Towards the Magic Radioactive Bullet: Improving Targeted Radionuclide Therapy by Reducing the Renal Retention of Radioligands
Abstract
1. Introduction
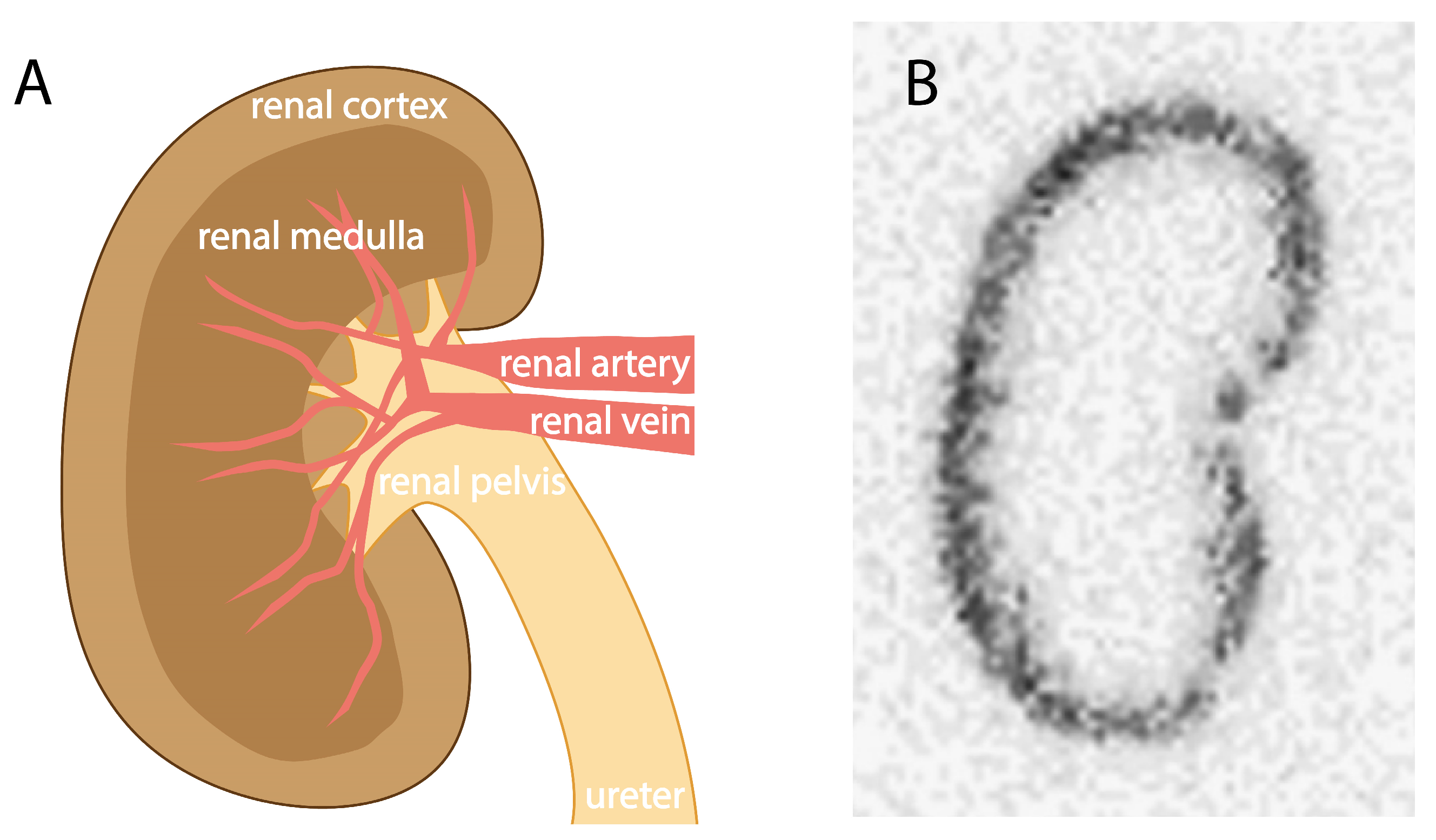
2. Kidney Retention of Low-to-Moderate-Molecular-Weight Molecules
3. Radionuclide-Dependent Kidney Uptake Reduction
4. Competitive Inhibition in Proximal Tubules
4.1. Charged Amino Acids
4.2. Gelofusine
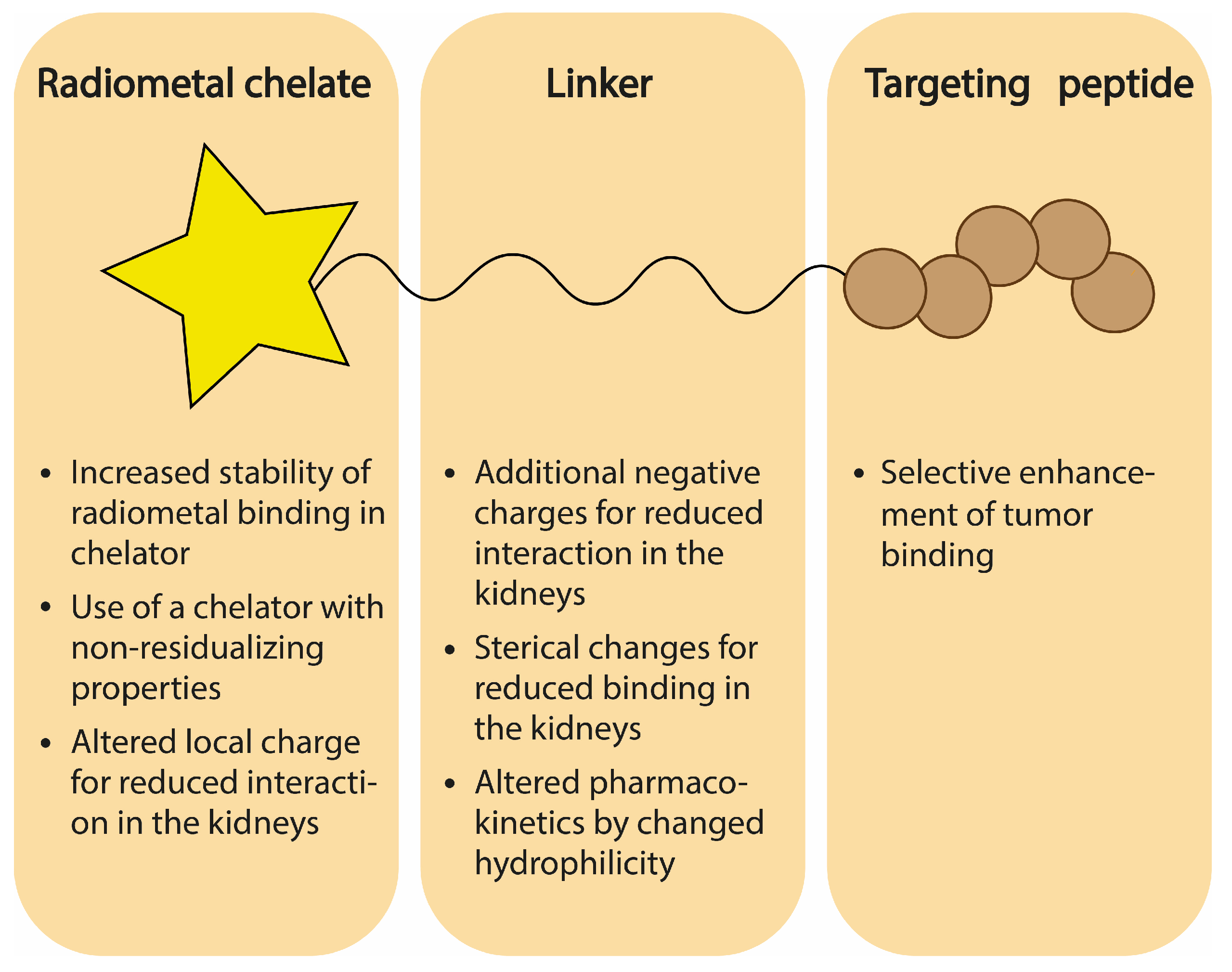
5. Chemical Design of Radioligand
5.1. Influence of Chelator
5.1.1. Stability
5.1.2. Residualizing Activity
5.1.3. Charge
5.2. Influence of Linker Charge
5.3. Influence of Amino Acid Sterics
5.4. Influence of Linker Length and Type
6. Cleavable Linkers
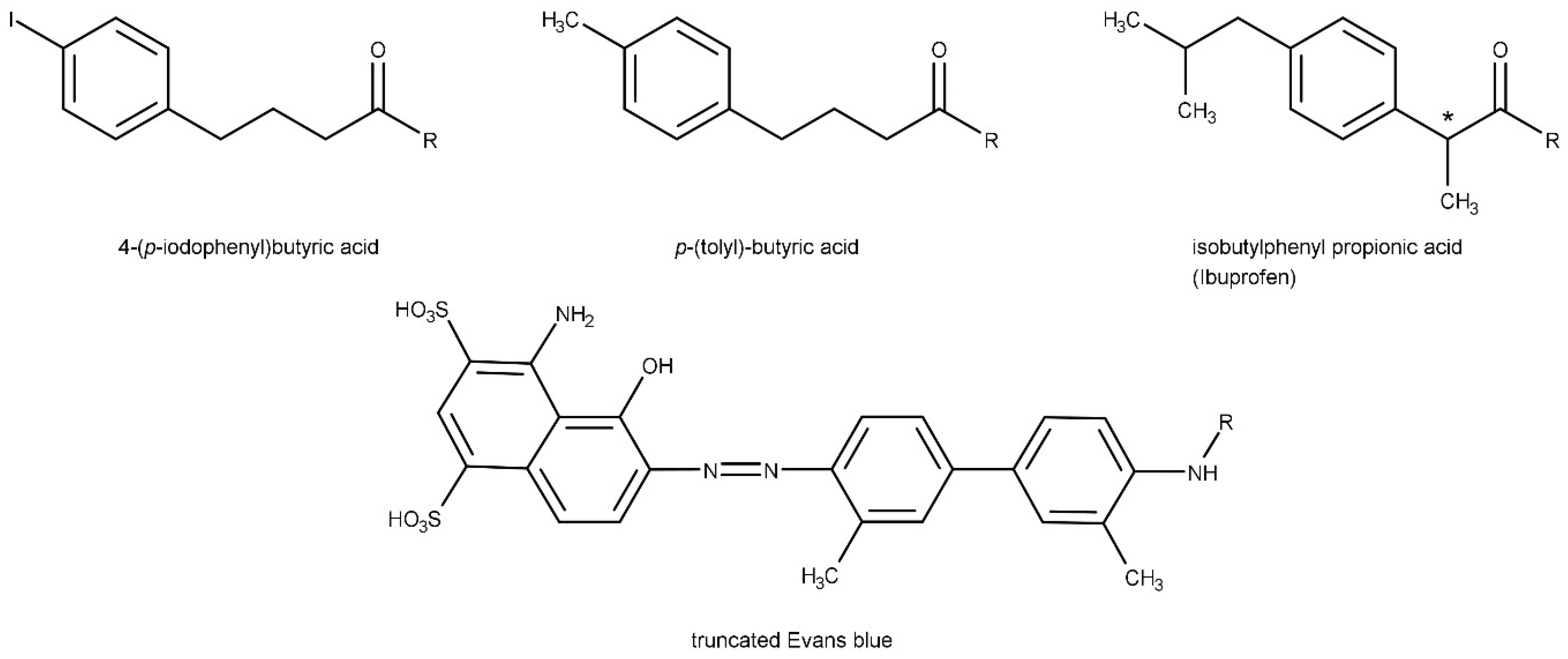
7. Albumin-Binding Radioligands
8. Discussion, Conclusions, and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| AUC | area under the curve |
| CCK1R | cholecystokinin type 1 receptor |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DAB | diaminobutyric acid |
| DOTA | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DOTAGA | 1,4,7,10-tetraazacyclododececane-1-(glutaric acid)-4,7,10-triacetic acid |
| EB | Evans Blue |
| EMA | European Medicines Agency |
| FAP | fibroblast activation protein |
| FAPI | fibroblast activation protein inhibitor |
| FDA | Food and Drug Administration |
| GFK | glycine phenylalanine-lysine |
| GLP-1R | glucagon-like peptide-1 receptor |
| GY | glycine-tyrosine |
| HBED-CC | N,N′-bis-[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N′-diacetic acid |
| HE | histidine-glutamic acid |
| Her2/neu | human epidermal growth factor receptor 2 |
| HYNIC | hydrazinonicotinic acid |
| kDa | kilodalton |
| mAb | monoclonal antibody |
| MAG3 | mercaptoacetylglycylglycylglycin |
| MC1R | melanocortin-1 receptor |
| mCRPC | metastatic castration resistant prostate cancer |
| α-MSH | melanocyte stimulating hormone |
| MVK | methionine-valine-leucine |
| NEP | neutral endopeptidase |
| NODAGA | 1,4,7-triazacyclononane-1-(glutaric acid)-4,7-diacetic acid |
| NOTA | 1,4,7-triazacyclononane-1,4,7-triacetic acid |
| NOTA-p-SCN | S-2-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid |
| PEG | polyethylene glycol |
| PET | positron emission tomography |
| PGA | polyglutamic acid |
| p.i. | post injection |
| PSMA | prostate specific membrane antigen |
| RGD | arginine-glycine-aspartic acid |
| sdAb | single-domain antibody-fragment |
| SPECT/CT | single photon emission computed tomography/computed tomography |
| TE2A | 1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4,11-diacetic acid |
| TRT | targeted radionuclide therapy |
| uPAR | urokinase-type plasminogen receptor |
References
- Bodei, L.; Cremonesi, M.; Ferrari, M.; Pacifici, M.; Grana, C.M.; Bartolomei, M.; Baio, S.M.; Sansovini, M.; Paganelli, G. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: The role of associated risk factors. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1847–1856. [Google Scholar] [CrossRef]
- Geenen, L.; Geenen, L.; Nonnekens, J.; Konijnenberg, M.; Baatout, S.; De Jong, M.; Aerts, A. Overcoming nephrotoxicity in peptide receptor radionuclide therapy using [(177)Lu]Lu-DOTA-TATE for the treatment of neuroendocrine tumours. Nucl. Med. Biol. 2021, 102–103, 1–11. [Google Scholar] [CrossRef]
- Sandstrom, M.; Garske-Roman, U.; Granberg, D.; Johansson, S.; Widstrom, C.; Eriksson, B.; Sundin, A.; Lundqvist, H.; Lubberink, M. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J. Nucl. Med. 2013, 54, 33–41. [Google Scholar] [CrossRef]
- Konijnenberg, M.; Melis, M.; Valkema, R.; Krenning, E.; de Jong, M. Radiation dose distribution in human kidneys by octreotides in peptide receptor radionuclide therapy. J. Nucl. Med. 2007, 48, 134–142. [Google Scholar] [PubMed]
- Wahl, R.L.; Sgouros, G.; Iravani, A.; Jacene, H.; Pryma, D.; Saboury, B.; Capala, J.; Graves, S.A. Normal-Tissue Tolerance to Radiopharmaceutical Therapies, the Knowns and the Unknowns. J. Nucl. Med. 2021, 62 (Suppl. S3), 23S–35S. [Google Scholar] [CrossRef]
- Edeani, A.; Cohen, E.P. Radiation Nephropathy. In Onco-Nephrology Curriculum; Perazella, M.A., Ed.; American Society of Nephrology: Washington, DC, USA, 2016. [Google Scholar]
- Gotthardt, M.; van Eerd-Vismale, J.; Oyen, W.J.; de Jong, M.; Zhang, H.; Rolleman, E.; Maecke, H.R.; Behe, M.; Boerman, O. Indication for different mechanisms of kidney uptake of radiolabeled peptides. J. Nucl. Med. 2007, 48, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.; Braunlich, H. Factors Determining the Relationship between Renal and Hepatic Excretion of Xenobiotics. Arzneim.-Forsch./Drug Res. 1990, 40, 942–946. [Google Scholar]
- Christensen, E.I.; Nielsen, R. Role of megalin and cubilin in renal physiology and pathophysiology. Rev. Physiol. Biochem. Pharmacol. 2007, 158, 1–22. [Google Scholar] [PubMed]
- Vegt, E.; Melis, M.; Eek, A.; de Visser, M.; Brom, M.; Oyen, W.J.; Gotthardt, M.; de Jong, M.; Boerman, O.C. Renal uptake of different radiolabelled peptides is mediated by megalin: SPECT and biodistribution studies in megalin-deficient mice. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 623–632. [Google Scholar] [CrossRef]
- Orlando, R.A.; Exner, M.; Czekay, R.P.; Yamazaki, H.; Saito, A.; Ullrich, R.; Kerjaschki, D.; Farquhar, M.G. Identification of the second cluster of ligand-binding repeats in megalin as a site for receptor-ligand interactions. Proc. Natl. Acad. Sci. USA 1997, 94, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, S.K.; Kozyraki, R.; Kristiansen, M.; Kaysen, J.H.; Rasmussen, H.H.; Brault, D.; Pontillon, F.; Goda, F.O.; Christensen, E.I.; Hammond, T.G.; et al. The intrinsic factor-vitamin B12 receptor and target of teratogenic antibodies is a megalin-binding peripheral membrane protein with homology to developmental proteins. J. Biol. Chem. 1998, 273, 5235–5242. [Google Scholar] [CrossRef]
