Comparative Analysis of the Sedative and Hypnotic Effects among Various Parts of Zizyphus spinosus Hu and Their Chemical Analysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Pharmacodynamic Evaluation Results of Different Parts of ZS
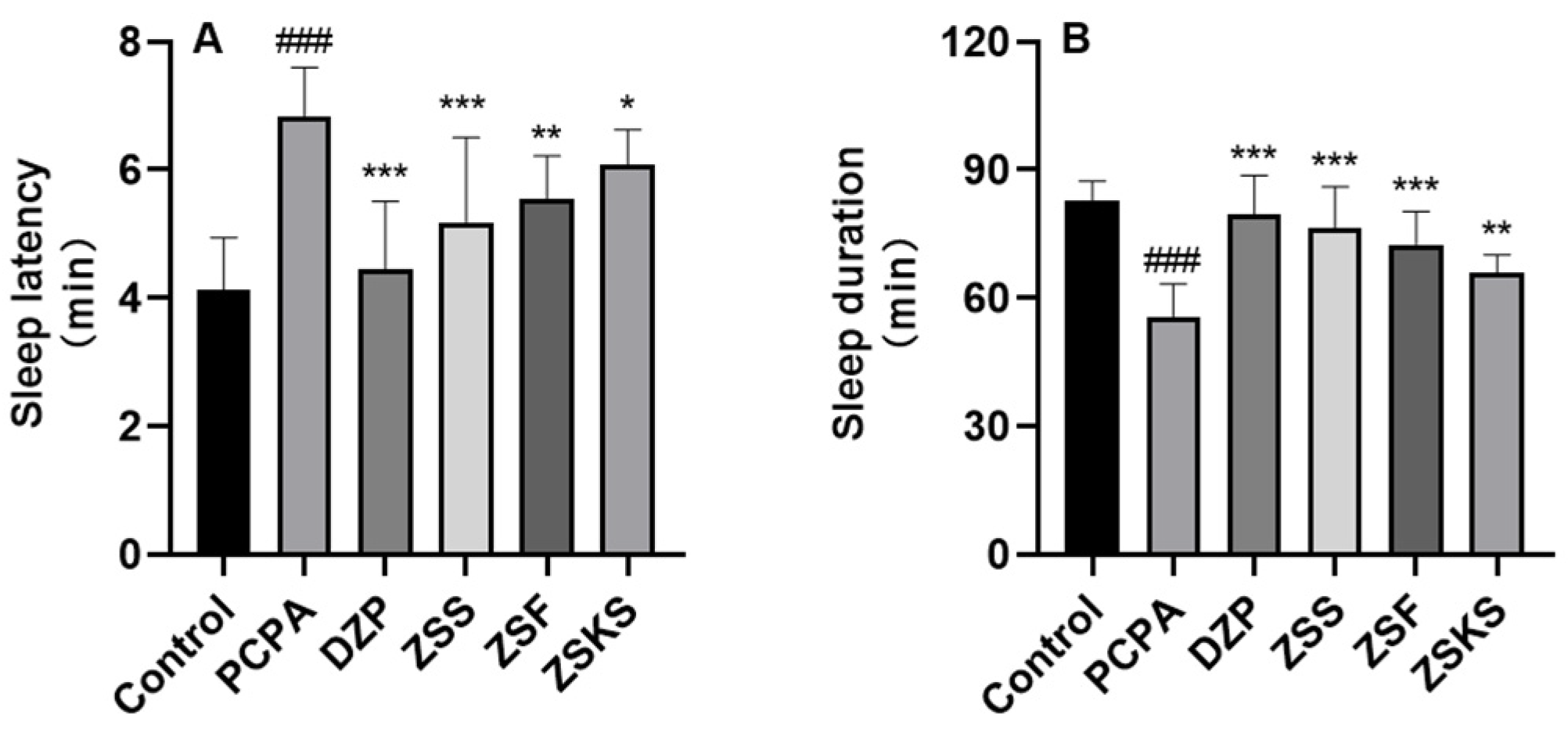
2.1.1. Sleep Latency and Sleep Duration
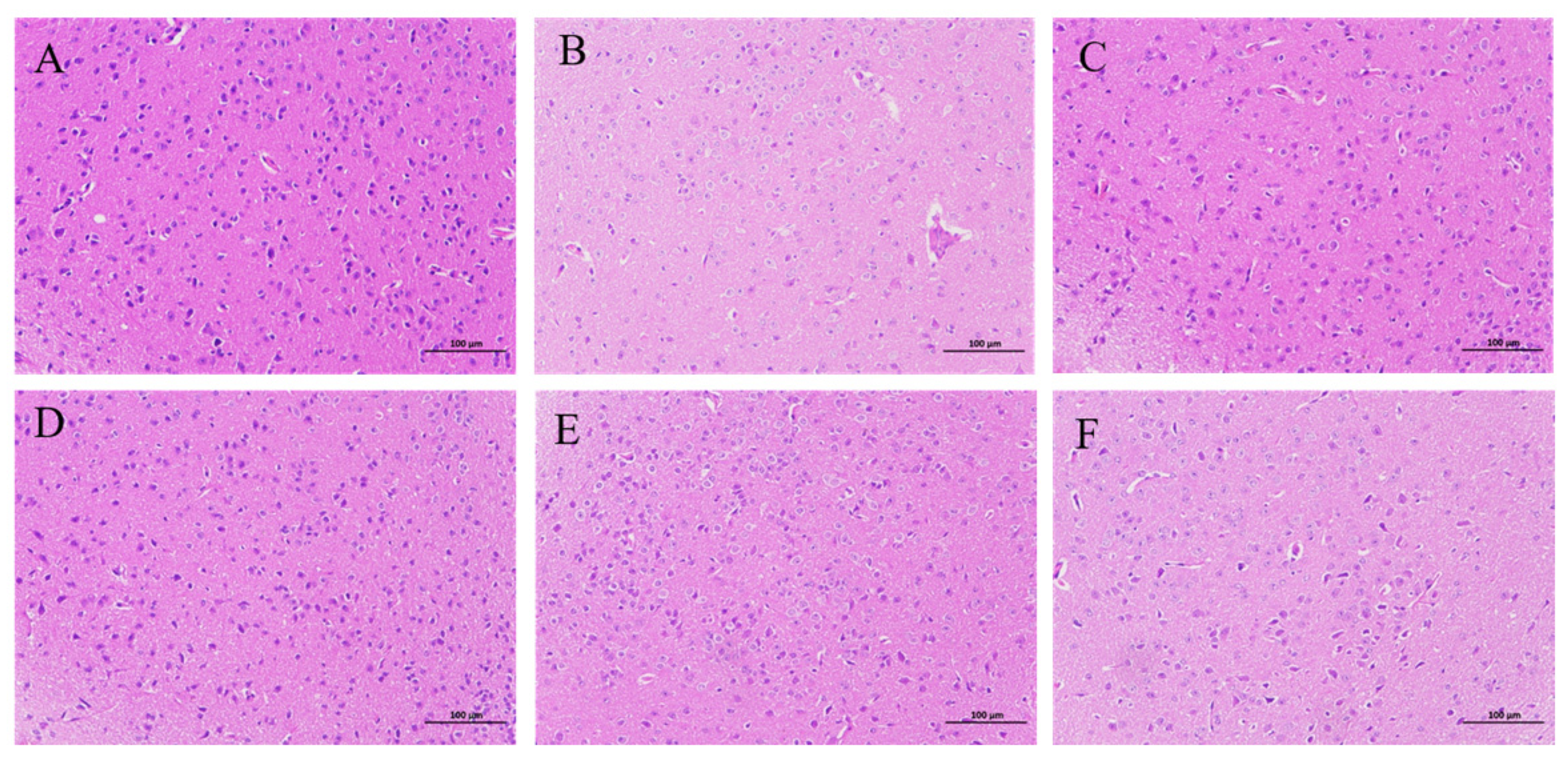
2.1.2. HE Staining
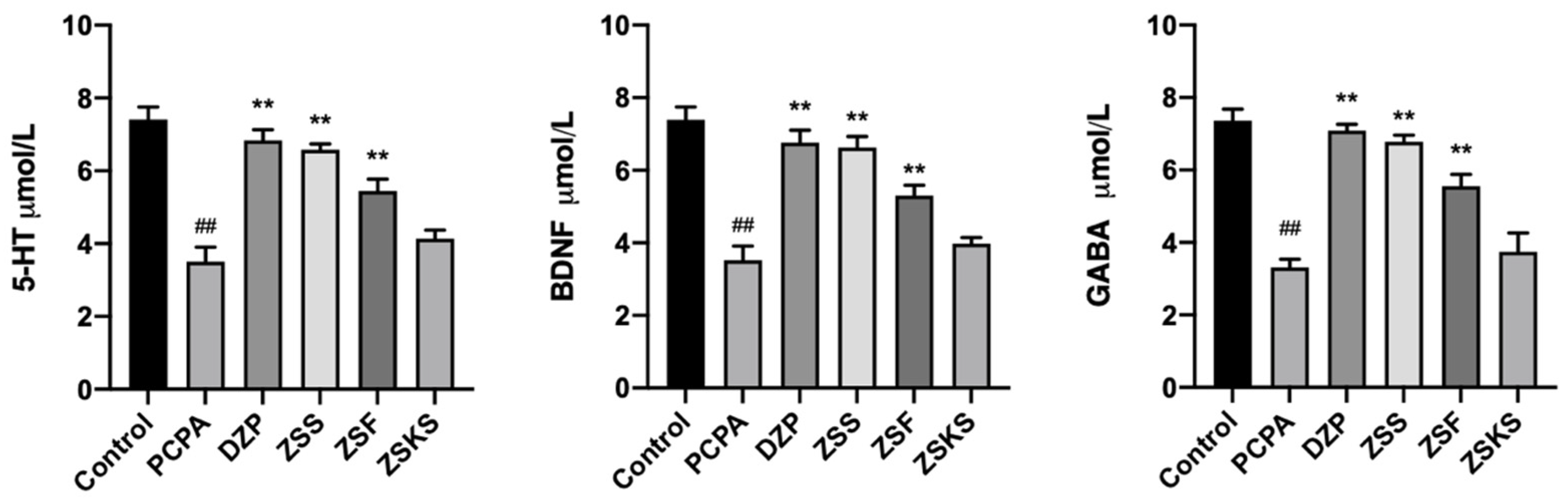
2.1.3. ELISA Analysis
2.1.4. PCR Analysis
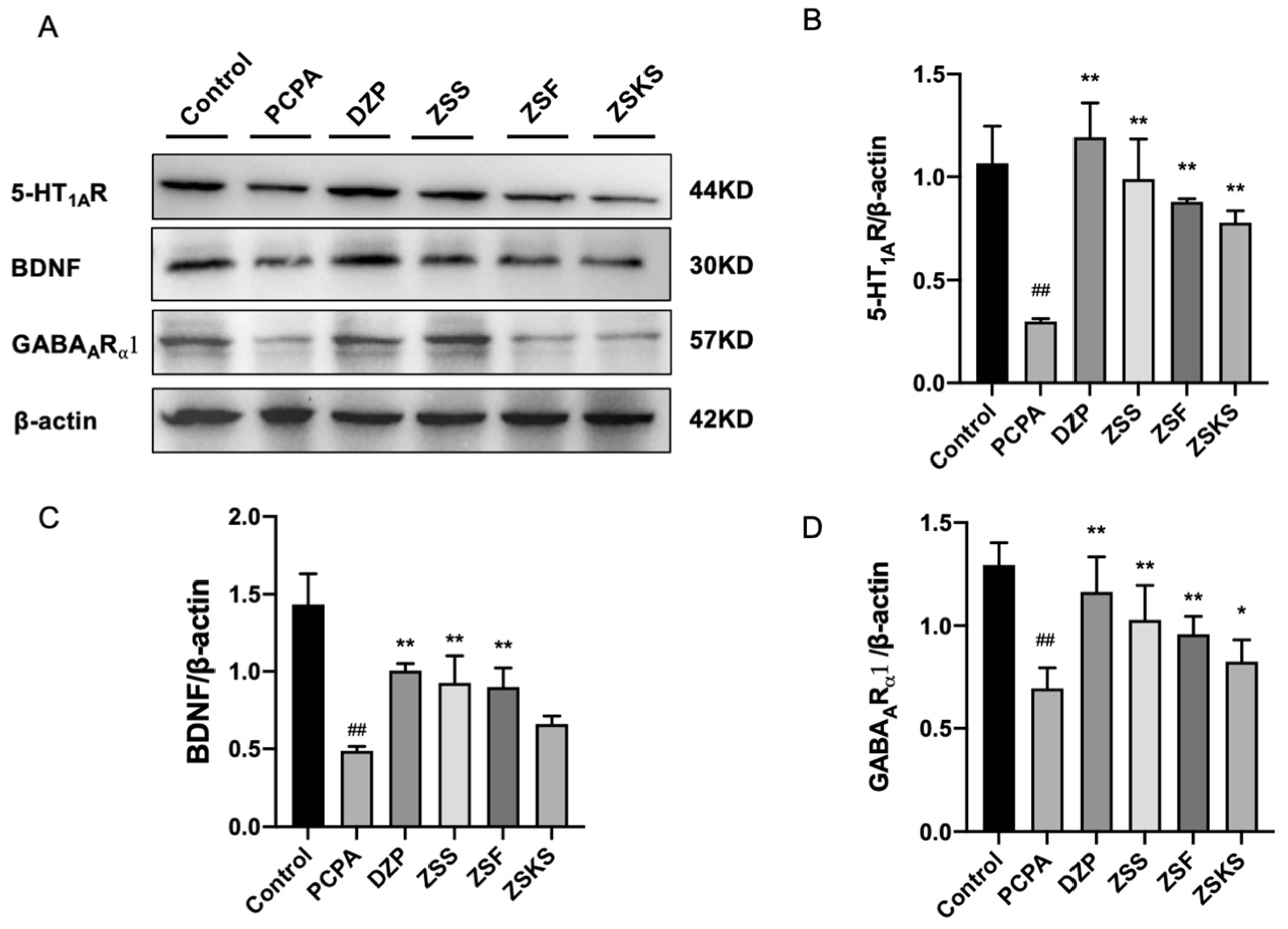
2.1.5. Western Blotting
2.2. The Constituents Analysis of Different Parts of ZS
2.2.1. Alkaloid
2.2.2. Triterpenoid
2.2.3. Flavonoid
2.2.4. Saponin
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Gavage Solution
3.2.1. Preparation of PCPA Suspensions
3.2.2. Preparation of Gavage Liquid from Different Parts of ZS
3.2.3. Preparation of Diazepam Gavage Solution
3.3. Animals
3.4. Animal Modeling and Drug Administration
3.5. Sodium Pentobarbital Synergistic Sleep Experiment
3.6. HE Staining
3.7. ELISA Assay
3.8. RT-qPCR Assay
3.9. Western Blotting Analysis
3.10. Preparation of Control and Test Solution
3.11. UPLC-QTOF-MS Analysis
3.12. Compound Structure Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Edinger, J.D.; Arnedt, J.T.; Bertisch, S.M.; Carney, C.E.; Harrington, J.J.; Lichstein, K.L.; Sateia, M.J.; Troxel, W.M.; Zhou, E.S.; Kazmi, U.; et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2021, 17, 255–262. [Google Scholar] [CrossRef]
- Rocha, R.B.; Bomtempo, F.F.; Nager, G.B.; Cenci, G.I.; Telles, J.P.M. Dual orexin receptor antagonists for the treatment of insomnia: Systematic review and network meta-analysis. Arq. Neuropsiquiatr. 2023, 81, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Perlis, M.L.; Posner, D.; Riemann, D.; Bastien, C.H.; Teel, J.; Thase, M. Insomnia. Lancet 2022, 400, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Benz, F.; Dressle, R.J.; Espie, C.A.; Johann, A.F.; Blanken, T.F.; Leerssen, J.; Wassing, R.; Henry, A.L.; Kyle, S.D.; et al. Insomnia disorder: State of the science and challenges for the future. J. Sleep Res. 2022, 31, e13604. [Google Scholar] [CrossRef]
