Therapeutic Potential of Targeting the PERK Signaling Pathway in Ischemic Stroke
Abstract
1. Introduction
2. General Overview of the UPR in Ischemic Stroke
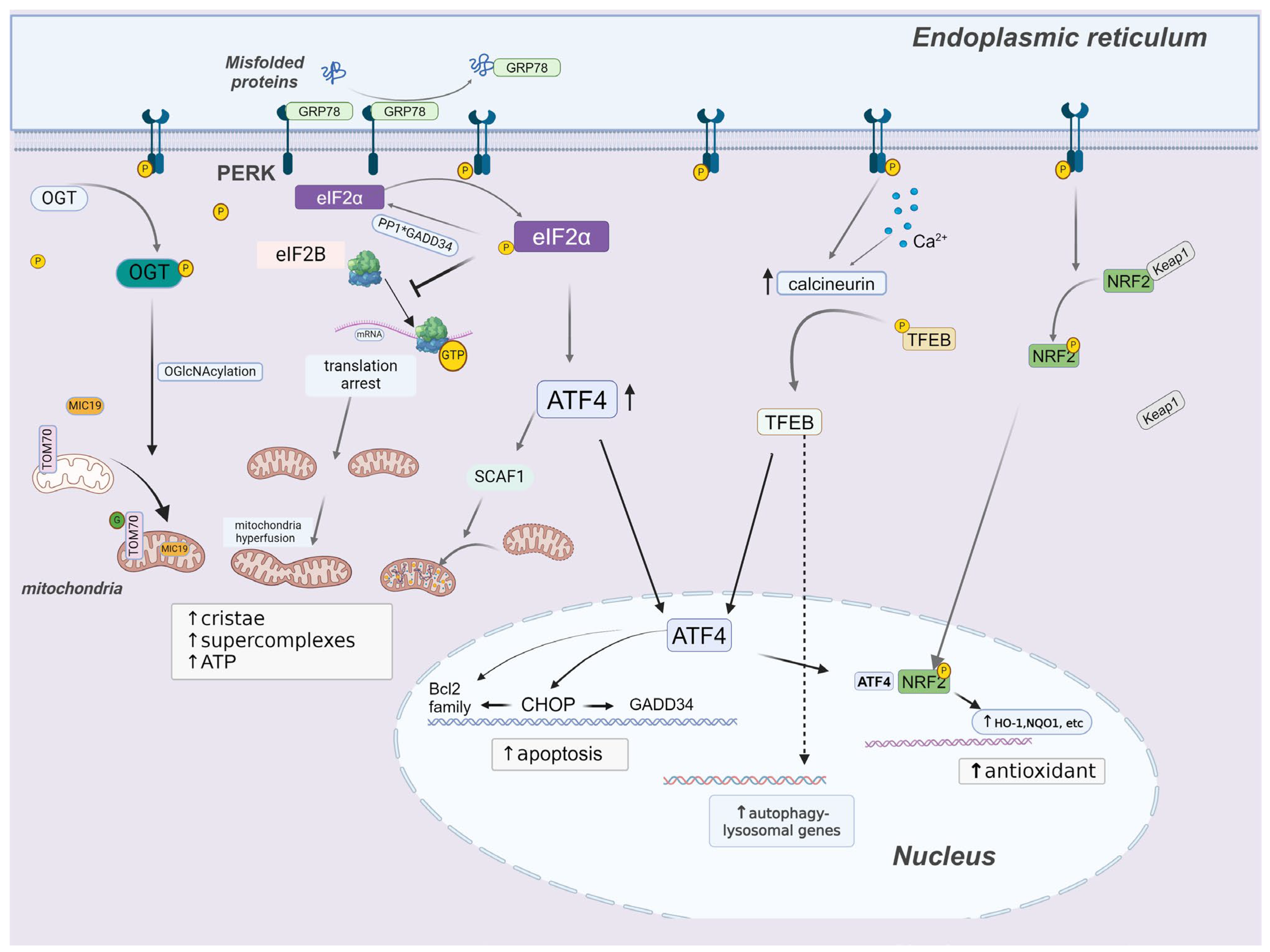
3. PERK Signaling
3.1. PERK and p-eIF2α
3.2. PERK and Autophagy
3.3. PERK and Redox Homeostasis
3.4. PERK and Mitochondrial Homeostasis
3.5. PERK and Cell Death
4. The PERK Pathway in Ischemic Stroke
4.1. Experimental Evidence from PERK-Specific Mouse Models
4.2. Experimental Evidence from Pharmacologic Tools
4.2.1. PERK Inhibitors
4.2.2. PERK Activators
4.2.3. eIF2α Phosphatase Inhibitors
4.2.4. eIF2B Activators
5. The PERK Pathway in Memory, Aging, and Neurodegenerative Diseases
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, W. Impaired capacity to restore proteostasis in the aged brain after ischemia: Implications for translational brain ischemia research. Neurochem. Int. 2019, 127, 87–93. [Google Scholar] [CrossRef]
- Yang, W.; Paschen, W. Unfolded protein response in brain ischemia: A timely update. J. Cereb. Blood Flow. Metab. 2016, 36, 2044–2050. [Google Scholar] [CrossRef]
- Li, X.; Yang, W. An update on the unfolded protein response in brain ischemia: Experimental evidence and therapeutic opportunities. Neurochem. Int. 2021, 151, 105218. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Zhang, X.; Ye, Y.; Xiong, X.; Zhang, S.; Gu, L.; Jian, Z.; Wang, H. Endoplasmic Reticulum Stress and the Unfolded Protein Response in Cerebral Ischemia/Reperfusion Injury. Front. Cell. Neurosci. 2022, 16, 864426. [Google Scholar] [CrossRef]
- Iurlaro, R.; Munoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Chambers, J.E.; Ron, D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov. 2022, 21, 115–140. [Google Scholar] [CrossRef]
- Lin, J.H.; Li, H.; Yasumura, D.; Cohen, H.R.; Zhang, C.; Panning, B.; Shokat, K.M.; Lavail, M.M.; Walter, P. IRE1 signaling affects cell fate during the unfolded protein response. Science 2007, 318, 944–949. [Google Scholar] [CrossRef]
- Almeida, L.M.; Pinho, B.R.; Duchen, M.R.; Oliveira, J.M.A. The PERKs of mitochondria protection during stress: Insights for PERK modulation in neurodegenerative and metabolic diseases. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1737–1748. [Google Scholar] [CrossRef]
- Talukdar, G.; Orr, H.T.; Lei, Z. The PERK pathway: Beneficial or detrimental for neurodegenerative diseases and tumor growth and cancer. Hum. Mol. Genet. 2023, 32, 2545–2557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.V.; Deng, Y.; Gao, N.; Pedrozo, Z.; Li, D.L.; Morales, C.R.; Criollo, A.; Luo, X.; Tan, W.; Jiang, N.; et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 2014, 156, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Yu, S.; Yu, Z.; Sheng, H.X.; Li, Y.; Liu, S.; Warner, D.S.; Paschen, W.; Yang, W. XBP1 (X-Box-Binding Protein-1)-Dependent O-GlcNAcylation Is Neuroprotective in Ischemic Stroke in Young Mice and Its Impairment in Aged Mice Is Rescued by Thiamet-G. Stroke 2017, 48, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Spasojevic, I.; Lu, L.; Shen, Y.; Qu, X.; Hoffmann, U.; Warner, D.S.; Paschen, W.; Sheng, H.; et al. Increasing O-GlcNAcylation is neuroprotective in young and aged brains after ischemic stroke. Exp. Neurol. 2021, 339, 113646. [Google Scholar] [CrossRef] [PubMed]
