347. [Google Scholar] [PubMed]
- Nutt, D.J. Benzodiazepine dependence in the clinic: A cause for anxiety? Trends. Pharmacol. Sci. 1986, 7, 457–460. [Google Scholar] [CrossRef]
- Nutt, D.J. Pharmacological mechanisms of benzodiazepine withdrawal. J. Psychiatr. Res. 1990, 24 (Suppl. 2), 105–110. [Google Scholar] [CrossRef]
- Ashton, H. The diagnosis and management of benzodiazepine dependence. Curr. Opin. Psychiatry 2005, 18, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Heberlein, A.; Bleich, S.; Kornhuber, J.; Hillemacher, T. Benzodiazepine dependence: Causalities and treatment options. Fortschr. Neurol. Psychiatr. 2009, 77, 7–15. [Google Scholar] [CrossRef]
- Dubovsky, S.L.; Marshall, D. Benzodiazepines remain important therapeutic options in psychiatric practice. Psychother. Psychosom. 2022, 91, 307–334. [Google Scholar] [CrossRef]
- Asehinde, S.; Ajayi, A.; Bakre, A.; Omorogbe, O.; Adebesin, A.; Umukoro, S. Effects of Jobelyn® on isoniazid-induced seizures, biomarkers of oxidative stress and glutamate decarboxylase activity in mice. Basic Clin. Neurosci. 2018, 9, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.J. GABA-A receptor subtypes in the brain: A paradigm for CNS drug discovery? Drug Discov. Today 2003, 8, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Corda, M.G.; Costa, E.; Guidotti, A. Specific proconvulsant action of an imidazobenzodiazepine (RO 15-1788) on isoniazid convulsions. Neuropharmacology 1982, 21, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.M.; Donevan, S.D.; Rogawski, M.A. Direct activation of GABAA receptors by barbiturates in cultured rat hippocampal neurons. J. Physiol. 1997, 497, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.A.R. Advantages of an antagonist: Bicuculline and other GABA antagonists. Br. J. Pharmacol. 2013, 169, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Brohan, J.; Goudra, B.G. The Role of GABA Receptor agonists in anesthesia and sedation. CNS Drugs 2017, 31, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Franks, N.P.; Lieb, W.R. Which molecular targets are most relevant to general anaesthesia? Toxicol. Lett. 1998, 100–101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khanna, J.M.; Kalant, H.; Weiner, J.; Shah, G. Rapid tolerance and cross-tolerance as predictors of chronic tolerance and cross-tolerance. Pharmacol. Biochem. Behav. 1992, 41, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.M.; Baier, L.D.; Zhang, X. Effects of lorazepam tolerance and withdrawal on GABAA receptor-operated chloride channels. J. Pharmacol. Exp. Ther. 1992, 261, 395–402. [Google Scholar]
- Rooke, K.C. The use of flurazepam (dalmane) as a substitute for barbiturates and methaqualone/diphenhydramine (mandrax) in general practice. J. Int. Med. Res. 1976, 4, 3559. [Google Scholar] [CrossRef]
- Reddy, D.S.; Rogawski, M.A. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J. Pharmacol. Exp. Ther. 2000, 295, 1241–1248. [Google Scholar] [PubMed]
- Allison, C.; Pratt, J.A. Neuroadaptive processes in GABAergic and glutamatergic systems in benzodiazepine dependence. Pharmacol. Ther. 2003, 98, 171–195. [Google Scholar] [CrossRef]
- Pitzele, H.Z.; Tolia, V.M. Twenty per hour: Altered mental state due to ethanol abuse and withdrawal. Emerg. Med. Clin. N. Am. 2010, 28, 683–705. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic liver disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef]
- Diamond, I.; Messing, R.O. Neurologic effects of alcoholism. West J. Med. 1994, 161, 279–287. [Google Scholar]
- Noble, J.M.; Weimer, L.H. Neurologic complications of alcoholism. Continuum 2014, 20, 624–641. [Google Scholar] [CrossRef] [PubMed]
- Prkacin, I.; Separovic, J.; Aralicia, G.; Perovic, D.; Gjurasin, M.; Lovric-Bencic, M.; Stancic-Rokotov, D.; Staresinic, M.; Anic, T.; Mikus, D.; et al. Portal hypertension and liver lesions in chronically alcohol drinking rats prevented and reversed by stable gastric pentadecapeptide BPC 157 (PL-10, PLD-116), and propranolol, but not ranitidine. J. Physiol. 2001, 95, 315–324. [Google Scholar] [CrossRef]
- Prkacin, I.; Aralica, G.; Perovic, D.; Separovic, J.; Gjurasin, M.; Lovric-Bencic, M.; Stancic-Rokotov, D.; Ziger, T.; Anic, T.; Sikiric, P.; et al. Chronic cytoprotection: Pentadecapeptide BPC 157, ranitidine and propranolol prevent, attenuate and reverse the gastric lesions appearance in chronic alcohol drinking rats. J. Physiol. 2001, 95, 295–301. [Google Scholar] [CrossRef]