- Riemann, D.; Espie, C.A.; Altena, E.; Arnardottir, E.S.; Baglioni, C.; Bassetti, C.L.A.; Bastien, C.; Berzina, N.; Bjorvatn, B.; Dikeos, D.; et al. The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023. J. Sleep Res. 2023, 32, e14035. [Google Scholar] [CrossRef] [PubMed]
- Hieu, T.H.; Dibas, M.; Surya Dila, K.A.; Sherif, N.A.; Hashmi, M.U.; Mahmoud, M.; Trang, N.T.T.; Abdullah, L.; Nghia, T.L.B.; Nhu, M.Y.; et al. Therapeutic efficacy and safety of chamomile for state anxiety, generalized anxiety disorder, insomnia, and sleep quality: A systematic review and meta-analysis of randomized trials and quasi-randomized trials. Phytother. Res. 2019, 33, 1604–1615. [Google Scholar] [CrossRef]
- Bollu, P.C.; Kaur, H. Sleep Medicine: Insomnia and Sleep. Mo. Med. 2019, 116, 68–75. [Google Scholar] [PubMed]
- Yan, Y.; Li, Q.; Du, H.Z.; Shen, C.X.; Li, A.P.; Pei, X.P.; Du, C.H.; Qin, X.M. Determination of five neurotransmitters in the rat brain for the study of the hypnotic effects of Ziziphi Spinosae Semen aqueous extract on insomnia rat model by UPLC-MS/MS. Chin. J. Nat. Med. 2019, 17, 551–560. [Google Scholar] [CrossRef]
- Wang, H.; Gu, Y.; Khalid, R.; Chen, X.; Han, T. Herbal medicines for insomnia through regulating 5-hydroxytryptamine receptors: A systematic review. Chin. J. Nat. Med. 2023, 21, 483–498. [Google Scholar] [CrossRef]
- Hui, P.; Yang, J.; Wang, J.; Zhao, L.; Wang, X.; Su, X.; Wang, J.; Ma, W.; Fan, J.; Chen, W.; et al. Association between 5-hydroxytryptamine gene polymorphism rs140700 and primary insomnia in Chinese population. Intern. Med. J. 2021, 51, 732–738. [Google Scholar] [CrossRef]
- Tang, L.; You, F.; Hu, X.; Li, Y.F. Electroacupuncture improves insomnia by down-regulating peripheral benzodiazepine receptor expression in hippocampus, and up-regulating 5-HT, 5-HIAA, TNF-α and IL-1β contents in hypothalamus in insomnia rats. Zhen Ci Yan Jiu 2019, 44, 560–565. [Google Scholar]