- Bates, C.M.; Kegg, H.; Petrevski, C.; Grady, S. Expression of somatostatin receptors 3, 4, and 5 in mouse kidney proximal tubules. Kidney Int. 2003, 63, 53–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhandari, S.; Watson, N.; Long, E.; Sharpe, S.; Zhong, W.; Xu, S.Z.; Atkin, S.L. Expression of somatostatin and somatostatin receptor subtypes 1–5 in human normal and diseased kidney. J. Histochem. Cytochem. 2008, 56, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Rolleman, E.J.; Kooij, P.P.; de Herder, W.W.; Valkema, R.; Krenning, E.P.; de Jong, M. Somatostatin receptor subtype 2-mediated uptake of radiolabelled somatostatin analogues in the human kidney. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1854–1860. [Google Scholar] [CrossRef][Green Version]
- Sokoloff, R.L.; Norton, K.C.; Gasior, C.L.; Marker, K.M.; Grauer, L.S. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: Levels in tissues, seminal fluid and urine. The Prostate 2000, 43, 150–157. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Leotta, K.; Eder, M.; Hoppe-Tich, T.; Youssoufian, H.; Kopka, K.; Babich, J.W.; Haberkorn, U. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J. Nucl. Med. 2015, 56, 293–298. [Google Scholar] [CrossRef]
- Campos, R.V.; Lee, Y.C.; Drucker, D.J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 1994, 134, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, P.; Beglinger, C.; Drewe, J.; Gutmann, H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul. Pept. 2007, 141, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Mojsov, S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: Brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995, 358, 219–224. [Google Scholar] [CrossRef]
- de Weerth, A.; Jonas, L.; Schade, R.; Schoneberg, T.; Wolf, G.; Pace, A.; Kirchhoff, F.; Schulz, M.; Heinig, T.; Greten, H.; et al. Gastrin/cholecystokinin type B receptors in the kidney: Molecular, pharmacological, functional characterization, and localization. Eur. J. Clin. Investig. 1998, 28, 592–601. [Google Scholar] [CrossRef]
- Monstein, H.J.; Nylander, A.G.; Salehi, A.; Chen, D.; Lundquist, I.; Hakanson, R. Cholecystokinin-A and cholecystokinin-B/gastrin receptor mRNA expression in the gastrointestinal tract and pancreas of the rat and man. A polymerase chain reaction study. Scand. J. Gastroenterol. 1996, 31, 383–390. [Google Scholar] [CrossRef]
- Lay, J.M.; Jenkins, C.; Friis-Hansen, L.; Samuelson, L.C. Structure and developmental expression of the mouse CCK-B receptor gene. Biochem. Biophys. Res. Commun. 2000, 272, 837–842. [Google Scholar] [CrossRef]
- von Schrenck, T.; de Weerth, A.; Bechtel, S.; Eschenhagen, T.; Weil, J.; Wolf, G.; Schulz, M.; Greten, H. Evidence for CCK(B) receptors in the guinea-pig kidney: Localization and characterization by [125I]gastrin binding studies and by RT-PCR. Naunyn Schmiedebergs Arch. Pharmacol. 1998, 358, 287–292. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Jestin, E.; Olafsen, T.; Wu, A.M.; Zalutsky, M.R. Evaluation of an anti-p185(HER2) (scFv-CH2-CH3)2 fragment following radioiodination using two different residualizing labels: SGMIB and IB-Mal-D-GEEEK. Nucl. Med. Biol. 2009, 36, 671–680. [Google Scholar] [CrossRef][Green Version]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx YG, J.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T.; et al. (131)I-labeled Anti-HER2 Camelid sdAb as a Theranostic Tool in Cancer Treatment. Clin. Cancer Res. 2017, 23, 6616–6628. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; Vos, J.; Caveliers, V.; Vaneycken, I.; Heemskerk, J.; Duhoux, F.P.; Fontaine, C.; Vanhoeij, M.; Windhorst, A.; van der Aa, F.; et al. Phase I Trial of (131)I-GMIB-Anti-HER2-VHH1, a New Promising Candidate for HER2-Targeted Radionuclide Therapy in Breast Cancer Patients. J. Nucl. Med. 2021, 62, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Birn, H. Megalin and cubilin: Synergistic endocytic receptors in renal proximal tubule. Am. J. Physiol. Renal Physiol. 2001, 280, F562–F573. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Barone, R.; Krenning, E.; Bernard, B.; Melis, M.; Visser, T.; Gekle, M.; Willnow, T.E.; Walrand, S.; Jamar, F.; et al. Megalin is essential for renal proximal tubule reabsorption of (111)In-DTPA-octreotide. J. Nucl. Med. 2005, 46, 1696–1700. [Google Scholar] [PubMed]
- Gainkam, L.O.; Caveliers, V.; Devoogdt, N.; Vanhove, C.; Xavier, C.; Boerman, O.; Muyldermans, S.; Bossuyt, A.; Lahoutte, T. Localization, mechanism and reduction of renal retention of technetium-99m labeled epidermal growth factor receptor-specific nanobody in mice. Contrast Media Mol. Imaging 2011, 6, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Pimm, M.V.; Gribben, S.J. Prevention of renal tubule re-absorption of radiometal (indium-111) labelled Fab fragment of a monoclonal antibody in mice by systemic administration of lysine. Eur. J. Nucl. Med. 1994, 21, 663–665. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Rolleman, E.J.; Bernard, B.F.; Visser, T.J.; Bakker, W.H.; Breeman, W.A.; Krenning, E.P. Inhibition of renal uptake of indium-111-DTPA-octreotide in vivo. J. Nucl. Med. 1996, 37, 1388–1392. [Google Scholar]
- Hammond, P.J.; Wade, A.F.; Gwilliam, M.E.; Peters, A.M.; Myers, M.J.; Gilbey, S.G.; Bloom, S.R.; Calam, J. Amino acid infusion blocks renal tubular uptake of an indium-labelled somatostatin analogue. Br. J. Cancer 1993, 67, 1437–1439. [Google Scholar] [CrossRef][Green Version]
- Rolleman, E.J.; Valkema, R.; de Jong, M.; Kooij, P.P.; Krenning, E.P. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 9–15. [Google Scholar] [CrossRef]
- Rolleman, E.J.; de Jong, M.; Valkema, R.; Kwekkeboom, D.; Kam, B.; Krenning, E.P. Inhibition of kidney uptake of radiolabeled somatostatin analogs: Amino acids or gelofusine? J. Nucl. Med. 2006, 47, 1730–1731. [Google Scholar]
