The 125th Anniversary of Aspirin—The Story Continues
Abstract
1. Introduction
2. Pharmacodynamic Properties of Aspirin—Recent Insights
3. Aspirin as an Anti-Inflammatory, Analgesic, and Antipyretic Agent
3.1. Anti-Inflammatory Action—Clinical Experience
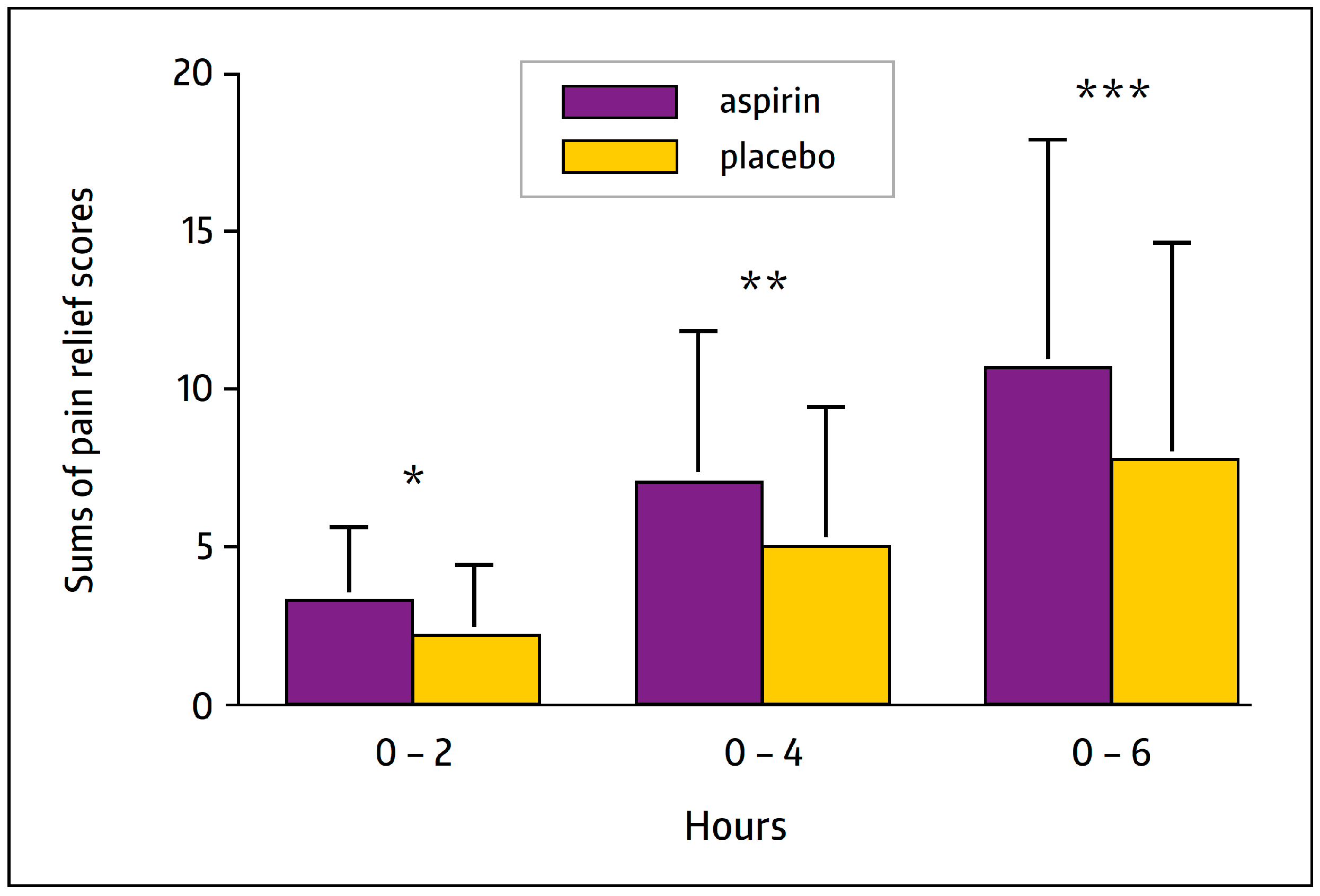
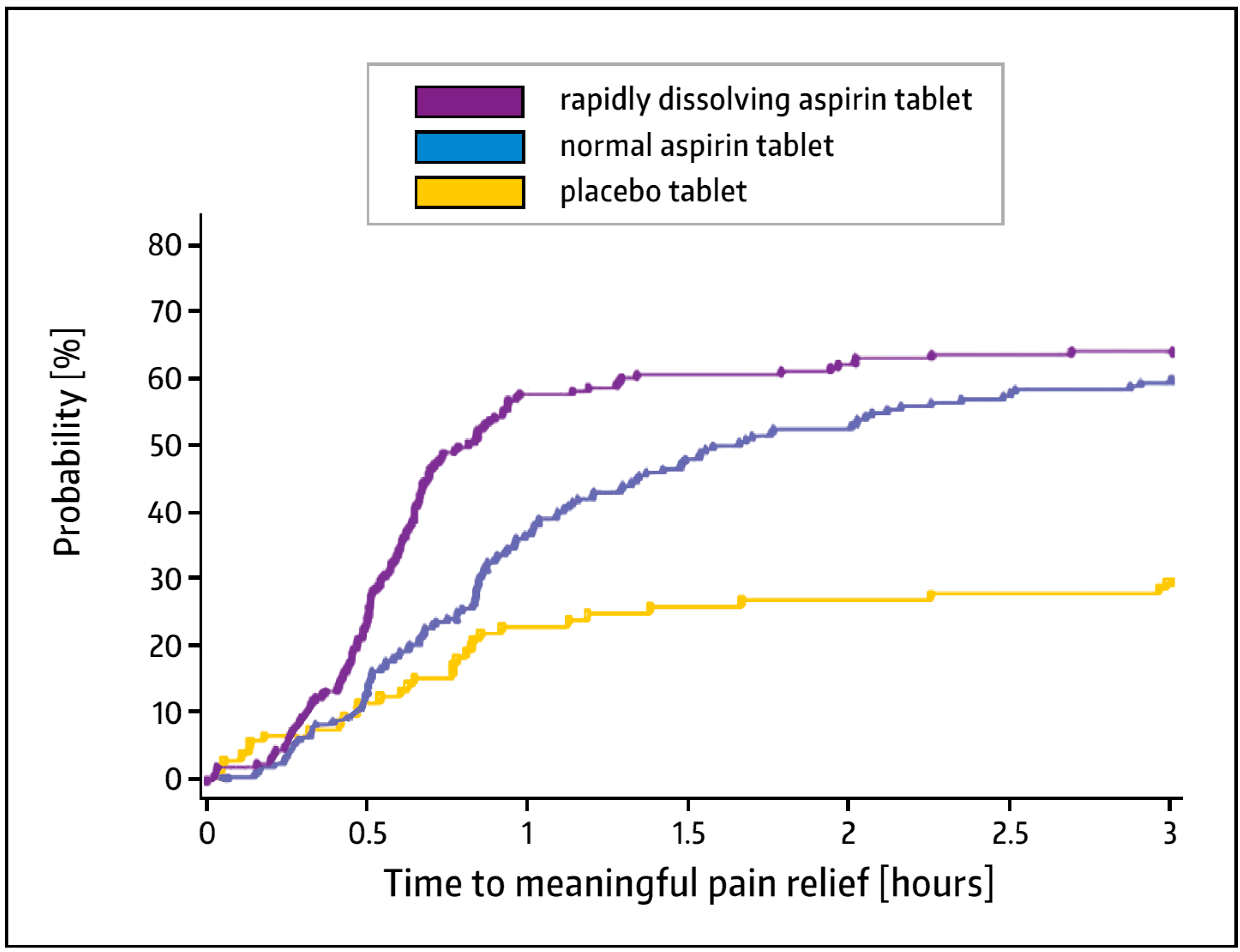
3.2. Analgesic Action—Clinical Experience
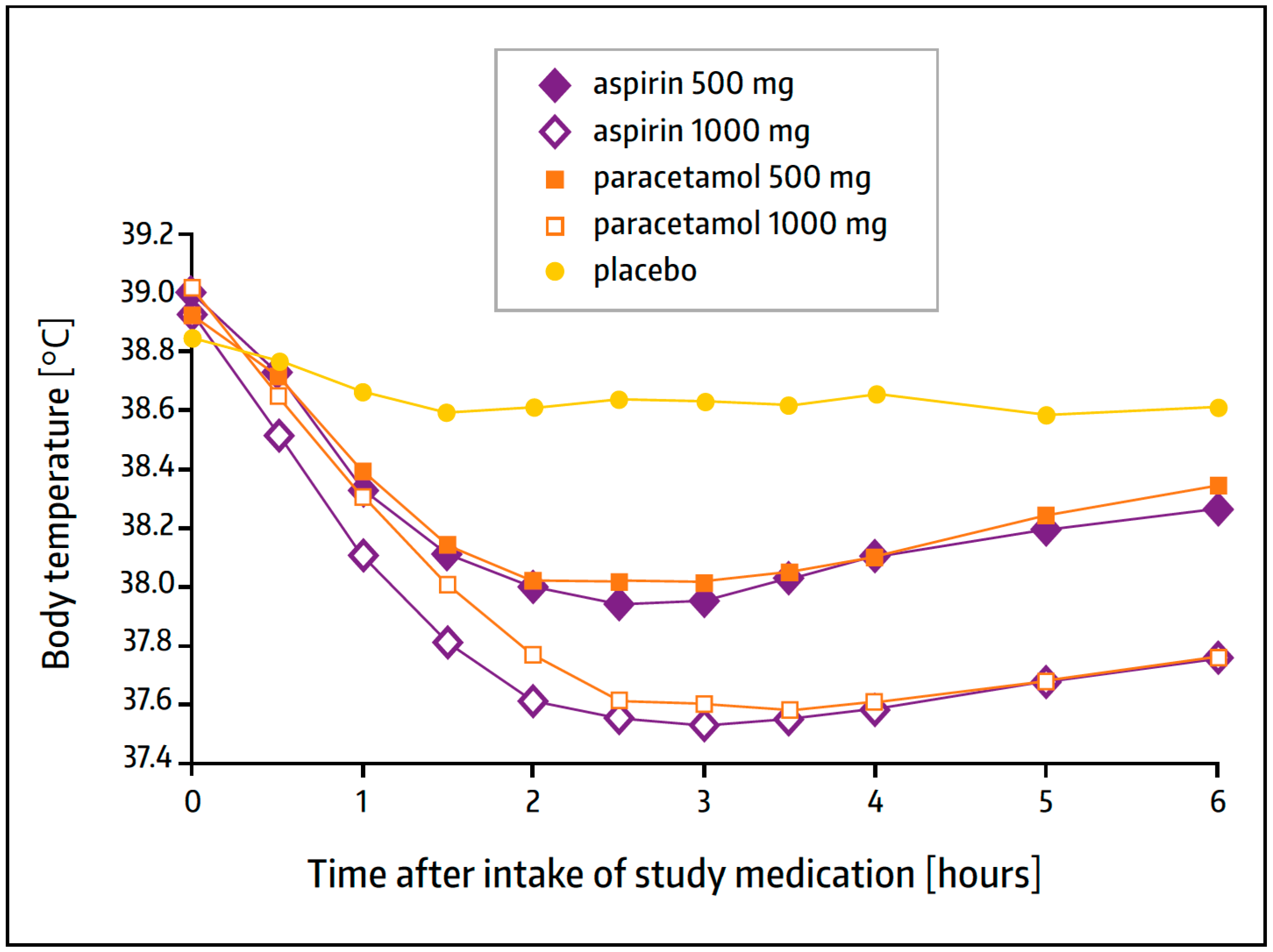
3.3. Antipyretic Action—Clinical Experience
4. Aspirin as an Antiplatelet/Antithrombotic Agent
5. Aspirin and Tumor Prevention
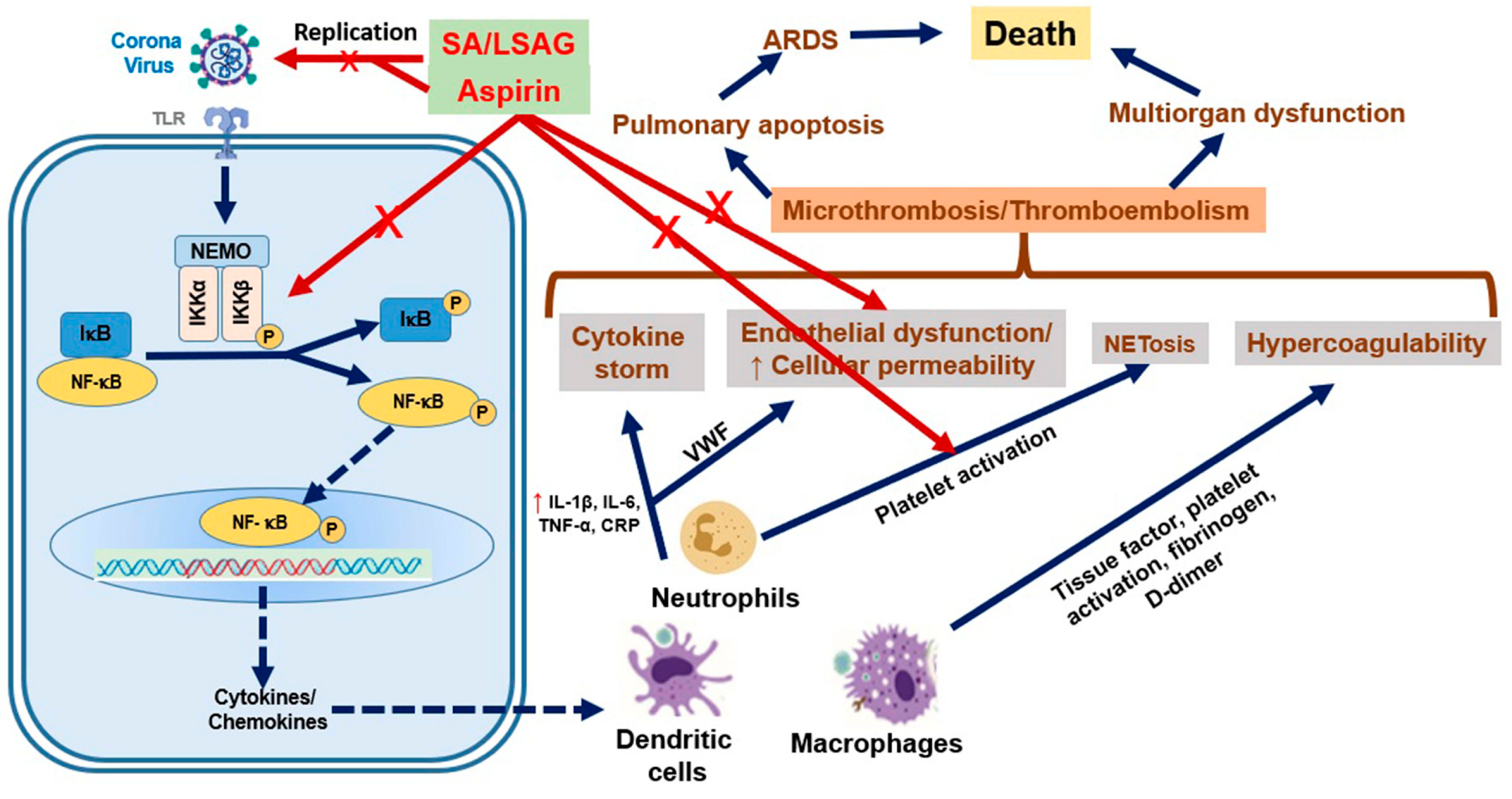
6. Aspirin and Infections
7. Safety of Aspirin
8. Aspirin Nowadays
9. Future Perspectives for Aspirin
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuster, V.; Sweeny, J.M. Aspirin: A historical and contemporary therapeutic overview. Circulation 2011, 123, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Montinari, M.R.; Minelli, S.; De Caterina, R. The first 3500 years of aspirin history from its roots-A concise summary. Vascul. Pharmacol. 2019, 113, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jack, D.B. One hundred years of aspirin. Lancet 1997, 350, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Desborough, M.J.; Keeling, D.M. The aspirin story-from willow to wonder drug. Br. J. Haematol. 2017, 177, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Schrör, K. Acetylsalicylic Acid, 3rd ed.; Walter de Gruyter GmbH: Berlin, Germany, 2022; pp. 1–630. [Google Scholar] [CrossRef]
- Patrono, C.; Rocca, B. Aspirin, 110 years later. J. Thromb. Haemost. 2009, 7 (Suppl. 1), 258–261. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.; Härter, M.; Haß, B.; Schmeck, C.; Baerfacker, L. Small molecules and their impact in drug discovery: A perspective on the occasion of the 125th anniversary of the Bayer Chemical Research Laboratory. Drug Discov. Today 2022, 27, 1560–1574. [Google Scholar] [CrossRef]
- Patrono, C. Aspirin: 1A @ 125. Eur. Heart J. 2022, 43, 3194–3195. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C. Fifty years with aspirin and platelets. Br. J. Pharmacol. 2023, 180, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Tantry, U.S.; Schrör, K.; Navarese, E.P.; Jeong, Y.H.; Kubica, J.; Bliden, K.P.; Gurbel, P.A. Aspirin as an adjunctive pharmacologic therapy option for COVID-19: Anti-Inflammatory, antithrombotic, and antiviral effects all in one agent. J. Exp. Pharmacol. 2021, 13, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Geiger, N.; König, E.M.; Oberwinkler, H.; Roll, V.; Diesendorf, V.; Fähr, S.; Obernolte, H.; Sewald, K.; Wronski, S.; Steinke, M.; et al. Acetylsalicylic acid and salicylic acid inhibit SARS-CoV-2 replication in precision-cut lung slices. Vaccines 2022, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Arif, H.; Aggarwal, S. Salicylic acid (aspirin). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; pp. 1–6. [Google Scholar]
- Jang, Y.; Kim, M.; Hwang, S.W. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J. Neuroinflamm. 2020, 17, 30. [Google Scholar] [CrossRef]
- Bianconi, V.; Violi, F.; Fallarino, F.; Pignatelli, P.; Sahebkar, A.; Pirro, M. Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID-19? Drugs 2020, 80, 1383–1396. [Google Scholar] [CrossRef]
- Cattaneo, M. Aspirin in essential thrombocythemia. For whom? What formulation? What regimen? Haematologica 2023, 108, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Mekaj, Y.H.; Daci, F.T.; Mekaj, A.Y. New insights into the mechanisms of action of aspirin and its use in the prevention and treatment of arterial and venous thromboembolism. Ther. Clin. Risk Manag. 2015, 11, 1449–1456. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.; Attur, M.G.; Pillinger, M.; Abramson, S.B. The pleiotropic functions of aspirin: Mechanisms of action. Cell. Mol. Life Sci. 1999, 56, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Javeed, A.; Ashraf, M.; Zhao, Y.; Mukhtar, M.M.; Rehman, M.U. Aspirin and immune system. Int. Immunopharmacol. 2012, 12, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Kata, D.; Földesi, I.; Feher, L.Z.; Hackler, L., Jr.; Puskas, L.G.; Gulya, K. A novel pleiotropic effect of aspirin: Beneficial regulation of pro- and anti-inflammatory mechanisms in microglial cells. Brain Res. Bull. 2017, 132, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.T.; Dietsch, E. Anti-influenza viral activity of aspirin in cell culture. N. Engl. J. Med. 1988, 319, 797. [Google Scholar] [CrossRef] [PubMed]
- Bitko, V.; Velazquez, A.; Yang, L.; Yang, Y.C.; Barik, S. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-κB and is inhibited by sodium salicylate and aspirin. Virology 1997, 232, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Speir, E.; Yu, Z.X.; Ferrans, V.J.; Huang, E.S.; Epstein, S.E. Aspirin attenuates cytomegalovirus infectivity and gene expression mediated by cyclooxygenase-2 in coronary artery smooth muscle cells. Circ. Res. 1998, 83, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Walz-Cicconi, M.A.; Weller, T.H. Dose-related effect of acetylsalicylic acid on replication of varicella zoster virus in vitro. Proc. Natl. Acad. Sci. USA 1984, 81, 5223–5226. [Google Scholar] [CrossRef] [PubMed]