- Kokot, A. Atropine, Pilocarpine, NO-System, BPC 157 in Mydriasis in Rats. Ph.D. Thesis, Medical Faculty Osijek, University Juraj Strossmayer, Osijek, Croatia, 2015. [Google Scholar]
- Donati, F. Neuromuscular blocking drugs for the new millennium: Current practice, future trends—Comparative pharmacology of neuromuscular blocking drugs. Anesth. Analg. 2000, 90 (Suppl. 5), S2–S6. [Google Scholar] [CrossRef]
- Vélez, P.A.; Lara-Erazo, V.; Caballero-Lozada, A.F.; Botero, A.; Lozada, G.; Velásquez, A.F.; Villegas, L.M.; Zorrilla-Vaca, A. Preoperative pregabalin prevents succinylcholine-induced fasciculation and myalgia: A meta-analysis of randomized trials. Rev. Esp. Anestesiol. Reanim. 2023, in press. [Google Scholar] [CrossRef]
- Van den Bersselaar, L.R.; Snoeck, M.M.J.; Gubbels, M.; Riazi, S.; Kamsteeg, E.J.; Jungbluth, H.; Voermans, N.C. Anaesthesia and neuromuscular disorders: What a neurologist needs to know. Pract. Neurol. 2020, 21, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Manno, F.A. 3rd Pupillometry in mice: Sex and strain-dependent phenotypes of pupillary functioning. Optom. Vis. Sci. 2009, 86, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Mitchelson, F. Muscarinic receptor agonists and antagonists: Effects on ocular function. Handb. Exp. Pharmacol. 2012, 208, 263–298. [Google Scholar] [CrossRef]
- Himmel, H.M.; Eriksson Faelker, T.M. Pupillary function test in rat: Establishment of imaging setup and pharmacological validation within modified Irwin test. J. Pharmacol. Toxicol. Methods 2019, 99, 106588. [Google Scholar] [CrossRef] [PubMed]
- Amic, F.; Drmic, D.; Bilic, Z.; Krezic, I.; Zizek, H.; Peklic, M.; Klicek, R.; Pajtak, A.; Amic, E.; Vidovic, T.; et al. Bypassing major venous occlusion and duodenal lesions in rats, and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5366–5378. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H.; Bondonno, C.P.; Croft, K.D.; Puddey, I.B.; Woodman, R.J.; Rich, L.; Ward, N.C.; Vita, J.A.; Hodgson, J.M. Effects of a nitrate-rich meal on arterial stiffness and blood pressure in healthy volunteers. Nitric Oxide 2013, 35, 123–130. [Google Scholar] [CrossRef]
- Green, S.J. Nitric oxide in mucosal immunity. Nat. Med. 1995, 1, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.J.; Lee, J.S.; Sillberg, V.A.; Wells, G.A. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst. Rev. 2008, 2, CD002788. [Google Scholar] [CrossRef]
- Martyn, J.A.; Richtsfeld, M. Succinylcholine-induced hyperkalemia in acquired pathologic states: Etiologic factors and molecular mechanisms. Anesthesiology 2006, 104, 158–169. [Google Scholar] [CrossRef]
- Bartolone, R.S.; Rao, T.L. Dysrhythmias following muscle relaxant administration in patients receiving digitalis. Anesthesiology 1983, 58, 567–569. [Google Scholar] [CrossRef]
- Bowman, W.C. Prejunctional and postjunctional cholinoceptors at the neuromuscular junction. Anesth. Analg. 1980, 59, 935–943. [Google Scholar] [CrossRef]
- Walker, F.O.; Scott, G.E.; Butterworth, J. Sustained focal effects of low-dose intramuscular succinylcholine. Muscle Nerve 1993, 16, 181–187. [Google Scholar] [CrossRef]
- Foldes, F.F.; Brown, I.M. The possible dangers of intramuscular succinylcholine. JAMA 1961, 177, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Mazze, R.I.; Dunbar, R.W. Intralingual succinylcholine administration in children: An alternative to intravenous and intramuscular routes? Anesth. Analg. 1968, 47, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Krivic, A.; Anic, T.; Seiwerth, S.; Huljev, D.; Sikiric, P. Achilles detachment in rat and stable gastric pentadecapeptide BPC 157: Promoted tendon-to-bone healing and opposed corticosteroid aggravation. J. Orthop. Res. 2006, 24, 982–989. [Google Scholar] [CrossRef]
- Krivic, A.; Majerovic, M.; Jelic, I.; Seiwerth, S.; Sikiric, P. Modulation of early functional recovery of Achilles tendon to bone unit after transection by BPC 157 and methylprednisolone. Inflamm. Res. 2008, 57, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Cerovecki, T.; Bojanic, I.; Brcic, L.; Radic, B.; Vukoja, I.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 (PL 14736) improves ligament healing in the rat. J. Orthop. Res. 2010, 28, 1155–1161. [Google Scholar] [CrossRef]