- Stallons TA, R.; Saidi, A.; Tworowska, I.; Delpassand, E.S.; Torgue, J.J. Preclinical Investigation of (212)Pb-DOTAMTATE for Peptide Receptor Radionuclide Therapy in a Neuroendocrine Tumor Model. Mol. Cancer Ther. 2019, 18, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; Konijnenberg, M.W.; Daniels, T.; Nysus, M.; Makvandi, M.; de Blois, E.; Breeman, W.A.; Atcher, R.W.; de Jong, M.; Norenberg, J.P. Improved safety and efficacy of (213)Bi-DOTATATE-targeted alpha therapy of somatostatin receptor-expressing neuroendocrine tumors in mice pre-treated with L-lysine. EJNMMI Res. 2016, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. (2)(1)(3)Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Behe, M.; Kluge, G.; Becker, W.; Gotthardt, M.; Behr, T.M. Use of polyglutamic acids to reduce uptake of radiometal-labeled minigastrin in the kidneys. J. Nucl. Med. 2005, 46, 1012–1015. [Google Scholar] [PubMed]
- Veldman, B.A.; Schepkens, H.L.; Vervoort, G.; Klasen, I.; Wetzels, J.F. Low concentrations of intravenous polygelines promote low-molecular weight proteinuria. Eur. J. Clin. Invest. 2003, 33, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Park, E.A.; Graves, S.A.; Menda, Y. The Impact of Radiopharmaceutical Therapy on Renal Function. Semin. Nucl. Med. 2022, 52, 467–474. [Google Scholar] [CrossRef]
- van Eerd, J.E.; Vegt, E.; Wetzels, J.F.; Russel, F.G.; Masereeuw, R.; Corstens, F.H.; Oyen, W.J.; Boerman, O.C. Gelatin-based plasma expander effectively reduces renal uptake of 111In-octreotide in mice and rats. J. Nucl. Med. 2006, 47, 528–533. [Google Scholar] [PubMed]
- Melis, M.; Bijster, M.; de Visser, M.; Konijnenberg, M.W.; de Swart, J.; Rolleman, E.J.; Boerman, O.C.; Krenning, E.P.; de Jong, M. Dose-response effect of Gelofusine on renal uptake and retention of radiolabelled octreotate in rats with CA20948 tumours. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Konijnenberg, M.W.; KolencPeitl, P.; Garnuszek, P.; Nock, B.A.; Kaloudi, A.; Kroselj, M.; Zaletel, K.; Maecke, H.; Mansi, R.; et al. Preclinical pharmacokinetics, biodistribution, radiation dosimetry and toxicity studies required for regulatory approval of a phase I clinical trial with (111)In-CP04 in medullary thyroid carcinoma patients. Eur. J. Pharm. Sci. 2016, 91, 236–242. [Google Scholar] [CrossRef]
- Briat, A.; Wenk, C.H.; Ahmadi, M.; Claron, M.; Boturyn, D.; Josserand, V.; Dumy, P.; Fagret, D.; Coll, J.L.; Ghezzi, C.; et al. Reduction of renal uptake of 111In-DOTA-labeled and A700-labeled RAFT-RGD during integrin alphavbeta3 targeting using single photon emission computed tomography and optical imaging. Cancer Sci. 2012, 103, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.H.; Furukawa, T.; Sogawa, C.; Claron, M.; Aung, W.; Tsuji, A.B.; Wakizaka, H.; Zhang, M.R.; Boturyn, D.; Dumy, P.; et al. PET imaging and biodistribution analysis of the effects of succinylated gelatin combined with L-lysine on renal uptake and retention of (6)(4)Cu-cyclam-RAFT-c(-RGDfK-)(4) in vivo. Eur. J. Pharm. Biopharm. 2014, 86, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Vegt, E.; Wetzels, J.F.; Russel, F.G.; Masereeuw, R.; Boerman, O.C.; van Eerd, J.E.; Corstens, F.H.; Oyen, W.J. Renal uptake of radiolabeled octreotide in human subjects is efficiently inhibited by succinylated gelatin. J. Nucl. Med. 2006, 47, 432–436. [Google Scholar] [PubMed]
- Buitinga, M.; Jansen, T.; van der Kroon, I.; Woliner-van der Weg, W.; Boss, M.; Janssen, M.; Aarntzen, E.; Behe, M.; Wild, D.; Visser, E.; et al. Succinylated Gelatin Improves the Theranostic Potential of Radiolabeled Exendin-4 in Insulinoma Patients. J. Nucl. Med. 2019, 60, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Pan, Q.; Li, F. Decreased 68Ga-NOTA-exendin-4 renal uptake in patients pretreated with Gelofusine infusion: A randomized controlled study. J. Pancreatol. 2020, 3, 161–166. [Google Scholar] [CrossRef]
- Knor, S.; Sato, S.; Huber, T.; Morgenstern, A.; Bruchertseifer, F.; Schmitt, M.; Kessler, H.; Senekowitsch-Schmidtke, R.; Magdolen, V.; Seidl, C. Development and evaluation of peptidic ligands targeting tumour-associated urokinase plasminogen activator receptor (uPAR) for use in alpha-emitter therapy for disseminated ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 53–64. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N.; et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708–720. [Google Scholar] [CrossRef]
- Dekempeneer, Y.; Caveliers, V.; Ooms, M.; Maertens, D.; Gysemans, M.; Lahoutte, T.; Xavier, C.; Lecocq, Q.; Maes, K.; Covens, P.; et al. Therapeutic Efficacy of (213)Bi-labeled sdAbs in a Preclinical Model of Ovarian Cancer. Mol. Pharm. 2020, 17, 3553–3566. [Google Scholar] [CrossRef]
- Pruszynski, M.; D’Huyvetter, M.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T. Evaluation of an Anti-HER2 Nanobody Labeled with (225)Ac for Targeted alpha-Particle Therapy of Cancer. Mol. Pharm. 2018, 15, 1457–1466. [Google Scholar] [CrossRef]
- Rodak, M.; Dekempeneer, Y.; Wojewodzka, M.; Caveliers, V.; Covens, P.; Miller, B.W.; Sevenois, M.B.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T.; et al. Preclinical Evaluation of 225Ac-Labeled Single-Domain Antibody for the Treatment of HER2pos Cancer. Mol. Cancer Ther. 2022, 21, 1835–1845. [Google Scholar] [CrossRef]
- Rolleman, E.J.; Bernard, B.F.; Breeman, W.A.; Forrer, F.; de Blois, E.; Hoppin, J.; Gotthardt, M.; Boerman, O.C.; Krenning, E.P.; de Jong, M. Molecular imaging of reduced renal uptake of radiolabelled [DOTA0,Tyr3]octreotate by the combination of lysine and Gelofusine in rats. Nuklearmedizin 2008, 47, 110–115. [Google Scholar]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Copper chelation chemistry and its role in copper radiopharmaceuticals. Curr. Pharm. Des. 2007, 13, 3–16. [Google Scholar] [CrossRef]