- Primache, V.; Binda, S.; De Benedittis, G.; Barbi, M. In vitro activity of acetylsalicylic acid on replication of varicella-zoster virus. New Microbiol. 1998, 21, 397–401. [Google Scholar] [PubMed]
- Chen, C.J.; Raung, S.L.; Kuo, M.D.; Wang, Y.M. Suppression of Japanese encephalitis virus infection by non-steroidal anti-inflammatory drugs. J. Gen. Virol. 2002, 83, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Wang, H.; Li, Z.J.; Deng, X.Y.; Xiang, H.; Tao, Y.G.; Li, W.; Tang, M.; Cao, Y. Aspirin induces lytic cytotoxicity in Epstein-Barr virus-positive cells. Eur. J. Pharmacol. 2008, 589, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Murillo, K.; Rincón-Sánchez, A.R.; Martínez-Rodríguez, H.; Bosques-Padilla, F.; Ramos-Jiménez, J.; Barrera-Saldaña, H.A.; Rojkind, M.; Rivas-Estilla, A.M. Acetylsalicylic acid inhibits hepatitis C virus RNA and protein expression through cyclooxygenase 2 signaling pathways. Hepatology 2008, 47, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Estilla, A.M.; Bryan-Marrugo, O.L.; Trujillo-Murillo, K.; Pérez-Ibave, D.; Charles-Niño, C.; Pedroza-Roldan, C.; Ríos-Ibarra, C.; Ramírez-Valles, E.; Ortiz-López, R.; Islas-Carbajal, M.C.; et al. Cu/Zn superoxide dismutase (SOD1) induction is implicated in the antioxidative and antiviral activity of acetylsalicylic acid in HCV-expressing cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1264–G1273. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, A.; Ríos-Ibarra, C.P.; Rincón-Sánchez, A.R.; Ortiz-López, R.; Garza-Juárez, A.; Morlett-Chávez, J.; Martínez-Rodríguez, H.; Rivas-Estilla, A.M. Use of proteomic analysis tools to identify HCV-proteins down-regulated by acetylsalicylic acid. Ann. Hepatol. 2013, 12, 725–732. [Google Scholar] [CrossRef]
- Ríos-Ibarra, C.P.; Lozano-Sepulveda, S.; Muñoz-Espinosa, L.; Rincón-Sánchez, A.R.; Cordova-Fletes, C.; Rivas-Estilla, A.M. Downregulation of inducible nitric oxide synthase (iNOS) expression is implicated in the antiviral activity of acetylsalicylic acid in HCV-expressing cells. Arch. Virol. 2014, 159, 3321–3328. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, L. Aspirin inhibits hepatitis C virus entry by downregulating claudin-1. J. Viral Hepat. 2016, 23, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Peng, Z.; Tan, L.; Zou, F.; Zhou, N.; Liu, B.; Liang, L.; Chen, C.; Liu, J.; Wu, L.; et al. Nonsteroidal anti-inflammatory drugs potently inhibit the replication of Zika viruses by inducing the degradation of AXL. J. Virol. 2018, 92, e01018-18. [Google Scholar] [CrossRef] [PubMed]
- Glatthaar-Saalmüller, B.; Mair, K.H.; Saalmüller, A. Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study. Influenza Other Respir. Viruses 2017, 11, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Glatthaar-Saalmüller, B.; Saalmüller, A.; Mair, K.H. Anti-viral activity of acetylsalicylic acid against human rhinovirus 14 infection involves suppression of VP3 expression and infection-dependent down-regulation of CD54. Curr. Res. Virol. Sci. 2022, 3, 100022. [Google Scholar] [CrossRef]
- Fernández-Sánchez, S.Y.; Cerón-Carrasco, J.P.; Risco, C.; Fernández de Castro, I. Antiviral activity of acetylsalicylic acid against Bunyamwera birus in cell culture. Viruses 2023, 15, 948. [Google Scholar] [CrossRef] [PubMed]
- Mazur, I.; Wurzer, W.J.; Ehrhardt, C.; Pleschka, S.; Puthavathana, P.; Silberzahn, T.; Wolff, T.; Planz, O.; Ludwig, S. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-κB-inhibiting activity. Cell Microbiol. 2007, 9, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Z.; Yu, M.L.; Liu, Y.; Liu, C.C.; Jia, X.J.; Liu, M.Q.; Li, Y.G. Aspirin inhibits rotavirus replication and alters rat gut microbial composition. Virol. J. 2023, 20, 237. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Qadeer, A.; Xie, Y.; Jin, Y.; Li, Q.; Xiao, Y.; She, K.; Zheng, X.; Li, J.; Ji, S.; et al. Dietary Supplementation of aspirin promotes Drosophila defense against viral infection. Molecules 2023, 28, 5300. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.L.; Lin, Y.L.; Wu, B.C.; Tsao, C.H.; Wang, M.C.; Liu, C.I.; Huang, Y.L.; Chen, J.H.; Wang, J.P.; Chen, L.K. Salicylates inhibit flavivirus replication independently of blocking nuclear factor kappa B activation. J. Virol. 2001, 75, 7828–7839. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Bella, S.; Luzzati, R.; Principe, L.; Zerbato, V.; Meroni, E.; Giuffrè, M.; Crocè, L.S.; Merlo, M.; Perotto, M.; Dolso, E.; et al. Aspirin and infection: A narrative review. Biomedicines 2022, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Hart, F.D.; Huskisson, E.C. Non-steroidal anti-inflammatory drugs. Current status and rational therapeutic use. Drugs 1984, 27, 232–255. [Google Scholar] [CrossRef] [PubMed]