- Fahie, K.M.M.; Papanicolaou, K.N.; Zachara, N.E. Integration of O-GlcNAc into Stress Response Pathways. Cells 2022, 11, 3509. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, A.; Kamide, T.; Hashida, K.; Ta, H.M.; Inahata, Y.; Takarada-Iemata, M.; Hattori, T.; Mori, K.; Takahashi, R.; Matsuyama, T.; et al. Deletion of Atf6alpha impairs astroglial activation and enhances neuronal death following brain ischemia in mice. J. Neurochem. 2015, 132, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sheng, H.; Liu, S.; Zhao, S.; Glembotski, C.C.; Warner, D.S.; Paschen, W.; Yang, W. Activation of the ATF6 branch of the unfolded protein response in neurons improves stroke outcome. J. Cereb. Blood Flow. Metab. 2017, 37, 1069–1079. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Lu, L.; Dhar, A.; Sheng, H.; Yang, W. Beneficial effects of neuronal ATF6 activation in permanent ischemic stroke. Front. Cell. Neurosci. 2022, 16, 1016391. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Kolls, B.J.; Mace, B.; Yu, S.; Li, X.; Liu, W.; Chaparro, E.; Shen, Y.; Dang, L.; et al. Sustained overexpression of spliced X-box-binding protein-1 in neurons leads to spontaneous seizures and sudden death in mice. Commun. Biol. 2023, 6, 252. [Google Scholar] [CrossRef]
- Wang, Y.C.; Li, X.; Shen, Y.; Lyu, J.; Sheng, H.; Paschen, W.; Yang, W. PERK (Protein Kinase RNA-Like ER Kinase) Branch of the Unfolded Protein Response Confers Neuroprotection in Ischemic Stroke by Suppressing Protein Synthesis. Stroke 2020, 51, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yan, B.; Zhao, Q.; Wang, Z.; Wu, J.; Ren, J.; Wang, W.; Yu, S.; Sheng, H.; Crowley, S.D. Aging is associated with impaired activation of protein homeostasis-related pathways after cardiac arrest in mice. J. Am. Heart Assoc. 2018, 7, e009634. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sheng, H.; Yu, Z.; Paschen, W.; Yang, W. O-linked beta-N-acetylglucosamine modification of proteins is activated in post-ischemic brains of young but not aged mice: Implications for impaired functional recovery from ischemic stress. J. Cereb. Blood Flow. Metab. 2016, 36, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.R.; Kumar, R.; Zhang, P.; McGrath, B.C.; Cavener, D.R.; Krause, G.S. PERK is responsible for the increased phosphorylation of eIF2alpha and the severe inhibition of protein synthesis after transient global brain ischemia. J. Neurochem. 2005, 94, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G.; Lorsch, J.R. The mechanism of eukaryotic translation initiation: New insights and challenges. Cold Spring Harb. Perspect. Biol. 2012, 4, a011544. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Zhang, Y.; Bertolotti, A.; Zeng, H.; Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 2000, 5, 897–904. [Google Scholar] [CrossRef]
- Boyce, M.; Bryant, K.F.; Jousse, C.; Long, K.; Harding, H.P.; Scheuner, D.; Kaufman, R.J.; Ma, D.; Coen, D.M.; Ron, D.; et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 2005, 307, 935–939. [Google Scholar] [CrossRef]
- Raben, N.; Puertollano, R. TFEB and TFE3: Linking Lysosomes to Cellular Adaptation to Stress. Annu. Rev. Cell Dev. Biol. 2016, 32, 255–278. [Google Scholar] [CrossRef]