- Dumont, R.A.; Deininger, F.; Haubner, R.; Maecke, H.R.; Weber, W.A.; Fani, M. Novel (64)Cu- and (68)Ga-labeled RGD conjugates show improved PET imaging of alpha(nu)beta(3) integrin expression and facile radiosynthesis. J. Nucl. Med. 2011, 52, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Miao, Y. Cu-64-labeled lactam bridge-cyclized alpha-MSH peptides for PET imaging of melanoma. Mol. Pharm. 2012, 9, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Garrison, J.C.; Rold, T.L.; Sieckman, G.L.; Figueroa, S.D.; Volkert, W.A.; Jurisson, S.S.; Hoffman, T.J. In vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using 64Cu bombesin analogs: Side-by-side comparison of the CB-TE2A and DOTA chelation systems. J. Nucl. Med. 2007, 48, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Wallberg, H.; Orlova, A.; Altai, M.; Hosseinimehr, S.J.; Widstrom, C.; Malmberg, J.; Stahl, S.; Tolmachev, V. Molecular design and optimization of 99mTc-labeled recombinant affibody molecules improves their biodistribution and imaging properties. J. Nucl. Med. 2011, 52, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Altai, M.; Honarvar, H.; Wallberg, H.; Strand, J.; Varasteh, Z.; Rosestedt, M.; Orlova, A.; Dunas, F.; Sandstrom, M.; Lofblom, J.; et al. Selection of an optimal cysteine-containing peptide-based chelator for labeling of affibody molecules with (188)Re. Eur. J. Med. Chem. 2014, 87, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Zhang, Z.; Zeisler, J.; Kuo, H.T.; Lin, K.S.; Benard, F. Reducing the Kidney Uptake of High Contrast CXCR4 PET Imaging Agents via Linker Modifications. Pharmaceutics 2022, 14, 1502. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Welch, M.J.; Lapi, S.E. Effects of chelator modifications on (68)Ga-labeled [Tyr (3)]octreotide conjugates. Mol. Imaging Biol. 2013, 15, 606–613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitran, B.; Varasteh, Z.; Selvaraju, R.K.; Lindeberg, G.; Sorensen, J.; Larhed, M.; Tolmachev, V.; Rosenstrom, U.; Orlova, A. Selection of optimal chelator improves the contrast of GRPR imaging using bombesin analogue RM26. Int. J. Oncol. 2016, 48, 2124–2134. [Google Scholar] [CrossRef]
- Varasteh, Z.; Rosenstrom, U.; Velikyan, I.; Mitran, B.; Altai, M.; Honarvar, H.; Rosestedt, M.; Lindeberg, G.; Sorensen, J.; Larhed, M.; et al. The effect of macrocyclic chelators on the targeting properties of the 68Ga-labeled gastrin releasing peptide receptor antagonist PEG2-RM26. Nucl. Med. Biol. 2015, 42, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xia, L.; Cai, P.; Wang, Y.; Feng, Y.; Zhang, W.; Liu, N.; Chen, Y.; Zhou, Z. In vitro and in vivo comparative study of 68Ga-labeled DOTA-, NOTA-, and HBEDCC-chelated radiotracers targeting prostate-specific membrane antigen. J. Radioanal. Nucl. Chem. 2023, 332, 617–628. [Google Scholar] [CrossRef]
- Gourni, E.; Mansi, R.; Jamous, M.; Waser, B.; Smerling, C.; Burian, A.; Buchegger, F.; Reubi, J.C.; Maecke, H.R. N-terminal modifications improve the receptor affinity and pharmacokinetics of radiolabeled peptidic gastrin-releasing peptide receptor antagonists: Examples of 68Ga- and 64Cu-labeled peptides for PET imaging. J. Nucl. Med. 2014, 55, 1719–1725. [Google Scholar] [CrossRef]
- Good, S.; Walter, M.A.; Waser, B.; Wang, X.; Muller-Brand, J.; Behe, M.P.; Reubi, J.C.; Maecke, H.R. Macrocyclic chelator-coupled gastrin-based radiopharmaceuticals for targeting of gastrin receptor-expressing tumours. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1868–1877. [Google Scholar] [CrossRef]
- Eder, M.; Lohr, T.; Bauder-Wust, U.; Reber, M.; Mier, W.; Schafer, M.; Haberkorn, U.; Eisenhut, M. Pharmacokinetic properties of peptidic radiopharmaceuticals: Reduced uptake of (EH)3-conjugates in important organs. J. Nucl. Med. 2013, 54, 1327–1330. [Google Scholar] [CrossRef][Green Version]
- Hofstrom, C.; Orlova, A.; Altai, M.; Wangsell, F.; Graslund, T.; Tolmachev, V. Use of a HEHEHE purification tag instead of a hexahistidine tag improves biodistribution of affibody molecules site-specifically labeled with (99m)Tc, (111)In, and (125)I. J. Med. Chem. 2011, 54, 3817–3826. [Google Scholar] [CrossRef]
- Sadeghzadeh, N.; Ahmadzadeh, M.; Erfani, M. Evaluation of a new radiolabeled bombesin derivative with (99m)Tc as potential targeted tumor imaging agent. J. Radioanal. Nucl. Chem. 2013, 298, 287–293. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Rennke, H.G. The structural and molecular basis of glomerular filtration. Circ. Res. 1978, 43, 337–347. [Google Scholar] [CrossRef]
- Akizawa, H.; Saito, M.; Tsukamoto, I.; Ohkura, T.; Shimizu, T.; Kitamura, Y.; Mifune, M.; Saito, Y.; Arano, Y.; Saji, H. Effect of molecular charges on renal uptake of 111In-DTPA-conjugated peptides. Nucl. Med. Biol. 2001, 28, 761–768. [Google Scholar] [CrossRef]
- Oshima, N.; Akizawa, H.; Kitaura, H.; Kawashima, H.; Zhao, S.; Zhao, Y.; Nishijima, K.I.; Kitamura, Y.; Arano, Y.; Kuge, Y.; et al. (111)In-DTPA-d-Phe(-1)-Asp(0)-d-Phe(1)-octreotide exhibits higher tumor accumulation and lower renal radioactivity than (111)In-DTPA-d-Phe(1)-octreotide. Nucl. Med. Biol. 2017, 54, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Akizawa, H.; Saito, M.; Tsukamoto, I.; Ohkura, T.; Shimizu, T.; Kitamura, Y.; Mifune, M.; Saito, Y.; Arano, Y.; Saji, H. Effect of carboxyl-group of D-glutamic acid or gamma-carboxy-D-glutamic acid as N-terminal amino acid of (111)in-diethylenetriaminepentaacetic acid-octreotide on accumulation of radioactivity in kidney. Biol. Pharm. Bull. 2007, 30, 2226–2228. [Google Scholar] [CrossRef] [PubMed]
- Oshima, N.; Akizawa, H.; Zhao, S.; Zhao, Y.; Nishijima, K.; Kitamura, Y.; Arano, Y.; Kuge, Y.; Ohkura, K. Design, synthesis and biological evaluation of negatively charged (1)(1)(1)In-DTPA-octreotide derivatives. Bioorg Med. Chem. 2014, 22, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Oshima, N.; Akizawa, H.; Kawashima, H.; Zhao, S.; Zhao, Y.; Nishijima, K.I.; Kitamura, Y.; Arano, Y.; Kuge, Y.; Ohkura, K. Redesign of negatively charged (111)In-DTPA-octreotide derivative to reduce renal radioactivity. Nucl. Med. Biol. 2017, 48, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yang, J.; Gallazzi, F.; Miao, Y. Effects of the amino acid linkers on the melanoma-targeting and pharmacokinetic properties of 111In-labeled lactam bridge-cyclized alpha-MSH peptides. J. Nucl. Med. 2011, 52, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Fisher, D.R.; Quinn, T.P. Reducing renal uptake of 90Y- and 177Lu-labeled alpha-melanocyte stimulating hormone peptide analogues. Nucl. Med. Biol. 2006, 33, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Gallazzi, F.; Guo, H.; Quinn, T.P. 111In-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide analogues for melanoma imaging. Bioconjug. Chem. 2008, 19, 539–547. [Google Scholar] [CrossRef][Green Version]
