Abstract
Ammodaucus leucotrichus exhibits promising pharmacological activity, hinting at anti-inflammatory and anti-arthritic effects. This study investigated seed extracts from Ammodaucus leucotrichus using methanol and n-hexane, focusing on anti-inflammatory and anti-arthritic properties. The methanol extract outperformed the n-hexane extract and diclofenac, a reference anti-inflammatory drug, in trypsin inhibition (85% vs. 30% and 64.67% at 125 μg/mL). For trypsin inhibition, the IC50 values were 82.97 μg/mL (methanol), 202.70 μg/mL (n-hexane), and 97.04 μg/mL (diclofenac). Additionally, the n-hexane extract surpassed the methanol extract and diclofenac in BSA (bovine serum albumin) denaturation inhibition (90.4% vs. 22.0% and 51.4% at 62.5 μg/mL). The BSA denaturation IC50 values were 14.30 μg/mL (n-hexane), 5408 μg/mL (methanol), and 42.30 μg/mL (diclofenac). Gas chromatography–mass spectrometry (GC–MS) revealed 59 and 58 secondary metabolites in the methanol and n-hexane extracts, respectively. The higher therapeutic activity of the methanol extract was attributed to hydroxyacetic acid hydrazide, absent in the n-hexane extract. In silico docking studies identified 28 compounds with negative binding energies, indicating potential trypsin inhibition. The 2-hydroxyacetohydrazide displayed superior inhibitory effects compared to diclofenac. Further mechanistic studies are crucial to validate 2-hydroxyacetohydrazide as a potential drug candidate for rheumatoid arthritis treatment.
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disorder characterized by inflammatory changes in joint synovial tissue, cartilage, bone, and, occasionally, extra-articular locations [1]. The interaction between synovial-like fibroblasts, macrophages, and infiltrating lymphocytes leads to the production of inflammatory cytokines [2]. The inflammatory microenvironment can disrupt the balance between bone resorption by osteoclasts and bone production by osteoblasts, contributing to bone deterioration [3]. Various factors, such as ageing, smoking, immunogenicity, and genetic polymorphisms, may influence RA pathophysiology and contribute to its refractory nature [4]. Currently, non-steroidal anti-inflammatory drugs, immunosuppressive glucocorticoids, and disease-modifying antirheumatic drugs are used to alleviate RA symptoms and slow disease progression [5]. These drugs have been associated with important side effects, including gastrointestinal problems, hepatotoxicity, hepatitis, pneumonitis, and cardiovascular events [6]. An RA disability rate of 43–48% and a disease progression time of 5–10 years underscore the need for finding effective and safe treatments [4]. Plant-based therapeutics offer promising research and development opportunities because many of their active ingredients (e.g., triterpenoids, phenols, flavonoids, and polysaccharides) exhibit anti-inflammatory, analgesic, and immunomodulatory activities [7].
Medicinal plants for RA treatment have gained attention due to their potential therapeutic benefits and fewer side effects compared to conventional drugs [8]. Various plants with anti-inflammatory, analgesic, and immunomodulatory properties have been traditionally employed to alleviate RA symptoms. For instance, turmeric (Curcuma longa) contains the active compound curcumin, with anti-inflammatory effects [9]. Moreover, Boswellia serrata, commonly known as Indian frankincense, has recognized anti-inflammatory and analgesic properties [10]. Willow bark (Salix spp.) is another traditional remedy that contains salicin, a natural compound like aspirin with pain relief and anti-inflammatory effects [11]. The anti-arthritic potential of ginger (Zingiber officinale) has also been explored, and its active components exhibit anti-inflammatory and antioxidant properties [12]. Similarly, devil’s claw (Harpagophytum procumbens) and cat’s claw (Uncaria tomentosa) have been studied for managing RA symptoms [13]. However, more research and clinical studies are needed to establish their precise mechanisms and dosages for optimal therapeutic benefits in the context of RA treatment.
Ammodaucus leucotrichus, a plant within the Apiaceae family, stands out as a crucial element in Moroccan flora, with significant applications in traditional herbal medicine across North African nations. Known as ‘Kamune es sufi’ or ‘akâman’ in North African countries, and “Moudrayga” in Algeria, this glabrous annual plant thrives in the Saharan and sub-Saharan regions [14]. Its rich ethnobotanical uses include the treatment of various conditions, particularly noteworthy for its efficacy in addressing issues associated with RA. The plant has been traditionally employed to alleviate gastrointestinal problems, pulmonary diseases, labor pains, and various ailments in both children and adults [14]. Of particular relevance to RA treatment, Ammodaucus leucotrichus has demonstrated its effectiveness in managing conditions such as cystitis, nephritic colics, and kidney stones. Moreover, its role as a sugar regulator for diabetics further underscores its therapeutic potential [14]. The plant’s extracts, abundant in phytochemicals like perillaldehyde and limonene, exhibit a wide array of pharmacological activities, including anti-inflammatory effects [14]. This makes Ammodaucus leucotrichus a promising candidate for further research and development in the pharmaceutical industry, particularly in the quest for novel and effective treatments for rheumatoid arthritis.
This study addressed the limitations and side effects associated with the existing anti-inflammatory treatments for RA by focusing on innovative therapeutic strategies, with a particular emphasis on Ammodaucus leucotrichus extracts. This study explored the anti-inflammatory and anti-arthritic properties of Ammodaucus leucotrichus seed extracts obtained using methanol and n-hexane as solvents. The methodology involved comprehensive analyses, including gas chromatography–mass spectrometry (GC–MS), to identify the phytochemical constituents. In vitro assessments included protein denaturation assays using bovine serum albumin (BSA) and protease inhibition assays using trypsin, an inflammation enzyme. In silico molecular docking studies were used to assess the binding affinity of key compounds identified in the methanol extract for trypsin, an enzyme essential in RA-linked processes. Moreover, the interaction of the phytocompounds identified in the methanol extract with ten human enzymes was investigated by determining their binding affinity with the PASS tool, which was used to predict their biological activity, and ADME/T to predict their absorption, distribution, metabolism, excretion, and toxicity. This pivotal study explored the potential of Ammodaucus leucotrichus seed extracts for developing novel and safer treatments for RA, with the goal of overcoming the challenges associated with conventional drugs.
2. Results and Discussion
Seed extracts from Ammodaucus leucotrichus were investigated using methanol and n-hexane, focusing on anti-inflammatory and anti-arthritic properties. The methanol extract outperformed the n-hexane extract and diclofenac, a reference anti-inflammatory drug. The selection of solvents in our extraction process, namely methanol and n-hexane, was meticulously undertaken based on their distinct properties that allow for the selective extraction of specific classes of compounds from Ammodaucus leucotrichus seeds. Methanol, being a polar solvent, is proficient at extracting polar compounds such as phenolic compounds, flavonoids, and certain organic acids. On the other hand, n-hexane, being non-polar, excels in extracting lipophilic compounds like oils, fatty acids, and hydrophobic substances. The choice to avoid water as a solvent in the extraction of Ammodaucus leucotrichus seeds was driven by the nature of the target compounds. Water, being a polar solvent, might not effectively extract certain non-polar compounds present in the seeds, potentially leading to incomplete representation of the seed’s chemical composition. In contrast, the combination of methanol and n-hexane offers a comprehensive extraction approach, ensuring that a broader spectrum of compounds are captured in our analysis.
2.1. GC–MS Analysis of the Extracts
GC–MS analysis found 59 and 58 therapeutic secondary metabolites in the methanol and n-hexane extracts. Validation included peak area, molecular weight, retention time, PubChem CID number, and chemical formula. Details of the GC–MS analysis are summarized in Tables S1–S3 and Figure S1 (Supplementary Materials). These secondary metabolites were classified into different biological and pharmacological categories, such as ketones, amino alcohols, hydrazide derivatives, pyranone derivatives, furfural derivatives, aldehydes, phenol derivatives, sulfonate esters, cycloalkanols, carboxylic acids, tricyclic sesquiterpenes, sesquiterpene alcohols, heterocyclic compounds, steroids, spiro compounds, organosilicon compounds, tricyclic alcohols, phenone oximes, bicyclic terpenes, terpene alcohols, phthalate esters, siloxanes, sugar derivatives, nitrobenzofurans, cyclopropane derivatives, fatty acids, saturated hydrocarbons, and terpene esters.
Table S1 (Supplementary Materials) presents phytochemicals in methanol extract of Ammodaucus leucotrichus seed, highlighting the diverse chemical classes found in natural plant products. Major classes include fatty acids, terpenes, and mycotoxins [14]. Fatty acids play vital roles in biological processes, serving as lipid and signaling molecule components [15]. Terpenes, such as neophytadiene and isospathulenol, contribute to plant flavors and exhibit medicinal properties. The presence of trichothecenes suggests the potential existence of mycotoxins, known for their toxicity and potential medicinal uses [16]. This chemical diversity reflects the plant’s adaptation and defense mechanisms. The compounds offer potential biological activities, with the effects in RA depending on concentration, bioavailability, and interactions. Fatty acids may display anti-inflammatory properties, potentially alleviating RA symptoms [15]. Terpenes, including isospathulenol, studied for their anti-inflammatory and antioxidant effects, might aid in managing RA inflammation [17]. Incorporating docking studies could enhance understanding by investigating the interactions between these compounds and specific targets implicated in RA, providing valuable insights for future research and therapeutic development.