- Martina, J.A.; Diab, H.I.; Brady, O.A.; Puertollano, R. TFEB and TFE3 are novel components of the integrated stress response. EMBO J. 2016, 35, 479–495. [Google Scholar] [CrossRef]
- Kim, H.J.; Joe, Y.; Rah, S.Y.; Kim, S.K.; Park, S.U.; Park, J.; Kim, J.; Ryu, J.; Cho, G.J.; Surh, Y.J.; et al. Carbon monoxide induced TFEB nuclear translocation enhances mitophagy/mitochondrial biogenesis in hepatocytes and ameliorates inflammatory liver injury. Cell Death Dis. 2018, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Creamer, T.P. Calcineurin. Cell Commun. Signal. 2020, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, Y.; Huang, Q.; Xu, P.; Lenahan, C.; Lu, J.; Zheng, J.; Dong, X.; Shao, A.; Zhang, J. An updated review of autophagy in ischemic stroke: From mechanisms to therapies. Exp. Neurol. 2021, 340, 113684. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.; Logue, S.E. Unfolding the Interactions between Endoplasmic Reticulum Stress and Oxidative Stress. Antioxidants 2023, 12, 981. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Gordan, J.D.; Jin, J.; Harper, J.W.; Diehl, J.A. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: Oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 2004, 24, 8477–8486. [Google Scholar] [CrossRef]
- He, C.H.; Gong, P.; Hu, B.; Stewart, D.; Choi, M.E.; Choi, A.M.; Alam, J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 2001, 276, 20858–20865. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.A.; Li, R.C.; Thimmulappa, R.K.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Dore, S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience 2007, 147, 53–59. [Google Scholar] [CrossRef]
- Panahian, N.; Yoshiura, M.; Maines, M.D. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J. Neurochem. 1999, 72, 1187–1203. [Google Scholar] [CrossRef]
- Jiang, R.Q.; Li, Q.Q.; Sheng, R. Mitochondria associated ER membranes and cerebral ischemia: Molecular mechanisms and therapeutic strategies. Pharmacol. Res. 2023, 191, 106761. [Google Scholar] [CrossRef]
- Prinz, W.A.; Toulmay, A.; Balla, T. The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol. 2020, 21, 7–24. [Google Scholar] [CrossRef]
- Verfaillie, T.; Rubio, N.; Garg, A.D.; Bultynck, G.; Rizzuto, R.; Decuypere, J.P.; Piette, J.; Linehan, C.; Gupta, S.; Samali, A.; et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012, 19, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Fiorese, C.J.; Schulz, A.M.; Lin, Y.F.; Rosin, N.; Pellegrino, M.W.; Haynes, C.M. The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr. Biol. 2016, 26, 2037–2043. [Google Scholar] [CrossRef]
- Zhou, D.; Palam, L.R.; Jiang, L.; Narasimhan, J.; Staschke, K.A.; Wek, R.C. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J. Biol. Chem. 2008, 283, 7064–7073. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Di Benedetto, G.; Scorrano, L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 2011, 13, 589–598. [Google Scholar] [CrossRef]
- Balsa, E.; Soustek, M.S.; Thomas, A.; Cogliati, S.; Garcia-Poyatos, C.; Martin-Garcia, E.; Jedrychowski, M.; Gygi, S.P.; Enriquez, J.A.; Puigserver, P. ER and Nutrient Stress Promote Assembly of Respiratory Chain Supercomplexes through the PERK-eIF2alpha Axis. Mol. Cell 2019, 74, 877–890.e876. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, J.; Saunders, J.M.; Moraes, V.W.R.; Madhavan, A.; Madrazo, N.; Anthony, M.C.; Wiseman, R.L. The PERK Arm of the Unfolded Protein Response Regulates Mitochondrial Morphology during Acute Endoplasmic Reticulum Stress. Cell Rep. 2018, 22, 2827–2836. [Google Scholar] [CrossRef]