- Bapst, J.P.; Eberle, A.N. Receptor-Mediated Melanoma Targeting with Radiolabeled alpha-Melanocyte-Stimulating Hormone: Relevance of the Net Charge of the Ligand. Front. Endocrinol. 2017, 8, 93. [Google Scholar] [CrossRef]
- Jia, B.; Liu, Z.; Shi, J.; Yu, Z.; Yang, Z.; Zhao, H.; He, Z.; Liu, S.; Wang, F. Linker effects on biological properties of 111In-labeled DTPA conjugates of a cyclic RGDfK dimer. Bioconjug. Chem. 2008, 19, 201–210. [Google Scholar] [CrossRef][Green Version]
- Dijkgraaf, I.; Liu, S.; Kruijtzer, J.A.; Soede, A.C.; Oyen, W.J.; Liskamp, R.M.; Corstens, F.H.; Boerman, O.C. Effects of linker variation on the in vitro and in vivo characteristics of an 111In-labeled RGD peptide. Nucl. Med. Biol. 2007, 34, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Mather, S.J.; McKenzie, A.J.; Sosabowski, J.K.; Morris, T.M.; Ellison, D.; Watson, S.A. Selection of radiolabeled gastrin analogs for peptide receptor-targeted radionuclide therapy. J. Nucl. Med. 2007, 48, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Baranski, A.C.; Schafer, M.; Bauder-Wust, U.; Wacker, A.; Schmidt, J.; Liolios, C.; Mier, W.; Haberkorn, U.; Eisenhut, M.; Kopka, K.; et al. Improving the Imaging Contrast of (68)Ga-PSMA-11 by Targeted Linker Design: Charged Spacer Moieties Enhance the Pharmacokinetic Properties. Bioconjug. Chem. 2017, 28, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Liolios, C.; Schafer, M.; Haberkorn, U.; Eder, M.; Kopka, K. Novel Bispecific PSMA/GRPr Targeting Radioligands with Optimized Pharmacokinetics for Improved PET Imaging of Prostate Cancer. Bioconjug. Chem. 2016, 27, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Z.; Lin, K.S.; Pan, J.; Dude, I.; Hundal-Jabal, N.; Colpo, N.; Benard, F. Preclinical Melanoma Imaging with (68)Ga-Labeled alpha-Melanocyte-Stimulating Hormone Derivatives Using PET. Theranostics 2017, 7, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Laverman, P.; Joosten, L.; Eek, A.; Roosenburg, S.; Peitl, P.K.; Maina, T.; Macke, H.; Aloj, L.; von Guggenberg, E.; Sosabowski, J.K.; et al. Comparative biodistribution of 12 (1)(1)(1)In-labelled gastrin/CCK2 receptor-targeting peptides. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1410–1416. [Google Scholar] [CrossRef]
- Kuo, H.T.; Zhang, Z.; Merkens, H.; Zhang, C.; Lin, K.S.; Benard, F. Effects of replacing Glu in the PSMA-targeting Lys-urea-Glu pharmacophore of [68Ga]Ga-HTK03041 with a close derivative on the uptake of tumor xenograft, kidneys and salivary glands. J. Nucl. Med. 2022, 63 (Suppl. S2). [Google Scholar]
- Kuo, H.T.; Pan, J.; Zhang, Z.; Lau, J.; Merkens, H.; Zhang, C.; Colpo, N.; Lin, K.S.; Benard, F. Effects of Linker Modification on Tumor-to-Kidney Contrast of (68)Ga-Labeled PSMA-Targeted Imaging Probes. Mol. Pharm. 2018, 15, 3502–3511. [Google Scholar] [CrossRef]
- Garrison, J.C.; Rold, T.L.; Sieckman, G.L.; Naz, F.; Sublett, S.V.; Figueroa, S.D.; Volkert, W.A.; Hoffman, T.J. Evaluation of the pharmacokinetic effects of various linking group using the 111In-DOTA-X-BBN(7-14)NH2 structural paradigm in a prostate cancer model. Bioconjug. Chem. 2008, 19, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, I.; Tsvetanov, C.B. Oligomeric Poly(ethylene oxide)s. Functionalized Poly(ethylene glycol)s. PEGylation. In Polymer Science: A Comprehensive Reference; Elsevier B.V.: Amsterdam, The Netherlands, 2012; pp. 679–693. [Google Scholar]
- Chen, X.; Sievers, E.; Hou, Y.; Park, R.; Tohme, M.; Bart, R.; Bremner, R.; Bading, J.R.; Conti, P.S. Integrin alpha v beta 3-targeted imaging of lung cancer. Neoplasia 2005, 7, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hou, Y.; Tohme, M.; Park, R.; Khankaldyyan, V.; Gonzales-Gomez, I.; Bading, J.R.; Laug, W.E.; Conti, P.S. Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor alphavbeta3-integrin expression. J. Nucl. Med. 2004, 45, 1776–1783. [Google Scholar] [PubMed]
- Rogers, B.E.; Manna, D.D.; Safavy, A. In vitro and in vivo evaluation of a 64Cu-labeled polyethylene glycol-bombesin conjugate. Cancer Biother. Radiopharm. 2004, 19, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, Z.; Rosenstrom, U.; Velikyan, I.; Mitran, B.; Altai, M.; Honarvar, H.; Rosestedt, M.; Lindeberg, G.; Sorensen, J.; Larhed, M.; et al. The effect of mini-PEG-based spacer length on binding and pharmacokinetic properties of a 68Ga-labeled NOTA-conjugated antagonistic analog of bombesin. Molecules 2014, 19, 10455–10472. [Google Scholar] [CrossRef] [PubMed]
- Jamous, M.; Tamma, M.L.; Gourni, E.; Waser, B.; Reubi, J.C.; Maecke, H.R.; Mansi, R. PEG spacers of different length influence the biological profile of bombesin-based radiolabeled antagonists. Nucl. Med. Biol. 2014, 41, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Czerwinski, A.; Chakravarty, R.; Graves, S.A.; Yang, Y.; England, C.G.; Nickles, R.J.; Valenzuela, F.; Cai, W. Evaluation of two novel (6)(4)Cu-labeled RGD peptide radiotracers for enhanced PET imaging of tumor integrin alphavbeta(3). Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1859–1868. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haubner, R.; Decristoforo, C. Radiolabelled RGD peptides and peptidomimetics for tumour targeting. Front. Biosci. 2009, 14, 872–886. [Google Scholar] [CrossRef]