Table S2 (Supplementary Materials) presents phytochemicals in n-hexane extract of Ammodaucus leucotrichus seeds, highlighting the diverse chemical classes found in natural plant products. The n-hexane extract of Ammodaucus leucotrichus seeds contains a diverse array of compounds with potential implications for therapeutic applications. Notably, fatty acids such as 9-Octadecenoic acid (Octadec-9-enoic acid) and Octadeca-9,12-dienoic acid exhibit characteristics commonly associated with anti-inflammatory effects. Terpenoids like 3-Isopropyl-6,7-dimethyltricyclo [4.4.0.(2,8)]decane-9,10-diol and spathulenol have demonstrated anti-inflammatory and antioxidant properties, potentially contributing to the management of conditions like RA [18]. The presence of alcohols, esters, and hydrocarbons further diversifies the extract, highlighting the complex nature of the plant’s chemical composition. Molecular docking studies could shed light on the specific interactions between these compounds and molecular targets relevant to RA, potentially uncovering novel therapeutic avenues.
The differences in chemical structure and solvation power between the solvents n-hexane and methanol are explained by their distinct polarities [19,20]. The differences are evident in the peak area percentages of various compound classes for both extracts (Table 1). Notably, some compound classes that may have an effect on rheumatoid arthritis (e.g., amino alcohols, hydrazide derivatives, pyranone derivatives, furfural derivatives, sulfonate esters, cycloalkanols, tricyclic sesquiterpenes, heterocyclic compounds, steroids, organosilicon compounds, phenone oximes, bicyclic terpenes, phthalate esters, siloxanes, sugar derivatives, nitrobenzofurans, cyclopropane derivatives, and terpene esters) were absent in the n-hexane extract. In this class, hydrazide derivatives, heterocyclic compounds, and phenone oximes showed more significant effects in rheumatoid arthritis compared to diclofenac, as tested in silico afterward.
Analysis of the results in Table 1 showed that the methanol extract included mainly fatty acids (62.90%), followed by terpene alcohols (10.59%) and terpene esters (2.48%). The n-hexane extract also included mainly fatty acids (68.09%), lower percentages of tricyclic alcohols (3.65%), and terpene alcohols (0.11%). Moreover, n-hexadecanoic acid, with known anti-inflammatory effects, was identified in both extracts. Previous studies suggest that n-hexadecanoic acid has anti-inflammatory effects by inhibiting various inflammatory mediators, including phospholipase A2, prostaglandins E2, interleukin (IL)-6, IL-1, tumor necrosis factor, and nitric oxide synthase. Additionally, it exhibits hypocholesterolemic, nematicidal, and pesticidal effects [21,22,23,24,25,26]. The fatty acids identified are in line with the findings from a previous study [27]. Another noteworthy compound was 4-Prop-1-en-2-ylcyclohexene-1-carbaldehyde, commonly known as perillaldehyde. This chemical (molecular formula: C10H14O; molecular weight: 150.22 g/mol) was newly identified in both extracts and had a peak area of 0.35% in the methanol extract and 3.02% in the n-hexane extract.
A review by Idm’hand, Msanda, and Cherifi [14] offered comprehensive insights into the phytochemistry and pharmacological attributes of Ammodaucus leucotrichus. Their thorough analysis revealed an extensive phytochemical composition, comprising 129 compounds from diverse chemical classes, including terpenoids, aldehydes, alcohols, ketones, and other bioactive constituents. This array of compounds suggested broad pharmacological potential and versatile bioactive properties for potential therapeutic applications. The study detailed the phytochemicals isolated from various plant parts, referencing seeds, fruits, aerial parts, and others. The identified compounds ranged from well-known substances like limonene, perillaldehyde, and α-pinene to a variety of terpenoids, alcohols, and ketones. In contrast, our study took an experimental approach, focusing specifically on the anti-inflammatory and anti-arthritic properties of Ammodaucus leucotrichus seed extracts obtained using methanol and n-hexane as solvents. This targeted experimental work aimed to explore the potential therapeutic benefits of the plant’s seed extracts in the context of RA.

Table 1.
Comparative analysis of phytochemicals in the methanol and n-hexane extracts: peak area percentages from the GC–MS (gas chromatography–mass spectrometry) analysis and therapeutic effect on RA based on previous studies.
Table 1.
Comparative analysis of phytochemicals in the methanol and n-hexane extracts: peak area percentages from the GC–MS (gas chromatography–mass spectrometry) analysis and therapeutic effect on RA based on previous studies.
| Class | Methanol Extract | Peak Area (%) | n-Hexane Extract | Peak Area (%) | Anti-RA Effect |
|---|---|---|---|---|---|
| Fatty acids and derivatives | Tetradecanoic acid;Methyl (9Z,12Z)-octadeca-9,12-dienoate; (9Z, 12Z)-Octadeca-9,12-dienoic acid; 9-octadecenoic acid; octadecanoic acid; Methyl hexadecanoate; n-hexadecanoic acid; heptadecanoic acid; Methyl (9E)-octadec-9-enoate | 62.90 | Methyl (9Z,12Z)-octadeca-9,12-dienoate; (9Z, 12Z)-Octadeca-9,12-dienoic acid; 9-octadecenoic acid; octadecanoic acid; Methyl hexadecanoate; n-hexadecanoic acid; Methyl (9E)-octadec-9-enoate | 68.09 | Yes [28,29] |
| Terpene alcohols | 2,4,7,14-Tetramethyl-4-vinyl-tricyclo [5.4.3.0(1,8)]tetradecan-6-ol; bicyclo [4.4.0]dec-2-ene-4-ol, 2-methyl-9-(prop-1-en-3-ol-2)- | 10.59 | (S)-(-)-(4-isopropenyl-1-cyclohexenyl)methanol | 0.11 | Yes [30] |
| Terpene esters | Trans-(R,R)-chrysanthemyl (R)-2-methylbutanoate; kauren-19-yl-acetate; Methyl-5,9,13-trimethyltetradecanoate | 4.23 | Not present | 0.00 | Not tested |
| Phthalate esters | Phthalic acid, tetradecyl trans-dec-3-enyl ester; phthalic acid, butyl hept-4-yl ester | 2.42 | Not present | 0.00 | Not tested |
| Sesquiterpene alcohols | Isospathulenol, thunbergol, 1-(1,5-Dimethylhexyl)-10-hydroxy-3a,6,6,9a,11a-pentamethylhexadecahydrocyclopenta [7,8]- phenanthro [8a,9-b]oxiren-7-yl acetate, (7S)-1,1,7-Trimethyl-4-methylidene-1α,2,3,4α,5,6,7α,7β-octahydrocyclopropa[h]azulen-7-ol; methyl 16-R/S-hydroxy-cleroda-3,13(14)-Z-dien-15,16-olide | 2.39 | Isospathulenol, (7S)-1,1,7-Trimethyl-4-methylidene-1α,2,3,4α,5,6,7α,7β-octahydrocyclopropa[h]azulen-7-o, methyl 16-R/S-hydroxy-cleroda-3,13(14)-Z-dien-15,16-olide; (1R,7S,E)-7-isopropyl-4,10-dimethylenecyclodec-5-enol | 2.72 | Not tested |
| Hydrazide derivatives | 2-hydroxyacetohydrazide; 3-hydroxy-3-methyl-butyric acid, hydrazide | 1.91 | Not present | 0.00 | Yes [31] |
| Bicyclic terpenes | 2-Ethylidene-1,7,7-trimethylbicyclo [2.2.1]heptane; 8-isopropyl-1,5-dimethyltricyclo [4.4.0.02,7]dec-4-en-3-one | 1.08 | Not present | 0.00 | Not tested |
| Ketones | 4,4-Dimethylpentan-2-one | 1.36 | 4-Isopropenylcyclohexanone | 0.46 | Yes [20,32,33] |
| Tricyclic alcohols | 3-Isopropyl-6,7-dimethyltricyclo [4.4.0.0(2,8)]decane-9,10-diol | 0.97 | 3-Isopropyl-6,7-dimethyltricyclo [4.4.0.0(2,8)]decane-9,10-diol | 3.65 | Yes [34] |