- Scorrano, L.; Ashiya, M.; Buttle, K.; Weiler, S.; Oakes, S.A.; Mannella, C.A.; Korsmeyer, S.J. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell 2002, 2, 55–67. [Google Scholar] [CrossRef]
- Latorre-Muro, P.; O’Malley, K.E.; Bennett, C.F.; Perry, E.A.; Balsa, E.; Tavares, C.D.J.; Jedrychowski, M.; Gygi, S.P.; Puigserver, P. A cold-stress-inducible PERK/OGT axis controls TOM70-assisted mitochondrial protein import and cristae formation. Cell Metab. 2021, 33, 598–614.e597. [Google Scholar] [CrossRef]
- Sassano, M.L.; van Vliet, A.R.; Vervoort, E.; Van Eygen, S.; Van den Haute, C.; Pavie, B.; Roels, J.; Swinnen, J.V.; Spinazzi, M.; Moens, L.; et al. PERK recruits E-Syt1 at ER-mitochondria contacts for mitochondrial lipid transport and respiration. J. Cell Biol. 2023, 222, e202206008. [Google Scholar] [CrossRef]
- McCullough, K.D.; Martindale, J.L.; Klotz, L.O.; Aw, T.Y.; Holbrook, N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001, 21, 1249–1259. [Google Scholar] [CrossRef]
- Puthalakath, H.; O’Reilly, L.A.; Gunn, P.; Lee, L.; Kelly, P.N.; Huntington, N.D.; Hughes, P.D.; Michalak, E.M.; McKimm-Breschkin, J.; Motoyama, N.; et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 2007, 129, 1337–1349. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef]
- Novoa, I.; Zhang, Y.; Zeng, H.; Jungreis, R.; Harding, H.P.; Ron, D. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 2003, 22, 1180–1187. [Google Scholar] [CrossRef]
- Brush, M.H.; Weiser, D.C.; Shenolikar, S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol. Cell. Biol. 2003, 23, 1292–1303. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Tajiri, S.; Oyadomari, S.; Yano, S.; Morioka, M.; Gotoh, T.; Hamada, J.I.; Ushio, Y.; Mori, M. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004, 11, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.H.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M.; et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- B’Chir, W.; Maurin, A.C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013, 41, 7683–7699. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, C.; Adajar, R.C.; DeZwaan-McCabe, D.; Thomas Rutkowski, D. A beneficial adaptive role for CHOP in driving cell fate selection during ER stress. EMBO Rep. 2024, 25, 228–253. [Google Scholar] [CrossRef]
- Li, H.Q.; Xia, S.N.; Xu, S.Y.; Liu, P.Y.; Gu, Y.; Bao, X.Y.; Xu, Y.; Cao, X. γ-Glutamylcysteine Alleviates Ischemic Stroke-Induced Neuronal Apoptosis by Inhibiting ROS-Mediated Endoplasmic Reticulum Stress. Oxidative Med. Cell. Longev. 2021, 2021, 2961079. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, X.; Chen, S.; Deng, L.; Jiang, L.; Yang, S.; Dong, Z. Geraniol-Mediated Suppression of Endoplasmic Reticulum Stress Protects against Cerebral Ischemia-Reperfusion Injury via the PERK-ATF4-CHOP Pathway. Int. J. Mol. Sci. 2022, 24, 544. [Google Scholar] [CrossRef]
- Nakka, V.P.; Gogada, R.; Simhadri, P.K.; Qadeer, M.A.; Phanithi, P.B. Post-treatment with apocynin at a lower dose regulates the UPR branch of eIF2α and XBP-1 pathways after stroke. Brain Res. Bull. 2022, 182, 1–11. [Google Scholar] [CrossRef]