- Winsky-Sommerer, R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur. J. Neurosci. 2009, 29, 1779–1794. [Google Scholar] [CrossRef]
- Kim, S.; Jo, K.; Hong, K.B.; Han, S.H.; Suh, H.J. GABA and l-theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 2019, 57, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Bruni, O.; Ferini-Strambi, L.; Giacomoni, E.; Pellegrino, P. Herbal Remedies and Their Possible Effect on the GABAergic System and Sleep. Nutrients 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Ballesio, A.; Zagaria, A.; Curti, D.G.; Moran, R.; Goadsby, P.J.; Rosenzweig, I.; Lombardo, C. Peripheral brain-derived neurotrophic factor (BDNF) in insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2023, 67, 101738. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Rahmani, F.; Rezaei, N. The Brain-Derived Neurotrophic Factor: Missing Link Between Sleep Deprivation, Insomnia, and Depression. Neurochem. Res. 2020, 45, 221–231. [Google Scholar] [CrossRef]
- Mikoteit, T.; Brand, S.; Eckert, A.; Holsboer-Trachsler, E.; Beck, J. Brain-derived neurotrophic factor is a biomarker for subjective insomnia but not objectively assessable poor sleep continuity. J. Psychiatr. Res. 2019, 110, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Shaha, D.P. Insomnia Management: A Review and Update. J. Fam. Pract. 2023, 72 (Suppl. S6), S31–S36. [Google Scholar] [CrossRef]
- Roach, M.; Juday, T.; Tuly, R.; Chou, J.W.; Jena, A.B.; Doghramji, P.P. Challenges and opportunities in insomnia disorder. Int. J. Neurosci. 2021, 131, 1058–1065. [Google Scholar] [CrossRef]
- Liao, Y.H.; Chen, L.Y.; Liao, K.M.; Chen, C.Y. Drug Safety of Benzodiazepines in Asian Patients With Chronic Obstructive Pulmonary Disease. Front. Pharmacol. 2020, 11, 592910. [Google Scholar] [CrossRef]
- Rosenberg, R.P. Sleep maintenance insomnia: Strengths and weaknesses of current pharmacologic therapies. Ann. Clin. Psychiatry 2006, 18, 49–56. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Li, J.J.; Zhou, Y.; Xu, X.Y. Review for sedative and hypnotic mechanism of sedative traditional Chinese medicine and relative active components on neurotransmitters. Zhongguo Zhong Yao Za Zhi 2016, 41, 4320–4327. [Google Scholar] [PubMed]