| Furfural derivatives | 5-Hydroxymethylfurfural | 0.87 | Not present | 0.00 | Yes [32] |
| Cyclopropane derivatives | Cyclopropanebutanoic acid, Methyl-4-[2-[[2-[[2-[(2-pentylcyclopropyl)methyl]cyclopropyl]methyl]cyclopropyl]methyl]-cyclopropyl]butanoate | 0.71 | Not present | 0.00 | Not tested |
| Saturated hydrocarbons | Tetracontane | 0.68 | 3-Methylheptane; 3-Methylhexane; 2,2-Dimethylhexane; heptane | 1.68 | Not tested |
| Nitrobenzofurans | 5-nitrobenzofuran-2(3H)-one | 0.64 | Not present | 0.00 | Not tested |
| Amino alcohols | (2R/2S)-2-Aminopropan-1-ol | 0.57 | Not present | 0.00 | Yes [35,36] |
| Tricyclic sesquiterpenes | (1R,4R,6R,10S)-4,12,12-Trimethyl-9-methylene-5-oxatricyclo [8.2.0.04,6]dodecane | 0.55 | Not present | 0.00 | Yes [37] |
| Carboxylic acids and derivatives | 4-Prop-1-en-2-ylcyclohexene-1-carbaldehyde; Undecyl methanoate | 0.48 | Dodecanoic acid, 2-(Tricyclo [3.3.1.13,7]dec-1-yl)propanoic acid | 0.40 | Yes [38,39,40] |
| Spiro compounds | Spiro [5.6]dodecan-7-one, spiro [5.5]undeca-1,7-diene | 0.45 | 5,5-Diethyl-4-methyl-6-spiro [2.3] hexane | 1.05 | Not tested |
| Steroids | 3beta-trimethylsiloxy-5alpha,6alpha-epoxycholestane | 0.43 | Not present | 0.00 | Not tested |
| Heterocyclic compounds | 2,2-bis(oxidanylidene)-1,5-dihydroimidazo [4,5-c][1,2,6]thiadiazin-4-one | 0.38 | Not present | 0.00 | Not tested |
| Organosilicon compounds | Trichloro(dodecyl)silane | 0.38 | Not present | 0.00 | Not tested |
| Aldehydes | 4-Prop-1-en-2-ylcyclohexene-1-carboxylic acid | 0.35 | 4-Prop-1-en-2-ylcyclohexene-1-carboxylic acid | 3.07 | Not tested |
| Siloxanes | Octadecamethyl-cyclononasiloxane | 0.33 | Not present | 0.00 | Not tested |
| Sugar derivatives | 1,2,3,4,5-Penta-O-acetyl-D-xylitol | 0.27 | Not present | 0.00 | Not tested |
| Phenol derivatives | Thymol | 0.25 | 2,6-Dimethoxy-4-(prop-2-en-1-yl)phenol | 0.79 | Yes [41] |
| Phenone oximes | 4’-Hydroxybutyrophenone oxime | 0.22 | Not present | 0.00 | Yes [42] |
| Pyranone derivatives | 3,5-Dihydroxy-6-methyl-2,3-dihydropyran-4-one | 0.20 | Not present | 0.00 | Yes [32] |
| Cycloalkanols | Cyclopentanol | 0.19 | Not present | 0.00 | Not tested |
| Sulfonate esters | [(Z)-4-Methylsulfonyloxybut-2-enyl] 2-(tert-butoxycarbonylamino)acetate | 0.13 | Not present | 0.00 | Yes [33] |
2.2. Protein Denaturation Assay
The anti-inflammatory properties of methanol and n-hexane extracts were assessed using in vitro protein denaturation and protease activity (crucial factors in chronic inflammatory conditions like RA) inhibition assays. Protein denaturation, which is associated with inflammation, was evaluated using a BSA denaturation assay. The strongest BSA denaturation inhibition was obtained with the n-hexane extract at 62.5 µg/mL: 90.36% vs. 51.36% for diclofenac (the reference drug) at the same concentration (Figure 1A). Conversely, the methanol extract exhibited a modest effect, reaching an IC50 of 5408.00 µg/mL, compared with the n-hexane extract (IC50 = 14.30 µg/mL) and also diclofenac (IC50 = 42.30 µg/mL) (Figure 1 and Table S4). This emphasizes the remarkable effectiveness of the n-hexane extract in inhibiting BSA denaturation compared with the methanol extract and also diclofenac, suggesting its potential to inhibit the release of lysosomal material by neutrophils at the inflammation site [43,44].
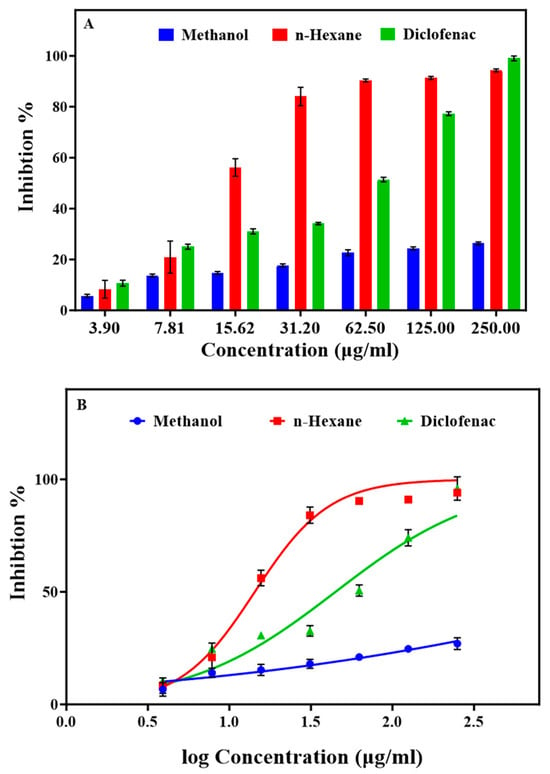
Figure 1.
Effect of different concentrations of the Ammodaucus leucotrichus seed methanol and n-hexane extracts on BSA denaturation. (A) Comparison of the extracts with diclofenac in a protein denaturation assay and (B) IC50 values (μg/mL) for BSA denaturation inhibition. Data are the mean ± standard error of the mean.
2.3. Protease Inhibition Activity
Figure 2A illustrates the significant trypsin inhibition demonstrated by both extracts at various concentrations, implying their potential for RA treatment (Table S5 in the Supplementary Materials). The methanol extracts, along with diclofenac, achieved complete inhibition (100%) at a concentration of 250 μg/mL. The n-hexane extract exhibited lower (51%), but still significant inhibition, at the same concentration. The IC50 values (Figure 2B and Table S5) were 82.97 μg/mL for the methanol extract, 97.04 μg/mL for diclofenac, and 202.7 μg/mL for the n-hexane extract. The high trypsin inhibition by the methanol extract suggests considerable anti-inflammatory effects, as reported by Biswajita, P et al. [45] and Mane, M. P et al. [46] using other plant extracts (Enteromorpha intestinalis and Polygala arvensis). Protease inhibitors have demonstrated efficacy in various clinical diseases, including cancer, AIDS, RA, pancreatitis, and thrombosis [47]. Therefore, the methanol extract holds promise for RA by effectively inhibiting proteases, offering a natural alternative approach to diclofenac, a non-steroidal anti-inflammatory drug with important side effects.
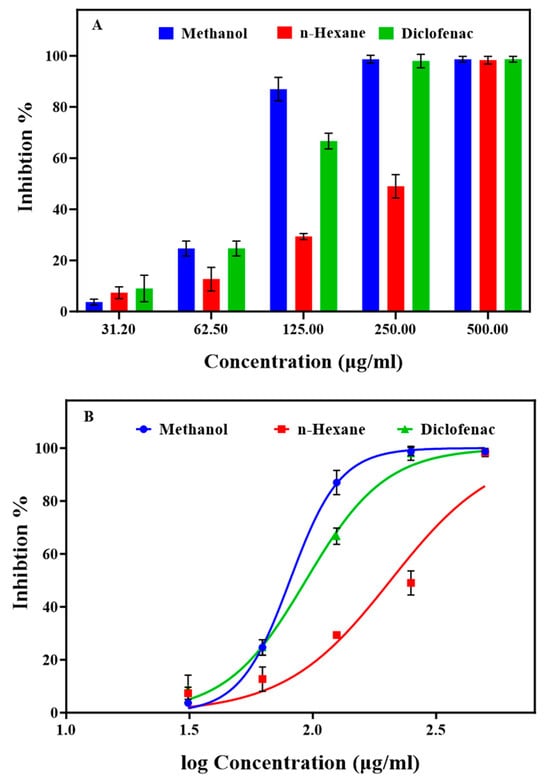
Figure 2.
Influence of various concentrations of the methanol and n-hexane extracts from Ammodaucus leucotrichus seeds on protease (trypsin) inhibition. (A) Percentage of trypsin inhibition compared with diclofenac, the reference drug, and (B) IC50 values (μg/mL) for trypsin inhibition. Data are the mean ± standard error of the mean.
2.4. Molecular Docking
Earlier assays clearly demonstrated the exclusive anti-protease efficacy of the methanol extract, specifically against trypsin. To elucidate the responsible compounds, an in silico screening of the 59 identified phytochemicals within the methanol extract was conducted, utilizing drug design tools crucial for advancing drug development [48]. Molecular docking simulations with trypsin unveiled a diverse range of affinities among the 59 key compounds, with the hydrazide group, including 2-hydroxyacetohydrazide and 3-Hydroxy-3-methyl-butyric acid hydrazide (S1 and S2 in Table S6), standing out for its notable inhibitory potential against RA. The substantial binding energies of these hydrazides highlight their promising candidacy for further exploration in RA inhibition.
Notably, 2-hydroxyacetohydrazide exhibited a more negative binding energy than diclofenac, emphasizing its inhibitory potency. Visual analysis underscores the rational orientation of 2-hydroxyacetohydrazide and diclofenac within the trypsin active site (Figure 3). Remarkably, 2-hydroxyacetohydrazide formed seven hydrogen bonds with crucial residues (Cys191, Ser195, Ser190, and Gly219), indicating a robust inhibitory potential. In contrast, diclofenac formed only two hydrogen bonds (Figure 4) [48]. The predictive analysis unveiled multi-faceted actions, encompassing sphinganine kinase inhibition, G-protein-coupled receptor kinase inhibition, JAK2 expression inhibition, NF-kB activation, immunosuppressant effects, and complement factor D inhibition. These insights provide a comprehensive foundation for guiding future experimental investigations into the therapeutic potential of Ammodaucus leucotrichus seed extract, positioning it as a promising source for developing effective anti-RA agents.