- Lahiri, A.; Walton, J.C.; Zhang, N.; Billington, N.; DeVries, A.C.; Meares, G.P. Astrocytic deletion of protein kinase R-like ER kinase (PERK) does not affect learning and memory in aged mice but worsens outcome from experimental stroke. J. Neurosci. Res. 2023, 101, 1586–1610. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.S.; Chavez, J.C.; Pinto, J.T.; Coppola, G.; Sun, C.W.; Townes, T.M.; Geschwind, D.H.; Ratan, R.R. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J. Exp. Med. 2008, 205, 1227–1242. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Axten, J.M.; Patterson, J.B. Pharmacological targeting of the unfolded protein response for disease intervention. Nat. Chem. Biol. 2019, 15, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Dhir, N.; Jain, A.; Sharma, A.R.; Prakash, A.; Radotra, B.D.; Medhi, B. PERK inhibitor, GSK2606414, ameliorates neuropathological damage, memory and motor functional impairments in cerebral ischemia via PERK/p-eIF2α/ATF4/CHOP signaling. Metab. Brain Dis. 2023, 38, 1177–1192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Fu, H.; Huang, H.; Lu, Q.; Qin, H.; Wu, Y.; Huang, H.; Mao, G.; Wei, Z.; et al. Hes1 Knockdown Exacerbates Ischemic Stroke Following tMCAO by Increasing ER Stress-Dependent Apoptosis via the PERK/eIF2α/ATF4/CHOP Signaling Pathway. Neurosci. Bull. 2020, 36, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Xiang, P.; Luo, W.; Tan, X.; Gu, C.; Liu, M.; Chen, M.; Wang, Q.; Yang, J. CTRP1 Attenuates Cerebral Ischemia/Reperfusion Injury via the PERK Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 700854. [Google Scholar] [CrossRef]
- Nakka, V.P.; Gusain, A.; Raghubir, R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox. Res. 2010, 17, 189–202. [Google Scholar] [CrossRef]
- Anuncibay-Soto, B.; Perez-Rodriguez, D.; Santos-Galdiano, M.; Font, E.; Regueiro-Purrinos, M.; Fernandez-Lopez, A. Post-ischemic salubrinal treatment results in a neuroprotective role in global cerebral ischemia. J. Neurochem. 2016, 138, 295–306. [Google Scholar] [CrossRef]
- Anuncibay-Soto, B.; Perez-Rodriguez, D.; Santos-Galdiano, M.; Font-Belmonte, E.; Ugidos, I.F.; Gonzalez-Rodriguez, P.; Regueiro-Purrinos, M.; Fernandez-Lopez, A. Salubrinal and robenacoxib treatment after global cerebral ischemia. Exploring the interactions between ER stress and inflammation. Biochem. Pharmacol. 2018, 151, 26–37. [Google Scholar] [CrossRef]
- Font-Belmonte, E.; Ugidos, I.F.; Santos-Galdiano, M.; Gonzalez-Rodriguez, P.; Anuncibay-Soto, B.; Perez-Rodriguez, D.; Gonzalo-Orden, J.M.; Fernandez-Lopez, A. Post-ischemic salubrinal administration reduces necroptosis in a rat model of global cerebral ischemia. J. Neurochem. 2019, 151, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Dhir, N.; Jain, A.; Sharma, A.R.; Sharma, S.; Mahendru, D.; Patial, A.; Malik, D.; Prakash, A.; Attri, S.V.; Bhattacharyya, S.; et al. Rat BM-MSCs secretome alone and in combination with stiripentol and ISRIB, ameliorated microglial activation and apoptosis in experimental stroke. Behav. Brain Res. 2023, 449, 114471. [Google Scholar] [CrossRef]