- Riera, E.; Olivé, A.; Narváez, J.; Holgado, S.; Santo, P.; Mateo, L.; Bianchi, M.M.; Nolla, J.M. Adult onset Still’s disease: Review of 41 cases. Clin. Exp. Rheumatol. 2011, 29, 331–336. [Google Scholar] [PubMed]
- Sakulchit, T.; Benseler, S.M.; Goldman, R.D. Acetylsalicylic acid for children with Kawasaki disease. Can. Fam. Physician 2017, 63, 607–609. [Google Scholar] [PubMed]
- Ralph, A.P.; Noonan, S.; Boardman, C.; Halkon, C.; Currie, B.J. Prescribing for people with acute rheumatic fever. Aust. Prescr. 2017, 40, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Cilliers, A.; Adler, A.J.; Saloojee, H. Anti-inflammatory treatment for carditis in acute rheumatic fever. Cochrane Database Syst. Rev. 2015, 5, CD003176. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Marques, C. Acute recurrent pericarditis: From pathophysiology towards new treatment strategy. Heart 2020, 106, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.; Shi, H.; Zhang, X.; Shao, Y.; Hang, B.; Xu, Z.; Rong, X.; Chu, M.; Qiu, H. Effect of different doses of aspirin on the prognosis of Kawasaki disease. Pediatr. Rheumatol. Online J. 2020, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Carestia, A.; Davis, R.P.; Grosjean, H.; Lau, M.W.; Jenne, C.N. Acetylsalicylic acid inhibits intravascular coagulation during Staphylococcus aureus-induced sepsis in mice. Blood 2020, 135, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Ho-Tin-Noé, B. Acetylsalicylic acid to fight thrombosis in sepsis. Blood 2020, 135, 1195–1196. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.; Kohli, P.; Nasserifar, S.; Sheehan, J.; Herrera, Y.; Poon, J.; Narasimhan, B.; Wu, L.; Salonia, J. Halting sepsis with aspirin? Am. J. Respir. Crit. Care Med. 2020, 201, A6012. [Google Scholar] [CrossRef]
- Ouyang, Y.; Wang, Y.; Liu, B.; Ma, X.; Ding, R. Effects of antiplatelet therapy on the mortality rate of patients with sepsis: A meta-analysis. J. Crit. Care. 2019, 50, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Otto, G.P.; Sossdorf, M.; Boettel, J.; Kabisch, B.; Breuel, H.; Winning, J.; Lösche, W. Effects of low-dose acetylsalicylic acid and atherosclerotic vascular diseases on the outcome in patients with severe sepsis or septic shock. Platelets 2013, 24, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Lösche, W.; Boettel, J.; Kabisch, B.; Winning, J.; Claus, R.A.; Bauer, M. Do aspirin and other antiplatelet drugs reduce the mortality in critically ill patients? Thrombosis 2012, 2012, 720254. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D.P.; Reid, D.; McBryde, E.S. Acetyl salicylic acid usage and mortality in critically ill patients with the systemic inflammatory response syndrome and sepsis. Crit. Care Med. 2012, 40, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.; McGuire, A.; Young, L.; Mackay, A. Aspirin and statin therapy in sepsis, a red herring? Intensive Care Med. Exp. 2015, 3 (Suppl. 1), A227. [Google Scholar] [CrossRef]
- Al Harbi, S.A.; Tamim, H.M.; Al-Dorzi, H.M.; Sadat, M.; Arabi, Y.M. Association between aspirin therapy and the outcome in critically ill patients: A nested cohort study. BMC Pharmacol. Toxicol. 2016, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Sossdorf, M.; Otto, G.P.; Boettel, J.; Winning, J.; Lösche, W. Benefit of low-dose aspirin and non-steroidal anti-inflammatory drugs in septic patients. Crit. Care. 2013, 17, 402. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Donnelly, J.P.; Chaudhary, N.S.; Moore, J.X.; Safford, M.M.; Kim, J.; Wang, H.E. Aspirin use and long-term rates of sepsis: A population-based cohort study. PLoS ONE 2018, 13, e0194829. [Google Scholar] [CrossRef] [PubMed]
- Trauer, J.; Muhi, S.; McBryde, E.S.; Al Harbi, S.A.; Arabi, Y.M.; Boyle, A.J.; Cartin-Ceba, R.; Chen, W.; Chen, Y.T.; Falcone, M.; et al. Quantifying the effects of prior acetyl-salicylic acid on sepsis-related deaths: An individual patient data meta-analysis using propensity matching. Crit. Care Med. 2017, 45, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D.P.; Leder, K.; Woods, R.L.; Lockery, J.E.; McGuinness, S.L.; Wolfe, R.; Pilcher, D.; Moore, E.M.; Shastry, A.; Nelson, M.R.; et al. Effect of aspirin on deaths associated with sepsis in healthy older people (ANTISEPSIS): A randomised, double-blind, placebo-controlled primary prevention trial. Lancet Respir. Med. 2021, 9, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ni, Y.N.; Liang, Z.A.; Liang, B.M.; Wang, Y. The effect of aspirin in preventing the acute respiratory distress syndrome/acute lung injury: A meta-analysis. Am. J. Emerg. Med. 2018, 36, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Panka, B.A.; de Grooth, H.J.; Spoelstra-de Man, A.M.; Looney, M.R.; Tuinman, P.R. Prevention or treatment of ARDS with aspirin: A review of preclinical models and meta-analysis of clinical studies. Shock 2017, 47, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ding, X.; Li, H.; Li, L.; Sun, T. Association between prior aspirin use and acute respiratory distress syndrome incidence in at-risk patients: A systematic review and meta-analysis. Front. Pharmacol. 