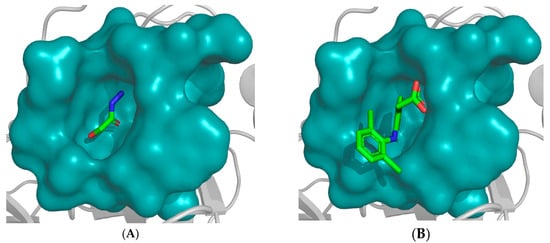
Figure 3.
Placement of (A) 2-hydroxyacetohydrazide and (B) diclofenac within trypsin active site. The most plausible conformation of each compound, obtained by FlexX, is illustrated. The active site is represented in cyan as a ‘surface mode’, with the ligand atoms color-coded: carbon in green, oxygen in red, and nitrogen in blue.
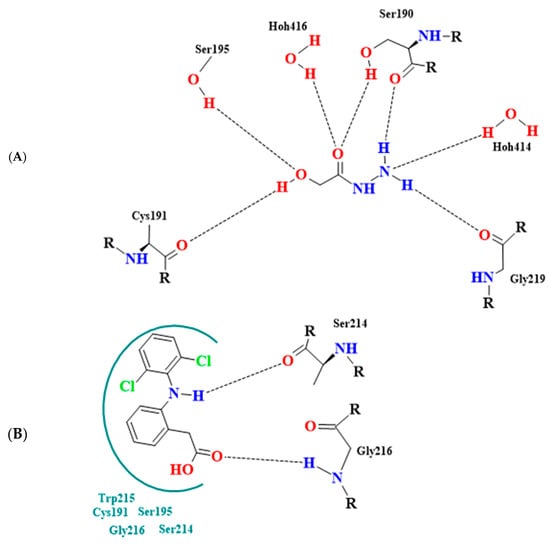
Figure 4.
Binding mode interactions of (A) 2-hydroxyacetohydrazide from the extract and (B) diclofenac (reference drug) within trypsin active site (inflammation-related enzyme); hydrogen bonds depicted by broken lines.
Furthermore, the maintenance of bone and cartilage integrity crucially depends on balanced proteolytic activity, and various enzymes contribute to pro-inflammatory functions in RA [49]. Serine proteases, such as trypsin, play a role in the complement cascade, extending beyond the traditional roles of human collagenases in collagen breakdown [50]. The molecular docking results accentuate that 2-hydroxyacetohydrazide from Ammodaucus leucotrichus exhibits tighter binding to the target protein trypsin than diclofenac. This underscores 2-hydroxyacetohydrazide as a promising candidate for future RA research, showcasing its potential in modulating proteolytic activity associated with RA pathology.
2.5. Prediction of Potential Protein Targets and Druglikeness Analysis
The screening of the 59 phytochemicals from the methanol extract of Ammodaucus leucotrichus seeds also involved using the online PASS tool to predict their biological activity spectrum (Figure 5). This tool provides insights into the potential pharmacological effects, mechanisms of action, and toxicity based on the compound chemical structure [51]. The diversity of predicted activities encompassed anti-inflammatory effects and targeting of key molecules associated with RA. The tool successfully identified 2-hydroxyacetohydrazide in Table S7 (Supplementary Materials) as a potent agent with anti-inflammatory effects (Pa: 0.004) and sphinganine kinase inhibition activity. This positions 2-hydroxyacetohydrazide as a promising multi-faceted candidate for RA treatment. Notably, the tool encountered challenges predicting outcomes for 2-hydroxyacetohydrazide, possibly attributable to its relatively short carbon chain.
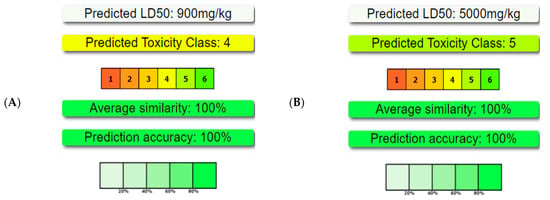
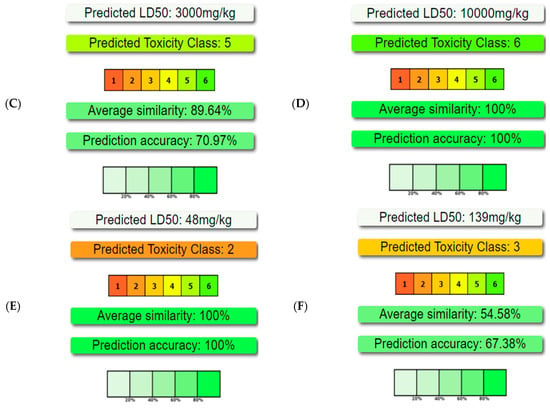
Figure 5.
Toxicity, calculated by ProTox-II, of key compounds identified in the Ammodaucus leucotrichus seed methanol extract. The compounds include (A) n-hexadecanoic acid, (B) hexadecanoic acid methyl ester, (C) 9-octadecenoic acid methyl ester (E)-, (D) (9Z, 12Z)-Octadeca-9,12-dienoic acid, (E) 9-octadecenoic acid, and (F) 2-hydroxyacetohydrazide.
The identified phytochemicals from the methanol extract of Ammodaucus leucotrichus seeds target various key pathways associated with RA. Sphinganine kinase, a crucial enzyme in the regulation of inflammatory factors and immune responses, was targeted by several compounds, including 2-hydroxyacetohydrazide, emphasizing their potential in modulating sphingosine metabolism [52,53]. G-protein-coupled receptors, implicated in RA mechanisms, were also affected by multiple compounds, suggesting a role in modulating receptor signaling for therapeutic benefits [54,55,56]. Inhibition of Janus kinases (JAKs) by certain compounds aligns with the emerging class of JAK inhibitors in RA treatment, demonstrating potential efficacy in disease modification [57,58]. Compounds displaying immunosuppressant properties hold promise for mitigating chronic inflammation and pain associated with RA [59,60]. Furthermore, the prediction of matrix metalloproteinase (MMP) inhibition, particularly MMP9, suggests a potential avenue for joint protection in RA [61,62]. Transcription factor NF-κB, a key regulator of inflammation, was targeted by several compounds, providing insights into potential anti-inflammatory effects [63,64]. Notably, the hydrazide of 2-hydroxyacetohydrazide stands out, possibly due to its unique structure.
Six selected compounds, including 2-hydroxyacetohydrazide, n-hexadecanoic acid, hexadecanoic acid methyl ester, (9Z,12Z)-Octadeca-9,12-dienoic acid, and (E) 9-octadecenoic acid, demonstrated promising effects on multiple RA therapeutic targets. This selection process, involving in vitro and in silico assessments along with PASS predictions (Figure 5), underscores a comprehensive approach to identifying potential anti-RA candidates [65]. To further evaluate these compounds, pharmacokinetic properties and toxicity were assessed. SwissADME and ProTox-II results indicated low toxicity for n-hexadecanoic acid, hexadecanoic acid methyl ester, (9Z,12Z)-Octadeca-9,12-dienoic acid, and (E) 9-octadecenoic acid, while 9-octadecenoic acid and 2-hydroxyacetohydrazide exhibited higher toxicity, highlighting the importance of considering safety profiles in drug development [66]. Additionally, Swiss Target Prediction revealed potential interactions with enzymes, fatty-acid-binding proteins, G-protein-coupled receptors, and nuclear receptors, providing valuable insights into the biological activity of these compounds [65]. Overall, this comprehensive analysis offers a foundation for further experimental exploration and potential drug development for RA treatment.
The bioavailability radar charts generated by SwissADME [66] stressed the favorable characteristics of the compounds: high scores for size, polarity, solubility, and saturation, and low scores for flexibility and lipophilicity. Moreover, 2-hydroxyacetohydrazide was within the optimal range for all the tested characteristics (Figure 6 and Table 2). The BOILED-Egg method was used to predict blood–brain barrier (BBB) access and passive gastrointestinal absorption. Key compounds (n-hexadecanoic acid, hexadecanoic acid methyl ester, and 9-octadecenoic acid) were inside the yolk, suggesting high BBB permeation. Only 2-hydroxyacetohydrazide was in the white part, suggesting favorable gastrointestinal absorption due to its lower WLOGP value (a lipophilicity indicator) and higher Total Polar Surface Area (TPSA) value compared with the other compounds. The outer gray region indicates molecules with low absorption and limited BBB penetration: 9-octadecenoic acid methyl ester (E)- and (9Z, 12Z)-Octadeca-9,12-dienoic acid. The SwissADME results suggest a minimal impact of P-glycoproteins (P-gp) in the central nervous system on the main substances present in the methanol extract, as indicated by the red dots. SwissADME also predicted that most of the compounds but n-hexadecanoic acid and 9-octadecenoic acid should not inhibit major cytochrome P450 (CYP) isoforms (CYP2C19, CYP2D6, CYP3A4, and CYP2C9). Conversely, all the tested compounds but 2-hydroxyacetohydrazide should inhibit CYP1A2 (Table 2). The physicochemical properties, crucial for efficacy, safety, and metabolism, were assessed using six rule-based methods, including Lipinski’s Rule of Five (RO5), confirming full compliance, with molecular mass <500 daltons, hydrogen bond donors (HBD) <5, hydrogen bond acceptors (HBA) <10, and octanol–water partition coefficient (Clog P) ≤5 for all the selected molecules (Table 2).