- Hoffman, T.J.; Hariprasad Gali, C.; Smith, J.; Sieckman, G.L.; Hayes, D.L.; Owen, N.K.; Volkert, W.A. Novel Series of 111In-Labeled Bombesin Analogs as Potential Radiopharmaceuticals for Specific Targeting of Gastrin-Releasing Peptide Receptors Expressed on Human Prostate Cancer Cells. J. Nucl. Med. 2003, 44, 823–831. [Google Scholar]
- Weber, R.W.; Boutin, R.H.; Nedelman, M.A.; Lister-James, J.; Dean, R.T. Enhanced kidney clearance with an ester-linked 99mTc-radiolabeled antibody Fab’-chelator conjugate. Bioconjug. Chem. 1990, 1, 431–437. [Google Scholar] [CrossRef]
- Tibben, J.G.; Massuger, L.F.; Boerman, O.C.; Claessens, R.A.; Borm, G.F.; Pak, K.Y.; Koenders, E.B.; Corstens, F.H. Decreased kidney uptake of technetium-99m-labelled Fab’ fragments in ovarian carcinoma bearing nude mice using a cleavable chelator. Nucl. Med. Biol. 1994, 21, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bridger, G.J.; Abrams, M.J.; Padmanabhan, S.; Gaul, F.; Larsen, S.; Henson, G.W.; Schwartz, D.A.; Longley, C.B.; Burton, C.A.; Ultee, M.E. A comparison of cleavable and noncleavable hydrazinopyridine linkers for the 99mTc labeling of Fab’ monoclonal antibody fragments. Bioconjug. Chem. 1996, 7, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Skidgel, R.A. Basic carboxypeptidases: Regulators of peptide hormone activity. Trends Pharmacol. Sci. 1988, 9, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Arano, Y.; Fujioka, Y.; Akizawa, H.; Ono, M.; Uehara, T.; Wakisaka, K.; Nakayama, M.; Sakahara, H.; Konishi, J.; Saji, H. Chemical design of radiolabeled antibody fragments for low renal radioactivity levels. Cancer Res. 1999, 59, 128–134. [Google Scholar] [PubMed]
- Fujioka, Y.; Satake, S.; Uehara, T.; Mukai, T.; Akizawa, H.; Ogawa, K.; Saji, H.; Endo, K.; Arano, Y. In vitro system to estimate renal brush border enzyme-mediated cleavage of Peptide linkages for designing radiolabeled antibody fragments of low renal radioactivity levels. Bioconjug. Chem. 2005, 16, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Akizawa, H.; Imajima, M.; Hanaoka, H.; Uehara, T.; Satake, S.; Arano, Y. Renal brush border enzyme-cleavable linkages for low renal radioactivity levels of radiolabeled antibody fragments. Bioconjug. Chem. 2013, 24, 291–299. [Google Scholar] [CrossRef]
- Yim, C.B.; Mikkola, K.; Fagerholm, V.; Elomaa, V.V.; Ishizu, T.; Rajander, J.; Schlesinger, J.; Roivainen, A.; Nuutila, P.; Solin, O. Synthesis and preclinical characterization of [64Cu]NODAGA-MAL-exendin-4 with a Nepsilon-maleoyl-L-lysyl-glycine linkage. Nucl. Med. Biol. 2013, 40, 1006–1012. [Google Scholar] [CrossRef]
- Sterchi, E.E.; Stocker, W.; Bond, J.S. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol. Aspects Med. 2008, 29, 309–328. [Google Scholar] [CrossRef]
- Jodal, A.; Pape, F.; Becker-Pauly, C.; Maas, O.; Schibli, R.; Behe, M. Evaluation of (1)(1)(1)in-labelled exendin-4 derivatives containing different meprin beta-specific cleavable linkers. PLoS ONE 2015, 10, e0123443. [Google Scholar] [CrossRef]
- Kanazawa, M.; Johnston, C.I. Distribution and inhibition of neutral metalloendopeptidase (NEP) (EC 3.4.24.11), the major degradative enzyme for atrial natriuretic peptide, in the rat kidney. Clin. Exp. Pharmacol. Physiol. 1991, 18, 449–453. [Google Scholar] [CrossRef]
- Uehara, T.; Rokugawa, T.; Kinoshita, M.; Nemoto, S.; Fransisco Lazaro, G.G.; Hanaoka, H.; Arano, Y. (67/68)Ga-labeling agent that liberates (67/68)Ga-NOTA-methionine by lysosomal proteolysis of parental low molecular weight polypeptides to reduce renal radioactivity levels. Bioconjug. Chem. 2014, 25, 2038–2045. [Google Scholar] [CrossRef]
- Uehara, T.; Yokoyama, M.; Suzuki, H.; Hanaoka, H.; Arano, Y. A Gallium-67/68-Labeled Antibody Fragment for Immuno-SPECT/PET Shows Low Renal Radioactivity Without Loss of Tumor Uptake. Clin. Cancer Res. 2018, 24, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Kanazawa, N.; Suzuki, C.; Mizuno, Y.; Suzuki, H.; Hanaoka, H.; Arano, Y. Renal Handling of (99m)Tc-Labeled Antibody Fab Fragments with a Linkage Cleavable by Enzymes on Brush Border Membrane. Bioconjug. Chem. 2020, 31, 2618–2627. [Google Scholar] [CrossRef]
- Suzuki, C.; Uehara, T.; Kanazawa, N.; Wada, S.; Suzuki, H.; Arano, Y. Preferential Cleavage of a Tripeptide Linkage by Enzymes on Renal Brush Border Membrane To Reduce Renal Radioactivity Levels of Radiolabeled Antibody Fragments. J. Med. Chem. 2018, 61, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; Kang, C.M.; McDougald, D.; Minn, I.; Brummet, M.; Pomper, M.G.; Zalutsky, M.R. Brush border enzyme-cleavable linkers: Evaluation for reducing renal uptake of radiolabeled prostate-specific membrane antigen inhibitors. Nucl. Med. Biol. 2018, 62–63, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Devoogdt, N.; Zalutsky, M.R.; Vaidyanathan, G. An Efficient Method for Labeling Single Domain Antibody Fragments with (18)F Using Tetrazine- Trans-Cyclooctene Ligation and a Renal Brush Border Enzyme-Cleavable Linker. Bioconjug. Chem. 2018, 29, 4090–4103. [Google Scholar] [CrossRef]
- Zhou, Z.; Zalutsky, M.R.; Vaidyanathan, G. Labeling a TCO-functionalized single domain antibody fragment with (18)F via inverse electron demand Diels Alder cycloaddition using a fluoronicotinyl moiety-bearing tetrazine derivative. Bioorg. Med. Chem. 2020, 28, 115634. [Google Scholar] [CrossRef]
- Christ, E.; Antwi, K.; Fani, M.; Wild, D. Innovative imaging of insulinoma: The end of sampling? A review. Endocr. Relat. Cancer 2020, 27, R79–R92. [Google Scholar] [CrossRef]
- Boss, M.; Rottenburger, C.; Brenner, W.; Blankenstein, O.; Prasad, V.; Prasad, S.; Coppi, P.; Kuhnen, P.; Buitinga, M.; Nuutila, P.; et al. (68)Ga-NODAGA-Exendin-4 PET/CT Improves the Detection of Focal Congenital Hyperinsulinism. J. Nucl. Med. 2022, 63, 310–315. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, J.; Xie, Z.; Yan, Y.; Wang, J.; Chen, X. Optimization of Enzymolysis Clearance Strategy To Enhance Renal Clearance of Radioligands. Bioconjug. Chem. 2021, 32, 2108–2116. [Google Scholar] [CrossRef]