- Sebecić, B.; Nikolić, V.; Sikirić, P.; Seiwerth, S.; Sosa, T.; Patrlj, L.; Grabarević, Z.; Rucman, R.; Petek, M.; Konjevoda, P.; et al. Osteogenic effect of a gastric pentadecapeptide, BPC-157, on the healing of segmental bone defect in rabbits: A comparison with bone marrow and autologous cortical bone implantation. Bone 1999, 24, 195–202. [Google Scholar] [CrossRef]
- Keremi, B.; Lohinai, Z.; Komora, P.; Duhaj, S.; Borsi, K.; Jobbagy-Ovari, G.; Kallo, K.; Szekely, A.D.; Fazekas, A.; Dobo-Nagy, C.; et al. Antiinflammatory effect of BPC 157 on experimental periodontitis in rats. J. Physiol. Pharmacol. 2009, 60 (Suppl 7), 115–122. [Google Scholar]
- Zimmermann, M. Pathobiology of neuropathic pain. Eur. J. Pharmacol. 2001, 429, 23–37. [Google Scholar] [CrossRef]
- Sikirić, P.; Seiwerth, S.; Grabarević, Z.; Rucman, R.; Petek, M.; Jagić, V.; Turković, B.; Rotkvić, I.; Mise, S.; Zoricić, I.; et al. Beneficial effect of a novel pentadecapeptide BPC 157 on gastric lesions induced by restraint stress, ethanol, indomethacin, and capsaicin neurotoxicity. Dig. Dis. Sci. 1996, 41, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.G.; Silvestroff, L.; Soto, E.F.; Pasquini, J.M. Thyroid hormones promote differentiation of oligodendrocyte progenitor cells and improve remyelination after cuprizone-induced demyelination. Exp. Neurol. 2008, 212, 458–467. [Google Scholar] [CrossRef]
- Guyenet, S.J.; Furrer, S.A.; Damian, V.M.; Baughan, T.D.; La Spada, A.R.; Garden, G.A. A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J. Vis. Exp. 2010, 39, 1787. [Google Scholar] [CrossRef]
- Fadini, G.P.; Pauletto, P.; Avogaro, A.; Rattazzi, M. The good and the bad in the link between insulin resistance and vascular calcification. Atherosclerosis 2007, 193, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Velísek, L.; Velísková, J.; Chudomel, O.; Poon, K.L.; Robeson, K.; Marshall, B.; Sharma, A.; Moshé, S.L. Metabolic environment in substantia nigra reticulata is critical for the expression and control of hypoglycemia-induced seizures. J. Neurosci. 2008, 28, 9349–9362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Avila, G.; Sandoval, A.; Felix, R. Intramembrane charge movement associated with endogenous K+ channel activity in HEK-293 cells. Cell. Mol. Neurobiol. 2004, 24, 317–330. [Google Scholar] [CrossRef]
- Kass, R.S.; Freeman, L.C. Potassium channels in the heart Cellular, molecular, and clinical implications. Trends Cardiovasc. Med. 1993, 3, 149–159. [Google Scholar] [CrossRef]
- Ukomadu, C.; Zhou, J.; Sigworth, F.J.; Agnew, W.S. muI Na+ channels expressed transiently in human embryonic kidney cells: Biochemical and biophysical properties. Neuron 1992, 8, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Berjukov, S.; Aczel, S.; Beyer, B.; Kimball, S.D.; Dichtl, M.; Hering, S.; Striessnig, J. Extra- and intracellular action of quaternary devapamil on muscle L-type Ca2+-channels. Br. J. Pharmacol. 1996, 119, 1197–1202. [Google Scholar] [CrossRef]
- Mirković, I.; Kralj, T.; Lozić, M.; Stambolija, V.; Kovačević, J.; Vrdoljak, L.; Zlatar, M.; Milanović, K.; Drmić, D.; Predović, J.; et al. Pentadecapeptide BPC 157 shortens duration of tetracaine- and oxybuprocaine-induced corneal anesthesia in rats. Acta Clin. Croat. 2020, 59, 394–406. [Google Scholar] [CrossRef]
- Barrantes, F.J. Modulation of a rapid neurotransmitter receptor-ion channel by membrane lipids. Front. Cell Dev. Biol. 2024, 11, 1328875. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, H.; Hollmann, M.W.; Stevens, M.F.; Lirk, P.; Brandenburger, T.; Piegeler, T.; Werdehausen, R. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: A narrative review. Br. J. Anaesth. 2019, 123, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Fozzard, H.A.; Sheets, M.F.; Hanck, D.A. The sodium channel as a target for local anesthetic drugs. Front. Pharmacol. 2011, 2, 68. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, D.B.; Zhorov, B.S. Mechanism of sodium channel block by local anesthetics, antiarrhythmics, and anticonvulsants. J. Gen. Physiol. 2017, 149, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Abelson, K.S.; Höglund, A.U. Intravenously administered lidocaine in therapeutic doses increases the intraspinal release of acetylcholine in rats. Neurosci. Lett. 2002, 317, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, H.; Shimizu, K.; Takenami, T.; Sugie, H.; Kawakami, T. Effects of clonidine on lidocaine-induced inhibition of axonal transport in cultured mouse dorsal root ganglion neurones. Br. J. Anaesth. 2008, 101, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Karnina, R.; Arif, S.K.; Hatta, M.; Bukhari, A. Molecular mechanisms of lidocaine. Ann. Med. Surg. 2021, 69, 102733. [Google Scholar] [CrossRef]