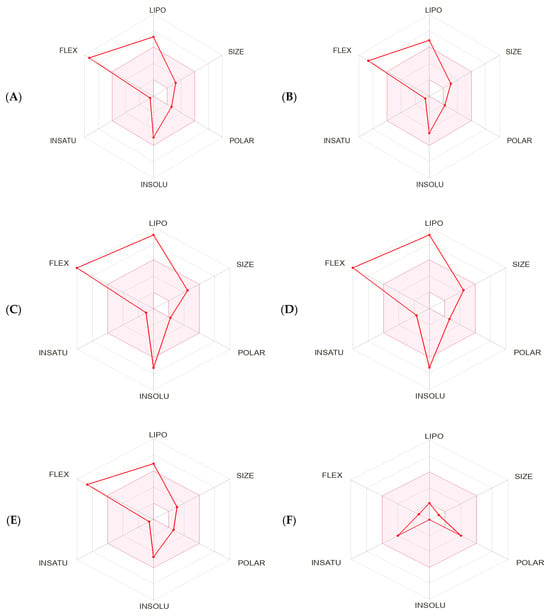
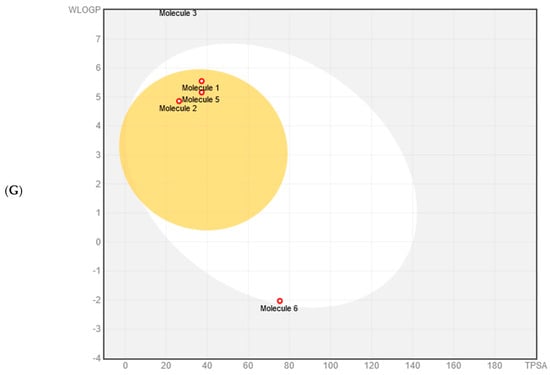
Figure 6.
Bioavailability radar charts within the ADME property domain borders, calculated by SwissADME, for the key compounds identified in the methanol extract of Ammodaucus leucotrichus seeds: (A) n-hexadecanoic acid, (B) hexadecanoic acid methyl ester, (C) 9-octadecenoic acid methyl ester (E)-, (D) (9Z, 12Z)-Octadeca-9,12-dienoic acid, (E) 9-octadecenoic acid, and (F) 2-hydroxyacetohydrazide. (G) BOILED-Egg model. The pink area in the radar chart shows the optimal range for the following features: lipophilicity (LIPO), size, polarity (POLAR), solubility (INSOLU), saturation (INSATU), and flexibility (FLEX).

Table 2.
In silico ADMET profiles of six phytocompounds identified in the methanol extract.
Table 2 provides the comprehensive in silico ADMET profile of the six key compounds identified in the methanol extract of Ammodaucus leucotrichus seeds. The TPSA values ranged from 26.30 to 75.35 Ų, and 2-hydroxyacetohydrazide had the highest TPSA. The consensus log Po/w, an indicator of lipophilicity, varied between 5.20 and −1.51; n-hexadecanoic acid displayed the highest lipophilicity and 2-hydroxyacetohydrazide was hydrophilic. All the compounds were predicted to have substantial gastrointestinal absorption and complied with Lipinski’s Rule of Five, indicating their druglike properties. BBB penetration was predicted for n-hexadecanoic acid and hexadecanoic acid methyl ester but not for 2-hydroxyacetohydrazide. Importantly, the lack of P-gp substrate prediction suggests favorable bioavailability. Although some compounds were predicted to inhibit specific CYP isoforms, the majority of them displayed interesting characteristics for effective drug development and therapeutic applications, underscoring their potential as promising drug candidates that deserve further exploration and experimental validation.
3. Materials and Methods
3.1. Phytochemical Extraction
Ammodaucus leucotrichus seeds were collected in the Bechar region (part of the arid Algerian Sahara) in the southwest of Algeria during the summer of 2021. Professor Sabah Charmat from Ferhat Abbas University Setif 1, Algeria, confirmed their authenticity. An official voucher specimen was documented and assigned the reference number 220/SNV/DA/UFAS/21. The seed powder underwent solvent extraction using absolute methanol (CH3OH, ≥99.8%, Sigma-Aldrich, Burlington, MA, USA) or n-hexane (CH3(CH2)4CH3, ≥97.0%, Sigma-Aldrich) following the method outlined by Arrar et al. [67]. Briefly, 5 kg of seed powder was immersed in absolute methanol or n-hexane in a sealed container and left at room temperature (27 °C) with regular agitation for 3–7 days until complete dissolution of the soluble components. The solutions were filtered through Whatman No. 4.1 filter paper and concentrated under reduced pressure using a rotary evaporator at 40 °C, yielding the crude extracts, dissolved in distilled water and kept at 4 °C.
3.2. Phytochemical Analysis by GC–MS
GC–MS (gas chromatography–mass spectrometry) analysis was completed using a Hewlett-Packard 6890 system interfaced with a quadrupole mass spectrometer (model HP 5973), equipped with an HP5 MS capillary column (5% phenylmethyl siloxane in dimethylpolysiloxane, 30 m × 0.25 mm, 0.25 mm film thickness; PTAPC, BISKRA). The experimental setup included helium carrier gas flow rate of 0.5 mL/min, split ratio of 30, electron ionization system with ionization energy of 70 eV, and scan range of 30–550 atomic mass units. The GC–MS transfer line temperatures for the injector and detector were set at 250 °C and 280 °C, respectively, and the ion source temperature was maintained at 230 °C. The column temperature was at 60 °C for 8 min and then was increased gradually to 280 °C (2 °C/min) and held isothermal for 30 min. Then, 0.2 mL of n-hexane (HPLC grade 95%, Fisher Chemical) and methanol solution (HPLC grade, ≥99.9%, Sigma-Aldrich) was injected. Mass spectra were compared with the computer libraries Wiley 7N, National Institute of Standards and Technology (NIST) 02, and NIST 98 (NIST11 and Wiley 8) for compound identification [27].
3.3. Protein Denaturation Assay
The in vitro anti-inflammatory effects of methanol and n-hexane extracts were examined using a protein denaturation assay, based on BSA (bovine serum albumin) (pH 7, ≥98%, Sigma-Aldrich), as reported by Suresh, P [68]. Briefly, 0.2% BSA in Tris-HCl buffer (pH 6.8, Trizma® hydrochloride, >99%, Sigma-Aldrich) was combined with various extract concentrations (250, 125, 62.50, 31.20, 15.62, and 7.81, 3 μg/mL) solubilized in distilled water or the reference anti-inflammatory drug (diclofenac, DICLAMID®, 25 mg/mL). Mixtures were sealed and incubated at 37 °C in an oven for 15 min, followed by heating in a water bath at 70 °C for 5 min. Turbidity absorbance was measured at 660 nm using a UV–vis spectrophotometer (VIS-7220G). The protein denaturation inhibition percentage was calculated using the formula (Equation (1)):
where At is the absorbance of the test sample, and Ac is the absorbance of the control.
Protein denaturation inhibition (%) = (1 − At/Ac) × 100
3.4. Protease Inhibition Activity
The protease inhibition activity analysis was carried out following a modified version of the protocol described by Ahmad, S et al. [69]. The reaction mixture included 200 μL of extract at various concentrations (500, 250, 125, 62.5, and 31.2 μg/mL) solubilized in distilled water, 200 μL of 25 mM Tris-HCl buffer (Trizma® hydrochloride, >99%, Sigma-Aldrich) at pH 7, and 12 μL of 0.6 mg trypsin (1000–2000 units/mg solid, Sigma-Aldrich). After incubation at 37 °C for 5 min, 200 μL of 0.8% w/v ovalbumin (Sigma-Aldrich) was added to the mixtures that were then incubated at 37 °C for 20 min. The reaction was stopped by adding 400 μL of 70% v/v hydrochloric acid (HCl, 37%, Sigma-Aldrich). This was followed by centrifugation (refrigerated centrifuge 3–30 KS, Sigma-Aldrich, Darmstadt, Germany) at 5000 rpm for 5 min to collect the supernatant, and absorbance was measured at 280 nm with a UV–vis spectrophotometer (VIS-7220G). Diclofenac served as reference standard. The percentage of protease inhibition percentage was calculated for each extract and diclofenac using the following formula (Equation (2)):
where At is the absorbance of the test sample, and Ac is the absorbance of the control.
Protease inhibition (%) = (1 − At/Ac) × 100
3.5. In Silico Molecular Docking
To study the binding mechanisms of the compounds identified in the Ammodaucus leucotrichus seed methanol extract, a molecular docking study was conducted targeting the active sites of trypsin. The 3D coordinates for trypsin (ID: 2PTN) were obtained from the Protein Data Bank (https://www.rcsb.org, accessed on 5 Septembre 2023). Trypsin was prepared for docking using the LeadIT 2.1.8 software package (www.biosolveit.com, accessed on 5 Septembre 2023)). Before the docking simulation, all water molecules were removed, and polar hydrogen atoms were introduced [70]. Missing atoms were added, and formal charges were computed. Then, the resulting structure underwent rigorous minimization before being exported as mol2 files [71]. The 3D structure of each compound was retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/, accessed on 05 Septembre 2023) and prepared for docking simulations using Maestro, version 11.3, and the LigPrep module (Schrödinger software company, New York, NY, USA) [72]. This module streamlines the generation of all tautomeric forms, protonation states at pH = 7.4 ± 1, and enantiomers for each ligand [73]. Molecular docking calculations were completed with FlexX 2.1.8 and an incremental ligand construction approach [74]. Fragment selection was set in automatic mode and the standard algorithm for fragment placement was used. Phytochemicals in the extract were ranked using the FlexX scoring function, providing scores in terms of changes in Gibbs free energy (ΔG, in kJ/mol).