- Axten, J.M.; Romeril, S.P.; Shu, A.; Ralph, J.; Medina, J.R.; Feng, Y.; Li, W.H.; Grant, S.W.; Heerding, D.A.; Minthorn, E.; et al. Discovery of GSK2656157: An Optimized PERK Inhibitor Selected for Preclinical Development. ACS Med. Chem. Lett. 2013, 4, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Axten, J.M.; Medina, J.R.; Feng, Y.; Shu, A.; Romeril, S.P.; Grant, S.W.; Li, W.H.; Heerding, D.A.; Minthorn, E.; Mencken, T.; et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J. Med. Chem. 2012, 55, 7193–7207. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rivera, D.; Delvaeye, T.; Roelandt, R.; Nerinckx, W.; Augustyns, K.; Vandenabeele, P.; Bertrand, M.J.M. When PERK inhibitors turn out to be new potent RIPK1 inhibitors: Critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ. 2017, 24, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Mahameed, M.; Wilhelm, T.; Darawshi, O.; Obiedat, A.; Tommy, W.S.; Chintha, C.; Schubert, T.; Samali, A.; Chevet, E.; Eriksson, L.A.; et al. The unfolded protein response modulators GSK2606414 and KIRA6 are potent KIT inhibitors. Cell Death Dis. 2019, 10, 300. [Google Scholar] [CrossRef]
- Smith, A.L.; Andrews, K.L.; Beckmann, H.; Bellon, S.F.; Beltran, P.J.; Booker, S.; Chen, H.; Chung, Y.A.; D’Angelo, N.D.; Dao, J.; et al. Discovery of 1H-pyrazol-3(2H)-ones as potent and selective inhibitors of protein kinase R-like endoplasmic reticulum kinase (PERK). J. Med. Chem. 2015, 58, 1426–1441. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Halliday, M.; Molloy, C.; Radford, H.; Verity, N.; Axten, J.M.; Ortori, C.A.; Willis, A.E.; Fischer, P.M.; Barrett, D.A.; et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 2013, 5, 206ra138. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, S.R.; Platt, G.; Barrie, S.E.; Zoumpoulidou, G.; Te Poele, R.H.; Aherne, G.W.; Wilson, S.C.; Sheldrake, P.; McDonald, E.; Venet, M.; et al. Mechanism-based screen for G1/S checkpoint activators identifies a selective activator of EIF2AK3/PERK signalling. PLoS ONE 2012, 7, e28568. [Google Scholar] [CrossRef] [PubMed]
- Ganz, J.; Shacham, T.; Kramer, M.; Shenkman, M.; Eiger, H.; Weinberg, N.; Iancovici, O.; Roy, S.; Simhaev, L.; Da’adoosh, B.; et al. A novel specific PERK activator reduces toxicity and extends survival in Huntington’s disease models. Sci. Rep. 2020, 10, 6875. [Google Scholar] [CrossRef]
- Novoa, I.; Zeng, H.; Harding, H.P.; Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol. 2001, 153, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Jousse, C.; Oyadomari, S.; Novoa, I.; Lu, P.; Zhang, Y.; Harding, H.P.; Ron, D. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J. Cell Biol. 2003, 163, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Carrara, M.; Sigurdardottir, A.; Bertolotti, A. Decoding the selectivity of eIF2alpha holophosphatases and PPP1R15A inhibitors. Nat. Struct. Mol. Biol. 2017, 24, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Krzyzosiak, A.; Schneider, K.; Wrabetz, L.; D’Antonio, M.; Barry, N.; Sigurdardottir, A.; Bertolotti, A. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 2015, 348, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Tsaytler, P.; Harding, H.P.; Ron, D.; Bertolotti, A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 2011, 332, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Crespillo-Casado, A.; Chambers, J.E.; Fischer, P.M.; Marciniak, S.J.; Ron, D. PPP1R15A-mediated dephosphorylation of eIF2α is unaffected by Sephin1 or Guanabenz. eLife 2017, 6, e26109. [Google Scholar] [CrossRef]