- Shi, M.M.; Piao, J.H.; Xu, X.L.; Zhu, L.; Yang, L.; Lin, F.L.; Chen, J.; Jiang, J.G. Chinese medicines with sedative-hypnotic effects and their active components. Sleep Med. Rev. 2016, 29, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Lai, H.; Ning, J.; Liu, J.; Huang, J.; Yang, S.; Jin, J.; Liu, Y.; Liu, J.; Zhao, H.; et al. Traditional Chinese medicine for insomnia: Recommendation mapping of the global clinical guidelines. J. Ethnopharmacol. 2024, 322, 117601. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.J.; Tu, P.F.; Zhang, Q.Y. Research advances of Zaoren Anshen prescription preparations. Zhongguo Zhong Yao Za Zhi 2021, 46, 1301–1326. [Google Scholar] [PubMed]
- Liu, X.X.; Ma, Y.Q.; Wang, Y.G.; Zhong, F.X.; Yin, X.P.; Zhang, Q.M. Suanzaoren Decoction for the treatment of chronic insomnia: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8523–8533. [Google Scholar]
- Xiong, T.W.; Wu, Q.; Liu, J.; Liu, B.; Xu, Y.Y.; Wang, L.N.; Zhang, C.C.; Zhang, W.; Shi, J.S. Research progress of effect of anxiolytic traditional Chinese medicines and formulas on neurotransmitters. Zhongguo Zhong Yao Za Zhi 2020, 45, 14–19. [Google Scholar]
- Hua, Y.; Guo, S.; Xie, H.; Zhu, Y.; Yan, H.; Tao, W.W.; Shang, E.X.; Qian, D.W.; Duan, J.A. Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou Seed Ameliorates Insomnia in Rats by Regulating Metabolomics and Intestinal Flora Composition. Front. Pharmacol. 2021, 12, 653767. [Google Scholar] [CrossRef]
- Wang, D.; Ho, C.T.; Bai, N. Ziziphi Spinosae Semen: An updated review on pharmacological activity, quality control, and application. J. Food Biochem. 2022, 46, e14153. [Google Scholar] [CrossRef]