- Paganelli, M.A.; Popescu, G.K. Actions of bupivacaine, a widely used local anesthetic, on NMDA receptor responses. J. Neurosci. 2015, 35, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.E.; Reed, K.L. Local anesthetics: Review of pharmacological considerations. Anesth. Prog. 2012, 59, 90–101. [Google Scholar] [CrossRef]
- Cummins, T.R. Setting up for the block: The mechanism underlying lidocaine’s use-dependent inhibition of sodium channels. J. Physiol. 2007, 582 Pt 1, 11. [Google Scholar] [CrossRef]
- Braddock, M.; Houston, P.; Campbell, C.; Ashcroft, P. Born again bone: Tissue engineering for bone repair. News Physiol. Sci. 2001, 16, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Brcic, L.; Brcic, I.; Staresinic, M.; Novinscak, T.; Sikiric, P.; Seiwerth, S. Modulatory effect of gastric pentadecapeptide BPC 157 on angiogenesis in muscle and tendon healing. J. Physiol. Pharmacol. 2009, 60 (Suppl. 7), 191–196. [Google Scholar] [PubMed]
- Radeljak, S.; Seiwerth, S.; Sikiric, P. BPC 157 inhibits cell growth and VEGF signalling via the MAPK kinase pathway in the human melanoma cell line. Melanoma Res. 2004, 14, A14–A15. [Google Scholar] [CrossRef]
- Orton, R.J.; Sturm, O.E.; Vyshemirsky, V.; Calder, M.; Gilbert, D.R.; Kolch, W. Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochem. J. 2005, 392 Pt 2, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zhan, Q.; Xiao, W.; Tang, X.; Li, J.; Dong, H.; Sha, W.; Zhang, X. Altered serum levels of vascular endothelial growth factor in first-episode drug-naïve and chronic medicated schizophrenia. Psychiatry Res. 2018, 264, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.; Howell, K.R.; Ahmed, A.O.; Weinberg, D.; Allen, K.M.; Bruggemann, J.; Lenroot, R.; Liu, D.; Galletly, C.; Weickert, C.S.; et al. Association of serum VEGF levels with prefrontal cortex volume in schizophrenia. Mol. Psychiatry 2016, 21, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Stramecki, F.; Stanczykiewicz, B.; Frydecka, D.; Lubeiro, A. Vascular endothelial growth factor in patients with schizophrenia: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 24–29. [Google Scholar] [CrossRef]
- Rampino, A.; Annese, T.; Torretta, S.; Tamma, R.; Facone, R.M.; Ribatti, D. Involvement of vascular endothelial growth factor in schizophrenia. Neurosci. Lett. 2021, 760, 136093. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Chakroborty, D.; Goswami, S.; Fan, H.; Mo, X.; Basu, S. VEGF-A controls the expression of its regulator of angiogenic functions, dopamine D2 receptor, on endothelial cells. J. Cell Sci. 2022, 135, jcs259617. [Google Scholar] [CrossRef] [PubMed]
- Udo, H.; Hamasu, K.; Furuse, M.; Sugiyama, H. VEGF-induced antidepressant effects involve modulation of norepinephrine and serotonin systems. Behav. Brain. Res. 2014, 275, 107–113. [Google Scholar] [CrossRef]
- Warner-Schmidt, J.L.; Duman, R.S. VEGF as a potential target for therapeutic intervention in depression. Curr. Opin. Pharmacol. 2008, 8, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Warner-Schmidt, J.L.; Duman, R.S. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc. Natl. Acad. Sci. USA 2007, 104, 4647–4652. [Google Scholar] [CrossRef] [PubMed]
- Castro-Torres, R.D.; Ureña-Guerrero, M.E.; Morales-Chacón, L.M.; Lorigados-Pedre, L.; Estupiñan-Díaz, B.; Rocha, L.; Orozco-Suárez, S.; Rivera-Cervantes, M.C.; Alonso-Vanegas, M.; Beas-Zárate, C.J. New aspects of VEGF, GABA, and glutamate signaling in the neocortex of human temporal lobe pharmacoresistant epilepsy revealed by RT-qPCR arrays. Mol. Neurosci. 2020, 70, 916–929. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, D.P.; Croll, S.D.; Scharfman, H.E. Depression of synaptic transmission by vascular endothelial growth factor in adult rat hippocampus and evidence for increased efficacy after chronic seizures. J. Neurosci. 2005, 25, 8889–8897. [Google Scholar] [CrossRef]