- Crespillo-Casado, A.; Claes, Z.; Choy, M.S.; Peti, W.; Bollen, M.; Ron, D. A Sephin1-insensitive tripartite holophosphatase dephosphorylates translation initiation factor 2α. J. Biol. Chem. 2018, 293, 7766–7776. [Google Scholar] [CrossRef]
- Zyryanova, A.F.; Kashiwagi, K.; Rato, C.; Harding, H.P.; Crespillo-Casado, A.; Perera, L.A.; Sakamoto, A.; Nishimoto, M.; Yonemochi, M.; Shirouzu, M.; et al. ISRIB Blunts the Integrated Stress Response by Allosterically Antagonising the Inhibitory Effect of Phosphorylated eIF2 on eIF2B. Mol. Cell 2021, 81, 88–103.e106. [Google Scholar] [CrossRef]
- Marlin, E.; Valencia, M.; Peregrín, N.; Ferrero, R.; Nicolás, M.J.; Vinueza-Gavilanes, R.; Pineda-Lucena, A.; Artieda, J.; Arrasate, M.; Aragón, T. Pharmacological inhibition of the integrated stress response accelerates disease progression in an amyotrophic lateral sclerosis mouse model. Br. J. Pharmacol. 2023, 181, 495–508. [Google Scholar] [CrossRef]
- Wong, Y.L.; LeBon, L.; Basso, A.M.; Kohlhaas, K.L.; Nikkel, A.L.; Robb, H.M.; Donnelly-Roberts, D.L.; Prakash, J.; Swensen, A.M.; Rubinstein, N.D.; et al. eIF2B activator prevents neurological defects caused by a chronic integrated stress response. eLife 2019, 8, e42940. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Klann, E. Spatiotemporally resolved protein synthesis as a molecular framework for memory consolidation. Trends Neurosci. 2022, 45, 297–311. [Google Scholar] [CrossRef]
- Sharma, V.; Ounallah-Saad, H.; Chakraborty, D.; Hleihil, M.; Sood, R.; Barrera, I.; Edry, E.; Kolatt Chandran, S.; Ben Tabou de Leon, S.; Kaphzan, H.; et al. Local Inhibition of PERK Enhances Memory and Reverses Age-Related Deterioration of Cognitive and Neuronal Properties. J. Neurosci. 2018, 38, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Gobert, D.; Stern, E.; Gamache, K.; Colina, R.; Cuello, C.; Sossin, W.; Kaufman, R.; Pelletier, J.; Rosenblum, K.; et al. eIF2α phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 2007, 129, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sood, R.; Khlaifia, A.; Eslamizade, M.J.; Hung, T.Y.; Lou, D.; Asgarihafshejani, A.; Lalzar, M.; Kiniry, S.J.; Stokes, M.P.; et al. eIF2α controls memory consolidation via excitatory and somatostatin neurons. Nature 2020, 586, 412–416. [Google Scholar] [CrossRef]
- Sidrauski, C.; Acosta-Alvear, D.; Khoutorsky, A.; Vedantham, P.; Hearn, B.R.; Li, H.; Gamache, K.; Gallagher, C.M.; Ang, K.K.; Wilson, C.; et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. eLife 2013, 2, e00498. [Google Scholar] [CrossRef]
- Shacham, T.; Patel, C.; Lederkremer, G.Z. PERK Pathway and Neurodegenerative Disease: To Inhibit or to Activate? Biomolecules 2021, 11, 354. [Google Scholar] [CrossRef]
- Devi, L.; Ohno, M. PERK mediates eIF2alpha phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2272–2281. [Google Scholar] [CrossRef]