- Hua, Y.; Xu, X.X.; Guo, S.; Xie, H.; Yan, H.; Ma, X.F.; Niu, Y.; Duan, J.A. Wild Jujube (Ziziphus jujuba var. spinosa): A Review of Its Phytonutrients, Health Benefits, Metabolism, and Applications. J. Agric. Food Chem. 2022, 70, 7871–7886. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, D.; Liu, P.; Zhi, X.; Shi, C.; Zhao, J.; Zhang, H. Study on the mechanism of regulating the hypothalamic cortical hormone releasing hormone/corticotropin releasing hormone type I receptor pathway by vibro-annular abdominal massage under the brain-intestine interaction in the treatment of insomnia. Medicine 2021, 100, e25854. [Google Scholar] [CrossRef]
- Bae, G.Y.; Ahn, Y.; Hong, K.B.; Jung, E.J.; Suh, H.J.; Jo, K. Sleep-Enhancing Effect of Water Extract from Jujube (Zizyphus jujuba Mill.) Seeds Fermented by Lactobacillus brevis L32. Foods 2023, 12, 2864. [Google Scholar] [CrossRef]
- Van Someren, E.J.W. Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef] [PubMed]
- Krystal, A.D.; Prather, A.A.; Ashbrook, L.H. The assessment and management of insomnia: An update. World Psychiatry 2019, 18, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E. Serotonin receptors and their function in sleep, anxiety disorders and depression. Psychother. Psychosom. 1996, 65, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Tartt, A.N.; Mariani, M.B.; Hen, R.; Mann, J.J.; Boldrini, M. Dysregulation of adult hippocampal neuroplasticity in major depression: Pathogenesis and therapeutic implications. Mol. Psychiatry 2022, 27, 2689–2699. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, P.; Srivastava, P.; Kakkar, D.; Pathak, M.; Tiwari, A.K. Development and challenges in the discovery of 5-HT(1A) and 5-HT(7) receptor ligands. Bioorg. Chem. 2023, 131, 106254. [Google Scholar] [CrossRef] [PubMed]
- Lorenz-Guertin, J.M.; Povysheva, N.; Chapman, C.A.; MacDonald, M.L.; Fazzari, M.; Nigam, A.; Nuwer, J.L.; Das, S.; Brady, M.L.; Vajn, K.; et al. Inhibitory and excitatory synaptic neuroadaptations in the diazepam tolerant brain. Neurobiol. Dis. 2023, 185, 106248. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Q.; Liu, R.; Xu, H.; Yin, Y.; Wang, Y.; Wang, H.; Bi, K. Quality control of Semen Ziziphi spinosae standard decoction based on determination of multi-components using TOF-MS/MS and UPLC-PDA technology. J. Pharm. Anal. 2019, 9, 406–413. [Google Scholar] [CrossRef]