- Guérit, S.; Allain, A.E.; Léon, C.; Cazenave, W.; Ferrara, N.; Branchereau, P.; Bikfalvi, A. VEGF modulates synaptic activity in the developing spinal cord. Dev. Neurobiol. 2014, 74, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Matsumoto, K.; Ohtake, H.; Oka, J.I.; Fujiwara, H. Endogenous acetylcholine regulates neuronal and astrocytic vascular endothelial growth factor expression levels via different acetylcholine receptor mechanisms. Neurochem. Int. 2018, 118, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Inada, C.; Niu, Y.; Matsumoto, K.; Le, X.T.; Fujiwara, H. Possible involvement of VEGF signaling system in rescuing effect of endogenous acetylcholine on NMDA-induced long-lasting hippocampal cell damage in organotypic hippocampal slice cultures. Neurochem. Int. 2014, 75, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Marko, S.B.; Damon, D.H. VEGF promotes vascular sympathetic innervation. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2646–H2652. [Google Scholar] [CrossRef] [PubMed]
- Long, J.B.; Jay, S.M.; Segal, S.S.; Madri, J.A. VEGF-A and Semaphorin3A: Modulators of vascular sympathetic innervation. Dev. Biol. 2009, 334, 119–132. [Google Scholar] [CrossRef]
- Van Beveren, N.J.; Schwarz, E.; Noll, R.; Guest, P.C.; Meijer, C.; de Haan, L.; Bahn, S. Evidence for disturbed insulin and growth hormone signaling as potential risk factors in the development of schizophrenia. Transl. Psychiatry. 2014, 4, e430. [Google Scholar] [CrossRef]
- Tena-Sempere, M.; Pinilla, L.; González, L.C.; Aguilar, E. Regulation of growth hormone (GH) secretion by different glutamate receptor subtypes in the rat. Amino Acids 2000, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.A.; Tena-Sempere, M.; Pinilla, L. Role of Excitatory amino acids in the control of growth hormone secretion. Endocrine 2006, 3, 295–302. [Google Scholar] [CrossRef]
- Powers, M. GABA supplementation and growth hormone response. Med. Sport Sci. 2012, 59, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Noaín, D.; Pérez-Millán, M.I.; Bello, E.P.; Luque, G.M.; Casas Cordero, R.; Gelman, D.M.; Peper, M.; Tornadu, I.G.; Low, M.J.; Becú-Villalobos, D.; et al. Central dopamine D2 receptors regulate growth-hormone-dependent body growth and pheromone signaling to conspecific males. J. Neurosci. 2013, 33, 5834–5842. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G. Serotonin and the regulation of hypothalamic-pituitary-adrenal axis function. Life Sci. 1996, 58, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, M.J.; Bertolus, C.; Ramanantsoa, N.; Saurini, F.; Callebert, J.; Sénamaud-Beaufort, C.; Ringot, M.; Bourgeois, T.; Matrot, B.; Collet, C.; et al. Acetylcholine modulates the hormones of the growth hormone/insulinlike growth factor-1 axis during development in mice. Endocrinology 2018, 159, 1844–1859. [Google Scholar] [CrossRef] [PubMed]
- Müller, E.E.; Locatelli, V.; Ghigo, E.; Cella, S.G.; Loche, S.; Pintor, C.; Camanni, F. Involvement of brain catecholamines and acetylcholine in growth hormone deficiency states. Pathophysiological, diagnostic and therapeutic implications. Drugs 1991, 41, 161–177. [Google Scholar] [CrossRef]
- Müller, E.E.; Rolla, M.; Ghigo, E.; Belliti, D.; Arvat, E.; Andreoni, A.; Torsello, A.; Locatelli, V.; Camanni, F. Involvement of brain catecholamines and acetylcholine in growth hormone hypersecretory states. Pathophysiological, diagnostic and therapeutic implications. Drugs 1995, 50, 805–837. [Google Scholar] [CrossRef]

| Effect | Specification | Ref. |
|---|---|---|
| Antidepressant effect (Porsolt’s test, open field) | BPC 157 therapy (Porsolt’s test, chronic stress, reduced duration of immobility) overwhelmed the effect of imipramine. | [65] |
| The regional serotonin synthesis (using alpha-[14C]methyl-L-tryptophan (α-MTrp) autoradiographic measurements) in the brain following peripheral (intraperitoneal) BPC 157 administration | There was an overall decrease in brain serotonin synthesis following acute treatment, which provided an overall increase in synthesis in chronically treated rats. In acute treatment, a significant decrease in the globus pallidus, dorsal and ventral hippocampus, dorsal thalamus, lateral geniculate body, and hypothalamus. Contrarily, the synthesis significantly increased in the medial anterior olfactory nucleus and substantia nigra reticulate. In chronic treatment, a significant decrease observed in the dorsal raphe is along with increases in the substantia nigra, the lateral caudate, and the accumbens nucleus. This can likely point to a particular serotonergic response that is timely related to BPC 157 applications. The substantia nigra’s (compacta and reticulata) structure (given serotonin synthesis significantly increased following both acute and chronic treatments) occurred as a particular point of pentadecapeptide BPC 157. | [67] |