2020, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Kor, D.J.; Carter, R.E.; Park, P.K.; Festic, E.; Banner-Goodspeed, V.M.; Hinds, R.; Talmor, D.; Gajic, O.; Ware, L.B.; Gong, M.N.; et al. Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: The LIPS-A randomized clinical trial. JAMA 2016, 315, 2406–2414. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Lu, H.C.; Tung, Y.T.; Chen, W. Antiplatelet therapy for acute respiratory distress syndrome. Biomedicines 2020, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Toner, P.; Boyle, A.J.; McNamee, J.J.; Callaghan, K.; Nutt, C.; Johnston, P.; Trinder, J.; McFarland, M.; Verghis, R.; McAuley, D.F.; et al. Aspirin as a treatment for ARDS: A randomized, placebo-controlled clinical trial. Chest 2022, 161, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.E.; Oldman, A.D.; Smith, L.A.; Carroll, D.; Wiffen, P.J.; McQuay, H.J.; Moore, A.R. Oral aspirin in postoperative pain: A quantitative systematic review. Pain 1999, 81, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Moore, R.A. Single dose oral aspirin for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2012, 4, CD002067. [Google Scholar] [CrossRef] [PubMed]
- McQuay, H.J.; Moore, R.A. Dose-response in direct comparisons of different doses of aspirin, ibuprofen and paracetamol (acetaminophen) in analgesic studies. Br. J. Clin. Pharmacol. 2007, 63, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Chuchalin, A.G.; Eisebitt, R.; Netayzhenko, V.Z.; Voelker, M. Aspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: A multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, single-dose, 6-h dose-ranging study. Clin. Ther. 2005, 27, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Voelker, M.; Schachtel, B.P.; Cooper, S.A.; Gatoulis, S.C. Efficacy of disintegrating aspirin in two different models for acute mild-to-moderate pain: Sore throat pain and dental pain. Inflammopharmacology 2016, 24, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Eccles, R.; Loose, I.; Jawad, M.; Nyman, L. Effects of acetylsalicylic acid on sore throat pain and other pain symptoms associated with acute upper respiratory tract infection. Pain Med. 2003, 4, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.A.; Desjardins, P.J. The value of the dental impaction pain model in drug development. Methods Mol. Biol. 2010, 617, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.A.; Voelker, M. Evaluation of onset of pain relief from micronized aspirin in a dental pain model. Inflammopharmacology 2012, 20, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Kirthi, V.; Derry, S.; Moore, R.A. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst. Rev. 2013, 4, CD008041. [Google Scholar] [CrossRef] [PubMed]
- Derry, C.J.; Derry, S.; Moore, R.A. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database Syst. Rev. 2014, 12, CD009281. [Google Scholar] [CrossRef] [PubMed]
- Schachtel, B.P.; Voelker, M.; Sanner, K.M.; Gagney, D.; Bey, M.; Schachtel, E.J.; Becka, M. Demonstration of the analgesic efficacy and dose-response of acetylsalicylic acid with pseudoephedrine. J. Clin. Pharmacol. 2010, 50, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Gaul, C.; Lehmacher, W.; Weiser, T. Aspirin, paracetamol (acetaminophen) and caffeine for the treatment of acute migraine attacks: A systemic review and meta-analysis of randomized placebo-controlled trials. Eur. J. Neurol. 2022, 29, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C.; Ciabattoni, G.; Patrignani, P.; Pugliese, F.; Filabozzi, P.; Catella, F.; Davì, G.; Forni, L. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation 1985, 72, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C.; García Rodríguez, L.A.; Landolfi, R.; Baigent, C. Low-dose aspirin for the prevention of atherothrombosis. N. Engl. J. Med. 2005, 353, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002, 324, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cook, N.R.; Lee, I.M.; Gordon, D.; Gaziano, J.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N. Engl. J. Med. 2005, 352, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Becattini, C.; Agnelli, G.; Schenone, A.; Eichinger, S.; Bucherini, E.; Silingardi, M.; Bianchi, M.; Moia, M.; Ageno, W.; Vandelli, M.R.; et al. Aspirin for preventing the recurrence of venous thromboembolism. N. Engl. J. Med. 2012, 366, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Brighton, T.A.; Eikelboom, J.W.; Mann, K.; Mister, R.; Gallus, A.; Ockelford, P.; Gibbs, H.; Hague, W.; Xavier, D.; Diaz, R.; et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N. Engl. J. Med. 2012, 367, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Simes, J.; Becattini, C.; Agnelli, G.; Eikelboom, J.W.; Kirby, A.C.; Mister, R.; Prandoni, P.; Brighton, T.A.; INSPIRE Study Investigators (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the prevention of recurrent venous thromboembolism: The INSPIRE collaboration. Circulation 2014, 130, 1062–1071. [Google Scholar] [CrossRef]