- Shang, J.; Chen, X.L.; Li, L.; Zhang, H.; Yang, L.; Yang, B.; Cao, L.; Wang, Z.Z.; Xiao, W. Identification of the absorptive constituents and their metabolites in vivo of Ziziphi spinosae Semen by UPLC-ESI-Q-TOF-MS/MS. Biomed. Chromatogr. 2020, 34, e4965. [Google Scholar] [CrossRef]
- Zhang, F.X.; Li, M.; Qiao, L.R.; Yao, Z.H.; Li, C.; Shen, X.Y.; Wang, Y.; Yu, K.; Yao, X.S.; Dai, Y. Rapid characterization of Ziziphi spinosae Semen by UPLC/Qtof MS with novel informatics platform and its application in evaluation of two seeds from Ziziphus species. J. Pharm. Biomed. Anal. 2016, 122, 59–80. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, X.; Pei, K.; Duan, Y.; Zhu, H.; Ma, J.; Xu, Y.; Wu, Z.; Zhou, Q.; Cai, B. Development of an analytical strategy to identify and classify the global chemical constituents of Ziziphi spinosae Semen by using UHPLC with quadrupole time-of-flight mass spectrometry combined with multiple data-processing approaches. J. Sep. Sci. 2018, 41, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiao, L.; Song, M.; Wang, L.; Xie, J.; Feng, H. Hplc-Esi-Ms/Ms Analysis of the Water-Soluble Extract from Ziziphi spinosae Semen and its Ameliorating Effect of Learning and Memory Performance in Mice. Pharmacogn. Mag. 2014, 10, 509–516. [Google Scholar] [PubMed]
- Du, B.; Tang, X.; Liu, F.; Zhang, C.; Zhao, G.; Ren, F.; Leng, X. Antidepressant-Like Effects of the Hydroalcoholic Extracts of Hemerocallis citrina and Its Potential Active Components. BMC Complement. Altern. Med. 2014, 14, 326. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.X.; Zhang, Q.Y.; Cui, S.Y.; Cui, X.Y.; Zhang, J.; Zhang, Y.H.; Bai, Y.J.; Zhao, Y.Y. Hypnotic Effect of Ju-jubosides from Semen Ziziphi spinosae. J. Ethnopharmacol. 2010, 130, 163–166. [Google Scholar] [CrossRef]