| Counteracting potential of BPC 157 therapy on serotonin syndrome | BPC 157 therapy counteracted serotonin syndrome initiation (i.e., counteracted pargyline effect). Then, in particular, BPC 157 counteracted the full serotonin syndrome crisis (attenuated the adverse effect of the subsequent L-tryptophan application). This effect may have been a particular effect, as BPC 157 counteracted each part of the serotonin syndrome presentations, and then, BPC 157 therapy might fully counteract serotonin syndrome [66] as a whole. Both temperature and behavioral changes in all these experiments were counteracted. | [66] |
| Inhibited release of enteric serotonin | Inhibited release of enteric serotonin and inhibited intestinal motility, the increased survival rate of cultured enteric neurons, and the increased proliferation of cultured enteric glial cells (EGCs) by BPC 157 application. | [119] |
| BPC 157 maintains platelet function in a particular way | Given in occlusion/occlusion-like syndromes, BPC 157 therapy eliminated/annihilated hemorrhage (i.e., brain, lung) and thrombosis, in particular of consideration of pulmonary embolism [87] and evidently, reversed already advanced Virchow triad circumstances [40,41,42,43,47,48,49,50,51,52,53,54,55,56]. In addition, there was the specifically maintained thrombocytes function (i.e., the opposed L-NAME-pro-thrombotic effect, opposed L-arginine-anti-thrombotic effect) [128], given the coagulation pathways not affected as also demonstrated in aggregometry and thromboelastometry studies [128,129,130], and counteracted prolonged bleeding following anticoagulants (heparin, warfarin) and anti-platelet agents (aspirin, clopidogrel, cilostazol) [126,127,128], organ perforation [49,173,174] or amputation of tail or foot [128,129,130]. | [126,127,128,129,130] |
| In conclusion, there is a restorative dimension of the BPC 157 therapy that can react with the serotonin system depending on the condition [65,66,67,119,126,127,128,129,130]. | The restorative dimension of the BPC 157 therapy, and thereby BPC 157 activity over the serotonin system, as a likely neurotransmitter of its own, can be based on the following consistent evidence providing a wide range of influence on various, even opposite, activities [1]. The arguments are its particular combination, such as antidepressant effect (Porsolt’s test, open field) [65] and region-specific influences on brain serotonin synthesis in rats given a particular increase in the substantia nigra [67] vs. counteraction of the serotonin syndrome as a whole [66]. Further, there is a reduction in enteric serotonin concentration, attenuated intestinal motility, increased survival of cultured enteric neurons, the proliferation of cultured EGCs [119], and a particular effect on maintaining platelets function [126,127,128,129,130]. | |
| Effect | Specification | Ref. |
|---|---|---|
| BPC 157 counteracted the effect of MK-801, a non-competitive antagonist of the NMDA receptor application in rats. | BPC 157 counteracted the effect of MK-801 locomotion, stereotyped sniffing, and ataxia as a sign of positive-like symptoms of schizophrenia [70]. | [70] |
| BPC 157 counteracted negative-like schizophrenia symptoms in rats | BPC 157 counteracted negative-like schizophrenia symptoms, ketamine-cognition dysfunction, social withdrawal, and anhedonia and exerted additional anxiolytic effects in rats. There was a distinctive ketamine dosage range, but all were counteracted using the same dosage range of BPC 157. The significance of such counteraction is further established by the additional application of NO-agents. Each of the negative-like symptoms differs from each other given their different responsibility to L-NAME and L-arginine given alone or together. | [59] |