- Warkentin, T.E. Aspirin for dual prevention of venous and arterial thrombosis. N. Engl. J. Med. 2012, 367, 2039–2041. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Brummel-Ziedins, K.; Mann, K.G. Why does aspirin decrease the risk of venous thromboembolism? On old and novel antithrombotic effects of acetyl salicylic acid. J. Thromb. Haemost. 2014, 12, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Haykal, T.; Kheiri, B.; Zayed, Y.; Barbarawi, M.; Miran, M.S.; Chahine, A.; Katato, K.; Bachuwa, G. Aspirin for venous thromboembolism prophylaxis after hip or knee arthroplasty: An updated meta-analysis of randomized controlled trials. J. Orthop. 2019, 16, 312–319. [Google Scholar] [CrossRef]
- Matharu, G.S.; Kunutsor, S.K.; Judge, A.; Blom, A.W.; Whitehouse, M.R. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: A aystematic review and meta-analysis of randomized clinical trials. JAMA Intern. Med. 2020, 180, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Coveney, E.I.; Hutton, C.; Patel, N.; Whitehouse, S.L.; Howell, J.R.; Wilson, M.J.; Hubble, M.J.; Charity, J.; Kassam, A.M. Incidence of symptomatic venous thromboembolism (VTE) in 8,885 elective total hip arthroplasty patients receiving post-operative aspirin VTE prophylaxis. Cureus 2023, 15, e36464. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Chan, N.C. Long-term management of venous thromboembolism: Lessons from EINSTEIN CHOICE and other extension trials. Thromb. Haemost. 2019, 119, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Walker, V.; Ip, S.; Cooper, J.A.; Bolton, T.; Keene, S.; Denholm, R.; Akbari, A.; Abbasizanjani, H.; Torabi, F.; et al. Association of COVID-19 with major arterial and venous thrombotic diseases: A population-wide cohort study of 48 million adults in England and Wales. Circulation 2022, 146, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Kune, G.A.; Kune, S.; Watson, L.F. Colorectal cancer risk, chronic illnesses, operations, and medications: Case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988, 48, 4399–4404. [Google Scholar] [CrossRef] [PubMed]
- Elwood, P.; Morgan, G.; Watkins, J.; Protty, M.; Mason, M.; Adams, R.; Dolwani, S.; Pickering, J.; Delon, C.; Longley, M. Aspirin and cancer treatment: Systematic reviews and meta-analyses of evidence: For and against. Br. J. Cancer 2023, 130, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, L.M. Using aspirin to prevent and treat cancer. Inflammopharmacology 2023, 32, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Elwood, P.C.; Morgan, G.; Delon, C.; Protty, M.; Galante, J.; Pickering, J.; Watkins, J.; Weightman, A.; Morris, D. Aspirin and cancer survival: A systematic review and meta-analyses of 118 observational studies of aspirin and 18 cancers. Ecancermedicalscience 2021, 15, 1258. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.; Cafferty, F.H.; Rowley, S.; MacKenzie, M.; Berkman, L.; Gupta, S.; Pramesh, C.S.; Gilbert, D.; Kynaston, H.; Cameron, D.; et al. ADD-ASPIRIN: A phase III, double-blind, placebo controlled, randomised trial assessing the effects of aspirin on disease recurrence and survival after primary therapy in common non-metastatic solid tumours. Contemp. Clin. Trials 2016, 51, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Joharatnam-Hogan, N.; Cafferty, F.; Hubner, R.; Swinson, D.; Sothi, S.; Gupta, K.; Falk, S.; Patel, K.; Warner, N.; Kunene, V.; et al. Aspirin as an adjuvant treatment for cancer: Feasibility results from the Add-Aspirin randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 854–862. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Cook, N.R.; Gaziano, J.M.; Price, J.F.; Belch, J.F.F.; Roncaglioni, M.C.; Morimoto, T.; Mehta, Z. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: Analysis of individual patient data from randomised trials. Lancet 2018, 392, 387–399. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Nelson, M.R.; Woods, R.L.; Lockery, J.E.; Wolfe, R.; Reid, C.M.; Kirpach, B.; Shah, R.C.; Ives, D.G.; Storey, E.; et al. Effect of aspirin on all-cause mortality in the healthy elderly. N. Engl. J. Med. 2018, 379, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Gibbs, P.; Orchard, S.G.; Lockery, J.E.; Bernstein, W.B.; Cao, Y.; Ford, L.; Haydon, A.; Kirpach, B.; Macrae, F.; et al. Effect of aspirin on cancer incidence and mortality in older adults. J. Natl. Cancer Inst. 2021, 113, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Kasza, J.; Woods, R.L.; Lockery, J.E.; Kirpach, B.; Reid, C.M.; Storey, E.; Nelson, M.R.; Shah, R.C.; Orchard, S.G.; et al. Compliance-adjusted estimates of aspirin effects among older persons in the ASPREE randomized trial. Am. J. Epidemiol. 