- Fan, L.; Gu, C.; Jiang, Y.; Cao, G.; Sun, L.; Ho, R.J.; Wu, D.; Han, Y.; Hong, Y. Screening of Different Chemical Components of Sedative and Hypnotic Effects of Ziziphi spinosae Semen Before and after Frying and Determina-tion of the Q-Marker. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1207, 123349. [Google Scholar] [CrossRef]

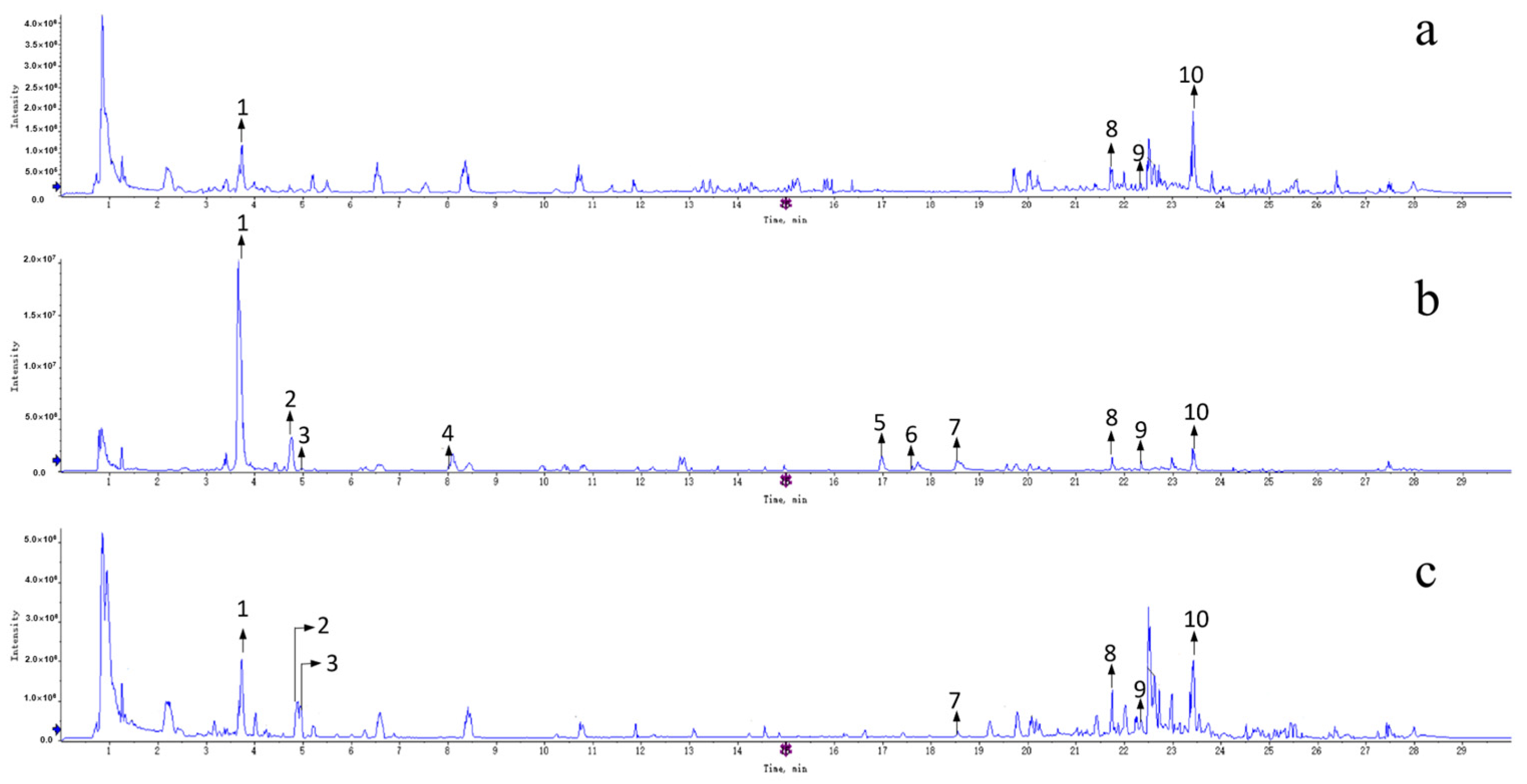
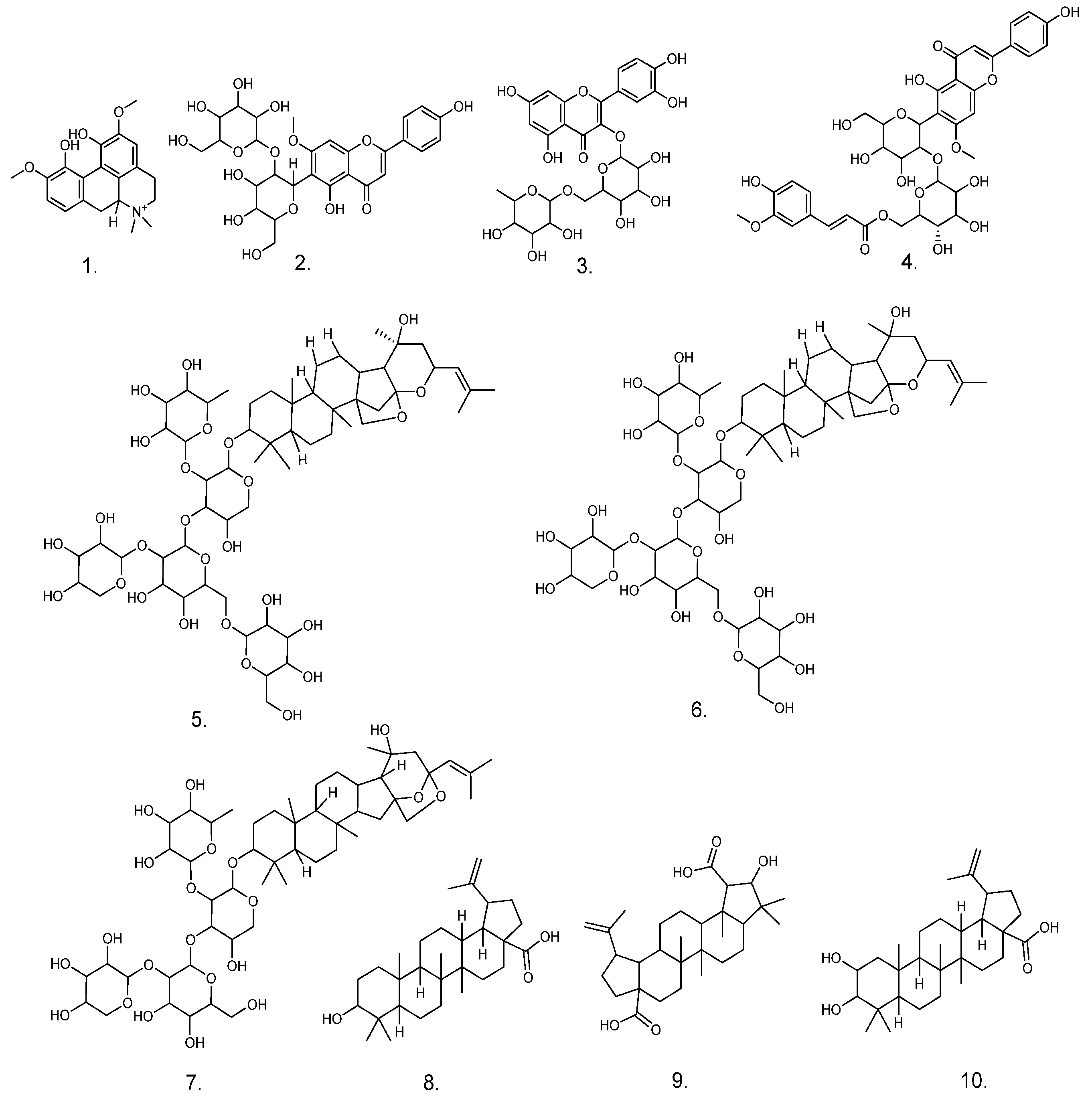
| No. | RT (min) | Molecular Formula | Ion Mode | Measured Value | Theoretical Value | Fragment Ion Information | Presumed Compound | Distributed Sites |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.735 | C20H24NO4 | [M+H]+ | 341.1673 | 341.1699 | 297.1102 [M+H-(CH3)2NH]+, 282.0872 [M+H-(CH3)3NH]+, 265.0842 [M+H-(CH3)3NH-OH]+, 237.0896 [M+H-(CH3)3NH-OH-CO]+, 222.0661 [M+H-(CH3)4NH-OH-CO]+ | Magnoflorine | ZSS ZSF ZSKS |
| 2 | 4.742 | C28H32O15 | [M+H]+ | 608.1777 | 608.1690 | 489.1125 [M+H-C4H8O4]+, 429.1166 [M+H-C4H8O4-C2H4O2]+, 351.0856 [M+H-(C4H8O4)2-H2O]+, 327.0852 [M+H-(C4H8O4)2-C2H2O], 297.0749 [M+H-(C4H8O4)2-C2H2O-CH2O]+ | Spinosin | ZSS ZSF |
| 3 | 4.942 | C27H30O16 | [M+Na]+ | 633.1382 | 633.145 | 463.0971 [M-Rha]−, 301.0486 [M-Rha-Glc]− | Rutin | ZSS ZSF |
| 4 | 8.074 | C38H40O18 | [M+H]+ | 784.225 | 784.2118 | 665.1028 [M+H-C4H8O4]+, 609.1147 [M+H-C4H8O4-C2O2]+, 429.1163 [M+H-C4H8O4-C2O2-OGlc]+, 351.0842 [M+H-C4H8O4-C2O2-OGlc-C2H2O2-H2O]+, 327.0846 [M+H-C4H8O4-C2O2-OGlc-C2H2O2-H2O-C2H2O]+, 177.0541 [M+H-C4H8O4-HCOOH-Glc-C2H2O2-H2O-C2H2O-C5H10O5]+ | 6‴-Feruloylspinosin | ZSS |
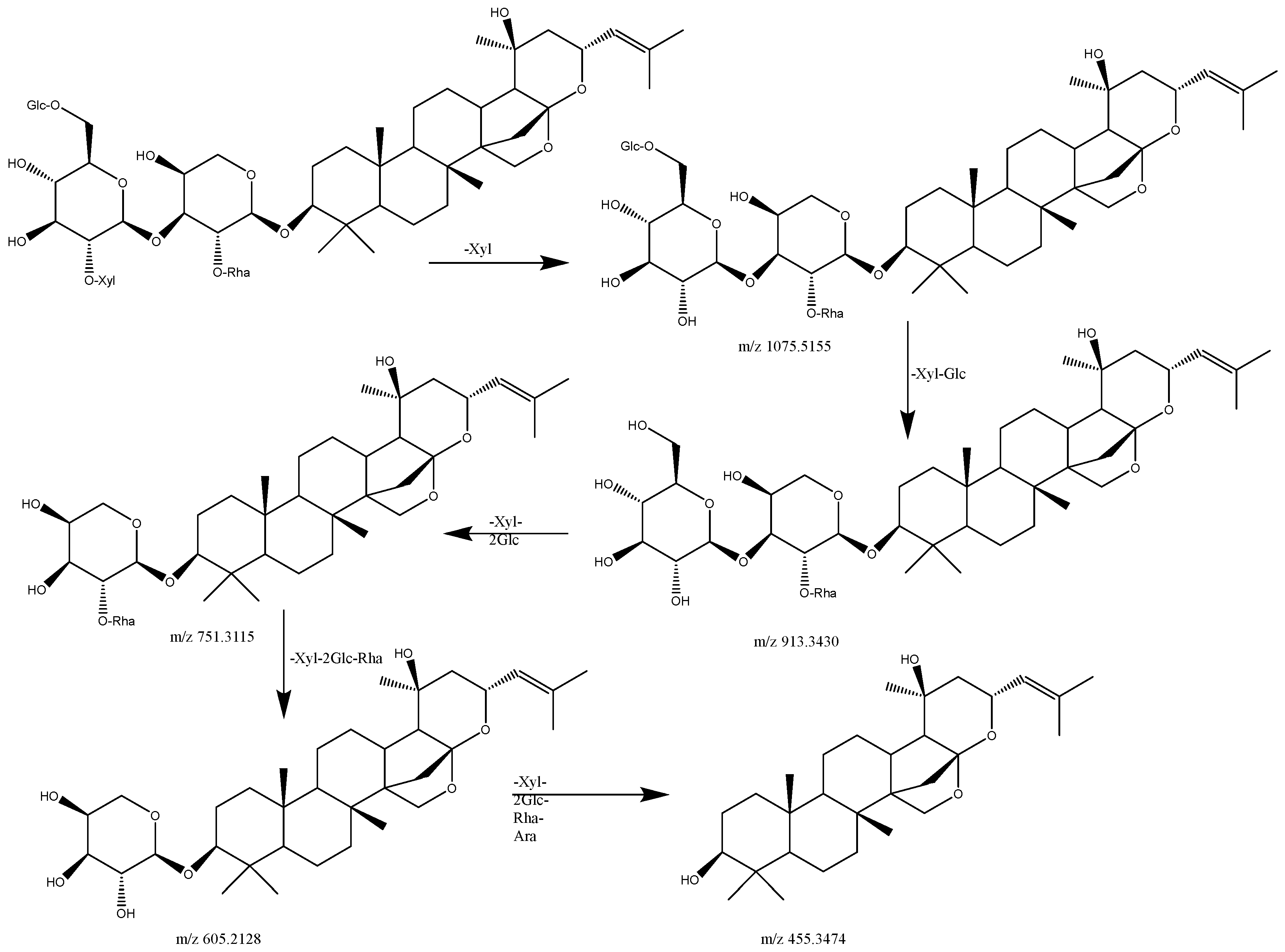
| 5 | 16.957 | C58H94O26 | [M+Na]2+ | 626.2857 | 626.2975 | 1075.5155 [M+Na-Xyl]+, 1057.4197 [M+Na-Xyl-H2O]+, 913.4130 [M+Na-Xyl-Glc]+, 751.2672 [M+Na-Xyl-2Glc]+, 605.1573 [M+Na-Xyl-2Glc-Rha]+, 455.3474 [M+Na-Xyl-2Glc-Rha-Ara]+ | Jujuboside A1 | ZSS |
| 6 | 17.587 | C58H94O26 | [M+Na]2+ | 626.2857 | 626.2975 | 1075.5024 [M+Na-Xyl]+, 1057.3259 [M+Na-Xyl-H2O]+, 913.3430 [M+Na-Xyl-Glc]+, 751.3115 [M+Na-Xyl-2Glc]+, 605.2128 [M+Na-Xyl-2Glc-Rha]+, 455.1573 [M+Na-Xyl-2Glc-Rha-Ara]+ | Jujuboside A | ZSS |
| 7 | 18.512 | C52H84O21 | [M+Na]2+ | 545.2591 | 545.271 | 913.4517 [M+Na-Xyl]+, 895.1880 [M+Na-Xyl-H2O]+, 751.2630 [M+Na-Xyl-H2O-Glu]+, 605.3415 [M+Na-Xyl-Glc-Glu]+ | Jujuboside B | ZSS ZSF |