| In conclusion, there is a restorative dimension of the BPC 157 therapy. It is evident that it can react with the glutamate system depending on the condition. | BPC 157/glutamate relation goes as a restorative dimension (i.e., neurotransmitter of its own over glutamate system) with the evidence that BPC 157 counteracted MK-801 positive-like symptoms of schizophrenia [70] and ketamine negative-like schizophrenia symptoms, ketamine-cognition dysfunction, social withdrawal, and anhedonia, and exerted additional anxiolytic effects in rats [59] | |
| Effect | Specification | Ref. |
|---|---|---|
| BPC 157 has a particular anxiolytic effect on its own | BPC 157 has a particular anxiolytic effect in light/dark and shock probe/burying tests. | [57] |
| BPC 157 has a particular anxiolytic effect on its own | BPC 157 has a particular additional anxiolytic effect in ketamine rats. | [59] |
| BPC 157 coadministration in chronically treated diazepam mice counteracts diazepam tolerance and withdrawal, postpones physical dependence, and prolongs residual diazepam anticonvulsive activity. | BPC 157 therapy also, on its own, counteracts isoniazid (GABA synthesis inhibitor)-, picrotoxin (non-completive channel blocker for GABAA receptors (GABAARs) chloride channels)-convulsions. | [58] |
| BPC 157 therapy counteracts bicuculline convulsions. | BPC 157 therapy counteracts bicuculline (completive antagonists of GABAARs)-convulsions. | [15] |
| BPC 157 therapy counteracts in rats the anesthetic effect of thiopental [218] | BPC 157 in doses of 10 ng/kg and 10 µg/kg, respectively, caused significant counteraction of loss of righting reflex produced by thiopental with a parallel shift of the dose–response curve for thiopental to the right. Illustratively, BPC 157 therapy also counteracts the effect of L-NAME, which increases the thiopental loss of the righting reflex seven times. | [218] |
| BPC 157 therapy exhibited the counteraction of both acute and chronic alcohol effects as a highlight of the BPC 157 particular potential consistently evidenced in mice that were either acutely intoxicated or physically dependent on alcohol [63,64]. | BPC 157 intraperitoneally or intragastrically strongly prevented and reversed the effects of acute intoxication (i.e., quickly produced and sustained anesthesia, hypothermia, increased ethanol blood values, 25% fatality, 90 min assessment period) when given before or after ethanol, and none of the mice died. When given after abrupt cessation of chronic ethanol (at 0 or 3 h withdrawal time), it attenuated withdrawal and handling induced withdrawal seizures. | [63,64] |
| Following acute absolute alcohol intragastric administration, BPC 157 therapy attenuated/eliminated the alcohol-occlusion/occlusion-like syndrome as a whole, major vascular and multiorgan failure [56], as described above [40,41,42,43,47,48,49,50,51,52,53,54,55,56]. | Intracranial, portal, and caval hypertension and aortal hypotension, lesions and hemorrhage in the brain, heart, lung, liver, and kidney, and thrombosis peripherally and centrally were all counteracted along with counteraction of the prime major stomach alcohol lesion. The therapy effect was ascribed to the counteraction of the congestion of major vessels and particularly to activation of the rescuing collaterals, i.e., azygos vein direct blood flow delivery. BPC 157 therapy effectiveness illustrates that brain swelling instantly decreases. A fall of intracranial hypertension occurs immediately upon BPC 157 therapy. The therapy effect was ascribed to the activation of the rescuing collaterals, i.e., azygos vein direct blood flow delivery, and to the counteraction of the congestion of major vessels that occurred instantly. | [56] |