3.6. Prediction of Potential Protein Targets and Druglikeness Analysis
To estimate the biological activity of the 59 compounds detected in the methanol extract, the PASS Online tool (way2drug.com/passonline/predict.php, accessed on 6 Septembre 2023) was employed (average accuracy >95%). The structures of the compounds were uploaded in “mol” format in PASS Online to obtain the probability to be active (Pa) and the probability to be inactive (Pi) values [75]. Then, the bioavailability and pharmacokinetic and toxicity properties of 2-hydroxyacetohydrazide were predicted using the SwissADME, BOILED-Egg, and ProTox-II—Prediction of toxicity of chemicals tools.
4. Conclusions
The evaluation of Ammodaucus leucotrichus seed extracts as potential anti-inflammatory and anti-arthritic agents involved employing methanol and n-hexane as solvents for the extraction of bioactive compounds. The extracts underwent assessment through protease (trypsin) and protein (BSA, bovine serum albumin) denaturation inhibition assays. The methanol extract demonstrated trypsin inhibition of 85%, surpassing both the n-hexane extract (30.0%) and diclofenac (64.67%) at 125 μg/mL. Conversely, the n-hexane extract exhibited the highest BSA denaturation inhibition rate at 90.4%, in comparison to the methanol extract (22.0%) and diclofenac (51.4%) at 62.5 μg/mL. GC–MS analysis revealed 59 and 58 secondary metabolites in the methanol and n-hexane extracts, respectively, indicating diverse bioactive compounds. In silico docking studies identified 28 compounds with negative binding energies, suggesting potential trypsin inhibition. Notably, 2-hydroxyacetohydrazide showed superior inhibitory effects (−17.13 Kj/mol) compared to diclofenac (−13.01 Kj/mol). The methanol extract, particularly 2-hydroxyacetohydrazide, emerged as a promising candidate for rheumatoid arthritis (RA) treatment due to potent trypsin inhibition. SwissADME analysis highlighted favorable bioavailability attributes, with optimal size, polarity, solubility, and saturation for 2-hydroxyacetohydrazide. The BOILED-Egg model predicted blood–brain barrier permeation and gastrointestinal absorption, providing insights into druglikeness and bioavailability. In summary, these findings propose the methanol extract as a promising candidate for anti-inflammatory applications, particularly in trypsin inhibition. However, we acknowledge the potential for alternative therapeutic approaches with the n-hexane extract, necessitating further investigation. The identified compound, 2-hydroxyacetohydrazide, shows promise as a potential drug candidate for rheumatoid arthritis treatment. Yet, rigorous mechanistic studies and validation are crucial to enhance our understanding of its therapeutic potential within the field of plant-based medicine.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph17030385/s1, Figure S1. Gas chromatography-mass spectrometry analysis of (A) the methanol and (B) n-hexane extracts of Ammodaucus leucotrichus seeds. The chromatograms show the abundance of the different compounds in relation to their retention time (in minutes); Figure S2. Data on the predicted target of the selected compounds identified in the Ammodaucus leucotrichus methanol extract; Table S1. Comprehensive list of the phytochemicals identified in the methanol extract by gas chromatography-mass spectrometry. The table includes compound name, molecular formula, molecular weight, retention time, peak area, and PubChem Compound Identifier (CID); Table S2. Comprehensive list of the phytochemicals identified in the n-hexane extract by gas chromatography-mass spectrometry. The table includes compound name, molecular formula, molecular weight, retention time, peak area, and PubChem Compound Identifier (CID); Table S3. Comparison of the key phytocomponents (with their peak area) present in the methanol and n-hexane extracts, identified by gas chromatography/mass spectrometry; Table S4: Impact of different concentrations of the methanol and n-hexane extracts from Ammodaucus leucotrichus seeds on BSA denaturation. Comparison with diclofenac (reference anti-inflammatory drug) in a BSA denaturation assay and IC50 values (μg/ml) for BSA denaturation inhibition. Data are the mean ± standard error of the mean; Table S5: Trypsin inhibition by the methanol and n-hexane extracts from Ammodaucus leucotrichus seeds compared with Diclofenac (reference drug) at different concentrations, and IC50 values (μg/ml) for trypsin inhibition. Data are the mean ± standard error of the mean; Table S6: Binding energies (in kJ/mol) of the 59 key compounds identified in the methanol extract from Ammodaucus leucotrichus seeds and of diclofenac during their interactions with trypsin. The different binding energy values reflect variations in the inhibitory potential of the compounds against trypsin; Table S7. PASS prediction of the bioactivities of the 59 phytochemicals identified in the methanol extract from Ammodaucus leucotrichus seeds.
Author Contributions
C.D., N.B., H.D., M.T., E.H.M., S.M., M.M., C.B., A.A., D.C., M.B., L.A. and A.B. collaboratively contributed to all aspects of the research, encompassing conception, data collection, analysis, drafting the article, critical revision, and final approval for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Joint Egyptian Japanese Scientific Cooperation (JEJSC, ID 42811), Egypt–France Joint Driver (Imhotep, Project No. 43990SF), and Researchers Supporting Project number (RSP2024-R78) at King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The Etiology of Rheumatoid Arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Kurowska-Stolarska, M.; Alivernini, S. Synovial Tissue Macrophages in Joint Homeostasis, Rheumatoid Arthritis and Disease Remission. Nat. Rev. Rheumatol. 2022, 18, 384–397. [Google Scholar] [CrossRef]
- Chimenti, M.S.; Triggianese, P.; Conigliaro, P.; Candi, E.; Melino, G.; Perricone, R. The Interplay between Inflammation and Metabolism in Rheumatoid Arthritis. Cell Death Dis. 2015, 6, e1887. [Google Scholar]
- Myasoedova, E.; Davis, J.; Matteson, E.L.; Crowson, C.S. Is the Epidemiology of Rheumatoid Arthritis Changing? Results from a Population-Based Incidence Study, 1985–2014. Ann. Rheum. Dis. 2020, 79, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef]
- Köhler, B.M.; Günther, J.; Kaudewitz, D.; Lorenz, H.M. Current Therapeutic Options in the Treatment of Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 938. [Google Scholar] [CrossRef]
- Satriotomo, I.; Bowen, K.K.; Vemuganti, R. JAK2 and STAT3 Activation Contributes to Neuronal Damage Following Transient Focal Cerebral Ischemia. J. Neurochem. 2006, 98, 1353–1368. [Google Scholar] [CrossRef]
- Qiang, R.; Huang, H.; Chen, J.; Shi, X.; Fan, Z.; Xu, G.; Qiu, H. Carbon quantum dots derived from herbal medicine as therapeutic nanoagents for rheumatoid arthritis with ultrahigh lubrication and anti-inflammation. ACS Appl. Mater. Interfaces 2023, 15, 38653–38664. [Google Scholar] [CrossRef]
- Qayyum, S.; Mehdi, A. Potential Efficacy of Turmeric as an Anti-Inflammatory Agent and Antioxidant in the Treatment of Osteoarthritis. Khyber Med. Univ. J. 2023, 15, 190–197. [Google Scholar] [CrossRef]
- Su, S.; Hua, Y.; Wang, Y.; Gu, W.; Zhou, W.; Duan, J.; Jiang, H.; Chen, T.; Tang, Y. Evaluation of the Anti-Inflammatory and Analgesic Properties of Individual and Combined Extracts from Commiphora Myrrha, and Boswellia Carterii. J. Ethnopharmacol. 2012, 139, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Gligorić, E.; Igić, R.; Srđenović Čonić, B.; Kladar, N.; Teofilović, B.; Grujić, N. Chemical Profiling and Biological Activities of “Green” Extracts of Willow Species (Salix L., Salicaceae): Experimental and Chemometric Approaches. Sustain. Chem. Pharm. 2023, 32, 100981. [Google Scholar] [CrossRef]