| 8 | 21.752 | C30H48O3 | [M+H]+ | 457.1672 | 457.352 | 439.1913 [M+H-H2O]+, 411.1404 [M+H-H2O-CO]+, 281.2958 [M+H-H2O-CO-C8H18O]+, 248.9924 [M+H-H2O-CO-C8H18O-(CH4)2]+, 202.9980 [M+H-H2O-CO-C8H10O-(CH4)2-C3H10]+, 119.0855 [M+H-H2O-CO-C8H10O-(CH4)2-C3H10-C6H12]+ | Betulinic acid | ZSS ZSF ZSKS |
| 9 | 22.35 | C30H46O5 | [M+H]+ | 487.3376 | 487.3262 | 459.0417 [M+H-CO]+, 312.3534 [M+H-CO-C7H14O3]+, 235.1782 [M+H-CO-C7H14O3-C5H17]+, 190.9958 [M+H-CO-C7H14O3-C5H17-C3H8]+ | Ceanothic acid | ZSS ZSF ZSKS |
| 10 | 23.416 | C30H48O4 | [M+Na]+ | 495.3442 | 495.3469 | 437.3412 [M+H-CH2O-CO]+, 338.3424 [M+H-CH2O-CO-C6H11O]+ | Alphitolic acid | ZSS ZSF ZSKS |
| Name of Primes | Upstream | Downstream |
|---|---|---|
| BDNF | GCCCATGAAAGAAGTAAACGTCC | AGTGTCAGCCAGTGATGTCGTC |
| 5-HT1AR | ACTCCACTTTCGGCGCTTTC | GGCTGACCATTCAGGCTCTTC |
| GABAARα1 | CCAAGTCTCCTTCTGGCTCAAC | CTTTTCTGGAACCACGCTTTTG |
| GAPDH | CCTCGTCCCGTAGACAAAATG | TGAGGTCAATGAAGGGGTCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Yang, Y.; Song, Z.; Tang, Z. Comparative Analysis of the Sedative and Hypnotic Effects among Various Parts of Zizyphus spinosus Hu and Their Chemical Analysis. Pharmaceuticals 2024, 17, 413. https://doi.org/10.3390/ph17040413
Li B, Yang Y, Song Z, Tang Z. Comparative Analysis of the Sedative and Hypnotic Effects among Various Parts of Zizyphus spinosus Hu and Their Chemical Analysis. Pharmaceuticals. 2024; 17(4):413. https://doi.org/10.3390/ph17040413
Chicago/Turabian StyleLi, Baojian, Yuangui Yang, Zhongxing Song, and Zhishu Tang. 2024. "Comparative Analysis of the Sedative and Hypnotic Effects among Various Parts of Zizyphus spinosus Hu and Their Chemical Analysis" Pharmaceuticals 17, no. 4: 413. https://doi.org/10.3390/ph17040413
APA StyleLi, B., Yang, Y., Song, Z., & Tang, Z. (2024). Comparative Analysis of the Sedative and Hypnotic Effects among Various Parts of Zizyphus spinosus Hu and Their Chemical Analysis. Pharmaceuticals, 17(4), 413. https://doi.org/10.3390/ph17040413