| In conclusion, there is a restorative dimension of the BPC 157 therapy. It is evident that it can react with the GABA system depending on the condition. | The restorative dimension of the BPC 157 therapy, and thereby BPC 157 activity over the GABA system, as a likely neurotransmitter of its own, can be based on the following consistent evidence providing a wide range of influence on various, even opposite, activities [1]. The anxiolytic effect on its own (light/dark, shock probe/burying [57], ketamine [59]) of BPC 157 therapy is a particular effect. In support, in diazepam tolerance/withdrawal and physical dependence/withdrawal studies [58], BPC 157 coadministration in chronically treated diazepam mice counteracts diazepam tolerance and withdrawal, postpones physical dependence, and prolongs residual diazepam anticonvulsive activity [58]. BPC 157 therapy also, on its own, counteracts isoniazid (GABA synthesis inhibitor)-, picrotoxin (non-completive channel blocker for GABAA receptors (GABAARs) chloride channels)- [58], and bicuculline (completive antagonists of GABAARs)-convulsions [15]. Finally, BPC 157 therapy counteracts the anesthetic effect of thiopental in rats [218], a prototype member of the barbiturate class of drugs. BPC 157 therapy exhibited the counteraction of both acute and chronic alcohol effects [63,64]. Following acute absolute alcohol intragastric administration, BPC 157 therapy attenuated/eliminated the alcohol-occlusion/occlusion-like syndrome as a whole, major vascular and multiorgan failure [56]. | |
| Effect | Specification | Ref. |
|---|---|---|
| BPC 157 exhibited counteraction of the succinylcholine and rocuronium (report in preparation). | In succinylcholine-rats, BPC 157 counteracted agitation before muscle disability, numerous twitches before complete loss of muscle tone, motionless prostration, and, subsequently, a painful reaction (violent screaming upon light touch). BPC 157 dose-dependently counteracted the effect of rocuronium (report in preparation). | [81] |
| BPC 157 counteracted the effect of muscarinic receptor agonist pilocarpine. | Additionally, BPC 157 counteracted the effect of muscarinic receptor agonist pilocarpine, miosis (and subsequent mydriasis in rats due to muscle disability), hypersalivation, and convulsions. | [244] |
| BPC 157 counteracted prototypical muscarinic receptor antagonist atropine, and mydriasis in rats and guinea pigs. | An equal counteracting effect following local, intragastric, and intraperitoneal application is consistent with an overall effect on acetylcholine system function. These effects appear to be NO-system related. | [86] |
| In conclusion, there is a restorative dimension of the BPC 157 therapy. It is evident that it can react with the acetylcholine system depending on the condition. | BPC 157/acetylcholine relation principle can be a particular way with the restorative dimension of the BPC 157 therapy, and thereby, BPC 157 activity over the acetylcholine system, as a likely neurotransmitter of its own. This can be based on the following consistent evidence providing a wide range of influence on various, even opposite, activities [1], noted in particular with BPC 157 counteraction of the succinylcholine [81], rocuronium (report in preparation), pilocarpine [244] and atropine [86] effects. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikiric, P.; Boban Blagaic, A.; Strbe, S.; Beketic Oreskovic, L.; Oreskovic, I.; Sikiric, S.; Staresinic, M.; Sever, M.; Kokot, A.; Jurjevic, I.; et al. The Stable Gastric Pentadecapeptide BPC 157 Pleiotropic Beneficial Activity and Its Possible Relations with Neurotransmitter Activity. Pharmaceuticals 2024, 17, 461. https://doi.org/10.3390/ph17040461
Sikiric P, Boban Blagaic A, Strbe S, Beketic Oreskovic L, Oreskovic I, Sikiric S, Staresinic M, Sever M, Kokot A, Jurjevic I, et al. The Stable Gastric Pentadecapeptide BPC 157 Pleiotropic Beneficial Activity and Its Possible Relations with Neurotransmitter Activity. Pharmaceuticals. 2024; 17(4):461. https://doi.org/10.3390/ph17040461
Chicago/Turabian StyleSikiric, Predrag, Alenka Boban Blagaic, Sanja Strbe, Lidija Beketic Oreskovic, Ivana Oreskovic, Suncana Sikiric, Mario Staresinic, Marko Sever, Antonio Kokot, Ivana Jurjevic, and et al. 2024. "The Stable Gastric Pentadecapeptide BPC 157 Pleiotropic Beneficial Activity and Its Possible Relations with Neurotransmitter Activity" Pharmaceuticals 17, no. 4: 461. https://doi.org/10.3390/ph17040461
APA StyleSikiric, P., Boban Blagaic, A., Strbe, S., Beketic Oreskovic, L., Oreskovic, I., Sikiric, S., Staresinic, M., Sever, M., Kokot, A., Jurjevic, I., Matek, D., Coric, L., Krezic, I., Tvrdeic, A., Luetic, K., Batelja Vuletic, L., Pavic, P., Mestrovic, T., Sjekavica, I., ... Seiwerth, S. (2024). The Stable Gastric Pentadecapeptide BPC 157 Pleiotropic Beneficial Activity and Its Possible Relations with Neurotransmitter Activity. Pharmaceuticals, 17(4), 461. https://doi.org/10.3390/ph17040461