2023, 192, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Toh, H.C.; Chia, W.K.; ASCOLT Trial Investigators. The utility of aspirin in Dukes C and high risk Dukes B colorectal cancer-the ASCOLT study: Study protocol for a randomized controlled trial. Trials 2011, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, E.; Francisci, D.; Schiaroli, E.; Minuz, P.; Orsini, S.; Malincarne, L.; Sebastiano, M.; Mezzasoma, A.M.; Pasticci, M.B.; Guglielmini, G.; et al. Effect of aspirin treatment on abacavir-associated platelet hyperreactivity in HIV-infected patients. Int. J. Cardiol. 2018, 263, 118–124. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Montenont, E.; Hu, L.; Nardi, M.A.; Valdes, V.; Merolla, M.; Gettenberg, G.; Cavanagh, K.; Aberg, J.A.; Bhardwaj, N.; et al. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: A pilot study. J. Acquir. Immune Defic. Syndr. 2013, 63, 280–288. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.P.; Hunt, P.W.; Kitch, D.W.; Klingman, K.; Stein, J.H.; Funderburg, N.T.; Berger, J.S.; Tebas, P.; Clagett, B.; Moisi, D.; et al. A randomized placebo controlled trial of aspirin effects on immune activation in chronically human immunodeficiency virus-infected adults on virologically suppressive antiretroviral therapy. Open Forum Infect. Dis. 2017, 4, ofw278. [Google Scholar] [CrossRef] [PubMed]
- Mystakelis, H.A.; Wilson, E.; Laidlaw, E.; Poole, A.; Krishnan, S.; Rupert, A.; Welker, J.L.; Gorelick, R.J.; Lisco, A.; Manion, M.; et al. An open label randomized controlled trial of atorvastatin versus aspirin in elite controllers and antiretroviral-treated people with HIV. AIDS 2023, 37, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Sayed Ahmed, H.A.; Merrell, E.; Ismail, M.; Joudeh, A.I.; Riley, J.B.; Shawkat, A.; Habeb, H.; Darling, E.; Goweda, R.A.; Shehata, M.H.; et al. Rationales and uncertainties for aspirin use in COVID-19: A narrative review. Fam. Med. Community Health 2021, 9, e000741. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Su, W.; Sun, C.; Lowe, S.; Zhou, Z.; Liu, H.; Qu, G.; Xia, W.; Xie, P.; Wu, B.; et al. Does aspirin have an effect on risk of death in patients with COVID-19? A meta-analysis. Eur. J. Clin. Pharmacol. 2022, 78, 1403–1420. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Mehta, J.L. Meta-analysis of the effect of aspirin on mortality in COVID-19. Am. J. Cardiol. 2021, 142, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Hasan, S.S. Use of antiplatelet drugs and the risk of mortality in patients with COVID-19: A meta-analysis. J. Thromb. Thrombolysis 2021, 52, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Kumar, A. Use of aspirin in reduction of mortality of COVID-19 patients: A meta-analysis. Int. J. Clin. Pract. 2021, 75, e14515. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, I.; Andhika, R.; Huang, I.; Purwiga, A.; Budiman, K.Y. The effects of aspirin on the outcome of COVID-19: A systematic review and meta-analysis. Clin. Epidemiol. Glob. Health 2021, 12, 100883. [Google Scholar] [CrossRef] [PubMed]
- Martha, J.W.; Pranata, R.; Lim, M.A.; Wibowo, A.; Akbar, M.R. Active prescription of low-dose aspirin during or prior to hospitalization and mortality in COVID-19: A systematic review and meta-analysis of adjusted effect estimates. Int. J. Infect. Dis. 2021, 108, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Brooks, M.M.; Sciurba, F.C.; Krishnan, J.A.; Bledsoe, J.R.; Kindzelski, A.; Baucom, A.L.; Kirwan, B.A.; Eng, H.; Martin, D.; et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: The ACTIV-4B randomized clinical trial. JAMA 2021, 326, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2022, 399, 143–151. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Jolly, S.S.; Belley-Cote, E.P.; Whitlock, R.P.; Rangarajan, S.; Xu, L.; Heenan, L.; Bangdiwala, S.I.; Tarhuni, W.M.; Hassany, M.; et al. Colchicine and aspirin in community patients with COVID-19 (ACT): An open-label, factorial, randomised, controlled trial. Lancet Respir. Med. 2022, 10, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- REMAP-CAP Writing Committee for the REMAP-CAP Investigators; Bradbury, C.A.; Lawler, P.R.; Stanworth, S.J.; McVerry, B.J.; McQuilten, Z.; Higgins, A.M.