- Tukiran, T.; Salma, N.A.; Sutoyo, S.; Sabila, F. Anti-Arthritic Activity of Combination of Caesalpinia Sappan and Zingiber Officinale Extracts in Complete Freund’s Adjuvant-Induced Arthritic in Rats. Trop. J. Nat. Prod. Res. 2023, 7, 5164–5171. [Google Scholar] [CrossRef]
- Pushpalatha, D.S.S.P.S.; Kumaraswamy, S.; Selvaraj, G.K.; Narayanaswamy, R.; Prabhakaran, V.-S.; Sivakumar, T.; Kesavan, A. Plant-Derived Bioactive Compounds: Promising Prospective Uses in the Chronic Inflammation. In Natural Products as Cancer Therapeutics; IGI Global: Hershey, PA, USA, 2023; pp. 254–274. [Google Scholar] [CrossRef]
- Idm’hand, E.; Msanda, F.; Cherifi, K. Medicinal Uses, Phytochemistry and Pharmacology of Ammodaucus leucotrichus. Clin. Phytoscience 2020, 6, 6. [Google Scholar] [CrossRef]
- Mustonen, A.-M.; Nieminen, P. Fatty Acids and Oxylipins in Osteoarthritis and Rheumatoid Arthritis—A Complex Field with Significant Potential for Future Treatments. Curr. Rheumatol. Rep. 2021, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Kizis, D.; Vichou, A.-E.; Natskoulis, P.I. Recent Advances in Mycotoxin Analysis and Detection of Mycotoxigenic Fungi in Grapes and Derived Products. Sustainability 2021, 13, 2537. [Google Scholar] [CrossRef]
- Yu, G.-W.; Nie, J.; Song, Z.-Y.; Li, Z.-G.; Lee, M.-R.; Wang, S.-P. Microwave-Assisted Simplified Simultaneous Distillation Coupled with Ionic Liquid Pretreatment for the Analysis of Essential Oil in Schisandra Sphenanthera. J. Chromatogr. Sci. 2017, 55, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives. Energy 2023, 16, 5852. [Google Scholar] [CrossRef]
- Zhou, C.; Dowlatshah, S.; Hansen, F.A.; Pedersen-Bjergaard, S. Generic conditions for electromembrane extraction of polar bases. Talanta 2024, 267, 125215. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-Inflammatory Property of n-Hexadecanoic Acid: Structural Evidence and Kinetic Assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.V.; Vargas, R.A.; Petricevich, V.L. Chemical Compounds and Biological Activity of an Extract from Bougainvillea x Buttiana (Var. Rose) Holttum and Standl. Int. J. Pharm. Pharm. Sci. 2017, 9, 42. [Google Scholar] [CrossRef]
- Shin, J.; Yang, S.J.; Lim, Y. Gamma-Tocopherol Supplementation Ameliorated Hyper-Inflammatory Response during the Early Cutaneous Wound Healing in Alloxan-Induced Diabetic Mice. Exp. Biol. Med. 2017, 242, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Godara, P.; Dulara, B.K.; Barwer, N.; Chaudhary, N.S. Comparative GC-MS Analysis of Bioactive Phytochemicals from Different Plant Parts and Callus of Leptadenia Reticulata Wight and Arn. Pharmacogn. J. 2019, 11, 129–140. [Google Scholar] [CrossRef]
- Joshua, P.E.; Anosike, C.J.; Asomadu, R.O.; Ekpo, D.E.; Uhuo, E.N.; Nwodo, O.F.C. Bioassay-Guided Fractionation, Phospholipase A2-Inhibitory Activity and Structure Elucidation of Compounds from Leaves of Schumanniophyton magnificum. Pharm. Biol. 2020, 58, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Mishra, P.; Meena, R.; Patni, V. GC-MS Analysis of Bioactive Compounds in Methanolic Extracts of Stem and Seed Samples of Distimake Spp. Int. J. Pharm. Res. Allied Sci. 2024, 13, 39–46. [Google Scholar] [CrossRef]
- Abdi Bellau, M.L.; Chiurato, M.A.; Maietti, A.; Fantin, G.; Tedeschi, P.; Marchetti, N.; Tacchini, M.; Sacchetti, G.; Guerrini, A. Nutrients and Main Secondary Metabolites Characterizing Extracts and Essential Oil from Fruits of Ammodaucus leucotrichus Coss. & Dur. (Western Sahara). Molecules 2022, 27, 5013. [Google Scholar] [CrossRef] [PubMed]
- Gnanasundaram, I.; Balakrishnan, K. Characterization of Bioactive Compounds in Ethanolic Extract of Cissus vitiginea Leaves Using GC-MS Technique. IOSR J. Appl. Chem. 2017, 10, 24–27. [Google Scholar]
- Liao, T.; Shen, F.; Zhu, H.; Mu, W.; Qian, H.; Liu, Y. Extracellular Polysaccharides from Sporidiobolus Pararoseus Alleviates Rheumatoid through Ameliorating Gut Barrier Function and Gut Microbiota. Int. J. Biol. Macromol. 2024, 260, 129436. [Google Scholar] [CrossRef]
- Kavitha, R.; Uduman Mohideen, A.M. Identification Of Bioactive Components And Its Biological Activities Of Abelmoschas Moschatus Flower Extrtact-A Gc-Ms Study. IOSR J. Appl. Chem. 2017, 10, 19–22. [Google Scholar]
- Xu, L.; Chang, C.; Jiang, P.; Wei, K.; Zhang, R.; Jin, Y.; Zhao, J.; Xu, L.; Shi, Y.; Guo, S.; et al. Metabolomics in Rheumatoid Arthritis: Advances and Review. Front. Immunol. 2022, 13, 961708. [Google Scholar] [CrossRef]
- Ciaffi, J.; Mitselman, D.; Mancarella, L.; Brusi, V.; Lisi, L.; Ruscitti, P.; Cipriani, P.; Meliconi, R.; Giacomelli, R.; Borghi, C.; et al. The Effect of Ketogenic Diet on Inflammatory Arthritis and Cardiovascular Health in Rheumatic Conditions: A Mini Review. Front. Med. 2021, 8, 2495. [Google Scholar] [CrossRef]
- Torequl Islam, M.; Quispe, C.; Herrera-Bravo, J.; Rahaman, M.M.; Hossain, R.; Sarkar, C.; Raihan, M.A.; Chowdhury, M.M.; Uddin, S.J.; Shilpi, J.A.; et al. Activities and Molecular Mechanisms of Diterpenes, Diterpenoids, and Their Derivatives in Rheumatoid Arthritis. Evid.-Based Complement. Altern. Med. 2022, 2022, 4787643. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, H.; Lin, Z.; Wang, Q.; Chen, L.; Liu, S.; Yang, X.; Zhu, F.; Huang, Y.; Cao, S.; et al. A Novel Tricyclic BTK Inhibitor Suppresses B Cell Responses and Osteoclastic Bone Erosion in Rheumatoid Arthritis. Acta Pharmacol. Sin. 2021, 42, 1653–1664. [Google Scholar] [CrossRef]
- Baoqi, Y.; Dan, M.; Xingxing, Z.; Xueqing, Z.; Yajing, W.; Ke, X.; Liyun, Z. Effect of Anti-Rheumatic Drugs on Cardiovascular Disease Events in Rheumatoid Arthritis. Front. Cardiovasc. Med. 2022, 8, 812631. [Google Scholar] [CrossRef]
- Azizov, V.; Zaiss, M.M. Alcohol Consumption in Rheumatoid Arthritis: A Path through the Immune System. Nutrients 2021, 13, 1324. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Yu, H.; Xie, Q.; Wang, B.; Jiang, S.; Su, W.; Mao, Y.; Li, B.; Peng, C.; et al. Sesquiterpenes from Kadsura Coccinea Attenuate Rheumatoid Arthritis-Related Inflammation by Inhibiting the NF-ΚB and JAK2/STAT3 Signal Pathways. Phytochemistry 2022, 194, 113018. [Google Scholar] [CrossRef]
- George, G.; Shyni, G.L.; Raghu, K.G. Current and Novel Therapeutic Targets in the Treatment of Rheumatoid Arthritis. Inflammopharmacology 2020, 28, 1457–1476. [Google Scholar] [CrossRef]
- Babaahmadi, M.; Tayebi, B.; Gholipour, N.M.; Kamardi, M.T.; Heidari, S.; Baharvand, H.; Eslaminejad, M.B.; Hajizadeh-Saffar, E.; Hassani, S.-N. Rheumatoid Arthritis: The Old Issue, the New Therapeutic Approach. Stem Cell Res. Ther. 2023, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Li, S.; Yang, G.; Luo, Y.; Qi, T.; Liang, Y.; Yang, T.; Zhang, L.; Wang, R.; Zhu, L.; et al. Design, Synthesis, Molecular Modeling, and Biological Evaluation of Acrylamide Derivatives as Potent Inhibitors of Human Dihydroorotate Dehydrogenase for the Treatment of Rheumatoid Arthritis. Acta Pharm. Sin. B 2021, 11, 795–809. [Google Scholar] [CrossRef]
- Apaza Ticona, L.; Siñani Callisaya, G.B.; Aguilar Rico, F.; Sánchez Sánchez-Corral, J.; Slowing, K. Anti-Inflammatory and Anti-Arthritic Effects of Compounds from Buddleja coriacea. Nat. Prod. Res. 2022, 36, 6324–6328. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Plotnikov, M.B.; Khlebnikov, A.I.; Plotnikova, T.M.; Quinn, M.T. Oximes: Novel Therapeutics with Anticancer and Anti-Inflammatory Potential. Biomolecules 2021, 11, 777. [Google Scholar] [CrossRef]