- Valpreda, G.; Trachsel, B.; Vogel, V.; Schibli, R.; Mu, L.; Behe, M. Dual MVK cleavable linkers effectively reduce renal retention of (111)In-fibronectin-binding peptides. Bioorg. Med. Chem. 2022, 73, 117040. [Google Scholar] [CrossRef]
- Arano, Y. Renal brush border strategy: A developing procedure to reduce renal radioactivity levels of radiolabeled polypeptides. Nucl. Med. Biol. 2021, 92, 149–155. [Google Scholar] [CrossRef]
- Sand, K.M.; Bern, M.; Nilsen, J.; Noordzij, H.T.; Sandlie, I.; Andersen, J.T. Unraveling the Interaction between FcRn and Albumin: Opportunities for Design of Albumin-Based Therapeutics. Front. Immunol. 2014, 5, 682. [Google Scholar] [CrossRef] [PubMed]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery--new applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Struthers, H.; Winiger, C.; Zhernosekov, K.; Schibli, R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J. Nucl. Med. 2013, 54, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Trussel, S.; Dumelin, C.; Frey, K.; Villa, A.; Buller, F.; Neri, D. New strategy for the extension of the serum half-life of antibody fragments. Bioconjug. Chem. 2009, 20, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Reber, J.; Brandt, S.; Bernhardt, P.; Groehn, V.; Schibli, R.; Muller, C. Folate receptor-targeted radionuclide therapy: Preclinical investigation of anti-tumor effects and potential radionephropathy. Nucl. Med. Biol. 2015, 42, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Kaeppeli SA, M.; Jodal, A.; Gotthardt, M.; Schibli, R.; Behe, M. Exendin-4 Derivatives with an Albumin-Binding Moiety Show Decreased Renal Retention and Improved GLP-1 Receptor Targeting. Mol. Pharm. 2019, 16, 3760–3769. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, E.; Lau, J.; Zhang, Z.; Uribe, C.F.; Kuo, H.T.; Zhang, C.; Zeisler, J.; Colpo, N.; Lin, K.S.; Benard, F. Effects of adding an albumin binder chain on [(177)Lu]Lu-DOTATATE. Nucl. Med. Biol. 2018, 66, 10–17. [Google Scholar] [CrossRef]
- Choy, C.J.; Ling, X.; Geruntho, J.J.; Beyer, S.K.; Latoche, J.D.; Langton-Webster, B.; Anderson, C.J.; Berkman, C.E. (177)Lu-Labeled Phosphoramidate-Based PSMA Inhibitors: The Effect of an Albumin Binder on Biodistribution and Therapeutic Efficacy in Prostate Tumor-Bearing Mice. Theranostics 2017, 7, 1928–1939. [Google Scholar] [CrossRef]
- Ling, X.; Latoche, J.D.; Choy, C.J.; Kurland, B.F.; Laymon, C.M.; Wu, Y.; Salamacha, N.; Shen, D.; Geruntho, J.J.; Rigatti, L.H.; et al. Preclinical Dosimetry, Imaging, and Targeted Radionuclide Therapy Studies of Lu-177-Labeled Albumin-Binding, PSMA-Targeted CTT1403. Mol. Imaging Biol. 2020, 22, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Benesova, M.; Umbricht, C.A.; Schibli, R.; Muller, C. Albumin-Binding PSMA Ligands: Optimization of the Tissue Distribution Profile. Mol. Pharm. 2018, 15, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.T.; Merkens, H.; Zhang, Z.; Uribe, C.F.; Lau, J.; Zhang, C.; Colpo, N.; Lin, K.S.; Benard, F. Enhancing Treatment Efficacy of (177)Lu-PSMA-617 with the Conjugation of an Albumin-Binding Motif: Preclinical Dosimetry and Endoradiotherapy Studies. Mol. Pharm. 2018, 15, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Nikolopoulou, A.; Wustemann, T.; Barelli, P.; Kim, D.; Williams, C.; Jr Zheng, X.; Bi, C.; Hu, B.; et al. Dual-Target Binding Ligands with Modulated Pharmacokinetics for Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2017, 58, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C.; Jr Schlyer, D.; Zhao, Y.; Kim, D.; Babich, J.W. Trifunctional PSMA-targeting constructs for prostate cancer with unprecedented localization to LNCaP tumors. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C.; Jr DiMagno, S.G.; Babich, J.W. Albumin-Binding PSMA Ligands: Implications for Expanding the Therapeutic Window. J. Nucl. Med. 2019, 60, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C., Jr.; Thiele, N.A.; Schlyer, D.; Wilson, J.J.; DiMagno, S.G.; Babich, J.W. A Single Dose of (225)Ac-RPS-074 Induces a Complete Tumor Response in an LNCaP Xenograft Model. J. Nucl. Med. 2019, 60, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted alpha-Therapy of Metastatic Castration-Resistant Prostate Cancer with (225)Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef]
- Benesova, M.; Guzik, P.; Deberle, L.M.; Busslinger, S.D.; Landolt, T.; Schibli, R.; Muller, C. Design and Evaluation of Novel Albumin-Binding Folate Radioconjugates: Systematic Approach of Varying the Linker Entities. Mol. Pharm. 2022, 19, 963–973. [Google Scholar] [CrossRef]
- Deberle, L.M.; Tschan, V.J.; Borgna, F.; Sozzi-Guo, F.; Bernhardt, P.; Schibli, R.; Muller, C. Albumin-Binding PSMA Radioligands: Impact of Minimal Structural Changes on the Tissue Distribution Profile. Molecules 2020, 25, 2542. [Google Scholar] [CrossRef]
- Iikuni, S.; Okada, Y.; Shimizu, Y.; Watanabe, H.; Ono, M. Modulation of the Pharmacokinetics of a Radioligand Targeting Carbonic Anhydrase-IX with Albumin-Binding Moieties. Mol. Pharm. 2021, 18, 966–975. [Google Scholar] [CrossRef]
- Siwowska, K.; Haller, S.; Bortoli, F.; Benesova, M.; Groehn, V.; Bernhardt, P.; Schibli, R.; Muller, C. Preclinical Comparison of Albumin-Binding Radiofolates: Impact of Linker Entities on the in Vitro and in Vivo Properties. Mol. Pharm. 2017, 14, 523–532. [Google Scholar] [CrossRef]
- Reissig, F.; Zarschler, K.; Novy, Z.; Petrik, M.; Bendova, K.; Kurfurstova, D.; Bouchal, J.; Ludik, M.C.; Brandt, F.; Kopka, K.; et al. Modulating the pharmacokinetic profile of Actinium-225-labeled macropa-derived radioconjugates by dual targeting of PSMA and albumin. Theranostics 2022, 12, 7203–7215. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, C.A.; Benesova, M.; Schibli, R.; Muller, C. Preclinical Development of Novel PSMA-Targeting Radioligands: Modulation of Albumin-Binding Properties To Improve Prostate Cancer Therapy. Mol. Pharm. 2018, 15, 2297–2306. [Google Scholar] [CrossRef]