- Ma, T.; Trinh, M.A.; Wexler, A.J.; Bourbon, C.; Gatti, E.; Pierre, P.; Cavener, D.R.; Klann, E. Suppression of eIF2alpha kinases alleviates Alzheimer’s disease-related plasticity and memory deficits. Nat. Neurosci. 2013, 16, 1299–1305. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Lourenco, M.V.; Longo, F.; Kasica, N.P.; Yang, W.; Ureta, G.; Ferreira, D.D.P.; Mendonca, P.H.J.; Bernales, S.; Ma, T.; et al. Correction of eIF2-dependent defects in brain protein synthesis, synaptic plasticity, and memory in mouse models of Alzheimer’s disease. Sci. Signal. 2021, 14, eabc5429. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yu, P.; Zhang, Y.; Yang, Y.; Zhu, M.; Qin, S.; Xu, J.T.; Duan, D.; Wu, Y.; Wang, D.; et al. Inhibition of the ISR abrogates mGluR5-dependent long-term depression and spatial memory deficits in a rat model of Alzheimer’s disease. Transl. Psychiatry 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; St George, B.; Fenton, M.; Firkins, L. Top ten research priorities relating to life after stroke. Lancet Neurol. 2012, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Leys, D.; Henon, H.; Mackowiak-Cordoliani, M.-A.; Pasquier, F. Poststroke dementia. Lancet Neurol. 2005, 4, 752–759. [Google Scholar] [CrossRef]
- Suenaga, J.; Hu, X.; Pu, H.; Shi, Y.; Hassan, S.H.; Xu, M.; Leak, R.K.; Stetler, R.A.; Gao, Y.; Chen, J. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp. Neurol. 2015, 272, 109–119. [Google Scholar] [CrossRef]
- Harding, H.P.; Zeng, H.Q.; Zhang, Y.H.; Jungries, R.; Chung, P.; Plesken, H.; Sabatini, D.D.; Ron, D. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 2001, 7, 1153–1163. [Google Scholar] [CrossRef]
| Compound | Animal | Ischemia Model | Dose | Intervention Regimen | Route | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| PERK inhibitors | |||||||
| GSK2606414 | Rats | tMCAO | 50, 100, or 150 mg/kg | Starting at 3 h post-MCAO, followed by 4 doses at a 24 h interval | Oral | Improved | [67] |
| Mice | tMCAO | 4 μL (20 μM) | 24 h before tMCAO | ICV | No effect | [68] | |
| PERK activator | |||||||
| CCT020312 | SD rats | tMCAO | Unspecified | Unspecified | ICV | No effect | [69] |
| eIF2α phosphatase inhibitor | |||||||
| Salubrinal | Mice | tMCAO | 1 mg/kg | 30 min after reperfusion | IP | Improved | [21] |
| SD rats | tMCAO | 1 mg/kg | 30 min before tMCAO | IP | Improved | [70] | |
| SD rats | GCI | 1 mg/kg | 1 h and 25 h after GCI | IP | Improved | [71] | |
| SD rats | GCI | 1 mg/kg | 1 h and 24 h after GCI | IP | Improved | [72] | |
| Rats | GCI | 1 mg/kg | 1 h and 24 h after GCI | IP | Improved | [73] | |
| eIF2B activator | |||||||
| ISRIB | Wistar rats | tMCAO | 2.5 mg/kg | 3 h post MCAO followed by 3 doses at a 12 h interval | IP | Improved | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Dang, L.; Zhang, R.; Yang, W. Therapeutic Potential of Targeting the PERK Signaling Pathway in Ischemic Stroke. Pharmaceuticals 2024, 17, 353. https://doi.org/10.3390/ph17030353
Yu X, Dang L, Zhang R, Yang W. Therapeutic Potential of Targeting the PERK Signaling Pathway in Ischemic Stroke. Pharmaceuticals. 2024; 17(3):353. https://doi.org/10.3390/ph17030353
Chicago/Turabian StyleYu, Xinyuan, Lihong Dang, Ran Zhang, and Wei Yang. 2024. "Therapeutic Potential of Targeting the PERK Signaling Pathway in Ischemic Stroke" Pharmaceuticals 17, no. 3: 353. https://doi.org/10.3390/ph17030353
APA StyleYu, X., Dang, L., Zhang, R., & Yang, W. (2024). Therapeutic Potential of Targeting the PERK Signaling Pathway in Ischemic Stroke. Pharmaceuticals, 17(3), 353. https://doi.org/10.3390/ph17030353