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; et al. Effect of antiplatelet therapy on survival and organ support-free days in critically ill patients with COVID-19: A randomized clinical trial. JAMA 2022, 327, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Rafaniello, C.; Ferrajolo, C.; Sullo, M.G.; Sessa, M.; Sportiello, L.; Balzano, A.; Manguso, F.; Aiezza, M.L.; Rossi, F.; Scarpignato, C.; et al. Risk of gastrointestinal complications associated to NSAIDs, low-dose aspirin and their combinations: Results of a pharmacovigilance reporting system. Pharmacol. Res. 2016, 104, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.; Kyaw, M.; Tanigawa, T.; Higuchi, K.; Fujimoto, K.; Cheong, P.K.; Lee, V.; Kinoshita, Y.; Naito, Y.; Watanabe, T.; et al. Similar efficacy of proton-pump inhibitors vs H2-receptor antagonists in reducing risk of upper gastrointestinal bleeding or ulcers in high-risk users of low-dose aspirin. Gastroenterology 2017, 152, 105–110.e1. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A.; McCarthy, D.; Voelker, M.; Brueckner, A.; Senn, S.; Baron, J.A. Short-term acetylsalicylic acid (aspirin) use for pain, fever, or colds-gastrointestinal adverse effects: A meta-analysis of randomized clinical trials. Drugs R D 2011, 11, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A.; Werz, O.; Mikhail, E. Comparison of gastrointestinal adverse events between fast release tablets and regular acetylsalicylic acid (aspirin) galenics after short-term use: A meta-analysis of randomized clinical trials. Inflammopharmacology 2023, 31, 2369–2381. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Arnold, J.K. Reye syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; pp. 1–6. [Google Scholar]
- Mills, J.A. Aspirin, the ageless remedy? N. Engl. J. Med. 1991, 325, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Feng, G.; Fang, Y. Association between aspirin use and risk of dementia: A systematic review and meta-analysis. Eur. Geriatr. Med. 2023, 15, 3–18. [Google Scholar] [CrossRef] [PubMed]


| Reference and Type of Study | Virus | Infection Model | Key Results of Aspirin Exposure |
|---|---|---|---|
| [21] In vitro | Influenza viruses (A/H7/N1 and A/H1N1/6/86) | MDCK cells | Inhibition of viral activity |
| [22] In vitro | RSV | A549 (human lung carci-noma epithelial cells) | Inhibition of RSV-mediated transcriptional induction of multiple cytokines |
| [23] In vitro | CMV | Coronary artery smooth muscle cells | Inhibition of CMV replication |
| [24] In vitro | VZV | Human embryonic lung cells | Reduction in VZV replication; the inhibition was partially reversible, depending on the concentration and exposure time |
| [25] In vitro | VZV | MRC-5 (human lung fibroblast) and Vero (monkey kidney epithelial) cells | High doses reduced VZV replication |
| [26] In vitro | JEV | N18 (mouse neuroblastoma cells) | Suppression of JEV propagation |
| [27] In vitro | EBV | EBV-positive B95.8 and Raji cells as well as EBV-negative BJAB cells | Induction of EBV lytic replication in host cells, which resulted in the killing of EBV-positive cells |
| [28] In vitro | HCV | Huh7 (human hepatoma) cells expressing nonstructural HCV proteins | Suppressive effects on HCV-RNA and protein expression partly caused by inhibiting COX-2 signaling pathways |
| [29] In vitro | HCV | Huh7 (human hepatoma) cells expressing nonstructural HCV proteins | Reduction in cellular oxidative stress and modulation of Cu/Zn SOD1 expression, thereby reducing the pathogenic effects of HCV |
| [30] In vitro | HCV | Huh7 (human hepatoma) cells expressing nonstructural HCV proteins | Overexpression of proteins that inhibit HCV replication |
| [31] In vitro | HCV | Huh7 (human hepatoma) cells expressing nonstructural HCV proteins | Reduction in iNOS expression and HCV-RNA replication |
| [32] In vitro | HCV | Huh7.5.1 (human hepatoma) cells | Downregulation of claudin-1 expression, an HCV receptor, thereby inhibiting HCV cell entry |
| [33] In vitro | Zika virus | Zika virus-sensitive cell lines, such as A549 (human lung carcinoma epithelial) and Vero (monkey kidney epithelial) cells | Inhibition of Zika virus replication by downregulating the viral entry cofactor AXL, a receptor tyrosine kinase known to regulate diverse cellular processes |
| [34] In vitro | Various respiratory RNA viruses, including influenza A H1N1 | MDCK, human epithelial, HeLa, and buffalo green monkey cells | Specific antiviral activity against influenza A virus, human rhinoviruses, and coxsackievirus (subtype 9) |
| [35] In vitro | Human rhinovirus type 14 | HeLa cells | Dose-dependent antiviral activity; the antiviral effect involved the suppression of VP3 expression, a major structural protein, and the infection-dependent downregulation of CD54 |
| [11] In vitro | SARS-CoV-2 | A549-ACE2 (human lung carcinoma epithelial cells expressing ACE2) as well as Huh7 (human hepatoma) and Vero (monkey kidney epithelial) cells Human precision-cut lung slices | Suppression of SARS-CoV-2 replication by up to two orders of magnitude; a lower viral RNA expression was observed in both models, indicating that post-entry pathways were inhibited |
| [36] In vitro | Bunyamwera | Vero (monkey kidney epi-thelial) cells | Inhibition of Bunyamwera infections by interfering with the biogenesis of its replication organelle |