- Kramer, V.; Fernandez, R.; Lehnert, W.; Jimenez-Franco, L.D.; Soza-Ried, C.; Eppard, E.; Ceballos, M.; Meckel, M.; Benesova, M.; Umbricht, C.A.; et al. Biodistribution and dosimetry of a single dose of albumin-binding ligand [(177)Lu]Lu-PSMA-ALB-56 in patients with mCRPC. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 893–903. [Google Scholar] [CrossRef]
- Deberle, L.M.; Benesova, M.; Umbricht, C.A.; Borgna, F.; Buchler, M.; Zhernosekov, K.; Schibli, R.; Muller, C. Development of a new class of PSMA radioligands comprising ibuprofen as an albumin-binding entity. Theranostics 2020, 10, 1678–1693. [Google Scholar] [CrossRef]
- Tschan, V.J.; Borgna, F.; Busslinger, S.D.; Stirn, M.; Rodriguez JM, M.; Bernhardt, P.; Schibli, R.; Muller, C. Preclinical investigations using [(177)Lu]Lu-Ibu-DAB-PSMA toward its clinical translation for radioligand therapy of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3639–3650. [Google Scholar] [CrossRef]
- Borgna, F.; Deberle, L.M.; Busslinger, S.D.; Tschan, V.J.; Walde, L.M.; Becker, A.E.; Schibli, R.; Muller, C. Preclinical Investigations to Explore the Difference between the Diastereomers [(177)Lu]Lu-SibuDAB and [(177)Lu]Lu-RibuDAB toward Prostate Cancer Therapy. Mol. Pharm. 2022, 19, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Busslinger, S.D.; Tschan, V.J.; Richard, O.K.; Talip, Z.; Schibli, R.; Muller, C. [(225)Ac]Ac-SibuDAB for Targeted Alpha Therapy of Prostate Cancer: Preclinical Evaluation and Comparison with [(225)Ac]Ac-PSMA-617. Cancers 2022, 14, 5651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tian, R.; Niu, G.; Ma, Y.; Lang, L.; Szajek, L.P.; Kiesewetter, D.O.; Jacobson, O.; Chen, X. Single Low-Dose Injection of Evans Blue Modified PSMA-617 Radioligand Therapy Eliminates Prostate-Specific Membrane Antigen Positive Tumors. Bioconjug. Chem. 2018, 29, 3213–3221. [Google Scholar] [CrossRef]
- Zang, J.; Fan, X.; Wang, H.; Liu, Q.; Wang, J.; Li, H.; Li, F.; Jacobson, O.; Niu, G.; Zhu, Z.; et al. First-in-human study of (177)Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 148–158. [Google Scholar] [CrossRef]
- Bandara, N.; Jacobson, O.; Mpoy, C.; Chen, X.; Rogers, B.E. Novel Structural Modification Based on Evans Blue Dye to Improve Pharmacokinetics of a Somastostatin-Receptor-Based Theranostic Agent. Bioconjug. Chem. 2018, 29, 2448–2454. [Google Scholar] [CrossRef]
- Tian, R.; Jacobson, O.; Niu, G.; Kiesewetter, D.O.; Wang, Z.; Zhu, G.; Ma, Y.; Liu, G.; Chen, X. Evans Blue Attachment Enhances Somatostatin Receptor Subtype-2 Imaging and Radiotherapy. Theranostics 2018, 8, 735–745. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Jacobson, O.; Cheng, Y.; Niu, G.; Li, F.; Bai, C.; Zhu, Z.; Chen, X. Safety, Pharmacokinetics, and Dosimetry of a Long-Acting Radiolabeled Somatostatin Analog (177)Lu-DOTA-EB-TATE in Patients with Advanced Metastatic Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 1699–1705. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Y.; Zhang, J.; Zang, J.; Li, H.; Liu, Q.; Wang, J.; Jacobson, O.; Li, F.; Zhu, Z.; et al. Response to Single Low-dose (177)Lu-DOTA-EB-TATE Treatment in Patients with Advanced Neuroendocrine Neoplasm: A Prospective Pilot Study. Theranostics 2018, 8, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xu, P.; Shi, M.; Liu, J.; Zeng, X.; Zhang, Y.; Shi, C.; Li, J.; Guo, Z.; Zhang, X.; et al. Evans blue-modified radiolabeled fibroblast activation protein inhibitor as long-acting cancer therapeutics. Theranostics 2022, 12, 422–433. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, P.; Ding, J.; Chen, J.; Huo, L.; Liu, Z. Albumin Binder-Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy. J. Nucl. Med. 2022, 63, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Jacobson, O.; Niu, G.; Lin, K.S.; Benard, F.; Chen, X. Bench to Bedside: Albumin Binders for Improved Cancer Radioligand Therapies. Bioconjug. Chem. 2019, 30, 487–502. [Google Scholar] [CrossRef]
- Rottenburger, C.; Nicolas, G.P.; McDougall, L.; Kaul, F.; Cachovan, M.; Vija, A.H.; Schibli, R.; Geistlich, S.; Schumann, A.; Rau, T.; et al. Cholecystokinin 2 Receptor Agonist (177)Lu-PP-F11N for Radionuclide Therapy of Medullary Thyroid Carcinoma: Results of the Lumed Phase 0a Study. J. Nucl. Med. 2020, 61, 520–526. [Google Scholar] [CrossRef] [PubMed]



| Sequence | One Letter Code | Enzyme |
|---|---|---|
| Gly-Lys | GK | Carboxypeptidase M |
| Met-Val-Lys | MVK | Neutral endopeptidase (NEP) |
| Gly-Phe-Lys | GFK | Neutral endopeptidase (NEP) |
| Gly-Tyr | GY | Carboxypeptidase M |
| Strategy | Advantages | Limitations and Disadvantages | Development Phase |
|---|---|---|---|
| Use of non-residualizing radionuclides |
|
| Clinical trials |
| Co-administration with competitive inhibitors |
|
| Applied in the clinic |
| Adaptation of the chelator or linker design on molecular level |
|
| Applied in the clinic |
| Cleavable linkers |
|
| Preclinical development |
| Albumin binding |
|
| Clinical trials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Roode, K.E.; Joosten, L.; Behe, M. Towards the Magic Radioactive Bullet: Improving Targeted Radionuclide Therapy by Reducing the Renal Retention of Radioligands. Pharmaceuticals 2024, 17, 256. https://doi.org/10.3390/ph17020256
de Roode KE, Joosten L, Behe M. Towards the Magic Radioactive Bullet: Improving Targeted Radionuclide Therapy by Reducing the Renal Retention of Radioligands. Pharmaceuticals. 2024; 17(2):256. https://doi.org/10.3390/ph17020256
Chicago/Turabian Stylede Roode, Kim E., Lieke Joosten, and Martin Behe. 2024. "Towards the Magic Radioactive Bullet: Improving Targeted Radionuclide Therapy by Reducing the Renal Retention of Radioligands" Pharmaceuticals 17, no. 2: 256. https://doi.org/10.3390/ph17020256
APA Stylede Roode, K. E., Joosten, L., & Behe, M. (2024). Towards the Magic Radioactive Bullet: Improving Targeted Radionuclide Therapy by Reducing the Renal Retention of Radioligands. Pharmaceuticals, 17(2), 256. https://doi.org/10.3390/ph17020256