- Khan, Z.U.R.; Assad, N.; Naeem-ul-Hassan, M.; Sher, M.; Alatawi, F.S.; Alatawi, M.S.; Omran, A.M.E.; Jame, R.M.A.; Adnan, M.; Khan, M.N.; et al. Aconitum Lycoctonum L. (Ranunculaceae) Mediated Biogenic Synthesis of Silver Nanoparticles as Potential Antioxidant, Anti-Inflammatory, Antimicrobial and Antidiabetic Agents. BMC Chem. 2023, 17, 128. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Jit, B.P.; Ragusa, A.; Jena, M. Preliminary Investigation of the Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activity of Enteromorpha Intestinalis Extracts. Molecules 2021, 26, 1171. [Google Scholar] [CrossRef]
- Mane, M.P.; Patil, R.S.; Magdum, A.B.; Kakade, S.S.; Patil, D.N.; Nimbalkar, M.S. Chemo-Profiling by UPLC-QTOF MS Analysis and in Vitro Assessment of Anti-Inflammatory Activity of Field Milkwort (Polygala arvensis Willd.). S. Afr. J. Bot. 2022, 149, 49–59. [Google Scholar] [CrossRef]
- Saldanha, E.; Pai, R.J.; George, T.; D’Souza, S.; Adnan, M.; Pais, M.; Naik, T.; D’Souza, R.C.C.; D’Cunha, R.; Shrinath Baliga, M. Health Effects of Various Dietary Agents and Phytochemicals (Therapy of Acute Pancreatitis). In Therapeutic, Probiotic, and Unconventional Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 303–314. [Google Scholar]
- Abbotto, E.; Casini, B.; Piacente, F.; Scarano, N.; Cerri, E.; Tonelli, M.; Astigiano, C.; Millo, E.; Sturla, L.; Bruzzone, S.; et al. Novel Thiazole-Based SIRT2 Inhibitors Discovered via Molecular Modelling Studies and Enzymatic Assays. Pharmaceuticals 2023, 16, 1316. [Google Scholar] [CrossRef]
- Oikonomopoulou, K.; Diamandis, E.P.; Hollenberg, M.D.; Chandran, V. Proteinases and Their Receptors in Inflammatory Arthritis: An Overview. Nat. Rev. Rheumatol. 2018, 14, 170–180. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, B. The Role of Inflammatory Mediators and Matrix Metalloproteinases (MMPs) in the Progression of Osteoarthritis. Biomater. Biosyst. 2024, 13, 100090. [Google Scholar] [CrossRef]
- Bu, Y.; Wu, H.; Deng, R.; Wang, Y. Therapeutic Potential of SphK1 Inhibitors Based on Abnormal Expression of SphK1 in Inflammatory Immune Related-Diseases. Front. Pharmacol. 2021, 12, 733387. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Fleischmann, R. Kinase Inhibitors: A New Approach to Rheumatoid Arthritis Treatment. Curr. Opin. Rheumatol. 2010, 22, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wei, K.; Jiang, P.; Chang, C.; Xu, L.; Xu, L.; Shi, Y.; Guo, S.; He, D. G-Protein-Coupled Receptors in Rheumatoid Arthritis: Recent Insights into Mechanisms and Functional Roles. Front. Immunol. 2022, 13, 907733. [Google Scholar] [CrossRef] [PubMed]
- Stegen, M.; Frey, U.H. The Role of G Protein-Coupled Receptor Kinase 6 Regulation in Inflammation and Pain. Int. J. Mol. Sci. 2022, 23, 15880. [Google Scholar] [CrossRef]
- Nakayamada, S.; Kubo, S.; Iwata, S.; Tanaka, Y. Recent Progress in JAK Inhibitors for the Treatment of Rheumatoid Arthritis. BioDrugs 2016, 30, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Laedermann, C.; Alten, R.; Feist, E.; Choy, E.; Haladyj, E.; De La Torre, I.; Richette, P.; Finckh, A.; Tanaka, Y. A JAK Inhibitor for Treatment of Rheumatoid Arthritis: The Baricitinib Experience. J. Clin. Med. 2023, 12, 4527. [Google Scholar] [CrossRef]
- Pieta, A.; Venetsanopoulou, A.I.; Kittas, C.; Christaki, E.; Voulgari, P.V. Recurrent Scedosporium Apiospermum Cutaneous Infection in a Patient with Rheumatoid Arthritis: The Potent Role of IL-6 Signaling Pathway Blockade: A Case-Based Review. J. Fungi 2023, 9, 683. [Google Scholar] [CrossRef]
- Radu, A.-F.; Bungau, S.G. Nanomedical Approaches in the Realm of Rheumatoid Arthritis. Ageing Res. Rev. 2023, 87, 101927. [Google Scholar] [CrossRef]
- Li, R.-L.; Duan, H.-X.; Liang, Q.; Huang, Y.-L.; Wang, L.-Y.; Zhang, Q.; Wu, C.-J.; Liu, S.-Q.; Peng, W. Targeting Matrix Metalloproteases: A Promising Strategy for Herbal Medicines to Treat Rheumatoid Arthritis. Front. Immunol. 2022, 13, 1046810. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, Y. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Synovial Fluids from Patients with Rheumatoid Arthritis or Osteoarthritis. Ann. Rheum. Dis. 2000, 59, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Nejatbakhsh Samimi, L.; Farhadi, E.; Tahmasebi, M.N.; Jamshidi, A.; Sharafat Vaziri, A.; Mahmoudi, M. NF-ΚB Signaling in Rheumatoid Arthritis with Focus on Fibroblast-like Synoviocytes. Autoimmun. Highlights 2020, 11, 11. [Google Scholar] [CrossRef]
- Brown, K.D.; Claudio, E.; Siebenlist, U. The Roles of the Classical and Alternative Nuclear Factor-KappaB Pathways: Potential Implications for Autoimmunity and Rheumatoid Arthritis. Arthritis Res. Ther. 2008, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Kawsar, S.M.A.; Kumer, A.; Munia, N.S.; Hosen, M.A.; Chakma, U.; Akash, S. Chemical Descriptors, PASS, Molecular Docking, Molecular Dynamics and ADMET Predictions of Glucopyranoside Derivatives as Inhibitors to Bacteria and Fungi Growth. Org. Commun. 2022, 15, 184–203. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Wen, Y.; Zhang, H.; Zhao, G.; Gao, Q. Signaling Pathways in Macrophages: Molecular Mechanisms and Therapeutic Targets. MedComm 2023, 4, e349. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Lagorce, D.; Douguet, D.; Miteva, M.A.; Villoutreix, B.O. Computational Analysis of Calculated Physicochemical and ADMET Properties of Protein-Protein Interaction Inhibitors. Sci. Rep. 2017, 7, 46277. [Google Scholar] [CrossRef]
- Arrar, L.; Benzidane, N.; Krache, I.; Charef, N.; Khennouf, S.; Baghiani, A. Comparison between Polyphenol Contents and Antioxidant Activities of Different Parts of Capparis Spinosa L. Pharmacogn. Commun. 2013, 3, 70–74. [Google Scholar] [CrossRef]
- Suresh, P. Anti-Inflammatory and Antioxidant Effects of Hydro-Alcoholic Extract of Dicliptera Cuneata Nees Aerial Parts. Bioinformation 2023, 19, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Saleem, M.; Riaz, N.; Lee, Y.S.; Diri, R.; Noor, A.; Almasri, D.; Bagalagel, A.; Elsebai, M.F. The Natural Polypeptides as Significant Elastase Inhibitors. Front. Pharmacol. 2020, 11, 531998. [Google Scholar] [CrossRef]
- Lasmari, S.; Ikhlef, S.; Boulcina, R.; Mokrani, E.H.; Bensouici, C.; Gürbüz, N.; Dündar, M.; Karcı, H.; Özdemir, İ.; Koç, A.; et al. New Silver Nheterocyclic Carbenes Complexes: Synthesis, Molecular Docking Study and Biological Activities Evaluation as Cholinesterase Inhibitors and Antimicrobials. J. Mol. Struct. 2021, 1238, 130399. [Google Scholar] [CrossRef]
- Boualia, I.; Derabli, C.; Boulcina, R.; Bensouici, C.; Yildirim, M.; Birinci Yildirim, A.; Mokrani, E.H.; Debache, A. Synthesis, Molecular Docking Studies, and Biological Evaluation of Novel Alkyl Bis(4-amino-5-cyanopyrimidine) Derivatives. Arch. Pharm. 2019, 352, 1900027. [Google Scholar] [CrossRef] [PubMed]
- Kakularam, K.R.; Karst, F.; Polamarasetty, A.; Ivanov, I.; Heydeck, D.; Kuhn, H. Paralog- and Ortholog-Specificity of Inhibitors of Human and Mouse Lipoxygenase-Isoforms. Biomed. Pharmacother. 2022, 145, 112434. [Google Scholar] [CrossRef]
- Demmak, R.G.; Bordage, S.; Bensegueni, A.; Boutaghane, N.; Hennebelle, T.; Mokrani, E.H.; Sahpaz, S. Chemical Constituents from Solenostemma argel and Their Cholinesterase Inhibitory Activity. Nat. Prod. Sci. 2019, 25, 115. [Google Scholar] [CrossRef]
- Rarey, M.; Kramer, B.; Lengauer, T.; Klebe, G. A Fast Flexible Docking Method Using an Incremental Construction Algorithm. J. Mol. Biol. 1996, 261, 470–489. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Z.; Xia, N.; Zhang, W.; Wei, Y.; Huang, J.; Ren, Z.; Meng, F.; Yang, L. Anti-Arthritic Activity of Ferulic Acid in Complete Freund’s Adjuvant (CFA)-Induced Arthritis in Rats: JAK2 Inhibition. Inflammopharmacology 2020, 28, 463–473. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).