| [37] In vitro and in vivo | Influenza viruses | MDCK and A549 (human lung carcinoma epithelial) cells Mice | Blocking of influenza virus replication in vitro and in vivo via NF-κB inhibition |
| [38] In vitro and in vivo | Rotavirus | MA104 (rhesus monkey kidney), Caco-2 (human colon adenocarci-noma), and CV-1 (fetal African green monkey kidney) cells Rats | Inhibition of rotavirus infection in cell lines and in rats Alteration of rat gut microbial composition |
| [39] In vivo | Several types of viruses | Drosophila | Higher resistances to viral infections largely through the mediation of the stimulator of interferon genes signaling pathway |
| Group | Cancer Mortality | All-Cause Mortality | ||
|---|---|---|---|---|
| No. of Studies | HRs (95% CIs) ORs (95% CIs) | No. of Studies | HRs (95% CIs) ORs (95% CIs) | |
| Colon cancer | 24 | HR 0.71 (0.62, 0.80) | 21 | HR 0.81 (0.73, 0.91) |
| 1 | OR 0.78 (0.66, 0.93) | 1 | OR 0.78 (0.65, 0.92) | |
| Breast cancer | 13 | HR 0.84 (0.72, 0.98) | 9 | HR 0.94 (0.70, 1.25) |
| 4 | OR 0.75 (0.36, 1.57) | None | - | |
| Prostate cancer | 15 | HR 0.89 (0.78, 1.02) | 6 | HR 1.00 (0.78, 1.27) |
| 1 | OR 1.02 (0.78, 1.34) | 1 | OR 1.06 (0.94, 1.19) | |
| 15 other cancers | 18 | HR 0.79 (0.70, 0.83) | 21 | HR 0.67 (0.60, 0.75) |
| 5 | OR 0.49 (0.26, 0.95) | 5 | OR 0.47 (0.26, 0.83) | |
| All 18 cancers | 70 | HR 0.77 (0.72, 0.83) | 56 | HR 0.79 (0.74, 0.86) |
| 11 | OR 0.67 (0.45, 1.00) | 7 | OR 0.57 (0.36, 0.89) |
| Symptom | Time Point (h) | Aspirin | Placebo | |
|---|---|---|---|---|
| 500 mg | 1000 mg | |||
| Headache | 0 | 6.44 (2.10) | 6.60 (2.05) | 6.12 (2.12) |
| 2 | 4.36 (1.94) # | 4.00 (1.85) # | 5.72 (1.93) | |
| 4 | 4.03 (1.99) # | 3.58 (2.01) # | 5.76 (2.14) | |
| 6 | 4.41 (2.18) # | 3.76 (2.26) # | 5.78 (2.06) | |
| Achiness | 0 | 6.12 (1.94) | 6.21 (2.37) | 5.62 (2.25) |
| 2 | 4.60 (1.85) $ | 3.65 (2.11) # | 5.36 (2.06) | |
| 4 | 4.31 (1.97) $ | 3.19 (2.25) # | 5.33 (2.21) | |
| 6 | 4.77 (2.04) | 3.36 (2.47) # | 5.26 (2.20) | |
| Feverish discomfort | 0 | 6.96 (1.43) | 7.14 (1.74) | 6.53 (1.54) |
| 2 | 5.00 (1.77) # | 4.12 (2.23) # | 6.21 (1.75) | |
| 4 | 4.75 (1.90) # | 3.49 (2.36) # | 6.08 (2.01) | |
| 6 | 5.29 (2.04) § | 3.71 (2.56) # | 6.00 (1.93) | |
| Reference | Study Population | Number of Studies/Patients | Key Results | Comment |
|---|---|---|---|---|
| [112] | Patients with confirmed COVID-19 | 3 studies/ n = 1054 | Mortality among aspirin users was 22.6% vs. 18.3% among non-aspirin users (RR: 1.12; 95% CI: 0.84–1.50) | No association was seen between aspirin use and mortality |
| [113] | Hospitalized COVID-19 patients | 6 studies/ n = 14,377 | Significantly reduced mortality risk in aspirin users relative to non-users (OR: 0.50; 95% CI: 0.32–0.77; HR: 0.50; 95% CI: 0.36–0.69) | Aspirin was associated with a reduced mortality risk but not antiplatelet drugs as a class |
| [114] | Adult patients with confirmed COVID-19 | 10 studies/ n = 56,696 | Significantly reduced mortality risk in aspirin users relative to non-users (OR: 0.70; 95% CI: 0.63–0.77) | No significant effect was seen after the exclusion of outlier studies in terms of sample size |
| [115] | Hospitalized, adult COVID-19 patients | 7 studies/ n = 34,415 | Significantly reduced mortality risk in aspirin users relative to non-users (RR: 0.56; 95% CI: 0.38–0.81; p = 0.002) | Effect of aspirin on the incidence of thrombosis and bleeding could not be judged due to low reporting in the studies |
| [116] | Hospitalized COVID-19 patients with low-dose aspirin (75–325 mg/day) before or during hospital stay | 6 studies/ n = 13,993 | Significantly reduced mortality risk in aspirin users relative to non-users (RR: 0.46; 95% CI: 0.35–0.61; p < 0.001) | Subgroup analysis on in-hospital aspirin use also revealed a significant mortality reduction |
| [111] | Hospitalized COVID-19 patients | 17 studies/ n = 49,041 | Significantly reduced mortality risk in aspirin users relative to non-users (adjusted RR: 0.69; 95% CI: 0.50–0.95; p < 0.001) | Subgroup analysis of low-dose aspirin use (80–100 mg/day) also showed a significant mortality reduction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werz, O.; Stettler, H.; Theurer, C.; Seibel, J. The 125th Anniversary of Aspirin—The Story Continues. Pharmaceuticals 2024, 17, 437. https://doi.org/10.3390/ph17040437
Werz O, Stettler H, Theurer C, Seibel J. The 125th Anniversary of Aspirin—The Story Continues. Pharmaceuticals. 2024; 17(4):437. https://doi.org/10.3390/ph17040437
Chicago/Turabian StyleWerz, Oliver, Hans Stettler, Christoph Theurer, and Jens Seibel. 2024. "The 125th Anniversary of Aspirin—The Story Continues" Pharmaceuticals 17, no. 4: 437. https://doi.org/10.3390/ph17040437
APA StyleWerz, O., Stettler, H., Theurer, C., & Seibel, J. (2024). The 125th Anniversary of Aspirin—The Story Continues. Pharmaceuticals, 17(4), 437. https://doi.org/10.3390/ph17040437







